ABSTRACT
Giardia lamblia is an important and ubiquitous cause of diarrheal disease. The primary agents in the treatment of giardiasis are nitroheterocyclic drugs, particularly the imidazoles metronidazole and tinidazole and the thiazole nitazoxanide. Although these drugs are generally effective, treatment failures occur in up to 20% of cases, and resistance has been demonstrated in vivo and in vitro. Prior work had suggested that side chain modifications of the imidazole core can lead to new effective 5-nitroimidazole drugs that can combat nitro drug resistance, but the full potential of nitroheterocycles other than imidazole to yield effective new antigiardial agents has not been explored. Here, we generated derivatives of two clinically utilized nitroheterocycles, nitrothiazole and nitrofuran, as well as a third heterocycle, nitropyrrole, which is related to nitroimidazole but has not been systematically investigated as an antimicrobial drug scaffold. Click chemistry was employed to synthesize 442 novel nitroheterocyclic compounds with extensive side chain modifications. Screening of this library against representative G. lamblia strains showed a wide spectrum of in vitro activities, with many of the compounds exhibiting superior activity relative to reference drugs and several showing >100-fold increase in potency and the ability to overcome existing forms of metronidazole resistance. The majority of new compounds displayed no cytotoxicity against human cells, and several compounds were orally active against murine giardiasis in vivo. These findings provide additional impetus for the systematic development of nitroheterocyclic compounds with nonimidazole cores as alternative and improved agents for the treatment of giardiasis and potentially other infectious agents.
KEYWORDS: antimicrobial agents, drug screening
INTRODUCTION
Giardia lamblia is an important and ubiquitous cause of diarrheal disease, infecting an estimated 5 to 10% of the world's population (1). In the United States, G. lamblia is one of the most common causes of waterborne diarrheal disease, with international travelers, hikers, and children in day care centers at particular risk (2). Infection with this anaerobic protozoan parasite is by the fecal-oral route through ingestion of infectious cysts in contaminated water or occasionally food. Following excystation, flagellated and motile trophozoites colonize mostly the lumen and epithelial surface of the upper small intestine. Infection can lead to villus atrophy, loss of brush border microvilli, digestive enzyme deficiencies, and epithelial barrier dysfunction (3). The duration of giardial infection is variable, but it is often protracted over weeks or even months. Symptomatic infection is characterized by diarrhea, malabsorption, dehydration, abdominal pain, and weight loss. In children, failure to thrive and cognitive impairment can ensue, while adults can develop postinfectious irritable bowel syndrome and chronic fatigue (4).
The leading antigiardial agent, metronidazole, is a synthetic 5-nitroimidazole derivative introduced in the 1950s. In addition to Giardia, metronidazole is active against Entamoeba histolytica and Trichomonas vaginalis, as well as several clinically important bacterial pathogens, including Clostridium difficile and Helicobacter pylori, making it a highly versatile antibiotic. Metronidazole is generally well tolerated, although it can have unpleasant adverse effects, such as a metallic taste in the mouth (5–7). Based on the therapeutic utility of metronidazole, several other 5-nitroimidazoles have been developed over the years. For example, tinidazole is a position 1 derivative of 5-nitroimidazole and is effective against giardiasis (8). Not all antigiardial drugs are based on the 5-nitroimidazole scaffold. Nitazoxanide belongs to an emerging class of nitrothiazole compounds with potent antigiardial activity (8, 9), and derivatives exhibit improved activity against other metronidazole-sensitive pathogens, including C. difficile (10). Other, structurally unrelated antigiardial agents exist, such as albendazole and quinacrine, which do not possess the characteristic 5-nitro functional group of metronidazole and nitazoxanide, but overall, nitro drugs have remained the most widely utilized drugs for treating giardiasis (9).
Although nitro drugs are generally effective against giardiasis, treatment failures occur in up to 20% of cases (11, 12). Clinical resistance of G. lamblia to existing nitro drugs has been proven (13), and in vitro resistance can be induced so resistant lines grow in concentrations similar to those found in sera from effectively treated patients (14, 15). In addition, resistance has been induced in vitro against all commonly used antigiardial drugs, further underlining the need for new compounds to stay ahead of the parasite's ability to develop resistance. The mechanisms of resistance are not fully understood, but they appear to be diverse. In sensitive cells, the nitro prodrug is first reduced to toxic free radical intermediates by low redox potential reactions present only in anaerobic pathogens (6). These short-lived free radicals cause lethal damage to the pathogen by inactivating critical macromolecules. Resistance is generally associated with diminished nitro prodrug activation by one or several electron-donating pathways, including pyruvate:ferredoxin oxidoreductase and ferredoxin (16), nitroreductases (17, 18), and flavin-dependent thioredoxin reductase (19), but the relative importance of specific pathways may depend on the particular form of drug resistance (12, 20). This apparent variability in nitro drug resistance mechanisms has the potential to be exploited in the development of alternative 5-nitroheterocyclic drugs that can combat different forms of drug resistance (21–23).
Prior reports suggested that extensive modifications at position 1 or 2 of the imidazole ring can lead to effective new 5-nitroimidazole compounds that can partially or even completely overcome nitro drug resistance (21, 22, 24). On the basis of these promising findings, we set out in the present study to explore the potential of structural modifications of the side chains of nitroheterocycles other than imidazole to yield effective new antigiardial agents.
RESULTS
Click chemistry facilitated structural diversification of nitrothiazole, nitrofuran, and nitropyrrole.
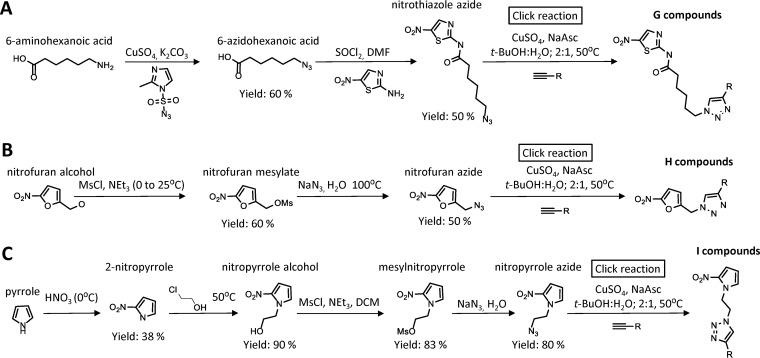
Imidazole constitutes the core of several widely used antimicrobial nitro drugs, including metronidazole and tinidazole, but other heterocyclic cores, most notably thiazole in nitazoxanide and furan in furazolidone, are present in clinically effective nitroheterocyclic compounds (25, 26). To determine whether broad structural diversification can improve the activity of nitroheterocyclic compounds other than nitroimidazole, we generated derivatives of the two nitroheterocycles, nitrothiazole and nitrofuran, as well as a third heterocycle, nitropyrrole, which is structurally related to nitroimidazole but has not been systematically explored as an antimicrobial drug scaffold. As a synthetic strategy, we utilized the copper(I)-catalyzed azide alkyne cycloaddition (“click reaction”), in which an azide and an alkyne are joined to yield a 1,4-substituted 1,2,3-triazole (27). As a first step, azido derivatives of the three heterocycles were synthesized by conventional approaches (Fig. 1). A library of ∼150 structurally diverse alkynes, which are shown in Table S1 in the supplemental material, was then assembled by requisition of commercially available compounds or de novo synthesis as described before (24). Almost all of the possible combinations of azides and alkynes were reacted by click chemistry to yield a total of 442 novel nitroheterocyclic triazoles in the three compound groups (Fig. 1). This compound library was subsequently screened for antimicrobial activity. We found in preliminary experiments that crude reaction mixtures could be used in the screens, because they showed antigiardial activities similar to those of selected purified compounds.
FIG 1.
Synthetic strategy. Azido derivatives of the nitroheterocycles, nitrothiazole (A), nitrofuran (B), and nitropyrrole (C) were synthesized following the depicted schemes. The azides were then reacted with one of ∼150 alkynes (see Table S1 in the supplemental material) utilizing the copper(I)-catalyzed azide alkyne cycloaddition (“click reaction”) to yield 1,4-substituted 1,2,3-triazoles. DMF, dimethylformamide; NaAsc, sodium ascorbate; MsCl, methanesulfonyl chloride; t-BuOH, tert-butyl alcohol; NEt3, triethylamine; DCM, dichloromethane.
Enhanced activity of modified nitroheterocycles against nitro drug-susceptible G. lamblia strains.
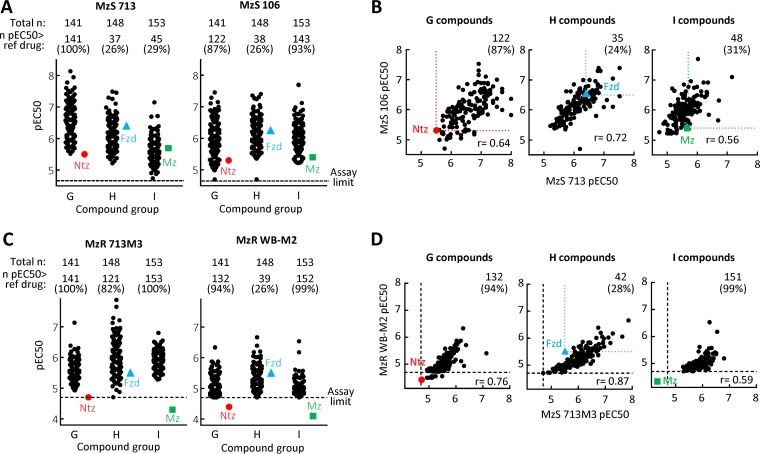
For activity testing, we employed different strains of G. lamblia, which is generally susceptible to nitro drugs, including metronidazole and nitazoxanide (28). Screening of the 442-compound library against two representative G. lamblia strains, BRIS/87/HEPU/713 (713) and BRIS/83/HEPU/106 (106), in a 48-h growth and survival assay showed a wide spectrum of activities, covering a >100-fold range of 50% effective concentrations (EC50s) (Fig. 2A and Tables S2 to S4). Almost all compounds exhibited significant antigiardial activity at 20 μM or less against both G. lamblia strains, and a substantial, albeit variable, proportion (average, 60%; range, 26 to 100%) had superior activity relative to their respective reference drugs, i.e., nitazoxanide for the nitrothiazoles (G compounds), furazolidone for the nitrofurans (H compounds), and metronidazole for the nitropyrroles (I compounds). The most active new compounds showed EC50 values of <10 nM (i.e., negative log10 of EC50 expressed in moles per liter [pEC50] of >8), which exceeded the potency of the reference drugs by >200-fold (Fig. 2A). The activities against the two G. lamblia strains showed generally good correlations for all three compound groups (correlation coefficients [r] of 0.56 to 0.72), although several compounds were found in each group that were >100-fold more active in one strain over the other strain (Fig. 2B), underlining that testing more than one strain of the target microbe is important for selecting the most promising compounds for further drug development.
FIG 2.
In vitro antigiardial activity of modified nitroheterocycles. (A to D) The activities of 442 newly synthesized nitroheterocyclic compounds were assayed against two metronidazole-sensitive (MzS) G. lamblia strains (713 and 106 [A and B]) and two metronidazole-resistant (MzR) G. lamblia strains (713M3 and WB-M2 [C and D]) in a 48-h growth and survival assay with ATP as a read-out. Panels A and C show activities separately against the indicated G. lamblia line, while panels B and D show the correlations between the two MzS strains and two MzR strains, respectively. Data are shown as means of the pEC50 values obtained in at least three independent experiments, with each point representing a single compound. Compounds are divided into groups with the same nitroheterocyclic cores: group G, nitrothiazoles; group H, nitrofurans; group I, nitropyrroles (see Fig. 1). The respective reference drugs are nitazoxanide (Ntz) (red circle), furazolidone (Fzd) (blue triangle), and metronidazole (Mz) (green square). The colored dotted lines in panels B and D show the activity of the respective reference compounds. The black dashed lines represent the assay sensitivity.
Modified nitroheterocycles can overcome existing nitro drug resistance in G. lamblia.
Resistance to the common clinical nitroheterocyclic drugs has been reported for G. lamblia in vitro and in vivo (12, 13, 20, 29), yet it has also been demonstrated that specific nitroimidazole derivatives can overcome existing forms of resistance to different degrees (22, 24), suggesting that resistance is relative and not a universal phenotype that equally impacts all nitroheterocyclic compounds. To evaluate the activity of the new nitroheterocyclic compounds against nitro drug-resistant G. lamblia, we tested two independently derived lines, 713M3 and WB-M2, which exhibit stable resistance in form of a 5- to 20-fold increase in EC50 compared to susceptible lines against the three reference drugs, nitazoxanide, furazolidone, and metronidazole (28) (Fig. 2C and D and Tables S2 to S4). Most of the new nitro compounds displayed excellent activity against both resistant G. lamblia strains, with 94% and 73% of all 442 compounds exceeding the activity of the respective reference drugs in the 713M3 and WB-M2 resistant lines, respectively (Fig. 2C and D). Several compounds had EC50 values close to or below 100 nM (pEC50 > 7) in the resistant strains, approaching those observed in the drug-sensitive strains. Overall, these results confirm and extend the notion that nitro drug resistance is a relative phenotype in G. lamblia which can be partially or even completely overcome by structurally diverse nitroheterocyclic compounds.
Evaluation of modified nitroheterocycles for cytotoxicity in human cells.
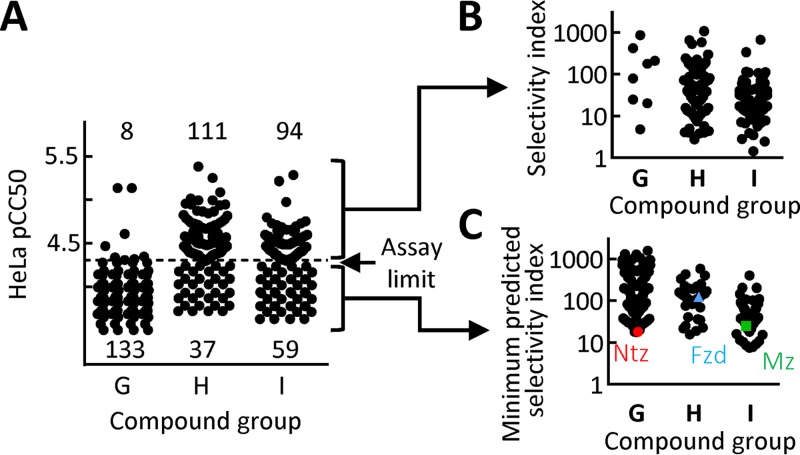
Nitroheterocyclic drugs, such as metronidazole and nitazoxanide, are generally well tolerated in humans, although unpleasant adverse effects, such as a metallic taste in the mouth, can occur (5, 7), and some in vitro studies have suggested mutagenic potential for certain nitro drugs (30, 31). However, safety may be affected by modifications of the core nitroheterocyclic and must therefore be freshly evaluated for new derivatives. To test for cytotoxicity against human cells, we employed HeLa cells, which are a common and sensitive model for initial drug safety evaluations (22, 32). Most (94%) of the new nitrothiazoles exhibited no cytotoxicity at the highest concentration (50 μM) we could test due to solubility limitations or interference with other components in the crude click reaction mixtures (Fig. 3A and Tables S2 to S4). In contrast, only 25% of the nitrofurans and 39% of the nitropyrroles showed no measurable cytotoxicity, while the majority of these nitroheterocycles had detectable 50% cytotoxic concentrations (CC50s) ranging from 1 to 50 μM. Calculation of the selectivity index (i.e., ratio of CC50 to EC50) for the compounds with measurable CC50 values revealed a spectrum from 2 to >1,000 (Fig. 3B). Similarly, the minimum predicted selectivity index of compounds with undetectable cytotoxicity in our assay spanned a range from 7 to >1,500 (Fig. 3C). The upper selectivity indices for the new compounds are close to the value we found previously for metronidazole under similar experimental conditions (∼2,300) (22). Moreover, parallel assays of nitazoxanide, nitrofuran, and metronidazole under the current conditions (with maximal compound concentrations for tests determined by the solubility characteristics of the new compounds) revealed selectivity indices for these approved drugs that were well within the range of the new compounds, suggesting that many of them are likely to have a favorable safety profile.
FIG 3.
Cytotoxicity assessment of new nitroheterocycles in human cells. Cytotoxicity of the 442 new nitroheterocyclic compounds was tested against human HeLa epithelial cells in a 48-h growth and survival assay, using alamarBlue dye reduction as a read-out. Data are shown as means of at least three independent experiments, with each point representing a single compound. Panel A shows the results for all compounds, of which 52% displayed no measureable cytotoxicity at the highest tested compound concentration (50 μM, equivalent to a pCC50 of 4.3; assay limit), while the remaining compounds had measurable cytotoxicity (i.e., pCC50 of >4.3). Panel B depicts the selectivity index (ratio of CC50 in HeLa cells versus EC50 in G. lamblia strain 713) for the compounds with measurable cytotoxicity, while panel C shows the minimum predicted selectivity index for those compounds that did not have measurable cytotoxicity (the threshold pCC50 of 4.3 was used for the calculations, but the actual pCC50 could be significantly lower, so the real selectivity index would be higher). Data obtained in parallel assays for the benchmark compounds nitazoxanide (Ntz) (red circle), furazolidone (Fzd) (blue triangle), and metronidazole (Mz) (green square) are shown for comparison. As for the other compounds with undetectable cytotoxicity (i.e., pCC50 of <4.3) in our assay, only the minimum predicted selectivity index could be shown for the benchmark compounds.
In vivo efficacy of nitrothiazole derivatives in a murine giardiasis model.
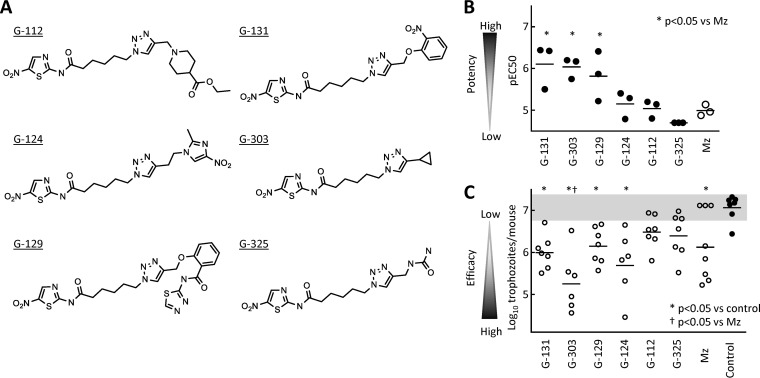
The nitrofuran and nitropyrrole derivatives were generally more cytotoxic than the nitrothiazoles, so we focused on the latter group for further evaluation. Based on the in vitro activity against drug-sensitive and -resistant G. lamblia strains, lack of detectable cytotoxicity (negative log10 of CC50 expressed in moles per liter [pCC50] of <4.3; selectivity index of >150) in human cells, and structural diversity, we selected six representative nitrothiazole compounds (Fig. 4A) and resynthesized and purified them to >90%. To retest the purified compounds for antigiardial activity, we employed another G. lamblia line, GS/M, which belongs to the human-pathogenic assemblage B of G. lamblia and can infect adult mice (33), whereas the 713 and 106 lines of G. lamblia used for the initial screens are members of the human-pathogenic assemblage A, and do not readily infect adult mice. All six compounds displayed good activity against the GS/M strain, with EC50 values similar to those in the other two G. lamblia lines (Fig. 4B).
FIG 4.
In vivo activity of selected modified nitrothiazoles in murine giardiasis. (A) Structures of the six representative nitrothiazoles that were selected for further tests, resynthesized, and purified to >90%. (B) In vitro activity of the depicted compounds against G. lamblia strain GS/M (a human-pathogenic strain belonging to assemblage B) was determined in a 48-h growth and survival assay. Data are shown as individual pEC50 values of three independent experiments, with metronidazole (Mz) shown as a reference drug. Mean pEC50 values that were significantly different (P < 0.05) from those for metronidazole are indicated by an asterisk. (C) The six compounds and Mz were subsequently tested for in vivo efficacy in mice infected with the G. lamblia strain GS/M. Two days after infection, compounds were orally administered to mice in two daily doses of 10 mg/kg for a total of 5 doses over a 3-day period or the mice were given solvent alone as controls, and trophozoite numbers in the small intestine were determined. Data are shown as individual log10-transformed counts with six to eight animals in each group. Horizontal lines depict the geometric mean for each group. The gray zone represents the 95% confidence interval of the counts in the controls.
Next, we tested the six compounds for in vivo efficacy against giardiasis in a murine infection model (28, 33). Adult C57BL/6 mice were orally inoculated with G. lamblia GS/M, and after 2 days (to allow establishment of infection), the mice were given the test compounds orally at a standard dose of 10 mg/kg of body weight twice daily for a total of five doses. Four of the six compounds led to a significant reduction in parasite burden, and one of the efficacious compounds was significantly more effective than the reference drug metronidazole given at the same dose (Fig. 4C). The in vitro and in vivo activities of the six compounds were not closely related, since the most active compound in vitro, G-131, was only moderately active in vivo, while G-303 had similar in vitro activity but proved to be the most efficacious compound in vivo (Fig. 4B and C). None of the mice treated with any of the six compounds died or exhibited clinically apparent adverse effects during the treatment period.
Analysis of the structure-activity relationships of modified nitroheterocycles.
A key feature of our synthetic approach in the current and prior work (24) was the use of click chemistry to join two building blocks, a nitroheterocyclic azide and a functionalized alkyne, with high yield in a combinatorial fashion. Most of the structural diversity in the resulting substituted 1,2,3-triazoles had come from the use of diverse alkynes, while the nitroheterocycles had been more limited in their diversity with only a few positions of the heterocycle available for modifications without fundamentally altering the core structure. This limitation had previously made it difficult to determine the relative contributions of the azide cores and alkyne groups to antimicrobial activity of the triazoles, yet such insights are important for further advancing the design and evaluation of the best antimicrobial compounds. With the synthesis of three new heterocyclic azides and our previously reported six imidazole-based heterocyclic azides (24), we had a total of nine different azide cores, all of which were combined with one of 83 identical alkynes. The resulting 747 nitroheterocycles represented adequate numbers of each reaction partner for a comprehensive analysis of structure-activity relationships (SAR), which was further helped by the fact that all these compounds were tested against the identical G. lamblia strains under similar experimental conditions.
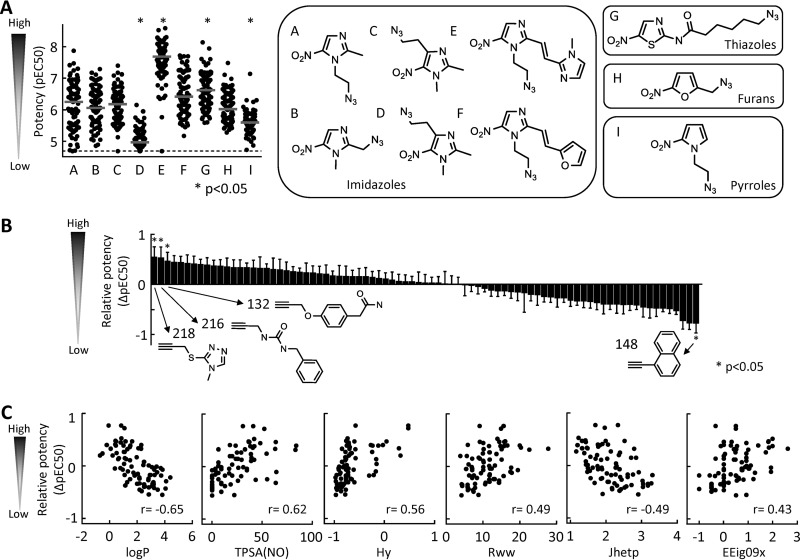
Comparison of the antigiardial activities of the sets of 83 triazoles generated from each of the nine available azide cores, cores A to F (24) and G to I (this study), showed marked and significant differences in average activity against the representative G. lamblia strain 713, with cores E and G leading to compounds with significantly increased activity and cores D and I leading to compounds with significantly reduced activity compared to all 747 compounds (Fig. 5A). These data demonstrate that the nitroheterocyclic azide core can contribute significantly to antigiardial activity independent of the alkyne used for triazole formation.
FIG 5.
SAR analysis of modified nitroheterocycles. The contributions of the azide cores (A) and alkynes (B and C) to the antigiardial activity of the resulting triazoles were analyzed. The nine azide cores used for the analysis are shown in panel A; six of these (A to F compounds) were reported previously (24), while the other three (G to I compounds) were synthesized in this study. The antigiardial activities of the triazoles generated by combining one of the nine azide cores with each of 83 identical alkynes are depicted in the left graph in panel A. Data are means of at least three independent experiments with G. lamblia strain 713. Each symbol represents one compound. The gray horizontal bars show the median values for the compound groups, and the dashed line depicts the assay sensitivity. Significance was evaluated by one-way ANOVA, followed by a post hoc Dunnett test with all compounds as a control. (B) The activity (pEC50) of each of 83 triazoles with the same azide core was normalized against the average activity of all compounds with that core. The normalized values were used to determine mean and SE for each alkyne and sorted from highest to lowest. Significance was evaluated by one-way ANOVA, followed by a post hoc Dunnett test with all compounds as a control. Structures are shown for the alkynes with significant contributions to the triazole activity. (C) All alkynes were subsequently analyzed by quantitative SAR using 1,666 chemical descriptors for correlation-based attribute subset evaluation to yield correlations between triazole activity and alkyne descriptors. The relationships with significant correlations are shown.
Conversely, to define the alkyne contributions, we normalized the activity of each of the 83 triazoles with any particular azide core against the average activity of all compounds with the same core, and then averaged the relative activities of each alkyne across all nine cores. The results were arranged in order of the greatest positive contribution to the greatest negative contribution of each alkyne (Fig. 5B). This analysis revealed that the top three alkynes and bottom alkyne had statistically significant impact on triazole activity, ranging from an average EC50 increase of 5.7-fold to an EC50 decrease of 8.4-fold. On the basis of these data, we conclude that 4 (5%) of the 83 tested alkynes contributed significantly to the antigiardial activity of the associated triazoles irrespective of the nitroheterocyclic partner. Furthermore, SAR analysis of all 83 alkynes revealed significant relationships between the relative alkyne contribution to the potency of the resulting triazoles for several calculated molecular parameters, including significant negative correlations with the octanol-water partition coefficient (logP) and the Balaban-type index from polarizability weighted distance matrix (Jhetp), and significant positive correlations with the topological polar surface area (TPSA), hydrophilic factor (Hy), reciprocal hyperdetour index (Rww), and the eigenvalue from edge-adjusted matrix weighted by edge degrees (EEig09x), as shown in Fig. 5C.
Taken together, these findings demonstrate that both components of the click reaction, azide and alkyne, can make independent contributions to overall triazole activity, and that at least for the alkynes, significant correlations exist between predicted molecular parameters and relative contributions to overall triazole potency.
DISCUSSION
This study demonstrates that superior activity against G. lamblia can be achieved by modifications of nitroheterocycles other than the previously reported imidazole (21, 22, 24). Superior activity in this context refers to the up to 200-fold-greater potency of several compounds against drug-sensitive strains of the parasite in vitro and their ability to overcome different forms of existing nitro drug resistance. Furthermore, several of the nitrothiazole compounds showed a high selectivity index without cytotoxicity and excellent in vivo activity against giardiasis after oral administration in mice, underlining the therapeutic potential of the compounds. These results confirm and extend our and other prior findings with nitroimidazoles that also revealed dramatic activity improvements of many of the derivatives (21, 24). Thus, based on a combined total of now >1,100 new nitroheterocyclic compounds, we can conclude that broad structural diversification is a valuable strategy for identifying new compounds in the nitro drug class with clear potential to contribute to the arsenal of drugs for control of giardiasis, and potentially for use against infections resistant to currently used drugs.
The mechanisms of enhanced antigiardial activity are unclear at this time. The general outline of nitro drug action is well understood, and it involves entry of the inactive prodrugs into target cells, activation by reduction, and formation of adducts with critical macromolecules, leading to their inactivation (6, 12). However, many of the details are not known in the target parasites. It is becoming increasingly evident that more than one reductase pathway can activate nitro drugs in G. lamblia and other microbes (16, 19, 34–37) and that multiple adduction targets exist (38). In addition, nitazoxanide has been shown to inhibit the interactions of a cofactor with its target enzyme, pyruvate:ferredoxin-flavodoxin oxidoreductase, and thus the activity of this critical enzyme, suggesting that nitro drugs may have other mechanisms of action beyond adduction of microbial target molecules (39). However, the relative contributions of specific activation pathways and targets of most nitro drugs remain elusive. Consequently, understanding the markedly increased activity of new nitro drugs presents a major challenge, as different mechanisms may be involved, including enhanced penetration or retention in target cells, more-effective activation by alternative reductases, enhanced adduct formation, or altered molecular target specificities. Despite this uncertainty, our data clearly show that multiple mechanisms must be at play, since the activity profiles of structurally different nitro compounds vary in respect to overcoming existent forms of nitro drug resistance, an observation that precludes a single mechanism of enhanced action for the different structural derivatives. As a corollary, these findings also suggest that excellent antigiardial activity does not require structurally convergent features but that it can be achieved in different structural domains.
Combined SAR analysis of 747 new and previously reported nitroheterocycles (24), representing all possible combinations of nine different azido-nitroheterocyclic cores and 83 alkynes, revealed that both partners of the click reaction can make significant independent contributions to the overall antigiardial activity of the resulting triazoles. This observation provides a strong basis for further exploration of our combinatorial approach to enhancing the activity of nitro antimicrobials. Practically, modification of the two reaction partners presents different synthetic challenges, as the azide cores offer fewer opportunities for structural diversification due to constraints of the core heterocycles and reactive nitro group (24), while the alkynes have an almost unlimited potential for modifications. Moreover, numerous alkynes are available commercially, further simplifying structural exploration in the alkyne space. For example, SAR analysis revealed that the most promising alkynes have logP values between 0 and 2, and topological polar surface areas (TPSAs) of 20 to 60, together indicating that modest lipophilicity and thus good passive diffusion through membranes are desirable alkyne features. By comparison, several of the topological indices, such as the reciprocal hyperdetour index Rww and the Balaban-type index from polarizability weighted distance matrix Jhetp, which also exhibited significant associations with superior alkyne contributions to antigiardial triazole activity, cannot be easily interpreted in terms of simple physicochemical quantities (40), and may be less immediately valuable for systematic selection or design of new alkynes for structural optimization.
The present work focused on G. lamblia as an important and ubiquitous human-pathogenic parasite, but it is likely that many of the new nitroheterocyclic compounds will show excellent activity against other protozoan pathogens, such as T. vaginalis or E. histolytica, that are generally susceptible to nitro drugs (24, 41). Future studies could also address the question whether superior broad-spectrum activity can be achieved against several pathogens or whether enhanced activity against any one pathogen diminishes the chances of better activity against other pathogens. Nonetheless, certain features of drug candidates are likely to be valuable for treating more than one infectious target. For example, good in vivo activity after oral administration, which we observed for several of the tested nitrothiazoles against giardiasis, should bode well for in vivo efficacy of these compounds against systemic infections, such as liver amebiasis, since we found in prior work that only compounds with good systemic absorption after oral dosing are effective against giardiasis (24). Furthermore, beyond potential broad-spectrum activity, other considerations such as estimated cost of synthesis, time to kill in vitro and in vivo, and optimal pharmacokinetic properties must be taken into account in selecting particular nitro compounds for further preclinical drug development. Taken together, the present studies provide additional impetus for the systematic optimization and development of nitroheterocyclic compounds with imidazole and nonimidazole cores as alternative and improved agents for the treatment of G. lamblia and potentially other infectious agents.
MATERIALS AND METHODS
Chemistry.
Three azido-alkyl-heterocycles were synthesized de novo from commercially available starting materials (Fig. 1). Briefly, to synthesize 5-nitrothiazole azide, 6-aminohexanoic acid was reacted with methylimidazole-1-sulfonyl azide hydrochloride to yield 6-azidohexanoic acid, which was purified and further reacted with thioyl chloride and 4-nitro-2-aminothiazole to generate the title compound. 5-Nitrofuran azide was generated by reaction of nitrofuran alcohol with mesylchloride, purification of the resulting nitrofuran mesylate, and further reaction of the mesylate with sodium azide. For synthesis of 5-nitropyrrole, pyrrole was reacted with nitric acid to yield the 5-nitroisomer after purification by column chromatography with hexane/ethyl acetate. Alkynes were purchased or synthesized de novo as described previously (24) and are shown in Table S1 in the supplemental material. The identities and purities of compounds were confirmed by 1H and 13C nuclear magnetic resonance (NMR) spectroscopy, melting point analysis, and high-resolution mass spectrometry.
To perform the copper(I)-catalyzed azide alkyne cycloaddition (“click reaction”), 100 mM (each) azide and alkyne in tert-butyl alcohol (t-BuOH)–H2O (2:1) were combined with 20 mM sodium ascorbate and 5 mM CuSO4. Reaction mixtures were heated at 50°C with stirring for 24 to 48 h until liquid chromatography/mass spectrometry indicated that reactions were complete. Reaction mixtures (containing ∼100 mM concentration of the respective triazole) were diluted with dimethyl sulfoxide (DMSO) to a final triazole concentration of 10 mM and used without further purification at a minimum dilution of 1:500 for the biological assays. The purity of the compounds in the crude reaction mixture ranged from 60 to 80%.
To ensure that the crude reaction mixtures did not interfere with the activity screens, we determined the potency of purified metronidazole and a representative new compound, G-303, in the presence or absence of the basic reaction mixture without azide or alkyne. No significant differences were observed between the potency (mean pEC50 ± standard error [SE]) for metronidazole or G-303 with and without added reaction mixture (for metronidazole, 5.89 ± 0.044 without reaction mixture versus 5.79 ± 0.028 with reaction mixture; for G-303, 7.43 ± 0.029 without reaction mixture versus 7.17 ± 0.018 with reaction mixture; n = 3 experiments). Triazole compounds for in vivo testing were further purified to >90% purity by column chromatography.
G. lamblia EC50 assays.
The following five G. lamblia lines were used: BRIS/87/HEPU/713 (713) (42), BRIS/83/HEPU/106 (106) (43), GS/M (ATCC 50580), 713M3 (42), and WB-M2 (28). The 713, 106, and GS/M lines are metronidazole sensitive, while 713M3 and WB-M2 are metronidazole resistant and were grown as described previously (22, 44, 45). For antimicrobial assays, the 10 mM stocks of the test compounds were diluted in phosphate-buffered saline (PBS) to 75 μM, serial 1:3 dilutions were made in 96-well plates, and 3 × 103 trophozoites/well were added in a final volume of 40 μl/well. Cultures were grown for 2 days at 37°C under anaerobic conditions (AnaeroPack-Anaero system; Remel). Cell growth and viability were determined by an ATP assay by adding BacTiter-Glo microbial cell viability assay reagent (Promega) and measuring ATP-dependent luminescence in a microplate reader (22). The 50% effective concentration (EC50) was derived from normalized concentration-response curves after 48-h drug exposure using BioAssay software (CambridgeSoft). pEC50 was calculated as the negative log10 of EC50 expressed in moles per liter. In preliminary studies, we confirmed that EC50 values showed good correlations with microscopically determined MICs after 48 h of drug exposure, but activity data are reported as EC50 values as more-precise measures of compound activity for quantitative comparisons.
Cytotoxicity assay in mammalian cells.
The human epithelial cell line, HeLa (ATCC CCL-2), was used to determine drug cytotoxicity in human cells (22, 32). Compounds were serially diluted (1:3) and added to HeLa cell cultures in 96-well plates. The cells were grown for 2 days, and viable-cell numbers were determined using alamarBlue reagent (Invitrogen). As done for the EC50 calculations, the 50% cytotoxic concentration (CC50) was derived from the normalized concentration-response curves using BioAssay software (CambridgeSoft).
Murine G. lamblia infections.
Adult C57BL/6 mice (The Jackson Laboratory) were infected by oral gavage of 106 G. lamblia GS/M trophozoites (28). After 2 days, mice were given test compound at a dose of 10 mg/kg in 0.1% hypromellose in PBS by oral gavage for a total of 5 doses over a 3-day period. Controls received only hypromellose-PBS. On day 5, animals were euthanized by controlled CO2 inhalation followed by cervical dislocation according to the guidelines of the American Veterinary Medical Association. The small intestine was removed, opened in 5 ml PBS, and chilled and shaken to release attached trophozoites. Live trophozoites were enumerated in a counting chamber. All animal studies were reviewed and approved by the UCSD Institutional Animal Care and Use Committee.
Data analysis.
EC50 and CC50 assays were repeated at least three times, and means and standard errors (SEs) were calculated from the results. For murine infections, trophozoite counts were log10 transformed for each animal, and the mean and SE were calculated. Significance was tested by Wilcoxon rank sum test, with a P value of <0.05 considered significant. To analyze the impact of the azide core on the activity of the resulting triazoles, the antigiardial activity (pEC50) of the triazoles made from one of nine azides were compared by azide core using one-way analysis of variance (ANOVA), followed by a post hoc Dunnett test with all compounds as the control. To evaluate the impact of alkynes on the activity of the resulting triazoles, we normalized the activity (pEC50) of each triazole with a particular azide core against the average activity of all compounds with the same core. The normalized values were used to determine the mean and SE for each alkyne and were compared by alkyne using one-way ANOVA, followed by a post hoc Dunnett test with all compounds as the control. For both analyses, a P value of <0.05 was considered significant. For the analysis of quantitative structure-activity relationships (QSAR), 1,666 chemical descriptors were calculated for each compound using E-dragon 1.0 from Virtual Computational Chemistry Laboratory (46). The measured EC50 and calculated chemical descriptor values were used as input attributes for correlation-based attribute subset evaluation (CfssubsetEval and BestFirst) in the Weka machine learning software to yield correlations between these parameters (47). Significance for individual correlations was evaluated by Pearson correlation (Graphpad Prism).
Supplementary Material
ACKNOWLEDGMENTS
We thank Lucia Hall and Elaine Hanson for technical assistance.
This work was supported by NIH grants AI114671 and DE020607.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02397-16.
REFERENCES
- 1.Baldursson S, Karanis P. 2011. Waterborne transmission of protozoan parasites: review of worldwide outbreaks-an update 2004-2010. Water Res 45:6603–6614. doi: 10.1016/j.watres.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Painter JE, Gargano JW, Collier SA, Yoder JS. 2015. Giardiasis surveillance–United States, 2011-2012. MMWR Suppl 64:15–25. [PubMed] [Google Scholar]
- 3.Troeger H, Epple HJ, Schneider T, Wahnschaffe U, Ullrich R, Burchard GD, Jelinek T, Zeitz M, Fromm M, Schulzke JD. 2007. Effect of chronic Giardia lamblia infection on epithelial transport and barrier function in human duodenum. Gut 56:328–335. doi: 10.1136/gut.2006.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanevik K, Wensaas K-A, Rortveit G, Eide GE, Morch K, Langeland N. 2014. Irritable bowel syndrome and chronic fatigue 6 years after Giardia infection: a controlled prospective cohort study. Clin Infect Dis 59:1394–1400. doi: 10.1093/cid/ciu629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman CD, Klutman NE, Lamp KC. 1997. Metronidazole. A therapeutic review and update. Drugs 54:679–708. [DOI] [PubMed] [Google Scholar]
- 6.Samuelson J. 1999. Why metronidazole is active against both bacteria and parasites. Antimicrob Agents Chemother 43:1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lofmark S, Edlund C, Nord CE. 2010. Metronidazole is still the drug of choice for treatment of anaerobic infections. Clin Infect Dis 50(Suppl 1):S16–S23. doi: 10.1086/647939. [DOI] [PubMed] [Google Scholar]
- 8.Escobedo AA, Alvarez G, Gonzalez ME, Almirall P, Canete R, Cimerman S, Ruiz A, Perez R. 2008. The treatment of giardiasis in children: single-dose tinidazole compared with 3 days of nitazoxanide. Ann Trop Med Parasitol 102:199–207. doi: 10.1179/136485908X267894. [DOI] [PubMed] [Google Scholar]
- 9.Miyamoto Y, Eckmann L. 2015. Drug development against the major diarrhea-causing parasites of the small intestine, Cryptosporidium and Giardia. Front Microbiol 6:1208. doi: 10.3389/fmicb.2015.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warren CA, van Opstal E, Ballard TE, Kennedy A, Wang X, Riggins M, Olekhnovich I, Warthan M, Kolling GL, Guerrant RL, Macdonald TL, Hoffman PS. 2012. Amixicile, a novel inhibitor of pyruvate:ferredoxin oxidoreductase, shows efficacy against Clostridium difficile in a mouse infection model. Antimicrob Agents Chemother 56:4103–4111. doi: 10.1128/AAC.00360-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lalle M. 2010. Giardiasis in the post genomic era: treatment, drug resistance and novel therapeutic perspectives. Infect Disord Drug Targets 10:283–294. doi: 10.2174/187152610791591610. [DOI] [PubMed] [Google Scholar]
- 12.Upcroft P, Upcroft JA. 2001. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin Microbiol Rev 14:150–164. doi: 10.1128/CMR.14.1.150-164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemee V, Zaharia I, Nevez G, Rabodonirina M, Brasseur P, Ballet JJ, Favennec L. 2000. Metronidazole and albendazole susceptibility of 11 clinical isolates of Giardia duodenalis from France. J Antimicrob Chemother 46:819–821. doi: 10.1093/jac/46.5.819. [DOI] [PubMed] [Google Scholar]
- 14.Escobedo AA, Cimerman S. 2007. Giardiasis: a pharmacotherapy review. Expert Opin Pharmacother 8:1885–1902. doi: 10.1517/14656566.8.12.1885. [DOI] [PubMed] [Google Scholar]
- 15.Nash TE, Ohl CA, Thomas E, Subramanian G, Keiser P, Moore TA. 2001. Treatment of patients with refractory giardiasis. Clin Infect Dis 33:22–28. doi: 10.1086/320886. [DOI] [PubMed] [Google Scholar]
- 16.Townson SM, Upcroft JA, Upcroft P. 1996. Characterisation and purification of pyruvate:ferredoxin oxidoreductase from Giardia duodenalis. Mol Biochem Parasitol 79:183–193. doi: 10.1016/0166-6851(96)02661-8. [DOI] [PubMed] [Google Scholar]
- 17.Pal D, Banerjee S, Cui J, Schwartz A, Ghosh SK, Samuelson J. 2009. Giardia, Entamoeba, and Trichomonas enzymes activate metronidazole (nitroreductases) and inactivate metronidazole (nitroimidazole reductases). Antimicrob Agents Chemother 53:458–464. doi: 10.1128/AAC.00909-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müller J, Schildknecht P, Müller N. 2013. Metabolism of nitro drugs metronidazole and nitazoxanide in Giardia lamblia: characterization of a novel nitroreductase (GlNR2). J Antimicrob Chemother 68:1781–1789. doi: 10.1093/jac/dkt106. [DOI] [PubMed] [Google Scholar]
- 19.Leitsch D, Burgess AG, Dunn LA, Krauer KG, Tan K, Duchene M, Upcroft P, Eckmann L, Upcroft JA. 2011. Pyruvate:ferredoxin oxidoreductase and thioredoxin reductase are involved in 5-nitroimidazole activation while flavin metabolism is linked to 5-nitroimidazole resistance in Giardia lamblia. J Antimicrob Chemother 66:1756–1765. doi: 10.1093/jac/dkr192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leitsch D. 2015. Drug resistance in the microaerophilic parasite Giardia lamblia. Curr Trop Med Rep 2:128–135. doi: 10.1007/s40475-015-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Upcroft JA, Dunn LA, Wright JM, Benakli K, Upcroft P, Vanelle P. 2006. 5-Nitroimidazole drugs effective against metronidazole-resistant Trichomonas vaginalis and Giardia duodenalis. Antimicrob Agents Chemother 50:344–347. doi: 10.1128/AAC.50.1.344-347.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valdez CA, Tripp JC, Miyamoto Y, Kalisiak J, Hruz P, Andersen YS, Brown SE, Kangas K, Arzu LV, Davids BJ, Gillin FD, Upcroft JA, Upcroft P, Fokin VV, Smith DK, Sharpless KB, Eckmann L. 2009. Synthesis and electrochemistry of 2-ethenyl and 2-ethanyl derivatives of 5-nitroimidazole and antimicrobial activity against Giardia lamblia. J Med Chem 52:4038–4053. doi: 10.1021/jm900356n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarrad AM, Debnath A, Miyamoto Y, Hansford KA, Pelingon R, Butler MS, Bains T, Karoli T, Blaskovich MA, Eckmann L, Cooper MA. 2016. Nitroimidazole carboxamides as antiparasitic agents targeting Giardia lamblia, Entamoeba histolytica and Trichomonas vaginalis. Eur J Med Chem 120:353–362. doi: 10.1016/j.ejmech.2016.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyamoto Y, Kalisiak J, Korthals K, Lauwaet T, Cheung DY, Lozano R, Cobo ER, Upcroft P, Upcroft JA, Berg DE, Gillin FD, Fokin VV, Sharpless KB, Eckmann L. 2013. Expanded therapeutic potential in activity space of next-generation 5-nitroimidazole antimicrobials with broad structural diversity. Proc Natl Acad Sci U S A 110:17564–17569. doi: 10.1073/pnas.1302664110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossignol J-F, Lopez-Chegne N, Julcamoro LM, Carrion ME, Bardin MC. 2012. Nitazoxanide for the empiric treatment of pediatric infectious diarrhea. Trans R Soc Trop Med Hyg 106:167–173. doi: 10.1016/j.trstmh.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Roghani HS, Massarrat S, Shirekhoda M, Butorab Z. 2003. Effect of different doses of furazolidone with amoxicillin and omeprazole on eradication of Helicobacter pylori. J Gastroenterol Hepatol 18:778–782. doi: 10.1046/j.1440-1746.2003.03058.x. [DOI] [PubMed] [Google Scholar]
- 27.Kolb HC, Finn MG, Sharpless KB. 2001. Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed Engl 40:2004–2021. doi:. [DOI] [PubMed] [Google Scholar]
- 28.Tejman-Yarden N, Millman M, Lauwaet T, Davids BJ, Gillin FD, Dunn L, Upcroft JA, Miyamoto Y, Eckmann L. 2011. Impaired parasite attachment as fitness cost of metronidazole resistance in Giardia lamblia. Antimicrob Agents Chemother 55:4643–4651. doi: 10.1128/AAC.00384-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Escobedo AA, Lalle M, Hrastnik NI, Rodriguez-Morales AJ, Castro-Sanchez E, Cimerman S, Almirall P, Jones J. 2016. Combination therapy in the management of giardiasis: what laboratory and clinical studies tell us, so far. Acta Trop 162:196–205. doi: 10.1016/j.actatropica.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 30.Re JL, De Meo MP, Laget M, Guiraud H, Castegnaro M, Vanelle P, Dumenil G. 1997. Evaluation of the genotoxic activity of metronidazole and dimetridazole in human lymphocytes by the comet assay. Mutat Res 375:147–155. doi: 10.1016/S0027-5107(97)00010-9. [DOI] [PubMed] [Google Scholar]
- 31.Menendez D, Rojas E, Herrera LA, Lopez MC, Sordo M, Elizondo G, Ostrosky-Wegman P. 2001. DNA breakage due to metronidazole treatment. Mutat Res 478:153–158. doi: 10.1016/S0027-5107(01)00136-1. [DOI] [PubMed] [Google Scholar]
- 32.Ekwall B, Johansson A. 1980. Preliminary studies on the validity of in vitro measurement of drug toxicity using HeLa cells. I. Comparative in vitro cytotoxicity of 27 drugs. Toxicol Lett 5:299–307. [DOI] [PubMed] [Google Scholar]
- 33.Byrd LG, Conrad JT, Nash TE. 1994. Giardia lamblia infections in adult mice. Infect Immun 62:3583–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leitsch D, Schlosser S, Burgess A, Duchene M. 2012. Nitroimidazole drugs vary in their mode of action in the human parasite Giardia lamblia. Int J Parasitol Drugs Drug Resist 2:166–170. doi: 10.1016/j.ijpddr.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leitsch D, Muller J, Muller N. 2016. Evaluation of Giardia lamblia thioredoxin reductase as drug activating enzyme and as drug target. Int J Parasitol Drugs Drug Resist 6:148–153. doi: 10.1016/j.ijpddr.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nillius D, Mueller J, Mueller N. 2011. Nitroreductase (GlNR1) increases susceptibility of Giardia lamblia and Escherichia coli to nitro drugs. J Antimicrob Chemother 66:1029–1035. doi: 10.1093/jac/dkr029. [DOI] [PubMed] [Google Scholar]
- 37.Sisson G, Goodwin A, Raudonikiene A, Hughes NJ, Mukhopadhyay AK, Berg DE, Hoffman PS. 2002. Enzymes associated with reductive activation and action of nitazoxanide, nitrofurans, and metronidazole in Helicobacter pylori. Antimicrob Agents Chemother 46:2116–2123. doi: 10.1128/AAC.46.7.2116-2123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leitsch D, Kolarich D, Wilson IB, Altmann F, Duchene M. 2007. Nitroimidazole action in Entamoeba histolytica: a central role for thioredoxin reductase. PLoS Biol 5:e211. doi: 10.1371/journal.pbio.0050211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffman PS, Sisson G, Croxen MA, Welch K, Harman WD, Cremades N, Morash MG. 2007. Antiparasitic drug nitazoxanide inhibits the pyruvate oxidoreductases of Helicobacter pylori, selected anaerobic bacteria and parasites, and Campylobacter jejuni. Antimicrob Agents Chemother 51:868–876. doi: 10.1128/AAC.01159-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Randic M, Zupan J. 2001. On interpretation of well-known topological indices. J Chem Infect Comput Sci 41:550–560. doi: 10.1021/ci000095o. [DOI] [PubMed] [Google Scholar]
- 41.Gonzales ML, Dans LF, Martinez EG. 2009. Antiamoebic drugs for treating amoebic colitis. Cochrane Database Syst Rev 2009:CD006085. doi: 10.1002/14651858.CD006085.pub2. [DOI] [PubMed] [Google Scholar]
- 42.Townson SM, Laqua H, Upcroft P, Boreham PF, Upcroft JA. 1992. Induction of metronidazole and furazolidone resistance in Giardia. Trans R Soc Trop Med Hyg 86:521–522. doi: 10.1016/0035-9203(92)90095-T. [DOI] [PubMed] [Google Scholar]
- 43.Boreham PF, Phillips RE, Shepherd RW. 1988. Altered uptake of metronidazole in vitro by stocks of Giardia intestinalis with different drug sensitivities. Trans R Soc Trop Med Hyg 82:104–106. doi: 10.1016/0035-9203(88)90278-7. [DOI] [PubMed] [Google Scholar]
- 44.Dunn LA, Burgess AG, Krauer KG, Eckmann L, Vanelle P, Crozet MD, Gillin FD, Upcroft P, Upcroft JA. 2010. A new-generation 5-nitroimidazole can induce highly metronidazole-resistant Giardia lamblia in vitro. Int J Antimicrob Agents 36:37–42. doi: 10.1016/j.ijantimicag.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clark CG, Diamond LS. 2002. Methods for cultivation of luminal parasitic protists of clinical importance. Clin Microbiol Rev 15:329–341. doi: 10.1128/CMR.15.3.329-341.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tetko IV, Gasteiger J, Todeschini R, Mauri A, Livingstone D, Ertl P, Palyulin VA, Radchenko EV, Zefirov NS, Makarenko AS, Tanchuk VY, Prokopenko VV. 2005. Virtual computational chemistry laboratory—design and description. J Comput Aided Mol Des 19:453–463. doi: 10.1007/s10822-005-8694-y. [DOI] [PubMed] [Google Scholar]
- 47.Frank E, Hall M, Trigg L, Holmes G, Witten IH. 2004. Data mining in bioinformatics using Weka. Bioinformatics 20:2479–2481. doi: 10.1093/bioinformatics/bth261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.