ABSTRACT
The integrase inhibitors elvitegravir (EVG) and dolutegravir (DTG) rapidly decrease the plasma HIV-1 viral load, a key factor in the prevention of maternal-to-fetal transmission of HIV-1. No data have been reported on the concentrations of these drugs in cord blood, maternal peripheral blood mononuclear cells (PBMCs), or placental tissue in pregnant women. We present in vivo pharmacokinetic data on antiretrovirals (ARV) within maternal and cord blood and within placentae from HIV-1-infected pregnant women. Maternal blood and cord blood were obtained from women receiving EVG, cobicistat, tenofovir disoproxil fumarate, and emtricitabine as a single fixed-dose combination formulation or DTG as part of a combination regimen. Plasma and PBMCs from maternal and cord blood were obtained along with villous placental samples. Drug concentrations were simultaneously determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Utilizing medians and ranges to interpret our data, we compared the drug concentration ratios between different matrices (maternal and cord blood plasma, PBMCs, and placenta). All five agents transferred from maternal into fetal circulation via the placenta. Concentration ratios for EVG, cobicistat, tenofovir, and emtricitabine (n = 10) and DTG (n = 3) were determined between cord plasma and placenta, cord and maternal plasma, and cord PBMCs and maternal PBMCs. TFV moves from maternal plasma through the placenta to the cord blood and then into cord PBMCs, where it is phosphorylated into its active forms (TFV diphosphate). These five ARVs were detected in each of the compartments, highlighting transfer of these agents from the maternal into the fetal circulation.
KEYWORDS: HIV-1, elvitegravir, dolutegravir, integrase inhibitors, pharmacokinetics, placental transfer, pregnancy
INTRODUCTION
Early initiation of combination antiretroviral therapy (cART) for maternal HIV-1 infection is the standard of care for the treatment of pregnant women and also prevents mother-to-child transmission (MTCT) of HIV-1 (1–3). The pharmacokinetics (PK) and placental transfer of a number of antiretroviral (ARV) drugs in pregnancy have been studied; however, there are limited PK data on the newer integrase strand transfer inhibitors (INSTIs) (4–8). A recent ex vivo study of dolutegravir (DTG) reported that in addition to precipitously decreasing maternal HIV-1 RNA levels, placental transfer of this ARV occurred in moderate to high concentrations, important for infant preexposure prophylaxis (8, 9). A few studies have measured the PK of INSTIs during pregnancy (6). Raltegravir (RTG) has been shown to cross the human placenta, with drug concentrations detected in maternal and cord blood plasma from mothers receiving RTG-based therapy during pregnancy (7, 11–13). Dolutegravir has also been shown to readily cross the blood-placenta barrier (8). Limited data are available to assess the transfer of elvitegravir (EVG) (14). To the best of our knowledge, there are no studies measuring the intracellular concentrations in placental cells for any ARV.
The primary purpose of this study was to determine placental transfer of the newer INSTIs by measuring the intracellular concentrations in placental tissue obtained from HIV-1-infected women. In addition, we compared the concentrations of these drugs in maternal plasma with those in cord blood at the time of delivery. After we determined these concentrations, we compared the drug concentration ratios between different matrices (maternal plasma, cord plasma, maternal peripheral blood mononuclear cells [PBMCs], cord PBMCs, and placental tissue) to illustrate if and in what concentrations these agents transfer from maternal to fetal circulation. For the PBMC matrix, we analyzed the active phosphorylated metabolites (tenofovir diphosphate [TFV-DP] and emtricitabine triphosphate [FTC-TP]). For the remainder of the matrices, we analyzed the parent FTC and TFV compound concentrations. These studies may be important in identifying pharmacologic correlates in placental cells associated with perinatal transmission and prevention.
RESULTS
Patient characteristics.
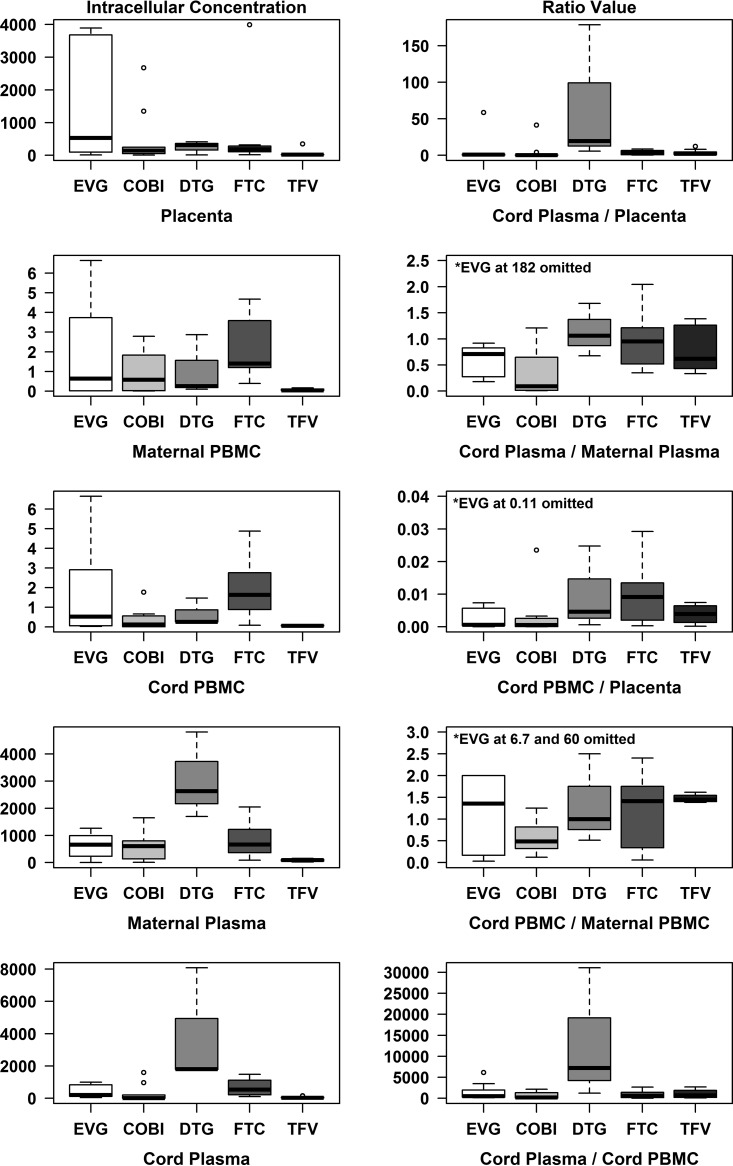
Thirteen patients were enrolled (10 patients on EVG, cobistat [COBI], tenofovir disoproxil fumarate [TDF], and FTC and 3 patients on DTG). Maternal characteristics are described in Table 1. Twelve of 13 (92.3%) mothers were black (non-Hispanic), with a median age of 29 years (interquartile range [IQR], 18 to 34). No congenital anomalies were identified by prenatal ultrasound or physical examination at the time of birth. Table 2 outlines neonatal outcomes in this study population. Nine women delivered at term, and four delivered prematurely. EVG and DTG, along with other ARV components (COBI, TFV, and FTC), were quantified by liquid chromatography-tandem mass spectrometry (LC-MS/MS) in 13 plasma samples (maternal and cord), PBMCs from maternal and cord blood, and placental tissue. Concentrations of EVG, DTG, COBI, FTC, and TFV are presented as medians and ranges and visually represented with box plots in Fig. 1.
TABLE 1.
Summary of the maternal characteristics of the study population
| Characteristica | No. (%) of patientsc |
|---|---|
| Age (yr) | 29 (18–34)b |
| Ethnicity | |
| Caucasian | 0 (0) |
| Asian | 1 (7.7) |
| Black (non-Hispanic) | 12 (92.3) |
| Hispanic | 0 (0) |
| Nulliparous | 2 (15.4) |
| Multiparous | 11 (84.6) |
| Pregnancy BMI (kg/m2) | 30 (22–45)b |
| cART during pregnancy | |
| Elvitegravir | 10 (76.9) |
| Dolutegravir | 3 (23.1) |
| Plasma viral load at entry of study (copies/ml) | |
| <40 | 4 (30.8) |
| 50–399 | 2 (15.4) |
| 400–999 | 5 (38.5) |
| 1,000–9,999 | 2 (15.4) |
| ≥10,000 | 0 (0) |
| HIV-1 RNA viral load at delivery (copies/ml) | |
| <40 | 12 (92.3) |
| 50–399 | 0 (0) |
| 400–999 | 0 (0) |
| 1,000–9,999 | 1 (7.7) |
| ≥10,000 | 0 (0) |
| CD4+ count at entry of study (cells/mm3) | |
| ≥500 | 5 (38.5) |
| 200–499 | 7 (53.8) |
| <200 | 1 (7.7) |
| CD4+ count at delivery (cells/mm3) | |
| ≥500 | 6 (46.2) |
| 200–499 | 7 (53.8) |
| <200 | 0 (0) |
| cART duration during pregnancy (wks) | 8 (3–22)b |
| Time from cART administration to sample (h) | 8 (2–16)b |
| Side effects or adverse reactions from cART | 0 (0) |
HIV, human immunodeficiency virus; BMI, body mass index; cART, combined antiretroviral therapy.
The median and interquartile range (IQR) are given.
Out of a total of 13 patients.
TABLE 2.
Summary of the neonatal outcomes of the study population, including prenatal ultrasound and genetic aneuploidy characteristics
| Characteristica | No. (%) of patientsb |
|---|---|
| Anomalies noted on prenatal ultrasound and/or physical exam at birth | 0 (0) |
| Aneuploidy | 0 (0) |
| Preterm birth | |
| Extremely preterm (<28 wks) | 1 (7.7) |
| Very preterm (28 to 32 wks) | 2 (15.4) |
| Moderate to late preterm (32 to <37 wks) | 1 (7.7) |
| Birth wt | |
| Extremely low (<1,000 g) | 2 (15.4) |
| Very low (<1500 g) | 1 (7.7) |
| Low (<2,500 g) | 1 (7.7) |
| Infant diagnostic HIV-1 positive testingc | 0 (0) |
HIV, human immunodeficiency virus.
Out of a total of 13 patients.
Infant diagnostic virologic testing for HIV-1 was performed at 14 to 21 days, 1 to 2 months, and 4 to 6 months of age. Definitive exclusion of HIV infection was based on two or more negative virologic tests at age ≥1 month and one at age ≥4 months or two negative HIV antibody tests from separate specimens obtained at age ≥6 months.
FIG 1.
Statistical significance was evaluated at a P of 0.05 significance level, and data analyses were performed using SAS v9.4 (SAS, Cary, NC) and R v3.3 (R Foundation for Statistical Computing, Vienna, Austria). Concentrations of EVG, DTG, and COBI, as well as FTC and TFV, were summarized using medians and interquartile ranges and minimums and maximums and are visually represented with box plots.
Drug concentrations within each matrix.
The concentrations of all five drugs within each matrix (placenta, maternal and cord PBMCs, maternal and cord plasma), including the drug concentration ratios between the different matrices, are illustrated in Table 3. Overall, all five drugs were detected within all five matrices. In addition, low concentration ratios for all five drugs were noted between cord plasma and placenta, cord plasma and maternal plasma, and cord PBMCs and maternal PBMCs.
TABLE 3.
Summary of drug concentrations and ratios between the placenta, PBMC, and plasma matrices
| Drug characteristic | Median value (minimum, maximum) for indicated druga |
||||
|---|---|---|---|---|---|
| EVG (n = 10) | COBI (n = 10) | DTG (n = 3) | FTC (n = 10) | TFV (n = 10) | |
| Concn in placenta (ng/g) | 532.5 (10.1, 3,891) | 146.5 (5.1, 2,673) | 318 (10.1, 411) | 188.5 (16.7, 3,990) | 21.9 (0.5, 345) |
| Concn in maternal PBMCs (ng/106 cells) | 0.64 (0.02, 6.64) | 0.58 (0, 2.79) | 0.26 (0.1, 2.87) | 1.4 (0.39, 4.68)b | 0.04 (0.03, 0.17)b |
| Concn in cord PBMCs (ng/106 cells) | 0.53 (0.02, 6.65) | 0.12 (0.01, 1.77) | 0.26 (0.25, 1.47) | 1.64 (0.08, 4.88)b | 0.06 (0.05, 0.13)b |
| Concn in maternal plasma (ng/ml) | 658.5 (1.1, 1,263) | 604 (8.19, 1,651) | 2,634 (1,697, 4,806) | 667 (89.6, 2,045) | 94.6 (22.6, 155) |
| Concn in cord plasma (ng/ml) | 210 (41.3, 1,001) | 11.5 (0.38, 1,595) | 1,803 (1,778, 8,078) | 545 (103, 1,487) | 38.5 (6.07, 155) |
| Cord plasma/placenta ratio | 1.01 (0.05, 58.5) | 0.29 (0, 41.4) | 19.7 (5.59, 178.5) | 4.22 (0.28, 8.56) | 1.98 (0.43, 12.1) |
| Cord plasma/maternal plasma ratio | 0.71 (0.18, 181.8) | 0.09 (0.01, 1.21) | 1.06 (0.68, 1.68) | 0.95 (0.35, 2.04) | 0.62 (0.33, 1.38) |
| Cord PBMC/placenta ratio | 0 (0, 0.11) | 0 (0, 0.02) | 0 (0, 0.02) | 0.01 (0, 0.03) | 0 (0, 0.01) |
| Cord PBMC/maternal PBMC ratio | 1.36 (0.03, 60) | 0.49 (0.12, 1.25) | 1 (0.51, 2.5) | 1.41 (0.06, 2.4) | 1.45 (1.38, 1.62) |
| Cord plasma/cord PBMC ratio | 537.3 (136.8, 6,150) | 210 (6, 2,149) | 7,212 (1,210, 31,069) | 571.1 (21.1, 2,675) | 721.6 (95.2, 2,691) |
COBI, cobicistat; EVG, elvitegravir; DTG, dolutegravir; FTC, emtricitabine; TFV, tenofovir; PBMCs, peripheral blood mononuclear cells.
These data represent the phosphorylated metabolites in PBMCs (tenofovir diphosphate and emtricitabine triphosphate).
EVG and DTG were detected within all five matrices; however, differences were noted when EVG and DTG concentration ratios were compared between the different matrices. Low concentration ratios for EVG and DTG were noted between cord plasma and placenta, cord plasma and maternal plasma, and cord PBMCs and maternal PBMCs. There are limited PK data on placental transfer of EVG (4–8). Distributions of all five drug concentrations for all the matrices were analyzed and are plotted in Fig. 1.
We also analyzed the concentration ratios of all five drugs between maternal PBMCs and cord PBMCs. All five drugs transfer from maternal plasma to placenta and then to cord blood. Our data also illustrate how TFV moves from maternal plasma through the placenta to the cord blood and then into cord PBMCs, where it is phosphorylated into its active form (TFV-DP). We show that the concentration of TFV-DP in PBMCs on the maternal side is similar to that on the fetal side. Overall, the transfer of these agents from the maternal into the fetal circulation was associated with relatively similar drug concentrations between the matrices. These agents did not accumulate at higher concentrations in one matrix than the other.
DISCUSSION
In this study, we analyzed the intracellular concentrations of EVG and DTG and other ARVs within placental tissue from HIV-1-infected women. In order to assess in vivo placental transfer of these ARVs, we also compared plasma concentrations from cord and maternal blood at the time of delivery. All five agents transferred from maternal circulation into fetal circulation via the placenta.
Previous studies have demonstrated placental transfer, with drug concentrations approaching 1 μg/ml in maternal and cord blood ratios for certain nucleoside (or nucleotide) reverse transcriptase inhibitors (NRTIs) (15–17, 32) with slightly lower ratios reported for non-NRTIs (18–20) and even lower ratios for the protease inhibitors (16, 21). Studies analyzing placental transfer of TFV have reported variable results, with maternal and cord blood concentration ratios ranging from 0.82 to 6.0 (16, 18). Protease inhibitors, such as atazanavir (22, 23) and lopinavir (20, 24) actively cross the placenta; however, undetectable concentrations in cord blood have been reported for nelfinavir, indinavir, and saquinavir (25, 26). Fusion/entry inhibitors cross poorly into cord blood (7, 11).
The human placenta plays a major role in maternal-to-fetal and fetal-to-maternal transfer of nutrients and oxygen and of waste products, respectively. Important drug-metabolizing enzymes, such as the cytochrome (CYP) P450 enzymes, fluctuate throughout gestation (27). The CYP3 family enzymes are detected more readily within the placenta than the other CYP family enzymes (27). Cobicistat is an important inhibitor of CYP3A, allowing ARV agents, such as EVG, to bypass first-pass metabolism in the liver and other tissues (9). A few studies have measured the PK of INSTIs during pregnancy (6). RTG has been shown to cross the placenta well, with drug concentrations detected in cord blood plasma from mothers receiving this ARV during pregnancy (11, 14). DTG has also been shown to readily cross the blood-placenta barrier (8). Limited data are available to assess the placental transfer of EVG (28). Given the paucity of data and the increasing use of EVG and DTG in clinical obstetrical practice, including the use of EVG-COBI-TDF-FTC as a single antiretroviral regimen (and now EVG-COBI-TAF-FTC [Stribild; Gilead], where TAF is tenofovir alafenamide), our study is helpful in understanding transplacental passage of INSTIs.
We show for the first time that all four drug components within a single combination regimen (EVG, TFV, FTC, and COBI), as well as DTG, were detected within maternal circulation and placental cells in vivo, crossing from the maternal circulation to the cord blood. We also noted, based on extracellular concentrations, that all of these agents readily crossed the placenta into the fetal circulation, which may be important in preventing vertical HIV-1 transmission. Low concentration ratios for EVG and DTG were noted between cord plasma and placenta, cord plasma and maternal plasma, and cord PBMCs and maternal PBMCs, indicating that not only were these ARVs detected in the matrices but, more importantly, that transfer of these agents from the maternal into the fetal circulation had occurred with relatively similar drug concentrations between the matrices and, further, that ARVs did not accumulate at higher concentrations in one matrix than the other.
The comparison between maternal PBMCs and cord PBMCs illustrates that both EVG and DTG transfer from maternal plasma to the placenta and then into cord blood. TFV moves from maternal plasma through the placenta to the cord blood and then into cord PBMCs, where it is phosphorylated into its active form (TFV-DP). We noted that the concentration of TFV-DP in PBMCs on the maternal side was similar to that on the fetal side. The transfer of all ARV agents from the maternal into the fetal circulation was associated with relatively similar drug concentrations between the matrices. These agents did not accumulate at higher concentrations in one matrix than the other. Variable detection of ARVs in the fetal circulation allude to different mechanisms of transplacental drug transfer.
No previous studies have analyzed the intracellular concentrations of any ARVs within the placenta. Detection of both INSTIs within homogenized placental tissue predicted the detectable maternal and cord plasma concentrations, emphasizing the importance of adequate ARV penetration intracellularly within placental cells.
Limitations and future directions.
Scarce data on placental transfer of DTG exist, with no prior studies previously conducted. The major drawback of our study includes a small sample size. Existing safety profile data of INSTIs are limited during pregnancy. Since our study had a small sample size, future larger prospective studies should evaluate intra- and extracellular placental concentrations of these ARVs. In addition, we did not measure unbound concentrations, thus limiting our ability to assess the toxicity of these compounds.
A recent study documented that cART, which includes an INSTI backbone, induces a more rapid reduction in HIV-1 RNA viral load than other regimens during pregnancy, which may be important if HIV-1-infected pregnant women present late to prenatal care or have failed to initiate an appropriately selected ARV regimen (29). Given our findings and previous data (7, 29, 30), consideration should be given to the use of the newer-generation INSTIs as part of a first-line preferred regimen during pregnancy.
We have previously studied the role of placental Hofbauer cells (placental macrophages) from HIV-1-infected pregnant subjects and their ability to reduce the replication and dissemination of CCR5-tropic HIV-1BaL in vitro, offsetting MTCT (31). With our extensive experience with this unique placental cell type, future studies could assess their potential association with transplacental passage of not only INSTIs but also other novel ARV agents, as a means of prevention of MTCT of HIV-1. This is the first study to characterize pharmacologic transfer of ARVs in the placenta at a cellular level.
MATERIALS AND METHODS
Subject enrollment procedure.
We conducted a prospective cohort pilot study of HIV-1-infected pregnant women receiving prenatal care at Grady Memorial Hospital in Atlanta, GA, between April 2015 and June 2016. The women were receiving 150 mg elvitegravir (EVG), 200 mg emtricitabine (FTC), 300 mg tenofovir disoproxil fumarate (TDF), and 150 mg cobicistat (COBI) (Stribild; Gilead) or 50 mg dolutegravir (DTG) within a combined ARV regimen. All of these women were managed in the Grady Memorial Hospital HIV prenatal clinic, which is staffed by maternal-fetal medicine physicians.
Eligibility criteria included pregnancy, HIV-1 infection, age of >18 years, receipt of prenatal care at Grady Memorial Hospital, and English speaking. Exclusion criteria included pregnant women with coexisting psychiatric illness or coexisting hepatitis B or C infection. The Emory University Institutional Review Board and Grady Research Oversight Committee approved this study. Given the once-daily dosing frequency of the newer INSTIs, our patients received a regimen containing either EVG or DTG as part of combination antiretroviral therapy (9).
Thirteen patients provided written informed consent during antenatal care visit and were enrolled in our study (10 patients on EVG-COBI-TDF-FTC and 3 patients on a DTG-containing regimen). Paired maternal and cord blood samples (10 ml) at the time of delivery were collected. Timing of cART was recorded in relation to when samples were collected at the time of delivery. Placentae from HIV-1-infected pregnant women receiving EVG or DTG, who delivered either vaginally or by cesarean delivery, were obtained.
Human placental isolation.
Term and preterm placentae were collected within 30 min of delivery. A segment of a placental cotyledon was excised into 2- by 2-cm pieces, drained of excess blood, and transported to the research laboratory. Membrane-free villous tissue was dissected from the placenta, rinsed in sterile phosphate-buffered saline (PBS) (31) to drain residual blood from tissue, and then stored at −80°C.
Isolation of mononuclear cells by Ficoll-Hypaque gradient centrifugation.
Cord blood mononuclear cells and peripheral blood mononuclear cells (PBMCs) were separated from heparinized whole-blood samples by density gradient centrifugation on Ficoll-Hypaque (Sigma Chemical Co., St. Louis, MO) and maintained in RPMI medium supplemented with 10% fetal bovine serum (FBS), 1 mM l-glutamine, and 1% penicillin/streptomycin (Mediatech, Manassas, VA). Cells were counted, and viability was determined by trypan blue exclusion.
Quantification of cART by LC-MS/MS.
Five million PBMCs were extracted with 500 μl of 70% methanol: 100 μl of human plasma was extracted with 500 μl of methanol, and 0.25 to 0.3 g of placental tissue was homogenized and extracted with 1 ml of acidified acetonitrile. The supernatant of each extraction was collected and divided into two equal fractions, air dried, and reconstituted in two different buffers, (i) 2 mM ammonium acetate with 0.1% formic acid and (ii) 2 mM ammonium acetate with 0.1% formic acid and methanol (40:60, vol/vol), and then subjected to LC-MS/MS analysis. A Dionex Ultimate 3000 high-performance liquid chromatography (HPLC) system (Thermo Scientific, Waltham, MA) coupled with an AB SCIEX API 5000 triple-quadrupole mass spectrometer (AB SCIEX, Framingham, MA) with an ESI interface operated in positive mode was used for the liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis.
Analytes were separated by a Kinetex XB-C18 column (100 by 2.1 mm) with a particle size of 2.6 μm (Phenomenex, Torrance, CA, USA) at a flow rate of 200 μl/min. Gradient elution was used for the separation with mobile phase A (2 mM ammonium acetate with 0.1% formic acid) and mobile phase B (acetonitrile). Target compounds were detected using a multiple reaction monitoring (MRM) mode. The precursors to product ion transitions were monitored at m/z 248 to 130, m/z 288 to 176, m/z 448.2 to 344.1, m/z 776.6 to 606.2, and m/z 420.1 to 136.1 for FTC, TFV, EVG, COBI, and DTG, respectively. Analyst software version 1.5.2 was used to operate the mass spectrometer and to perform data analysis. Calibration curves were generated from standards of cART by serial dilutions in blank biometric samples using the same extraction method described above. The calibration curves had r2 value greater than 0.99.
Statistical significance was evaluated at the 0.05 significance level, and data analysis was performed using SAS v9.4 (SAS, Cary, NC) and R v3.3 (R Foundation for Statistical Computing, Vienna, Austria).
ACKNOWLEDGMENTS
Rana Chakraborty is supported by the National Institute of Child Health and Human Development (NICHD) International Maternal, Pediatric, Adolescent AIDS Clinical Trials (IMPAACT) Network (HHSN275701300003C) and by NICHD grant 1U01AI131566-01. Erica Johnson is supported by NICCH grant 1U01AI131566-01. Martina Badell is supported by the NICHD IMPAACT Network (HHSN275701300003C). Raymond F. Schinazi is supported by CFAR grant NIH5P30-AI-50409. None of the other authors have any financial or funding disclosures to present.
Rana Chakraborty receives research support from Gilead. None of the other authors have any conflicts of interest.
REFERENCES
- 1.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JH, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, de Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Fleming TR, HPTN 052 Study Team . 2011. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zolopa A, Andersen J, Powderly W, Sanchez A, Sanne I, Suckow C, Hoqq E, Komarow L. 2009. Antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS One 4:e5575. doi: 10.1371/journal.pone.0005575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minkoff H, Ahdieh L, Watts H, Greenblatt RM, Schmidt J, Schneider M, Stek A. 2001. The relationship of pregnancy to the use of highly active antiretroviral therapy. Am J Obstet Gynecol 184:1221–1227. doi: 10.1067/mob.2001.113871. [DOI] [PubMed] [Google Scholar]
- 4.Nabekura T, Kawasaki T, Kamiya Y, Uwai Y. 2015. Effects of antiviral drugs on organic anion transport in human placental BeWo cells. Antimicrob Agents Chemother 59:7666–7670. doi: 10.1128/AAC.01634-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Else LJ, Taylor S, Back DJ, Khoo SH. 2011. Pharmacokinetics of antiretroviral drugs in anatomical sanctuary sites: the fetal compartment (placenta and amniotic fluid). Antivir Ther 16:1139–1147. doi: 10.3851/IMP1918. [DOI] [PubMed] [Google Scholar]
- 6.McCormack SA, Best BM. 2014. Protecting the fetus against HIV infection: a systematic review of placental transfer of antiretrovirals. Clin Pharmacokinet 53:989–1004. doi: 10.1007/s40262-014-0185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watts DH, Stek A, Best BM, Wang J, Capparelli EV, Cressey TR, Aweeka F, Lizak P, Kreitchmann R, Burchett SK, Shapiro DE, Hawkins E, Smith E, Mirochnick M, IMPAACT 1026s Study Team . 2014. Raltegravir pharmacokinetics during pregnancy. J Acquir Immune Defic Syndr 67:375–381. doi: 10.1097/QAI.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schalkwijk S, Greupink R, Colbers AP, Wouterse AC, Verweij VG, van Drongelen J, Teulen M, van den Oetelaar D, Burger DM, Russel FG. 2016. Placental transfer of the HIV integrase inhibitor dolutegravir in an ex vivo human cotyledon perfusion model. J Antimicrob Chemother 71:480–483. doi: 10.1093/jac/dkv358. [DOI] [PubMed] [Google Scholar]
- 9.Jeantils V, Alloui C, Rodrigues A, Bentata M, Peytavin G, Carbillon L. 2009. Use of enfurvitide in pregnancy in HIV positive women in seven cases. Gynecol Obstet Fertil 37:396–400. (In French.) doi: 10.1016/j.gyobfe.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Reference deleted.
- 11.Croci L, Trezzi M, Allegri MP, Carli T, Chigiotti S, Riccardi MP, Riccardi B, Toti M, Nencioni C. 2012. Pharmacokinetic and safety of raltegravir in pregnancy. Eur J Clin Pharmacol 68:1231–1232. doi: 10.1007/s00228-012-1250-5. [DOI] [PubMed] [Google Scholar]
- 12.McKeown DA, Rosenvinge M, Donaghy S, Sharland M, Holt DW, Cormack I, Hay P, Sadiq ST. 2010. High neonatal concentrations of raltegravir following transplacental transfer in HIV-1 positive pregnant women. AIDS 24:2416–2418. doi: 10.1097/QAD.0b013e32833d8a50. [DOI] [PubMed] [Google Scholar]
- 13.Hegazi A, McKeown D, Doerholt K, Donaghy S, Sadiq ST, Hay P. 2012. Raltegravir in the prevention of mother-to-child transmission of HIV-1: effective transplacental transfer and delayed plasma clearance observed in preterm neonates. AIDS 26:2421–2423. doi: 10.1097/QAD.0b013e32835a9aeb. [DOI] [PubMed] [Google Scholar]
- 14.Johnson EL, Chakraborty R. 2012. Placental Hofbauer cells limit HIV-1 replication and potentially offset mother to child transmission (MTCT) by induction of immunoregulatory cytokines. Retrovirology 9:101. doi: 10.1186/1742-4690-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chappuy H, Treluyer JM, Jullien V, Dimet J, Rey E, Fouche M, Firtion G, Pons G, Mandelbrot L. 2004. Maternal-fetal transfer and amniotic fluid accumulation of nucleoside analogue reverse transcriptase inhibitors in human immunodeficiency virus-infected pregnant women. Antimicrob Agents Chemother 48:4332–4336. doi: 10.1128/AAC.48.11.4332-4336.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colbers AP, Hawkins DA, Gingelmaier A, Kabeya K, Rockstroh JK, Wyen C, Weizsacker K, Sadiq ST, Ivanovic J, Giaquinto C, Taylor GP, Molto J, Burger DM. PANNA Network. 2013. The pharmacokinetics, safety and efficacy of tenofovir and emtricitabine in HIV-1-infected pregnant women. AIDS 27:739–748. doi: 10.1097/QAD.0b013e32835c208b. [DOI] [PubMed] [Google Scholar]
- 17.Cressey TR, Stek A, Capparelli E, Bowonwatanuwong C, Prommas S, Sirivatanapa P, Yuthavisuthi P, Neungton C, Huo Y, Smith E, Best BM, Mirochnick M, IMPAACT P1026s Team . 2012. Efavirenz pharmacokinetics during the third trimester of pregnancy and postpartum. J Acquir Immune Defic Syndr 59:245–252. doi: 10.1097/QAI.0b013e31823ff052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gandhi M, Mwesigwa J, Aweeka F, Plenty A, Charlebois E, Ruel TD, Huang Y, Clark T, Ades V, Natureeba P, Luwedde FA, Achan J, Kamya MR, Havlir DV, Cohan D, Prevention of Malaria and HIV Disease in Tororo (PROMOTE) Study. 2013. Hair and plasma data show that lopinavir, ritonvir and efavirenz all tranfer from mother to infant in utero, but efavirenz transfer via breastfeeding. J Acquir Immune Defic Syndr 63:578–584. doi: 10.1097/QAI.0b013e31829c48ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanovic J, Nicastri E, Anceshi MM, Ascenzi P, Signore F, Pisani G, Vallone C, Mattia E, Notari S, Tempestilli M, Puceiil LP, Narciso P, Pregnancy and Newborn Clinical Outcome Group in HIV Infection (PANCOH). 2009. Transplacental transfer of antiretroviral drugs and newborn birth weight in HIV-infected pregnant women. Curr HIV Res 7:620–625. doi: 10.2174/157016209789973628. [DOI] [PubMed] [Google Scholar]
- 20.Ripamonti D, Cattaneo D, Maggiolo F, Airoldi M, Frigerio L, Bertuletti P, Ruggeri M, Suter F. 2007. Atazanivir plus low-dose ritonavir in pregnancy: pharmacokinetics and placental transfer. AIDS 21:2409–2415. doi: 10.1097/QAD.0b013e32825a69d1. [DOI] [PubMed] [Google Scholar]
- 21.Lechelt M, Lyons F, Clarke A, Magaya V, Issa R, de Ruiter A. 2006. Human placental transfer of atazanavir: a case report. AIDS 20:307. doi: 10.1097/01.aids.0000202653.49020.dd. [DOI] [PubMed] [Google Scholar]
- 22.Gingelmaier A, Kurowski M, Kastner R, Eberle J, Mylonas I, Belohradsky BH, Friese K, Grubert TA. 2006. Placental transfer and pharmacokinetics of lopinavir and other protease inhibitors in combination with neverapine at delivery. AIDS 20:1737–1743. doi: 10.1097/01.aids.0000242820.67001.2c. [DOI] [PubMed] [Google Scholar]
- 23.Marzolini C, Rudin C, Decosterd LA, Telenti A, Schreyer A, Biollaz J, Buclin T; Swiss Mother + Child HIV Cohort Study. 2002. Transplacental passage of protease inhbitors at delivery. AIDS 16:889–893. doi: 10.1097/00002030-200204120-00008. [DOI] [PubMed] [Google Scholar]
- 24.Fayet-Mello A, Buclin T, Guignard N, Cruchon S, Cavassini M, Grawe C, Gremlich E, Popp KA, Schmid F, Eap CB, Telenti A, Biollaz J, Decosterd LA, Martinez de Tejada B, Swiss HIV Cohort Study, Mother & Child HIV Cohort Study. 2013. Free and total plasma levels of lopinavir during pregnancy, at delivery and postpartum: implications for dosage adjustments in pregnant women. Antivir Ther 18:171–182. doi: 10.3851/IMP2328. [DOI] [PubMed] [Google Scholar]
- 25.Syme MR, Paxton JW, Keelan JA. 2004. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet 43:487–514. doi: 10.2165/00003088-200443080-00001. [DOI] [PubMed] [Google Scholar]
- 26.Best BM, Capparelli EV, Stek A, Acosta E, Smith E, Chakhtoura N, Wang J, Hernandez A, Mirochnick M, IMPAACT P1026s Protocol Team . 2017. Elvitegravir/cobicistat pharmacokinetics in pregnancy and postpartum, abstr A755 24th Conference on Retroviruses and Opportunistic Infections, Seattle, WA, 13 to 16 February 2017. [Google Scholar]
- 27.Best BM, Burchett S, Li H, Stek A, Hu C, Wang J, Hawkins E, Byroads M, Watts DH, Smith E, Fletcher CV, Capparelli EV, Mirochnick M, International Maternal Pediatric and Adolescent AIDS Clinical Trials (IMPAACT) P1026s Team. 2015. Pharmacokinetics of tenofovir during pregnancy and postpartum. HIV Med 16:502–511. doi: 10.1111/hiv.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahangdale L, Cates J, Potter J, Badell ML, Seidman D, Miller ES, Coleman JS, Lazenby GB, Levison J, Short WR, Yawetz S, Ciaranello A, Livingston E, Duthley L, Rimawi BH, ANderson JR, Stringer EM; HOPES (HIV OB Pregnancy Education Study) Group. 2016. Integrase inhibitors in late pregnancy and rapid HIV viral load reduction. Am J Obstet Gynecol 214:385.e1-7. doi: 10.1016/j.ajog.2015.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blonk MI, Colbers AP, Hidalgo-Tenorio C, Kabeya K, Weizsäcker K, Haberl AE, Moltó J, Hawkins DA, van der Ende ME, Gingelmaier A, Taylor GP, Ivanovic J, Giaquinto C, Burger DM, Pharmacokinetics of Newly Developed Antiretroviral Agents in HIV-Infected Pregnant Women PANNA Network, PANNA Network. 2015. Raltegravir in HIV-1-infected pregnant women: pharmacokinetics, safety, and efficacy. Clin Infect Dis 61:809–816. doi: 10.1093/cid/civ366. [DOI] [PubMed] [Google Scholar]
- 30.Clavel-Osorio C, Cazassus F, Stegmann S, Huc-Anais P, Lecam D, Peytavin G. 2013. One-month transplacental pharmacokinetics of raltegravir in a premature newborn after short-course treatment of the HIV-1-infected mother. Antimicrob Agents Chemother 57:6393–6394. doi: 10.1128/AAC.01349-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fromentin E, Gavegnano C, Obikhod A, Schinazi RF. 2010. Simultaneous quantification of intracellular natural and antiretroviral nucleosides and nucleotides by liquid chromatography-tandem mass spectrometry. Anal Chem 82:1982–1989. doi: 10.1021/ac902737j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeh RF, Rezik NL, Kashuba AD, Dumond JB, Tappouni HL, Tien HC, Chen YC, Vourvahis M, Horton AL, Fiscus SA, Patterson KB. 2009. Genital tract, cord blood, and amniotic fluid exposures of seven antiretroviral drugs during and after pregnancy in human immunodeficiency virus type 1-infected women. Antimicrob Agents Chemother 53:2367–2374. doi: 10.1128/AAC.01523-08. [DOI] [PMC free article] [PubMed] [Google Scholar]