ABSTRACT
We tested 59 common and 27 uncommon Aspergillus species isolates for susceptibility to the mold-active azole antifungal agents itraconazole, voriconazole, and posaconazole using the Sensititre method. The overall essential agreement with the CLSI reference method was 96.5% for itraconazole and posaconazole and was 100% for voriconazole. By the Sensititre method as well as the CLSI reference method, all of 10 A. fumigatus isolates with a cyp51 mutant genotype were classified as being non-wild-type isolates (MIC > epidemiological cutoff value [ECV]) with respect to triazole susceptibility.
KEYWORDS: Aspergillus, Sensititre, antifungal susceptibility testing
TEXT
In contrast to other but emerging molds (1), Aspergillus species, particularly Aspergillus fumigatus, remain the most common causes of invasive fungal diseases in both North America and Europe (2, 3). Because of the availability of (tri)azole antifungal agents, survival of immunocompromised patients with invasive aspergillosis has improved dramatically and could be further improved by optimizing antifungal treatments (4). A key component of this optimization should be the regular in vitro antifungal susceptibility testing of the patients' A. fumigatus isolates to detect azole resistance (5). Unfortunately, in most clinical microbiology laboratories, antifungal susceptibility testing of aspergilli (and other molds) is not routinely performed (6), thus underestimating the true prevalence of fungal resistance (4).
The azole antifungal agents for clinical use include itraconazole, voriconazole, posaconazole, and, most recently, isavuconazole (7). Despite their role—unlike voriconazole, itraconazole and posaconazole are not approved as first-line agents—in treatment of invasive aspergillosis (4), the Clinical and Laboratory Standards Institute (CLSI) did not set clinical breakpoints (CBPs) for common Aspergillus species and mold-active triazoles, e.g., itraconazole and posaconazole (8), in contrast to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (9). However, CLSI-based epidemiological cutoff values (ECVs) were established—instead of CBPs—for Aspergillus species (A. fumigatus, A. flavus, A. terreus, A. niger, A. nidulans, and A. versicolor) and for triazoles to aid in the early identification of clinical isolates with acquired resistance mechanisms (10, 11). Isolates of these six Aspergillus species for which triazole MICs exceed the ECV are considered to be non-wild type (non-WT) and may harbor mutations in the cyp51a gene—the best-known mechanism of triazole resistance in the A. fumigatus species—or other mutations (12). Interestingly, whereas the significance of ECVs in clinical practice needs to be understood, the ECVs defined to date—albeit mainly for Candida species—are based not only on CLSI or EUCAST methods but also on the Sensititre YeastOne (SYO; Thermo Fisher Scientific, MA; reviewed in reference 13) method (8). Whereas we have shown previously that the SYO microdilution panel—with which amphotericin B, echinocandins, and triazoles can be tested in parallel—is a reliable tool for antifungal resistance surveillance in Candida species (14), only limited data have been reported for Aspergillus (and other mold) species (15, 16).
In the present study, we used the SYO method for determining the activities of itraconazole, voriconazole, and posaconazole against clinical Aspergillus isolates, including WT and non-WT (MIC > ECV) isolates, of common (59 isolates) and uncommon (27 isolates) Aspergillus species. All isolates were also tested against triazoles by the CLSI reference microdilution method, and data corresponding to species-specific and overall essential agreement (EA; ± 2 2-fold dilutions) were determined for each triazole.
Before testing was performed, a set of 86 Aspergillus isolates that represented either strains from clinical collections (held at the University Hospitals of Rome [Italy] and Nijmegen [The Netherlands]) or strains freshly isolated from clinical specimens were (re)identified at the species level by both molecular and proteomic analyses. First, comparative sequence analyses of the fungal ribosomal DNA internal transcribed spacer (ITS) region for intersection identification and of the beta-tubulin/calmodulin gene for intrasection identification (i.e., at the species level) were performed according to expert recommendations (17). Second, species-level identification was confirmed or exclusively obtained (e.g., for Aspergillus oryzae isolates) with matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry analysis as described previously (18; also see references 19 and 20), using an in-house database. Isolates with itraconazole and/or voriconazole CLSI MIC values of >1 μg/ml—the ECV developed for A. fumigatus, A. flavus, and A. terreus (10)—were submitted to cyp51 gene sequence analysis for detection of azole resistance-associated mutations (4, 12). The 86 Aspergillus species isolates were tested for in vitro susceptibility to the triazoles (itraconazole, voriconazole, and posaconazole) by using the broth microdilution method of CLSI (21) and the SYO manufacturer-recommended protocol. By the CLSI method, the final range of antifungal concentrations tested was 0.03 to 16 μg/ml for all triazoles; by the SYO method, the antifungal concentrations of the YO10 panel (i.e., the SYO-10 version that includes 10 antifungal agents) ranged from 0.008 to 8 μg/ml for voriconazole and posaconazole and from 0.015 to 16 μg/ml for itraconazole (14). The MIC results for all triazoles were read after 48 h of incubation, and the MIC values were determined visually as the lowest drug concentrations that caused complete (100%) inhibition of growth relative to that of the growth control. As prolonged incubation times (i.e., >24 h) of YO10 panels were required, visual readings of MICs obtained with the SYO method were performed regardless of colorimetric changes. To allow comparability between the methods, MIC values of 0.008 to 0.03 μg/ml for voriconazole and posaconazole and MIC values of 0.015 to 0.03 μg/ml for itraconazole, obtained with the SYO method, were reported as ≤0.03 μg/ml. Otherwise, MIC values of ≥16 μg/ml for voriconazole and posaconazole, obtained with the CLSI method, and similar values for itraconazole, obtained with the SYO method, were reported as >8 μg/ml. The SYO MIC results were compared with those of the CLSI method in order to determine the EA between MIC values. High off-scale MIC results were converted to the next highest concentration, and low off-scale MIC results were left unchanged. Discrepancies of at least ±2 2-fold dilutions among MIC results were used to calculate the EA (see Table S1 in the supplemental material). Thus, percent EA was calculated by using the number of test results in EA as the numerator and the total number of organisms tested as the denominator. Finally, according to the triazole ECVs established for A. fumigatus, A. flavus, A. terreus, A. niger, and A. nidulans (10), percentages of isolates from these species that were classified as WT (MIC ≤ ECV) or non-WT (MIC > ECV) with respect to each antifungal agent using either SYO or CLSI were calculated. MIC values of the triazoles for Candida krusei ATCC 6258, A. fumigatus ATCC MYA-3626, and A. flavus ATCC 204304, which were used as quality control isolates, were all within the expected ranges (data not shown).
Table 1 depicts the MIC distributions for posaconazole, voriconazole, and itraconazole for the 13 species (4 common and 9 uncommon) of Aspergillus tested by the SYO method. The numbers of cyp51 mutant strains detected in A. fumigatus and A. oryzae are listed in parentheses. Overall, 78 of 86 (90.7%) isolates from all Aspergillus species were captured at a posaconazole MIC of 0.5 μg/ml. The posaconazole MIC was 1 μg/ml for all 8 isolates of A. fumigatus characterized as being non-WT for posaconazole (ECV, 0.5 μg/ml). In contrast, 81 (94.2%) and 72 (83.7%) of 86 isolates from all Aspergillus species were captured at MICs of 1 μg/ml for itraconazole and voriconazole, respectively. Among the 14 Aspergillus isolates characterized as being non-WT for voriconazole (ECV, 1 μg/ml) or as having high voriconazole MIC values, the voriconazole MIC was ≥2 μg/ml for 9 A. fumigatus isolates (range, 2 to >8 μg/ml) and 3 A. lentulus isolates (MICs, 2; 2; and 4 μg/ml), 2 μg/ml for 1 A. (Neosartorya) udagawae isolate, and >8 μg/ml for 1 A. oryzae isolate. Only 4 of 9 A. fumigatus isolates were also classified as non-WT for itraconazole (ECV, 1 μg/ml), and the itraconazole MIC was 2 μg/ml for 1 isolate and >8 μg/ml for 3 isolates. The 1 remaining A. fumigatus isolate that was non-WT for itraconazole (MIC, >8 μg/ml) was instead WT for voriconazole. In summary, 2 of the 10 A. fumigatus isolates found to contain mutations in the cyp51a gene were WT for posaconazole (MIC, 0.5 μg/ml), whereas 5 and 1 of these isolates were WT for itraconazole (MICs, 0.5 to 1 μg/ml) and voriconazole (MIC, 1 μg/ml), respectively. Likewise, 1 A. oryzae isolate that contained the T788G mutation in the cyp51c gene exhibited a drug MIC value of 0.5 μg/ml for both posaconazole and itraconazole; such genetic alteration had previously been found in 1 A. flavus isolate exhibiting elevated CLSI voriconazole and itraconazole MICs (8 and 2 μg/ml, respectively) as described elsewhere (22). Taken together, our data indicate that the in vitro activity of posaconazole against both WT and cyp51 mutant strains of Aspergillus species was comparable to that of voriconazole and itraconazole tested by the SYO method. These findings are in agreement with those of Gheith et al., who found that the voriconazole and posaconazole MICs were below the ECVs for all 48 clinical Aspergillus isolates (17 A. niger isolates, 18 A. flavus isolates, 9 A. tubingensis isolates, 2 A. fumigatus isolates, 1 A. westerdijkiae isolate, and 1 A. ochraceus isolate) tested, whereas only 2 of these isolates (2 A. tubingensis isolates; 22%) exhibited itraconazole MICs that were >ECV (15). Although it is plausible that lower itraconazole susceptibility of A. tubingensis isolates is related to the occurrence of a cyp51a mutation—similarly to the mutation described in Aspergillus awamori (another species of the section Nigri; see reference 23), the finding of high voriconazole susceptibility in the Aspergillus species studied by Gheith et al. (15) argues for the use of voriconazole as the first-line treatment of invasive aspergillosis in hospital settings, in keeping with the international recommendations (24). However, these recommendations need to be cautiously assessed in confirmed cases of azole-resistant aspergillosis (25).
TABLE 1.
In vitro susceptibilities of 86 Aspergillus species isolates to azole antifungal agents as determined by the SYO methoda
| Species (no. of isolates tested) | Species complex or section | Antifungal agentc | No. of isolates (no. of mutantsb) with MIC (μg/ml) of: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | >8 | |||
| Common species | ||||||||||||
| A. fumigatus (21) | Fumigati | PSC | 5 | 6 | 2 (2) | 8 (8) | ||||||
| VRC | 2 | 5 | 4 | 1 (1) | 1 (1) | 2 (2) | 1 (1) | 5 (5) | ||||
| ITC | 2 | 7 | 3 (1) | 4 (4) | 1 (1) | 4 (4) | ||||||
| A. flavus (19) | Flavi | PSC | 7 | 12 | ||||||||
| VRC | 1 | 14 | 3 | 1 | ||||||||
| ITC | 1 | 9 | 8 | 1 | ||||||||
| A. terreus (12) | Terrei | PSC | 9 | 3 | ||||||||
| VRC | 1 | 8 | 3 | |||||||||
| ITC | 3 | 8 | 1 | |||||||||
| A. niger (7) | Nigri | PSC | 1 | 2 | 1 | 3 | ||||||
| VRC | 6 | 1 | ||||||||||
| ITC | 2 | 3 | 2 | |||||||||
| Uncommon species | ||||||||||||
| A. tubingensis (6) | Nigri | PSC | 4 | 2 | ||||||||
| VRC | 1 | 5 | ||||||||||
| ITC | 3 | 3 | ||||||||||
| A. nidulans (5) | Nidulantes | PSC | 3 | 2 | ||||||||
| VRC | 2 | 3 | ||||||||||
| ITC | 4 | 1 | ||||||||||
| A. oryzae (5) | Flavi | PSC | 2 | 2 | 1 (1) | |||||||
| VRC | 2 | 2 | 1 (1) | |||||||||
| ITC | 3 | 1 | 1 (1) | |||||||||
| A. lentulus (3) | Fumigati | PSC | 2 | 1 | ||||||||
| VRC | 2 | 1 | ||||||||||
| ITC | 1 | 2 | ||||||||||
| A. (Neosartorya) species (3)d | Fumigati | PSC | 2 | 1 | ||||||||
| VRC | 1 | 1 | 1 | |||||||||
| ITC | 2 | 1 | ||||||||||
| A. foetidus (3) | Nigri | PSC | 2 | 1 | ||||||||
| VRC | 2 | 1 | ||||||||||
| ITC | 3 | |||||||||||
| A. awamori (2) | Nigri | PSC | 2 | |||||||||
| VRC | 2 | |||||||||||
| ITC | 1 | 1 | ||||||||||
MICs were determined visually after 48 h of incubation and were defined as the antifungal concentrations at which complete (100%) inhibition of growth of the Aspergillus species isolates was observed. As a prolonged incubation (i.e., >24 h) of SYO colorimetric plates was required, visual readings of MICs were performed regardless of color changes.
Mutant isolates were defined as isolates carrying a cyp51a mutation (e.g., a leucine-for-histidine substitution), together with a tandem repeat of a 34-bp (or 46-bp) sequence in the gene promoter that is known to be associated with azole resistance in A. fumigatus (4, 12). One of 5 A. oryzae isolates was found to carry the T788G mutation in the cyp51c gene that has been reported as an azole resistance mechanism in the closely related species A. flavus (22).
PSC, posaconazole; VRC, voriconazole; ITC, itraconazole.
Data include 1 isolate each of A. (Neosartorya) hiratsukae, A. thermomutatus (Neosartorya pseudofischeri), and A. (Neosartorya) udagawae. In accordance with the recent taxonomists' recommendations for species for which a single-name nomenclature (i.e., keeping the name Aspergillus for all species of this genus) must be applied (17), the old teleomorphic name is indicated in brackets.
Table 2 shows the comparative levels of in vitro activity of the three azoles against the 13 Aspergillus species using the SYO and CLSI methods. Whereas the posaconazole MIC results were comparable for the two methods, the MIC values obtained for voriconazole were generally higher and for itraconazole were lower with the SYO method than with the CLSI method. The overall EA between SYO MICs and CLSI MICs was 100% for voriconazole and 96.5% for both itraconazole and posaconazole. Determined only for isolates of A. fumigatus (n = 21), A. flavus (n = 19), A. terreus (n = 12), and A. niger (n = 7), the EA value was unchanged for voriconazole (100%), whereas it increased for posaconazole (98.3%) and decreased for itraconazole (94.9%). As detailed (see Table S1 in the supplemental material), the lowest EA value (66.7%) was seen with 3 isolates of A. (Neosartorya) hiratsukae, A. thermomutatus (Neosartorya pseudofischeri), and A. (Neosartorya) udagawae. However, the N. udagawae isolate had SYO and CLSI MICs that disagreed for 3 2-fold dilutions, although 2 other isolates had SYO and CLSI MICs that were in agreement at ±0 2-fold dilutions. The categorical agreement between the methods was 96.9% (62/64 isolates) for posaconazole, 98.4% (63/64 isolates) for voriconazole, and 93.7% (60/64 isolates) for itraconazole in interpreting the MICs according to CLSI ECVs for the 21 A. fumigatus, 19 A. flavus, 12 A. terreus, 7 A. niger, and 5 A. nidulans isolates studied.
TABLE 2.
Comparison of in vitro activities of posaconazole, voriconazole, and itraconazole tested against Aspergillus species by SYO and CLSI methodsa
| Species (no. of isolates tested)b | Test method | MIC (μg/ml) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PSC |
VRC |
ITC |
||||||||
| Range | Mode(s) | % EA | Range | Mode(s) | % EA | Range | Mode(s) | % EA | ||
| A. fumigatus (21) | SYO | 0.06 to 1 | 1 | 100 | 0.125 to >8 | 0.25 | 100 | 0.125 to >8 | 0.25 | 95.2 |
| CLSI | ≤0.03 to 2 | 1 | 0.06 to >8 | 0.125 | 0.25 to >8 | 1 | ||||
| A. flavus (19) | SYO | 0.06 to 0.125 | 0.125 | 100 | 0.125 to 1 | 0.25 | 100 | 0.03 to 0.25 | 0.06 | 94.7 |
| CLSI | ≤0.03 to 0.25 | 0.125 | 0.06 to 0.25 | 0.125 | 0.06 to 0.5 | 0.125 | ||||
| A. terreus (12) | SYO | 0.06 to 0.125 | 0.06 | 100 | 0.06 to 0.25 | 0.125 | 100 | 0.06 to 0.25 | 0.125 | 91.7 |
| CLSI | ≤0.03 to 0.25 | 0.25 | 0.06 to 0.125 | 0.06 | 0.125 to 0.5 | 0.25 | ||||
| A. niger (7) | SYO | ≤0.03 to 0.25 | 0.25 | 85.7 | 0.25 to 0.5 | 0.25 | 100 | 0.125 to 0.5 | 0.25 | 100 |
| CLSI | 0.06 to 0.25 | 0.25 | 0.06 to 0.25 | 0.25 | 0.5 to 1 | 0.5 | ||||
| A. tubingensis (6) | SYO | 0.125 to 0.25 | 0.125 | 100 | 0.25 to 0.5 | 0.5 | 100 | 0.25 to 0.5 | 0.25, 0.5 | 100 |
| CLSI | 0.125 to 0.5 | 0.125, 0.25, 0.5 | 0.125 to 0.5 | 0.25 | 0.5 to 2 | 0.5 | ||||
| A. nidulans (5) | SYO | 0.06 to 0.125 | 0.06 | 100 | 0.125 to 0.25 | 0.25 | 100 | 0.125 to 0.25 | 0.125 | 100 |
| CLSI | ≤0.03 to 0.06 | 0.03 | 0.06 to 0.25 | 0.06, 0.125 | 0.25 to 1 | 0.5 | ||||
| A. oryzae (5) | SYO | 0.125 to 0.5 | 0.125, 0.25 | 80.0 | 0.5 to >8 | 0.5, 1 | 100 | 0.125 to 0.5 | 0.125 | 100 |
| CLSI | ≤0.03 to 1 | 0.03 | 0.125 to 2 | 0.125 | 0.25 to 1 | 0.25 | ||||
| A. lentulus (3) | SYO | 0.03 to 0.125 | 0.03 | 100 | 2 to 4 | 2 | 100 | 0.06 to 0.125 | 0.125 | 100 |
| CLSI | ≤0.03 to 0.06 | 0.03 | 0.5 to 2 | ND | 0.25 to 0.5 | 0.25 | ||||
| A. (Neosartorya) species (3)c | SYO | 0.125 to 0.25 | 0.125 | 66.7 | 0.5 to 2 | ND | 100 | 0.25 to 0.5 | 0.25 | 100 |
| CLSI | ≤0.03 to 0.125 | 0.125 | 0.5 to 1 | 1 | 0.5 to 1 | 0.5 | ||||
| A. foetidus (3) | SYO | 0.125 to 0.25 | 0.125 | 100 | 0.5 to 1 | 0.5 | 100 | 0.5 | 0.5 | 100 |
| CLSI | ≤0.03 to 0.25 | ND | 0.25 to 0.5 | 0.25 | 0.25 to 2 | ND | ||||
| A. awamori (2) | SYO | 0.06 | 0.06 | 100 | 0.25 | 0.25 | 100 | 0.125 to 0.25 | ND | 100 |
| CLSI | ≤0.03 to 0.125 | ND | 0.125 to 0.25 | ND | 0.25 to 0.5 | ND | ||||
Posaconazole (PSC), voriconazole (VRC), and itraconazole (ITC) MICs were defined as the antifungal concentrations at which complete (100%) inhibition of growth of the Aspergillus species isolates was observed and are reported as the range and mode(s) (i.e., most frequent MIC[s] for each species). ND, not determined. For each species, the essential agreement (EA) between MIC values (± 2 2-fold dilutions) was calculated by comparison of MIC results obtained with the SYO method to those obtained with the CLSI method.
Except for A. nidulans, all of the less common or cryptic Aspergillus species listed belonged to the following Aspergillus sections, per molecular and/or proteomic-based identification: Fumigati (A. lentulus and Aspergillus [Neosartorya] spp.), Flavi (A. oryzae), and Nigri (A. tubingensis, A. foetidus, and A. awamori).
Data include 1 isolate each of A. (Neosartorya) hiratsukae, A. thermomutatus (Neosartorya pseudofischeri), and A. (Neosartorya) udagawae. In accordance with the recent taxonomists' recommendations for species for which a single-name nomenclature (i.e., keeping the name Aspergillus for all species of this genus) must be applied (17), the old teleomorphic name is indicated in parentheses.
Table 3 summarizes the SYO and CLSI triazole MICs for 10 A. fumigatus isolates with cyp51 alterations. All but 2 isolates exhibited non-WT phenotypes for posaconazole and voriconazole (or itraconazole) according to their decreased susceptibilities (MIC > ECV) obtained with both the SYO and CLSI methods. It is worth noting that for 2 (v075-77 and v128-51) of 5 isolates with a TR34/L98H mutation, a posaconazole non-WT phenotype was determined by the CLSI method (MICs, 1 μg/ml) but not by the SYO method (MICs, 0.5 μg/ml). Interestingly, for the v128-51 isolate, a voriconazole non-WT phenotype was determined by the SYO method (MIC, 2 μg/ml) but not by the CLSI method (MIC, 1 μg/ml). In general, discrepancies between the methods—with respect to their capability of discriminating non-WT from WT isolates—were noticed among the A. fumigatus isolates for which ±1-dilution MIC differences fell into ranges of 0.5 to 1 μg/ml or 1 to 2 μg/ml and thus include the posaconazole or voriconazole (and itraconazole) ECVs of 0.5 and 1 μg/ml, respectively. Consistently, all of 5 A. fumigatus isolates with TR46/Y121F T289A mutations were found to have non-WT phenotypes for both posaconazole and voriconazole that were determined by both the SYO method (MICs of 1 and ≥8 μg/ml, respectively) and the CLSI method (MICs of ≥1 and >8 μg/ml, respectively). Once again, for 4 of these isolates, a non-WT phenotype for itraconazole was determined by the CLSI method (MICs, 2 μg/ml) but by not the SYO method (MICs, 0.5 to 1 μg/ml). Not surprisingly, MIC results showing discrepancies between commercial antifungal susceptibility methods (i.e., Etest and SYO) and the reference antifungal susceptibility method (i.e., EUCAST) (23), as well as between Etest and SYO methods (15), have been reported in previous evaluation studies. As outlined by Arendrup et al. (9), this issue can be related to the relatively low numbers of Aspergillus isolates with acquired resistance mechanisms that were tested in the single studies.
TABLE 3.
Triazole MICs for Aspergillus species isolates carrying a mutated cyp51 gene, as determined by SYO and CLSI methodsa
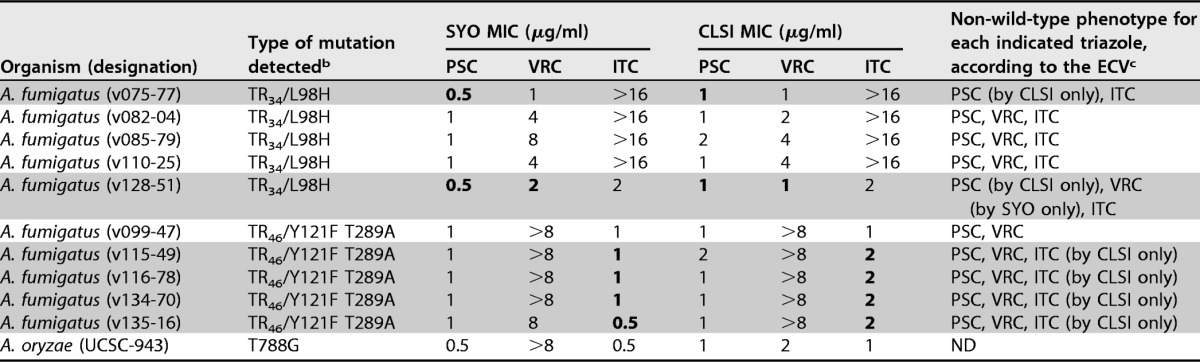
MICs of the triazoles posaconazole (PSC), voriconazole (VRC), and itraconazole (ITC) were determined as specified in the text (also see Tables 1 and 2 for details). MIC values of >16 μg/ml for itraconazole obtained by both methods were reported unchanged, whereas MIC values of ≥16 μg/ml for voriconazole obtained with the CLSI method were reported as >8 μg/ml, according to that specified in the text.
b Mutations occurring in the cyp51a gene of A. fumigatus and in the cyp51c gene (the homologue of cyp51a) of A. oryzae, which encode azole target enzyme, are indicated. The T788G missense mutation has been described, for the first time, in a clinical isolate of A. flavus (a species closely related to A. oryzae), with the data showing reduced in vitro susceptibility to voriconazole (MIC, 8 μg/ml) and itraconazole (MIC, 2 μg/ml) (22).
c ECVs were those published by Espinel-Ingroff et al. (10). Accordingly, PSC ECVs were used to identify non-wild-type (non-WT) isolates of A. fumigatus, A. terreus, and A. niger (ECV = >0.5 μg/ml), A. flavus (ECV = >0.25 μg/ml), and A. nidulans (ECV = >1 μg/ml); VRC ECVs were used to identify non-WT isolates of A. fumigatus, A. flavus, and A. terreus (ECV = >1 μg/ml) and of A. niger and A. nidulans (ECV = >2 μg/ml); and ITC ECVs were used to identify non-WT isolates of A. fumigatus, A. flavus, A. terreus, and A. nidulans (ECV = >1 μg/ml) and of A. niger (ECV = >2 μg/ml). Gray-shaded zones highlight those A. fumigatus isolates for which non-WT phenotypes were determined by only one of two methods (i.e., SYO or CLSI), where boldface denotes the MIC values that gave rise to the discrepancies between the methods. ND, not determined (because ECVs are lacking for the indicated species).
In conclusion, data originating from the present study support the claim that the SYO method is equivalent to the CLSI reference method for the identification of triazole resistance or decreased susceptibility (non-WT; MIC > ECV) in most Aspergillus species. Posaconazole MIC values of ≤0.5 μg/ml provided separation between WT strains of A. fumigatus (and A. flavus) species complexes and those harboring mutations in the cyp51 gene, as tested here (Table 1) and by others (26). However, the simultaneous testing of voriconazole and itraconazole—as allowed through use of the SYO antifungal panel—against these species was shown to enhance the identification of A. fumigatus strains with cyp51a gene alterations, especially the TR34/L98H and TR46/Y121F T289A mutations (the latter being associated with particularly high [≥16 μg/ml] voriconazole MICs; see reference 27) which confer triazole cross-resistance. Future studies are expected to clarify the clinical relevance of Aspergillus (or other mold) testing in the absence of CLSI CBPs for licensed triazoles, as well as the mechanisms of resistance in less-common non-A. fumigatus species.
Ultimately, while we agree that the SYO microdilution panel offers a practical alternative to the reference (CLSI or EUCAST) method for antifungal susceptibility testing of molds (16), clinical microbiologists who use SYO in the routine setting, as we do, are required to compare the MIC mode and range data for each mold species tested in their own laboratory with the MIC distributions freely available on line or in the published literature (9). This would guarantee that MIC endpoints generated in the laboratory for each mold species would mirror those of the reference antifungal susceptibility testing methods and thus would be able to be correctly used for clinical purposes. However, as variation between laboratories that use reference methods may occur, it is desirable that a quality control standard for MIC values should also be part of the CE marking of the SYO method.
Supplementary Material
ACKNOWLEDGMENTS
Università Cattolica del Sacro Cuore (UCSC) provided funding to B.P. under grant number Linea D1. Università Cattolica del Sacro Cuore (UCSC) provided funding to M.S. under grant number Linea D1.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00168-17.
REFERENCES
- 1.Miceli MH, Lee SA. 2011. Emerging moulds: epidemiological trends and antifungal resistance. Mycoses 54:e666–678. doi: 10.1111/j.1439-0507.2011.02032.x. [DOI] [PubMed] [Google Scholar]
- 2.Pfaller MA, Diekema DJ. 2010. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol 36:1–53. doi: 10.3109/10408410903241444. [DOI] [PubMed] [Google Scholar]
- 3.Drgona L, Khachatryan A, Stephens J, Charbonneau C, Kantecki M, Haider S, Barnes R. 2014. Clinical and economic burden of invasive fungal diseases in Europe: focus on pre-emptive and empirical treatment of Aspergillus and Candida species. Eur J Clin Microbiol Infect Dis 33:7–21. doi: 10.1007/s10096-013-1944-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verweij PE, Chowdhary A, Melchers WJ, Meis JF. 2016. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis 62:362–368. doi: 10.1093/cid/civ885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verweij PE, Lestrade PP, Melchers WJ, Meis JF. 2016. Azole resistance surveillance in Aspergillus fumigatus: beneficial or biased? J Antimicrob Chemother 71:2079–2082. doi: 10.1093/jac/dkw259. [DOI] [PubMed] [Google Scholar]
- 6.Posteraro B, Torelli R, De Carolis E, Posteraro P, Sanguinetti M. 2014. Antifungal susceptibility testing: current role from the clinical laboratory perspective. Mediterr J Hematol Infect Dis 6:e2014030. doi: 10.4084/mjhid.2014.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miceli MH, Kauffman CA. 2015. Isavuconazole: a new broad-spectrum triazole antifungal agent. Clin Infect Dis 61:1558–1565. doi: 10.1093/cid/civ571. [DOI] [PubMed] [Google Scholar]
- 8.Espinel-Ingroff A, Turnidge J. 2016. The role of epidemiological cutoff values (ECVs/ECOFFs) in antifungal susceptibility testing and interpretation for uncommon yeasts and moulds. Rev Iberoam Micol 33:63–75. doi: 10.1016/j.riam.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Arendrup MC, Cuenca-Estrella M, Lass-Flörl C, Hope WW. 2013. Breakpoints for antifungal agents: an update from EUCAST focussing on echinocandins against Candida spp. and triazoles against Aspergillus spp. Drug Resist Updat 16:81–95. doi: 10.1016/j.drup.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Espinel-Ingroff A, Diekema DJ, Fothergill A, Johnson E, Pelaez T, Pfaller MA, Rinaldi MG, Canton E, Turnidge J. 2010. Wild-type MIC distributions and epidemiological cutoff values for the triazoles and six Aspergillus spp. for the CLSI broth microdilution method (M38-A2 document). J Clin Microbiol 48:3251–3257. doi: 10.1128/JCM.00536-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinel-Ingroff A, Chowdhary A, Gonzalez GM, Lass-Flörl C, Martin-Mazuelos E, Meis J, Peláez T, Pfaller MA, Turnidge J. 2013. Multicenter study of isavuconazole MIC distributions and epidemiological cutoff values for Aspergillus spp. for the CLSI M38-A2 broth microdilution method. Antimicrob Agents Chemother 57:3823–3828. doi: 10.1128/AAC.00636-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfaller MA. 2012. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med 125:S3–13. doi: 10.1016/j.amjmed.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Posteraro B, Sanguinetti M. 2014. The future of fungal susceptibility testing. Future Microbiol 9:947–967. doi: 10.2217/fmb.14.55. [DOI] [PubMed] [Google Scholar]
- 14.Posteraro B, Spanu T, Fiori B, De Maio F, De Carolis E, Giaquinto A, Prete V, De Angelis G, Torelli R, D'Inzeo T, Vella A, De Luca A, Tumbarello M, Ricciardi W, Sanguinetti M. 2015. Antifungal susceptibility profiles of bloodstream yeast isolates by Sensititre YeastOne over nine years at a large Italian teaching hospital. Antimicrob Agents Chemother 59:3944–3955. doi: 10.1128/AAC.00285-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gheith S, Saghrouni F, Bannour W, Ben Youssef Y, Khelif A, Normand AC, Piarroux R, Ben Said M, Njah M, Ranque S. 2014. In vitro susceptibility to amphotericin B, itraconazole, voriconazole, posaconazole and caspofungin of Aspergillus spp. isolated from patients with haematological malignancies in Tunisia. Springerplus 3:19. doi: 10.1186/2193-1801-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halliday CL, Chen SC, Kidd SE, van Hal S, Chapman B, Heath CH, Lee A, Kennedy KJ, Daveson K, Sorrell TC, Morrissey CO, Marriott DJ, Slavin MA. 2016. Antifungal susceptibilities of non-Aspergillus filamentous fungi causing invasive infection in Australia: support for current antifungal guideline recommendations. Int J Antimicrob Agents 48:453–458. doi: 10.1016/j.ijantimicag.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Samson RA, Visagie CM, Houbraken J, Hong SB, Hubka V, Klaassen CH, Perrone G, Seifert KA, Susca A, Tanney JB, Varga J, Kocsubé S, Szigeti G, Yaguchi T, Frisvad JC. 2014. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud Mycol 78:141–173. doi: 10.1016/j.simyco.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Carolis E, Posteraro B, Lass-Flörl C, Vella A, Florio AR, Torelli R, Girmenia C, Colozza C, Tortorano AM, Sanguinetti M, Fadda G. 2012. Species identification of Aspergillus, Fusarium and Mucorales with direct surface analysis by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Microbiol Infect 18:475–484. doi: 10.1111/j.1469-0691.2011.03599.x. [DOI] [PubMed] [Google Scholar]
- 19.Sanguinetti M, Posteraro B. 2014. MALDI-TOF mass spectrometry: any use for Aspergilli? Mycopathologia 178:417–426. doi: 10.1007/s11046-014-9757-1. [DOI] [PubMed] [Google Scholar]
- 20.Gautier M, Normand AC, Ranque S. 2016. Previously unknown species of Aspergillus. Clin Microbiol Infect 22:662–669. doi: 10.1016/j.cmi.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi—2nd ed. CLSI document M38-A2 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 22.Liu W, Sun Y, Chen W, Liu W, Wan Z, Bu D, Li R. 2012. The T788G mutation in the cyp51C gene confers voriconazole resistance in Aspergillus flavus causing aspergillosis. Antimicrob Agents Chemother 56:2598–2603. doi: 10.1128/AAC.05477-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howard SJ, Harrison E, Bowyer P, Varga J, Denning DW. 2011. Cryptic species and azole resistance in the Aspergillus niger complex. Antimicrob Agents Chemother 55:4802–4809. doi: 10.1128/AAC.00304-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seyedmousavi S, Mouton JW, Verweij PE, Brüggemann RJ. 2013. Therapeutic drug monitoring of voriconazole and posaconazole for invasive aspergillosis. Expert Rev Anti Infect Ther 11:931–941. doi: 10.1586/14787210.2013.826989. [DOI] [PubMed] [Google Scholar]
- 25.Verweij PE, Ananda-Rajah M, Andes D, Arendrup MC, Brüggemann RJ, Chowdhary A, Cornely OA, Denning DW, Groll AH, Izumikawa K, Kullberg BJ, Lagrou K, Maertens J, Meis JF, Newton P, Page I, Seyedmousavi S, Sheppard DC, Viscoli C, Warris A, Donnelly JP. 2015. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist Updat 21–22:30–40. [DOI] [PubMed] [Google Scholar]
- 26.Pfaller MA, Messer SA, Woosley LN, Jones RN, Castanheira M. 2013. Echinocandin and triazole antifungal susceptibility profiles for clinical opportunistic yeast and mold isolates collected from 2010 to 2011: application of new CLSI clinical breakpoints and epidemiological cutoff values for characterization of geographic and temporal trends of antifungal resistance. J Clin Microbiol 51:2571–2581. doi: 10.1128/JCM.00308-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Linden JW, Camps SM, Kampinga GA, Arends JP, Debets-Ossenkopp YJ, Haas PJ, Rijnders BJ, Kuijper EJ, van Tiel FH, Varga J, Karawajczyk A, Zoll J, Melchers WJ, Verweij PE. 2013. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin Infect Dis 57:513–520. doi: 10.1093/cid/cit320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


