ABSTRACT
Fosfomycin is a broad-spectrum agent with activity against Gram-positive and Gram-negative bacteria, including drug-resistant strains, such as extended-spectrum-beta-lactamase (ESBL)-producing and carbapenem-resistant (CR) Gram-negative rods. In the present study, the pharmacokinetic/pharmacodynamic (PK/PD) activity of ZTI-01 (fosfomycin for injection) was evaluated in the neutropenic murine thigh infection model against 5 Escherichia coli, 3 Klebsiella pneumoniae, and 2 Pseudomonas aeruginosa strains, including a subset with ESBL and CR phenotypes. The pharmacokinetics of ZTI-01 were examined in mice after subcutaneous administration of 3.125, 12.5, 50, 200, 400, and 800 mg/kg of body weight. The half-life ranged from 0.51 to 1.1 h, area under the concentration-time curve (AUC0–∞) ranged from 1.4 to 87 mg · h/liter, and maximum concentrations ranged from 0.6 to 42.4 mg/liter. Dose fractionation demonstrated the AUC/MIC ratio to be the PK/PD index most closely linked to efficacy (R2 = 0.70). Net stasis and bactericidal activity were observed against all strains. Net stasis was observed at 24-h AUC/MIC ratio values of 24, 21, and 15 for E. coli, K., pneumoniae and P. aeruginosa, respectively. For the Enterobacteriaceae group, stasis was noted at mean 24-h AUC/MIC ratio targets of 23 and 1-log kill at 83. Survival in mice infected with E. coli 145 was maximal at 24-h AUC/MIC ratio exposures of 9 to 43, which is comparable to the stasis exposures identified in the PK/PD studies. These results should prove useful for the design of clinical dosing regimens for ZTI-01 in the treatment of serious infections due to Enterobacteriaceae and Pseudomonas.
KEYWORDS: fosfomycin, PK/PD
INTRODUCTION
Resistance to antibacterial agents is a growing threat to public health. Loss of all effective therapy is an increasing reality for patients with infections with multidrug-resistant (MDR) Enterobacteriaceae and Pseudomonas aeruginosa (1, 2). Novel agents are urgently sought to help combat the rising prevalence of drug resistance. One strategy to confront this challenge is the repurposing of antimicrobial drugs that have already been approved for clinical use but have not been optimized from a pharmacokinetic/pharmacodynamic (PK/PD) perspective to treat more serious infections due to these resistant pathogens (3, 4). Fosfomycin is an attractive candidate to address the therapy void for drug-resistant Enterobacteriaceae and P. aeruginosa infections, including extended-spectrum-beta-lactamase (ESBL)-producing and carbapenem-resistant (CR) organisms.
Fosfomycin is a phosphonic acid derivative that prevents the formation of N-acetylmuramic acid, a key component of the peptidoglycan chain involved in bacterial cell wall formation, through interaction with UDP-N-acetylglucosamine enolpyruvyl transferase (MurA) (5). It exhibits broad-spectrum activity and importantly remains highly active in vitro against multidrug-resistant (MDR) and extremely drug-resistant (XDR) Enterobacteriaceae and P. aeruginosa strains, including those with ESBL and CR phenotypes (6, 7). Thus, there is increasing interest in exploring the utility of fosfomycin to treat these drug-resistant pathogens due to this retained activity, the noted epidemic rise in antimicrobial resistance, and the diminishing number of novel therapies coming through traditional drug-discovery programs. In the United States, the oral formulation with limited bioavailability has been approved since 1996 to treat uncomplicated bacterial cystitis; however, in other parts of the world an intravenous formulation providing higher concentrations is available to treat more serious infections. ZTI-01 (fosfomycin for injection) is currently undergoing evaluation in the United States as an intravenous therapy for complicated urinary tract infection (UTI) or acute pyelonephritis (ClinicalTrials registration no. NCT 02753946 at clinicaltrials.gov).
The present studies sought to examine the in vivo PK/PD characteristics of ZTI-01 with the goal to provide a framework for further study and development of ZTI-01 dosing strategies for the treatment of systemic Gram-negative infections. Specifically, the impact of the dose and dosing regimen on the in vivo efficacy of this drug utilizing the neutropenic murine thigh infection model was assessed. Studies included those designed to (i) investigate the pharmacokinetic characteristics of escalating doses of ZTI-01 in mice, (ii) determine which PK/PD index (peak plasma level [Cmax]/MIC, area under the concentration-time curve [AUC]/MIC, or the duration of time that drug levels exceed the MIC [time above MIC {T>MIC}]) is most closely linked to efficacy via dose fractionation, and (iii) identify the magnitude of the PK/PD index required for microbiological efficacy among multiple strains of the three most common Gram-negative pathogens, including those with phenotypes of resistance to other commonly used antibiotics.
RESULTS
In vitro susceptibility testing.
The MICs of ZTI-01 by agar dilution for each of the strains are shown in Table 1 and ranged from 1 to 16 mg/liter. The presence of ESBL did not predictably affect the in vitro potency of ZTI-01, similarly to observations from other groups (6, 7).
TABLE 1.
In vitro activity of fosfomycin against select study strains
| Strain | Fosfomycin MIC by agar dilution (mg/liter) | Resistance mechanism |
|---|---|---|
| E. coli | ||
| ATCC 25922 | 1 | |
| 1-741-1 | 1 | ESBL+ |
| 681 | 16 | ESBL+ |
| 6042 | 8 | ESBL+ |
| 145 | 2 | ESBL+ |
| K. pneumoniae | ||
| 7023 | 16 | KPC-3 |
| 7068 | 8 | |
| 7040 | 4 | NDM-1 |
| P. aeruginosa | ||
| ATCC 27853 | 8 | |
| 9002 | 16 |
Plasma pharmacokinetics.
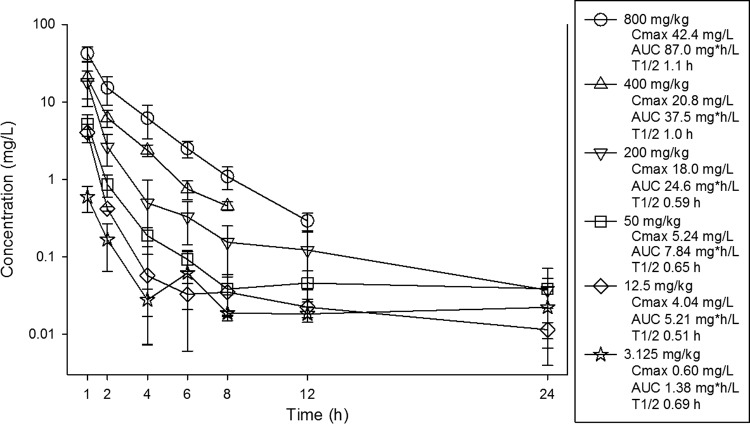
Single-dose pharmacokinetics of ZTI-01 in infected mice are shown in Fig. 1. At the doses studied, exposure to ZTI-01 increased in a dose-dependent manner across the dose range studies. Cmaxs ranged from 0.6 to 42.4 mg/liter. AUC0–∞ values ranged from 1.4 to 87 mg · h/liter. The AUCs and Cmaxs were linear over the dose range (R2 of 0.99 and 0.97, respectively). The elimination half-life (t1/2) ranged from 0.5 to 1.1 h. Total drug concentrations were utilized in PK/PD analyses, as plasma protein binding is considered negligible for fosfomycin (8, 9).
FIG 1.
Single-dose plasma pharmacokinetics of fosfomycin after subcutaneous administration in groups of three infected mice per dose and time point. The error bars represent the standard deviation. Pharmacokinetic parameters listed in the box include maximum drug concentrations (Cmax), the AUC from 0 to infinity (AUC), and elimination half-life (T1/2) for each dose.
Pharmacokinetic/pharmacodynamic index determination.
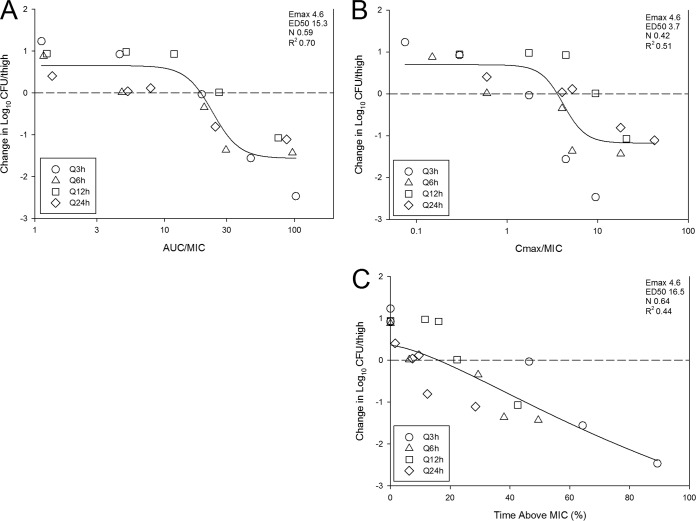
The relationships between log10 CFU per thigh and the AUC/MIC ratio, Cmax/MIC ratio, and the percentage of time that plasma levels exceeded the MIC are illustrated in Fig. 2A to C for E. coli 1-741-1, respectively. The PK/PD index that best predicted efficacy based on model fit and regression analysis was AUC/MIC ratio (R2 of 0.70). The Emax model data fit examining the exposure relationships for Cmax/MIC and the percentage of time that drug remained above the MIC (time above MIC) were less robust than AUC/MIC ratio.
FIG 2.
Impact of pharmacodynamic regression of the in vivo dose fractionation study with fosfomycin against E. coli 1-741-1. Each symbol represents the mean from four thighs. The dose data are expressed as either AUC/MIC ratio (A), Cmax/MIC (B), or percent time that drug concentrations exceed the MIC over the dosing period (time above MIC [%]) (C). The R2 represents the coefficient of determination. The ED50 represents the PD index associated with 50% of the maximal effect (Emax), and N is the slope of the relationship or the Hill coefficient. The line drawn through the data points is the best-fit line based upon the sigmoid Emax formula. The horizontal dashed line at 0 represents the burden of organisms in the thighs of mice at the start of therapy. Data points below the line represent killing, and points above the line represent growth.
Pharmacokinetic/pharmacodynamic index target for efficacy. (i) Escherichia coli experiments.
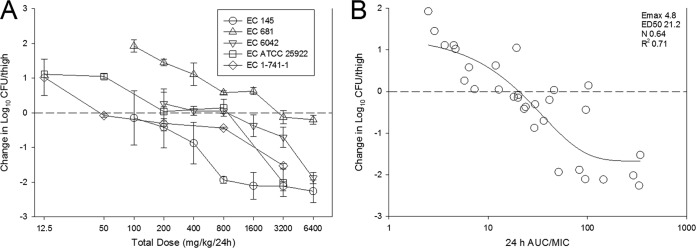
The dose-response data for each of the five E. coli strains are shown in Fig. 3A. In general, the dose-response relationships were similar among the strains, especially when MIC was considered. For example, the dose-response curves for strains 681 and 6042 were shifted to the right (more drug necessary), but they also exhibited the highest ZTI-01 MICs. Stasis was achieved against all strains, and a ≥1-log10 reduction was observed with strains with MICs of ≤8 mg/liter. The relationship between the PD index AUC/MIC ratio and treatment effect is shown in Fig. 3B. Similarly to the dose fractionation study, AUC/MIC ratio was a robust predictor of efficacy, with R2 being 0.71. Table 2 shows the static dose, 1-log kill dose (for studies that achieved that endpoint), associated AUC/MIC ratio, and time above MIC (T>MIC) values for each strain as well as mean, median, and standard deviation for the E. coli group. Net stasis was observed at AUC/MIC ratio values that ranged from 8.5 to 49.4 (mean, 23.7; median, 19.3) and at a T>MIC of 15 to 68% (mean, 38.6%; median, 37.7%). One-log kill was observed at AUC/MIC ratio values that ranged from 28 to 193 (mean, 98.9; median, 87.5) and at T>MIC values of 52 to 100% (mean, 75.8%; median, 75.3%).
FIG 3.
Dose-response curves (A) and 24-h AUC/MIC ratio in relation to treatment effect (B) against five select E. coli strains using a neutropenic mouse thigh model. Each symbol represents the mean from four thighs. The horizontal dashed line at 0 represents the burden of organisms in the thighs of mice at the start of therapy. Data points below the line represent killing, and points above the line represent growth. The R2 represents the coefficient of determination. The ED50 represents the AUC/MIC ratio associated with 50% of the maximal effect (Emax), and N is the slope of the relationship or the Hill coefficient. The line drawn through the data points is the best-fit line based upon the sigmoid Emax formula.
TABLE 2.
In vitro and in vivo efficacy of fosfomycin against E. coli, K. pneumoniae, and P. aeruginosa
| Organism group, strain, and/or parameter | Control growth (log10 CFU/ml) | MIC (mg/liter) | Static dose (mg/kg/24 h) | 24-h static dose AUC/MIC ratio | Static dose time above MIC (%) | 1-log kill dose (mg/kg/24 h) | 24-h 1-log kill AUC/MIC ratio | 1-log kill dose time above MIC (%) |
|---|---|---|---|---|---|---|---|---|
| E. coli | ||||||||
| 145 | 2.31 | 2 | 97.2 | 19.3 | 41.4 | 348 | 27.5 | 51.9 |
| 681 | 2.52 | 16 | 3,305 | 18.9 | 37.7 | NAa | ||
| 6042 | 1.81 | 8 | 491 | 8.5 | 15.0 | 3,572 | 41.6 | 75.9 |
| ATCC 25922 | 2.15 | 1 | 256 | 49.4 | 67.5 | 1,644 | 193 | 100 |
| 1-741-1 | 2.57 | 1 | 86.3 | 22.4 | 31.4 | 1,372 | 133 | 74.8 |
| Mean | 847 | 23.7 | 38.6 | 1,734 | 98.9 | 75.8 | ||
| Median | 256 | 19.3 | 37.7 | 1,508 | 87.5 | 75.3 | ||
| SD | 1,383 | 15.3 | 19.1 | 1,346 | 78.4 | 19.9 | ||
| K. pneumoniae | ||||||||
| 7023 | 1.265 | 16 | 1,122 | 8.61 | 12.8 | NA | ||
| 7068 | 1.8,375 | 8 | 3,707 | 43.6 | 78.0 | NA | ||
| 7040 | 1.25 | 4 | 171 | 11.1 | 26.5 | 652 | 21.5 | 42.6 |
| Mean | 1,667 | 21.1 | 39.1 | |||||
| Median | 1,122 | 11.1 | 26.5 | |||||
| SD | 1,830 | 19.5 | 34.4 | |||||
| Enterobacteriaceae group | ||||||||
| Mean | 1,154 | 22.7 | 38.8 | 1,518 | 83.3 | 69.0 | ||
| Median | 374 | 19.1 | 34.6 | 1,372 | 41.6 | 74.8 | ||
| SD | 1,494 | 15.6 | 23.4 | 1,262 | 76.1 | 22.5 | ||
| P. aeruginosa | ||||||||
| ATCC 27853 | 3.42 | 8 | 692 | 11.3 | 22.5 | 1,004 | 15.6 | 30.9 |
| 9002 | 3.52 | 16 | 3,148 | 17.9 | 35.4 | 6,310 | 40.8 | 76.4 |
NA, not achieved.
(ii) Klebsiella pneumoniae experiments.
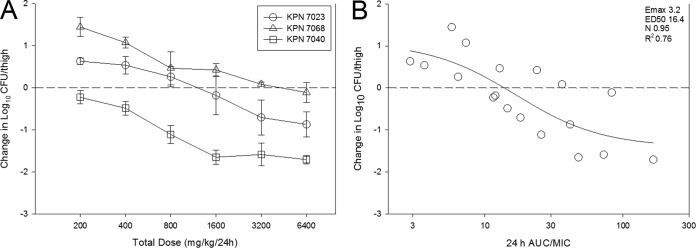
The dose-response data for each of the three K. pneumoniae strains are shown in Fig. 4A. Dose-dependent activity was observed against all three strains with stasis achieved against all strains and a ≥1-log10 reduction observed against strain 7040. The relationship between the PD index AUC/MIC ratio and treatment effect is shown in Fig. 4B. Similarly to E. coli studies, AUC/MIC ratio was a robust predictor of efficacy with R2 being 0.76. Table 2 shows the static dose, 1-log kill dose (for studies that achieved that endpoint), associated AUC/MIC ratio, and time above MIC (T>MIC) values for each strain as well as mean, median, and standard deviation for the K. pneumoniae group. Net stasis was observed at AUC/MIC ratio values that ranged from 8.6 to 43.6 (mean, 21.1; median, 11.1) and T>MIC values of 13 to 78% (mean, 39.1%; median, 26.5%). These values are remarkably congruent with those observed for E. coli (Mann-Whitney rank sum, P = 0.786). One-log kill was observed against a single K. pneumoniae strain (7040), and therefore, statistical analysis for this endpoint for K. pneumoniae was not able to be performed.
FIG 4.
The dose-response curves (A) and 24-h AUC/MIC ratio in relation to treatment effect (B) against three select K. pneumoniae strains using a neutropenic mouse thigh model. Each symbol represents the mean from four thighs. The horizontal dashed line at 0 represents the burden of organisms in the thighs of mice at the start of therapy. Data points below the line represent killing, and points above the line represent growth. The R2 represents the coefficient of determination. The ED50 represents the AUC/MIC ratio associated with 50% of the maximal effect (Emax), and N is the slope of the relationship or the Hill coefficient. The line drawn through the data points is the best-fit line based upon the sigmoid Emax formula.
(iii) Pseudomonas aeruginosa experiments.
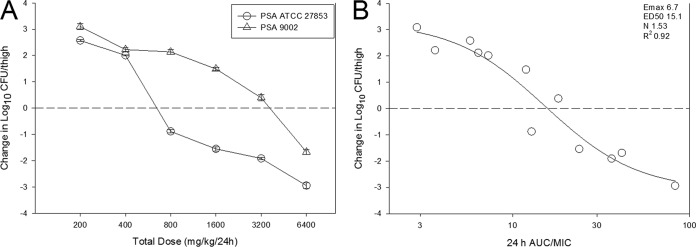
The dose-response data for each of the two P. aeruginosa strains are shown in Fig. 5A. Dose-dependent activity was observed with stasis, and a ≥1-log10 reduction was observed against both strains. The relationship between the PD index AUC/MIC ratio and treatment effect is shown in Fig. 5B. AUC/MIC ratio was a very strong predictor of efficacy, with R2 being 0.92. Table 2 shows the static dose, 1-log kill dose, associated AUC/MIC ratio, and time above MIC (T>MIC) values for both strains. Net stasis was observed at AUC/MIC ratio values of 11.3 and 17.9. The T>MIC values were 22.5% and 35.4%. One-log kill was observed at AUC/MIC ratio values of 15.6 and 40.8. T>MIC values associated with this endpoint were 30.9% and 76.4%.
FIG 5.
The dose-response curves (A) and 24-h AUC/MIC ratio in relation to treatment effect (B) against two select P. aeruginosa strains using a neutropenic mouse thigh model. Each symbol represents the mean from four thighs. The horizontal dashed line at 0 represents the burden of organisms in the thighs of mice at the start of therapy. Data points below the line represent killing, and points above the line represent growth. The R2 represents the coefficient of determination. The ED50 represents the AUC/MIC ratio associated with 50% of the maximal effect (Emax), and N is the slope of the relationship or the Hill coefficient. The line drawn through the data points is the best-fit line based upon the sigmoid Emax formula.
(iv) Combined results.
The PK/PD exposure-response data for all three organism were combined and plotted in Fig. 6. The relationships between AUC/MIC ratio and treatment effect were both strong (R2 of 0.64) and similar between each organism group.
FIG 6.
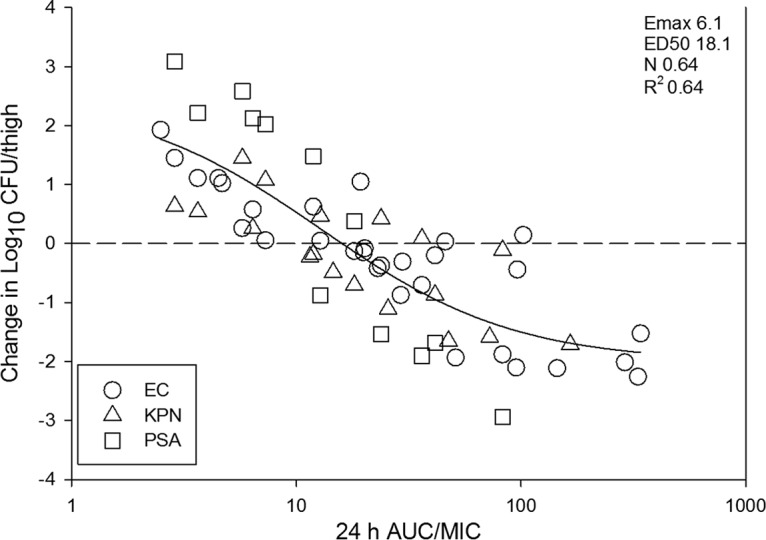
Relationship between the PD index AUC/MIC ratio and treatment efficacy of fosfomycin against all 10 isolates using a neutropenic mouse thigh model. The horizontal line at 0 represents the burden of organisms in the thighs of mice at the start of therapy. Data points below the line represent killing, and points above the line represent growth. The R2 represents the coefficient of determination. The ED50 represents 50% of the maximal effect (Emax), and N is the slope of the relationship or the Hill coefficient. The line drawn through the data points is the best-fit line based upon the sigmoid Emax formula.
Survival study.
The doses utilized in the survival study against E. coli 681 included 25, 100, 400, and 1,600 mg/kg of body weight administered every 6 h and correspond to AUC/MIC ratio exposures of 1.5, 3.2, 9.3, and 42.6 mg · h/liter, respectively. All eight animals in the two lowest-dose studies did not survive to the study endpoint (72 h). In contrast, 5 of 8 (62.5%) and 8 of 8 (100%) survived in the groups administered 400 and 1600 mg/kg/6 h, respectively. Therefore, maximal survival was observed at AUC/MIC ratio exposures between 9.3 and 42.6 mg · h/liter. Comparison of the survival analysis and PK/PD target analysis reveals that maximal survival was observed at AUC/MIC ratio exposures comparable to the stasis targets observed in the same infection model.
DISCUSSION
Drug resistance in Enterobacteriaceae and P. aeruginosa is a major threat to public health (1, 2, 10). A recent report from the Centers for Disease Control and Prevention highlighted this risk with the report of the death of a woman in Nevada due to a highly drug-resistant K. pneumoniae infection that was resistant to 26 antibiotics, including all beta-lactams, aminoglycosides, polymyxins, and tigecycline (11). In fact, this isolate was susceptible only to fosfomycin; however, intravenous formulations for severe infections are not available at this time in the United States. The development of an intravenous fosfomycin formulation for use in the United States is critically important, and currently, ZTI-01 (fosfomycin for injection) is undergoing clinical study for complicated UTI and pyelonephritis (ClinicalTrials registration no. NCT02753946 at clinicaltrials.gov).
Unfortunately, recent drug discovery efforts for novel antibacterial agents have not yet resulted in new clinically available therapeutics (1, 2, 12–18). The repurposing of older antibiotics is one potential strategy to fill the void of therapies for drug-resistant pathogens. Fosfomycin, which exhibits potent in vitro effects against Enterobacteriaceae and P. aeruginosa, including those with phenotypes of resistance to other commonly used drugs, is therefore an attractive candidate for repurposing. However, a critical step in the process of repurposing is a thorough understanding of the PK/PD characteristics of a candidate drug (19). This is necessary to design appropriate dosing regimens to be studied in clinical trials and set preliminary breakpoints for the new indications being sought. In the case of fosfomycin, there is a paucity of information on the PK/PD relationships necessary to optimize therapy and outcome for serious infections by Gram-negative rods such as Enterobacteriaceae and P. aeruginosa.
Prior investigations have suggested that fosfomycin exhibits concentration-independent activity, as escalating doses above the MIC did not lead to enhanced microbiological effect (20–22). Due to this, some have suggested a dosing design that optimizes the PK/PD index time above MIC (T>MIC). However, this assessment was often based on dose-escalation studies alone. As PK/PD indices are interdependent, when the dose is increased, each of the traditional indices (T>MIC, AUC/MIC ratio, and Cmax/MIC) rises, making it difficult to define the PK/PD index driver. Dose fractionation, however, reduces the interdependence of the PK/PD indices and is the preferred methodology for determining PK/PD index driver. The present study is the first in vivo PK/PD evaluation of fosfomycin using a validated murine model and dose fractionation study design to delineate the PK/PD index predictive of efficacy. Analysis demonstrates AUC/MIC ratio to be the PK/PD index most closely linked to efficacy in the murine model. A recently published in vitro dose fractionation study exists for comparison purposes (23). Hope and colleagues published a hollow-fiber study utilizing an ESBL-producing E. coli strain in which they observed the same rate and depth of kill irrespective of the dosing interval (23). This is consistent with AUC/MIC ratio as the pharmacodynamically linked index and supports our findings. The importance of the AUC/MIC ratio index for this compound with concentration-independent killing is reminiscent of PK/PD characteristics of the oxazolidinones and triazoles (24–26). One reason for this observed outcome in our dose fractionation study could be due to postantibiotic effects (PAEs). Modest PAEs have been observed in vitro (20) but were not evaluated in the current study, and this remains an important area for further investigation. Additionally, we recognize that previous in vitro reports have suggested optimizing time above MIC, including a recent study by VanScoy et al. demonstrating the relevance of this PK/PD index in relation to the prevention of resistant subpopulation growth (27). For this reason, we have reported those targets in addition to AUC/MIC ratio (Table 2).
An important element in PK/PD studies seeking to define the predictive PK/PD index magnitude or target is the inclusion of multiple strains to account for strain-to-strain variability. The current studies included 5 E. coli strains, including 4 ESBL-producing strains; 3 K. pneumoniae strains, including 2 carbapenem-resistant Enterobacteriaceae (CRE) strains; and 2 P. aeruginosa strains. ZTI-01 demonstrated dose-dependent in vivo efficacy with the achievement of bactericidal activity against all strains. The ZTI-01 24-h target AUC/MIC ratios associated with a net static and 1-log10 killing effect against all three organism groups were remarkably congruent. For example, the mean static dose AUC/MIC ratio target was 24, 21, and 15 for E. coli, K. pneumoniae, and P. aeruginosa, respectively.
Translation of preclinical PK/PD target assessments requires an understanding of which endpoint (i.e., stasis or 1-log10 kill) correlates with other measures of success such as survival or clinical success in patients. For example, in beta-lactams it has been shown that T>MIC values of at least 30 to 40% result in net stasis in preclinical animal models, 90 to 100% animal survival, and clinical success in patients (28, 29). In contrast, for fluoroquinolones the stasis targets are an AUC/MIC ratio of 25 to 50, but 100% animal survival and clinical success are often not noted unless AUC/MIC ratio exceeds 100, which is closer to the 1-log kill target exposures for that drug class (28, 29). Thus, the relevancies of stasis endpoints as they relate to survival are not necessarily similar for each drug class. We therefore included an additional survival study to compare which PK/PD exposures were associated with animal survival. Against a single E. coli strain, we observed that maximal survival (100%) occurred at AUC/MIC ratio exposures between 9.3 and 42.6 mg · h/liter, which encompasses our identified stasis target for the organism group, which was 23.7. This supports the relevance of stasis targets identified in our preclinical in vivo model; however, further studies with additional strains would be necessary to confirm this finding.
There are numerous remaining PK/PD questions. First, it will be interesting to explore the postexposure or postantibiotic effects for fosfomycin. Additional studies examining the protein binding characteristics across species will also be important; however, it is likely that the degrees of binding will be similar across mammalian species, and binding has been found to be negligible in humans (30). Finally, it will be important to assess PK/PD targets against additional strains, including Gram-positive pathogens.
In conclusion, the present studies demonstrate potent in vivo efficacy of ZTI-01 against Enterobacteriaceae and P. aeruginosa, including ESBL-producing and CRE strains, in a well-characterized neutropenic murine thigh infection model. The PK/PD index AUC/MIC ratio was most strongly linked to efficacy. Both static and killing endpoints were achieved, and the AUC/MIC ratio targets were similar across study strains. Maximal animal survival was observed at AUC/MIC ratio exposures similar to stasis targets. The PK/PD index and targets identified in these studies and human PK data should be useful to guide appropriate dosing regimen design for clinical trials, including the current study on complicated urinary tract infection and pyelonephritis.
MATERIALS AND METHODS
Organisms, medium, and antibiotic.
Five Escherichia coli (4 ESBL), 3 Klebsiella pneumoniae (2 CRE), and 2 P. aeruginosa strains were used for these studies (Table 1). Organisms were grown, subcultured, and quantified using cation- and glucose-6-phosphate (G-6-P)-adjusted Mueller-Hinton broth (MHB) and agar (Difco Laboratories, Detroit, MI). ZTI-01 was supplied by the sponsor in 4-g vials, reconstituted with sterile water, and diluted to appropriate concentrations prior to use.
In vitro susceptibility testing.
MICs were determined by agar dilution methods in cation-adjusted Mueller-Hinton medium (Difco Laboratories, Detroit, MI) supplemented with 25 mg/liter of glucose-6-phosphate (G-6-P; Sigma-Aldrich, St. Louis, MO) (31). All MIC assays were performed in duplicate on at least three occasions. The median MIC is presented in Table 1.
Murine thigh infection model.
Animals were maintained in accordance with the American Association for Accreditation of Laboratory Animal Care (AAALAC) criteria (32). All animal studies were approved by the Animal Research Committees of the William S. Middleton Memorial VA Hospital and the University of Wisconsin. Six-week-old, specific-pathogen-free, female ICR/Swiss mice weighing 24 to 27 g were used for all studies (Harlan Sprague-Dawley, Indianapolis, IN). Mice were rendered neutropenic (neutrophils, <100/mm3) by injecting cyclophosphamide (Mead Johnson Pharmaceuticals, Evansville, IN) intraperitoneally 4 days (150 mg/kg) and 1 day (100 mg/kg) before thigh infection. Previous studies have shown that this regimen produces neutropenia in this model for 5 days (33). Broth cultures of freshly plated bacteria were grown to logarithmic phase overnight to an absorbance of 0.3 at 580 nm (Spectronic 88; Bausch and Lomb, Rochester, NY). After a 1:10 dilution into fresh MHB, bacterial counts of the inoculum were 107.1 ± 100.2 CFU/ml. Thigh infections with each of the strains were produced by injection of 0.1 ml of inoculum into the thighs of isoflurane-anesthetized mice 2 h before therapy with ZTI-01.
Drug pharmacokinetics.
Single-dose plasma pharmacokinetics of ZTI-01 were assayed in infected neutropenic mice. Animals were administered a single subcutaneous dose (0.2 ml/dose) of ZTI-01 at dose levels of 3.125, 12.5, 50, 200, 400, and 800 mg/kg. Groups of three mice were sampled at each time point (7 time points) and dose level. Sampling times ranged from 1 to 24 h over a 24-h period. All samples were assayed by liquid chromatography-tandem mass spectrometry (LC/MS/MS). Standard curves were linear from 0.200 to 50.0 mg/liter for ZTI-01 (R2 values ranging from 0.990 to 0.998). The lower limit of quantification was 0.200 mg/liter. The interassay percent coefficients of variation (%CV) for the quality control samples at concentrations of 0.290, 1.50, 4.84, and 40.0 μg/ml were 8.31, 8.17, 8.14, and 7.08%, respectively. Pharmacokinetic constants (±standard deviation), including elimination half-life (t1/2), AUC, and Cmax, were calculated using a noncompartmental model. The half-life of ZTI-01 was determined by linear least-squares regression. The AUC0–∞ was calculated from the mean concentrations using the trapezoidal rule. Pharmacokinetic estimates for dose levels that were not measured were calculated using linear interpolation or extrapolation. Total drug concentrations were utilized in PK/PD analyses, as plasma protein binding is considered negligible for fosfomycin (8, 9).
Pharmacokinetic/pharmacodynamic index determination.
Neutropenic mice were infected with E. coli 1-741-1 as described above. Treatment with ZTI-01 by the subcutaneous route was initiated 2 h after infection. The dose fractionation experiment included five total drug concentrations that increased 4-fold from 3.125 to 800 mg/kg over a 24-h duration. The total doses were administered using 3-, 6-, 12-, and 24-hourly dosing intervals. Four thigh infections were included in each dosing group. After 24 h, the thighs were aseptically removed and processed for CFU determination.
To determine which PK/PD index was most closely linked with efficacy, the number of bacteria in the thigh at the end of therapy was correlated with (i) the Cmax/MIC ratio, (ii) the 24-hour AUC/MIC ratio, and (iii) the percentage of the dosing interval during which plasma levels exceed the MIC for each of the dosage regimens studied. The correlation between efficacy and each of the three PK/PD indices was determined by nonlinear least-squares multivariate regression (Sigma Plot version 12.3; Systat Software, San Jose, CA). The model is derived from the Hill equation: E = (Emax × DN)/(ED50N − DN), where E is the effect or, in this case, the log change in CFU per thigh between treated mice and untreated controls after the 24-h period of study, Emax is the maximum effect, D is the 24-h total dose, ED50 is the dose required to achieve 50% of Emax, and N is the slope of the dose-effect curve. The indices Emax, ED50, and N were calculated using nonlinear least-squares regression. The coefficient of determination (R2) was used to estimate the variance that could be due to regression with each of the PK/PD parameters.
Pharmacokinetic/pharmacodynamic index target for efficacy.
Dose-ranging efficacy studies to determine the PK/PD target exposures for net stasis and 1-log kill were then performed with each E. coli, K. pneumoniae, and P. aeruginosa strain using the murine neutropenic thigh infection model. Dosing regimens were chosen to yield drug exposures that varied from ineffective to maximally effective for each strain. In E. coli experiments, dosing regimens included five (4-fold) or seven (2-fold) increasing doses of ZTI-01. The total daily doses of ZTI-01 varied from 12.5 to 6,400 mg/kg/24 h administered in an every-3-h or every-6-h regimen. In K. pneumoniae and P. aeruginosa experiments, the dosing regimens included six (2-fold) increasing doses of ZTI-01 administered every 3 hours with total daily doses that varied from 200 to 6,400 mg/kg/24 h. All doses were administered by the subcutaneous route. Four thigh infections were included in each dosing regimen group. Therapy was initiated 2 h after infection. Animals were euthanized at 24 h after infection, and the thighs were aseptically removed and immediately processed for CFU determination. A sigmoid dose-response model derived from the four-parameter Hill equation was used to calculate the dose of ZTI-01 that produced a net bacteriostatic effect and 1-log10 kill over 24 h (static and 1-log kill doses) compared to the organism burden at the start of treatment. The PK/PD indices of interest were calculated for the static and 1-log kill doses using the sigmoid Emax model.
Survival study.
The neutropenic thigh model was used with a single strain, E. coli 681, to examine the PK/PD exposure associated with survival. Infection was induced in the thighs of neutropenic mice as described above with an inoculum of 107.0 CFU/ml 2 h prior to the initiation of drug administration. ZTI-01 was administered by the subcutaneous route in 4-fold-increasing doses from 25 to 1,600 mg/kg every 6 h to groups of eight mice. Survival was tracked over a 72-h treatment period. During this period, each mouse was monitored at least 4 times daily and moribund mice were euthanized by CO2 asphyxiation prior to the end of the study. The 24-h AUC/MIC ratio and percentage of the dosing interval during which plasma drug concentrations exceeded the MIC (percent time above MIC) associated with animal survival in each group were analyzed.
ACKNOWLEDGMENT
These studies were funded by Zavante.
REFERENCES
- 1.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 2.Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, Bartlett JG, Edwards J Jr, Infectious Diseases Society of America. 2008. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis 46:155–164. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 3.Cassir N, Rolain JM, Brouqui P. 2014. A new strategy to fight antimicrobial resistance: the revival of old antibiotics. Front Microbiol 5:551. doi: 10.3389/fmicb.2014.00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergen PJ, Landersdorfer CB, Lee HJ, Li J, Nation RL. 2012. ‘Old’ antibiotics for emerging multidrug-resistant bacteria. Curr Opin Infect Dis 25:626–633. doi: 10.1097/QCO.0b013e328358afe5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skarzynski T, Mistry A, Wonacott A, Hutchinson SE, Kelly VA, Duncan K. 1996. Structure of UDP-N-acetylglucosamine enolpyruvyl transferase, an enzyme essential for the synthesis of bacterial peptidoglycan, complexed with substrate UDP-N-acetylglucosamine and the drug fosfomycin. Structure 4:1465–1474. doi: 10.1016/S0969-2126(96)00153-0. [DOI] [PubMed] [Google Scholar]
- 6.Falagas ME, Maraki S, Karageorgopoulos DE, Kastoris AC, Mavromanolakis E, Samonis G. 2010. Antimicrobial susceptibility of multidrug-resistant (MDR) and extensively drug-resistant (XDR) Enterobacteriaceae isolates to fosfomycin. Int J Antimicrob Agents 35:240–243. doi: 10.1016/j.ijantimicag.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Falagas ME, Kastoris AC, Kapaskelis AM, Karageorgopoulos DE. 2010. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum beta-lactamase producing, Enterobacteriaceae infections: a systematic review. Lancet Infect Dis 10:43–50. doi: 10.1016/S1473-3099(09)70325-1. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez D, Schmidt S, Derendorf H. 2013. Importance of relating efficacy measures to unbound drug concentrations for anti-infective agents. Clin Microbiol Rev 26:274–288. doi: 10.1128/CMR.00092-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roussos N, Karageorgopoulos DE, Samonis G, Falagas ME. 2009. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections. Int J Antimicrob Agents 34:506–515. doi: 10.1016/j.ijantimicag.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Viale P, Giannella M, Tedeschi S, Lewis R. 2015. Treatment of MDR-Gram negative infections in the 21st century: a never ending threat for clinicians. Curr Opin Pharmacol 24:30–37. doi: 10.1016/j.coph.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Todd R, Kiehlbauch J, Walters M, Kallen A. 2017. Notes from the field: pan-resistant New Delhi metallo-beta-lactamase-producing Klebsiella pneumoniae—Washoe County, Nevada, 2016. MMWR Morb Mortal Wkly Rep 66:33. doi: 10.15585/mmwr.mm6601a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Infectious Diseases Society of America, Spellberg B, Blaser M, Guidos RJ, Boucher HW, Bradley JS, Eisenstein BI, Gerding D, Lynfield R, Reller LB, Rex J, Schwartz D, Septimus E, Tenover FC, Gilbert DN. 2011. Combating antimicrobial resistance: policy recommendations to save lives. Clin Infect Dis 52(Suppl 5):S397–S428. doi: 10.1093/cid/cir153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talbot GH. 2008. What is in the pipeline for Gram-negative pathogens? Expert Rev Anti Infect Ther 6:39–49. doi: 10.1586/14787210.6.1.39. [DOI] [PubMed] [Google Scholar]
- 14.Talbot GH, Bradley J, Edwards JE Jr, Gilbert D, Scheld M, Bartlett JG, Antimicrobial Availability Task Force of the Infectious Diseases Society of America. 2006. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis 42:657–668. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 15.Spellberg B. 2008. Antibiotic resistance and antibiotic development. Lancet Infect Dis 8:211–212. doi: 10.1016/S1473-3099(08)70048-3. [DOI] [PubMed] [Google Scholar]
- 16.Spellberg B, Bartlett JG, Gilbert DN. 2013. The future of antibiotics and resistance. N Engl J Med 368:299–302. doi: 10.1056/NEJMp1215093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spellberg B, Powers JH, Brass EP, Miller LG, Edwards JE Jr. 2004. Trends in antimicrobial drug development: implications for the future. Clin Infect Dis 38:1279–1286. doi: 10.1086/420937. [DOI] [PubMed] [Google Scholar]
- 18.Nathan C. 2004. Antibiotics at the crossroads. Nature 431:899–902. doi: 10.1038/431899a. [DOI] [PubMed] [Google Scholar]
- 19.Mouton JW, Ambrose PG, Canton R, Drusano GL, Harbarth S, MacGowan A, Theuretzbacher U, Turnidge J. 2011. Conserving antibiotics for the future: new ways to use old and new drugs from a pharmacokinetic and pharmacodynamic perspective. Drug Resist Updat 14:107–117. doi: 10.1016/j.drup.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Walsh CC, McIntosh MP, Peleg AY, Kirkpatrick CM, Bergen PJ. 2015. In vitro pharmacodynamics of fosfomycin against clinical isolates of Pseudomonas aeruginosa. J Antimicrob Chemother 70:3042–3050. doi: 10.1093/jac/dkv221. [DOI] [PubMed] [Google Scholar]
- 21.Parker S, Lipman J, Koulenti D, Dimopoulos G, Roberts JA. 2013. What is the relevance of fosfomycin pharmacokinetics in the treatment of serious infections in critically ill patients? A systematic review. Int J Antimicrob Agents 42:289–293. doi: 10.1016/j.ijantimicag.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Patel SS, Balfour JA, Bryson HM. 1997. Fosfomycin tromethamine. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy as a single-dose oral treatment for acute uncomplicated lower urinary tract infections. Drugs 53:637–656. [DOI] [PubMed] [Google Scholar]
- 23.Docobo-Perez F, Drusano GL, Johnson A, Goodwin J, Whalley S, Ramos-Martin V, Ballestero-Tellez M, Rodriguez-Martinez JM, Conejo MC, van Guilder M, Rodriguez-Bano J, Pascual A, Hope WW. 2015. Pharmacodynamics of fosfomycin: insights into clinical use for antimicrobial resistance. Antimicrob Agents Chemother 59:5602–5610. doi: 10.1128/AAC.00752-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andes D, van Ogtrop ML, Peng J, Craig WA. 2002. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob Agents Chemother 46:3484–3489. doi: 10.1128/AAC.46.11.3484-3489.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lepak AJ, Marchillo K, Pichereau S, Craig WA, Andes DR. 2012. Comparative pharmacodynamics of the new oxazolidinone tedizolid phosphate and linezolid in a neutropenic murine Staphylococcus aureus pneumonia model. Antimicrob Agents Chemother 56:5916–5922. doi: 10.1128/AAC.01303-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lepak AJ, Andes DR. 2014. Antifungal pharmacokinetics and pharmacodynamics. Cold Spring Harb Perspect Med 5:a019653. doi: 10.1101/cshperspect.a019653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.VanScoy BD, McCauley J, Ellis-Grosse EJ, Okusanya OO, Bhavnani SM, Forrest A, Ambrose PG. 2015. Exploration of the pharmacokinetic-pharmacodynamic relationships for fosfomycin efficacy using an in vitro infection model. Antimicrob Agents Chemother 59:7170–7177. doi: 10.1128/AAC.04955-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ambrose PG, Bhavnani SM, Rubino CM, Louie A, Gumbo T, Forrest A, Drusano GL. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin Infect Dis 44:79–86. doi: 10.1086/510079. [DOI] [PubMed] [Google Scholar]
- 29.Craig WA. 2007. Pharmacodynamics of antimicrobials: general concepts and applications, p 1–22. In Nightingale CH, Ambrose PG, Drusano GL, Murakawa T (ed), Antimicrobial pharmacodynamics in theory and clinical practice, 2nd ed Informa Healthcare USA, Inc, New York, NY. [Google Scholar]
- 30.Forest Pharmaceuticals, Inc. 2011. Monurol package insert. Forest Pharmaceuticals, Inc, St. Louis, MO: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/050717s007lbl.pdf. [Google Scholar]
- 31.Clinical and Laboratory Standards Institute. 2015. M07-A10. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 10th ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 32.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academies Press, Washington, DC. [Google Scholar]
- 33.Andes D, Craig WA. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob Agents Chemother 42:2375–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]