ABSTRACT
Tuberculosis (TB) treatment is long and requires multiple drugs, likely due to various phenotypes of TB bacilli with variable drug susceptibilities. Drugs with broad activity are urgently needed. This study aimed to evaluate delamanid's activity against growing or dormant bacilli in vitro as well as in vivo. Cultures of Mycobacterium bovis BCG Tokyo under aerobic and anaerobic conditions were used to study the activity of delamanid against growing and dormant bacilli, respectively. Delamanid exhibited significant bactericidal activity against replicating and dormant bacilli at or above concentrations of 0.016 and 0.4 mg/liter, respectively. To evaluate delamanid's antituberculosis activity in vivo, we used a guinea pig model of chronic TB infection in which the lung lesions were similar to those in human TB disease. In the guinea pig TB model, a daily dose of 100 mg delamanid/kg of body weight for 4 or 8 weeks demonstrated strong bactericidal activity against Mycobacterium tuberculosis. Importantly, histological examination revealed that delamanid killed TB bacilli within hypoxic lesions of the lung. The combination regimens containing delamanid with rifampin and pyrazinamide or delamanid with levofloxacin, ethionamide, pyrazinamide, and amikacin were more effective than the standard regimen (rifampin, isoniazid, and pyrazinamide). Our data show that delamanid is effective in killing both growing and dormant bacilli in vitro and in the guinea pig TB model. Adding delamanid to current TB regimens may improve treatment outcomes, as demonstrated in recent clinical trials with pulmonary multidrug-resistant (MDR) TB patients. Delamanid may be an important drug for consideration in the construction of new regimens to shorten TB treatment duration.
KEYWORDS: Mycobacterium tuberculosis, delamanid, dormant, guinea pig
INTRODUCTION
Tuberculosis (TB) remains one of the world's deadliest communicable diseases (1). TB is caused by Mycobacterium tuberculosis, an acid-fast bacillus that is transmitted primarily via the respiratory route. Although TB is treatable, the duration of treatment is at least 6 months for fully drug-susceptible TB with the 4 standard first-line drugs (i.e., isoniazid, rifampin, pyrazinamide, and ethambutol). The emergence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) mycobacterial strains and the epidemic of HIV/AIDS coinfections further complicate treatment (2). Even for uncomplicated MDR-TB treatment, current World Health Organization guidelines mandate at least 9 to 12 months of treatment with four or more second-line TB drugs with weaker activity and higher toxicity than the first-line drugs (3, 4). Several factors of the host and pathogen and interactions between them are thought to contribute to the difficulty of treating TB (5, 6). One such factor is the existence of dormancy; some bacilli replicate very slowly or not at all and have the ability to tolerate chemotherapy. The reasons underlying bacterial persistence are complex, but some environmental conditions, such as pH, low levels of oxygen, or nutrient starvation, are believed to induce M. tuberculosis to enter into a persistent state (7–9). New drugs that can kill persisters are needed to shorten TB treatment.
Delamanid (DLM; also called OPC-67683 or Deltyba) has demonstrated mycobacterium-specific antibacterial activity in vitro. In a mouse model, DLM exhibited strong bactericidal activity compared with those of first-line drugs in monotherapy and enhanced the eradication of TB bacilli when added to a regimen containing first-line drugs (10). However, the effect of DLM on persisters has not been specifically investigated. Moreover, recent publications have argued that traditional mouse models do not replicate the pathological lesions observed in humans; in particular, they lack well-formed granulomas leading to caseation and also lack hypoxic regions (11–13).
In the present study, we first evaluated the antibacterial activity of DLM against hypoxia-induced dormant bacilli in vitro using a modified Wayne model published by Wayne and Hayes (7). We then used a guinea pig model of chronic TB infection to determine the bactericidal activity of DLM against bacilli under hypoxic conditions in vivo. Previous studies have shown that guinea pigs infected with TB bacilli develop lesions with characteristics similar to those of human TB infections, i.e., subsequent necrosis and mineralization in lung granulomas as well as hypoxic lesions that can induce the dormancy of the infecting agent (12). It has been proposed that guinea pig models may be better than mouse models at predicting the sterilizing activity of new TB drugs against persisters (14, 15).
RESULTS
Bactericidal activity of delamanid against aerobically cultured and dormant mycobacteria.
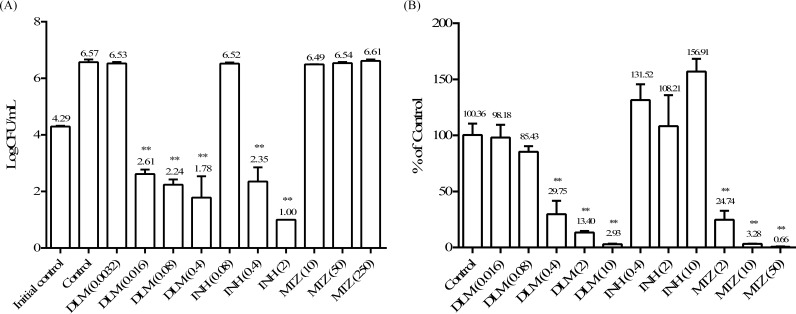
As shown in Fig. 1A, when cultured under the aerobic condition, bacteria grew robustly: during the 7-day period, bacterial numbers increased more than 2 logs. DLM had ≥4-log and ∼2-log kills relative, respectively, to numbers of bacilli in the growth control and the initial control at concentrations of 0.016, 0.08, and 0.4 mg/liter. Isoniazid (INH) also showed strong activities at concentrations of 0.4 mg/liter and higher. Metronidazole (MTZ), on the other hand, had absolutely no effect on Mycobacterium bovis BCG Tokyo growing under aerobic conditions. The MIC values against M. bovis BCG Tokyo were 0.012 mg/liter for DLM and 0.1 mg/liter for INH (10). Therefore, the 0.016-, 0.08-, and 0.4-mg/liter concentrations correspond to, respectively, 1.3-, 6.7-, and 33.3-fold the MIC of delamanid, and the 0.4- and 2-mg/liter concentrations correspond to 4- and 20-fold the MIC of INH. These results indicate that DLM and INH can kill rapidly replicating TB bacilli at similar magnitudes, but DLM kills at a much lower concentration, as evidenced by its lower MIC.
FIG 1.
Bactericidal activities of delamanid (DLM) against replicating and nonreplicating bacilli. (A) Bactericidal activities against replicating cells. One-day-cultured M. bovis BCG Tokyo bacilli were treated with delamanid at 0.0032, 0.016, 0.08, and 0.4 mg/liter, isoniazid (INH) at 0.08, 0.4, and 2 mg/liter, and metronidazole (MTZ) at 10, 50, 250 mg/liter for 7 days. The numbers of CFU per milliliter at the end of treatment were counted. DMSO was used as a control. The number of CFU per milliliter in day 0 culture was used as the initial control. (B) Bactericidal activities against nonreplicating M. bovis BCG Tokyo cells. M. bovis BCG Tokyo was cultured in a modified Wayne model, and nonreplicating bacilli were treated with DLM at 0.016, 0.08, 0.4, 2, and 10 mg/liter; INH at 0.4, 2, and 10 mg/liter; and MTZ at 2, 10, and 50 mg/liter for 7 days. Each value represents the mean of results from triplicate samples ± the standard deviation (SD) from three replicates. Treated groups were compared with the control by Dunnett's test. **, P < 0.01.
In Fig. 1B, the effect of drug treatment on bacteria cultured under anaerobic conditions is presented as a percentage of that of the control (dimethyl sulfoxide [DMSO]) treatment. The bactericidal activities of DLM at 0.016 and 0.08 mg/liter on dormant bacilli were fairly weak, but concentrations of 0.4 mg/liter and above demonstrated significant killing in this model. INH had no appreciable bactericidal effect at any tested concentration, while MTZ exhibited very strong killing. These observations are consistent with previous reports that dormant M. tuberculosis is phenotypically resistant to INH but can be killed by MTZ (16).
PK parameters of anti-TB drugs in guinea pigs.
Prior to the efficacy study, we evaluated the pharmacokinetic (PK) parameters of DLM and other anti-TB drugs in guinea pigs to choose the appropriate doses for each drug. PK parameters were determined after the administration of a single dose, except with rifampin (RIF), which was administered for 5 days.
In clinical studies, DLM demonstrated efficacy with dosing regimens of 200 mg or 400 mg daily (17, 18, 19). The PK results from these studies are summarized in Table 1 (18). In the guinea pigs, delamanid concentrations were lower after an oral dose of 10 mg/kg of body weight than those in humans after 100 mg twice a day (BID). Increasing the dose to 100 mg/kg produced the following PK parameters: a maximum concentration of the drug in serum (Cmax) of 0.53 mg/liter and an area under the concentration-time curve calculated to the last observable concentration at time t (AUCt) of 9.45 mg · h/liter (Table 1). These values are only slightly above those achieved with 100 mg BID in TB patients, indicating that 100 mg/kg is the human-equivalent dose for DLM in guinea pigs. The Cmax of 0.53 mg/liter is greater than the concentration of 0.4 mg/liter that resulted in a bactericidal effect on in vitro dormant bacilli (Fig. 1B). Therefore, 100 mg/kg was chosen for DLM in the guinea pig efficacy study.
TABLE 1.
Plasma levels of agents used for evaluating regimens in guinea pigs
| Agenta | Guinea pigsb |
Humans |
|||||
|---|---|---|---|---|---|---|---|
| Dose (mg/kg) | Cmax (mg/liter) | AUCt (mg · h/liter)c | Dose (mg) | Cmax (mg/liter) | AUCt (mg · h/liter) | Reference | |
| DLM | 10 | 0.21 | 2.32 | 100 BIDd | 0.414e | 7.925 | 18 |
| (0.4f) | |||||||
| 100 | 0.53 | 9.45 | 200 BID | 0.611e | 11.837 | ||
| (0.588f) | |||||||
| RIF | 25 | 6.76 | 28.28 | 450 | 5.5 | 29.7 | 33 |
| INH | 25 | 12.75 | 28.26 | 300 | 4.5 | 22.9 | 34 |
| PZA | 150 | 128.95 | 218.96 | 1,500 | 55.5 | 241 | 35 |
| LVX | 50 | 12.66 | 44.49 | 1,000 | 15.55 | NCg | 36 |
| ETO | 50 | 6.33 | 4.96 | 500 | NC | 3.95 | 37 |
PZA, pyrazinamide; LVX, levofloxacin; ETO, ethionamide.
The doses set up for using the plasma Cmax or AUCt achieved in the guinea pig model are equivalent to those achieved in humans at the clinical dose.
AUCts for guinea pig results were calculated by the trapezoidal rule up to 24 h.
BID, twice daily.
Maximum plasma concentration following the first daily dose.
Maximum plasma concentration following the second daily dose.
NC, not calculated.
For RIF, a previous study showed accumulation in guinea pigs after repeated administration (20), which was also observed in our preliminary studies (data not shown). We therefore determined the PK parameters for RIF after 5 days of administration. After a dose of 25 mg/kg daily for 5 days, the Cmax reached 6.76 mg/liter and the corresponding AUCt value was 28.28 mg · h/liter, which is very similar to the corresponding values after a 450-mg dose in TB patients (Table 1). Therefore, a 25-mg/kg dose was chosen for RIF.
Similarly, the human-equivalent doses chosen for INH, pyrazinamide (PZA), levofloxacin (LVX), and ethionamide (ETO) were 25 mg/kg, 150 mg/kg, 50 mg/kg, and 50 mg/kg, respectively, as the PK parameters following these doses were similar to or slightly greater than those observed in TB patients following clinical doses of these drugs (Table 1). The dose of amikacin (AMK) was based on that of a murine model (21).
Chemotherapy of M. tuberculosis-infected guinea pigs.
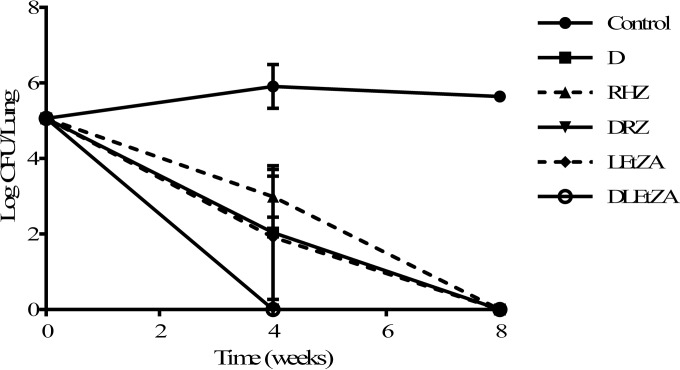
As shown in Fig. 2 and Table 2, 4 weeks postinfection and prior to the initiation of treatment (control at time zero), the bacterial load in animal lungs reached 5.06 log10 CFU. Without drug treatment (vehicle control), lung bacterial load stayed level, with only a slight increase at the subsequent 8-week time point.
FIG 2.
Numbers of CFU in the lungs of M. tuberculosis-infected guinea pigs treated with different regimens. Numbers of viable bacteria in whole lungs of M. tuberculosis Kurono-infected guinea pigs were counted after treatment with regimen D, RHZ, DRZ, LEtZA, or DLEtZA (D, DLM at 100 mg/kg; R, RIF at 25 mg/kg; H, INH at 25 mg/kg; Z, PZA at 150 mg/kg; L, LVX at 50 mg/kg; Et, ETO at 50 mg/kg; A, AMK at 150 mg/kg) for 4 and 8 weeks at 5 days per week (3 animals per group, except that there was 1 control guinea pig at 0 weeks and 2 control guinea pigs at 8 weeks because of animal deaths). In the DRZ group at 8 weeks, culture dishes (100 ml) of two samples were contaminated. No bacilli were detected in any 10-ml sample plates or in any sample plates of 4-week treatments. We considered that M. tuberculosis was totally eradicated in this treatment group. In the regimen D group after 8 weeks of treatment, there was one sample whose culture dish (100 ml) was contaminated. No bacteria were detected in the serially diluted 10-ml culture plates, the histopathological analysis sections, or the other two animals of that group. Therefore, we concluded that M. tuberculosis was totally killed in this group. Each value represents the mean of results from triplicate samples, and error bars are SDs from three replicates.
TABLE 2.
Numbers of CFU in the lungs of M. tuberculosis-infected guinea pigs treated with different regimens
| Regimena | Log10 no. of CFU in lungs (mean ± SD) atb: |
||
|---|---|---|---|
| 0 wk | 4 wk | 8 wk | |
| Control | 5.06c | 5.91 ± 0.58 | 5.64 ± 0.03d |
| D | NT | 2.04 ± 1.77 | 0 |
| RHZ | NT | 2.99 ± 0.54 | 0 |
| DRZ | NT | 0 | 0 |
| LEtZA | NT | 1.92 ± 1.80 | 0 |
| DLEtZA | NT | 0 | 0 |
All compounds except AMK were administered once daily for 5 days a week by oral gavage. The daily administration volume for each regimen group was calculated at 4 ml/kg of individual body weight. AMK was administered subcutaneously at 2 ml/kg of individual body weight.
Except in control week 0 and control week 8, the number of guinea pigs for each treatment was 3. Two animals died because the infection was missed, so only one animal was allocated into the initial control group. One animal died during the period of treatment in the 8-week control group. NT, not tested.
n = 1.
n = 2.
Regimen D (DLM alone at 100 mg/kg) eradicated lung CFU over time (2.04 log10 CFU at 4 weeks, 0 log10 CFU at 8 weeks), equivalent to the results with the LVX-ETO-PZA-AMK (LEtZA) regimen and the standard RIF-INH-PZA (RHZ) regimen (Fig. 2 and Table 2). Replacing INH with 100 mg/kg DLM in the RHZ regimen or adding 100 mg/kg DLM to the LEtZA regimen significantly accelerated eradication of bacilli, as lungs from guinea pigs treated with the DLM-RIF-PZA (DRZ) or DLM-LVX-ETO-PZA-AMK (DLEtZA) regimen resulted in no viable bacteria being detected at 4 weeks. These results indicate that DLM plays an important role in the eradication of M. tuberculosis in guinea pig lungs.
Morphological findings for guinea pig lung lesions and the effect of DLM.
All guinea pig lung samples in the above-described experiment were sectioned and stained with hematoxylin and eosin (H&E), auramine, or pimonidazole hydrochloride for histological evaluation and photography. Sections stained with H&E were ranked by microscopic examination in order of the severity of lesion burden. A typical section of guinea pig lung after 4 weeks of infection with M. tuberculosis is presented in Data Set S1A in the supplemental material. Necrotic cores, accumulations of epithelioid cells, and calcifications, the hallmarks of histological findings associated with human tuberculosis, were observed in the guinea pig lungs. Furthermore, after staining the sections with pimonidazole, an agent widely used in studies of hypoxic lesions (22), we found that all the well-developed lung granulomas had stained positively (Data Set S1B). Hypoxia was visible principally in regions surrounding the central necrotic lesions, as necrotic areas themselves cannot be stained by pimonidazole due to the lack of live cells. Bacteria (indicated by fluorescence imaging from the auramine staining) were found not only in the nonhypoxic areas but also in the hypoxic areas located in a microenvironment of necrosis with incomplete dystrophic calcification (Data Set S1A). The proportion of lesions (area of lesion per area of section) in all treated groups tended to decrease versus that in the control after 4 weeks of treatment.
We focused on the ability of each regimen to kill TB bacilli in the hypoxic regions within lung granulomas. After 4 weeks of treatment, some bacilli remained in the hypoxic regions in the D, RHZ, and LEtZA regimen groups (Data Set S2A, S2B, and S2C). Importantly, the hypoxic lesions were clearly absent in lungs treated with regimen DRZ, in which 100 mg/kg DLM replaced INH in the first-line regimen (Data Set S2D) or DLEtZA (100 mg/kg DLM with an MDR-TB regimen) (Data Set S2E). These results strongly suggest that DLM has bactericidal activity against M. tuberculosis in hypoxic lesions and can speed bacterial eradication.
DISCUSSION
We previously reported that DLM has potent antimycobacterial activity against drug-susceptible and -resistant type strains in vitro, as demonstrated by its low MICs (10). It has also been demonstrated that MICs of DLM for clinical isolates from DLM-naive patients with TB are very low (23). Moreover, DLM alone or in combination with other TB drugs is very effective in reducing the bacterial load in mice infected with M. tuberculosis (10). In clinical studies, DLM has been shown to have early bactericidal activity in drug-susceptible TB patients (17), and when added to an optimized background regimen for pulmonary MDR-TB patients, DLM increased the proportion of patients with sputum culture conversion after 2 months of treatment (18). Long-term outcomes were also improved in pulmonary MDR-TB patients when DLM was added to an optimized background regimen and administered for ≥6 months (19).
Accumulating evidence has suggested that a particular type of TB bacillus is the culprit in the high relapse rate and the need for prolonged treatment, even with multidrug therapy. TB bacillus populations are very heterogeneous, containing bacilli that replicate at different speeds or not at all, and some, often called dormant, develop phenotypic drug tolerance to currently available TB drugs (7–9). Drugs that can kill persisters may be able to shorten treatment duration (5, 6). In this study, we specifically investigated the effect of DLM on persisters in both in vitro and in vivo models.
First, we showed that DLM is bactericidal to replicating and dormant bacilli. We used an M. bovis BCG strain to evaluate the bactericidal activity of DLM against dormant bacilli mainly because many publications have reported that this slow-growing organism has physiological, morphological, and molecular behaviors similar to those of closely related pathogenic M. tuberculosis when induced by oxygen depletion (24–28). Many proteins related to the oxygen starvation-induced dormancy response of M. bovis BCG are highly identical (99.8 to 100%) to those of M. tuberculosis (24–26). The M. bovis BCG strain has also been reported to become MTZ sensitive in a manner similar to that of M. tuberculosis (29, 30). We therefore used this organism instead of M. tuberculosis in the present study. We used INH and MTZ as reference drugs, as published reports have shown that INH effectively kills only replicating bacilli, while MTZ kills only dormant ones (16). Using a modified Wayne model (7) to produce dormant bacilli, we showed that DLM kills dormant bacilli, although it requires higher concentrations than needed under aerobic conditions (Fig. 1).
Mice have often been used to evaluate TB drugs. However, traditional mouse strains do not quite mimic the pathological characteristics of TB infection observed in humans (11, 12). In particular, they do not develop caseous lesions in the lung (11). On the other hand, TB in species such as guinea pigs, rabbits, and primates possesses many similarities to human TB (6). In this study, we used guinea pigs, as they are relatively easy to house in our institute under biosafety level 3 (BSL3) conditions compared to larger animals.
Prior to the regimen study, we performed PK studies and decided the doses of these drugs based on the same criteria as those previously used to decide human-equivalent doses in mice (10). Various studies have reported different human-equivalent doses of INH, RIF, and other drugs in the treatment of M. tuberculosis-infected guinea pigs (31, 32). We noticed that when the results of PK studies of guinea pigs were compared with those of humans, the authors usually cited data from healthy people. To our knowledge, for some anti-TB drugs, the PK parameters of TB patients are quite different from those of healthy people. We therefore believed that it was better to simulate the clinical situation by using the same PK results from TB patients (33–37) in our animal models.
Indeed, as shown by histological examinations in this study, guinea pig lungs infected with M. tuberculosis possess human-like granulomas (i.e., necrotic core, accumulation of epithelioid cells, and calcification). In addition, hypoxic lesions were found in regions surrounding the central necrotic lesions and likely the whole granuloma, as necrotic areas themselves cannot be stained by pimonidazole due to the lack of live cells. Thus, the histological evidence points to the likelihood of dormant bacilli and persisters since hypoxia is one of the main inducers of these phenotypes (as supported by the in vitro data that INH was unable to kill bacilli under hypoxic conditions [Fig. 1B]). These observations are consistent with those reported by Lenaerts et al. (14) indicating that persisters may be prominent residents in guinea pig lung lesions and therefore that the guinea pig model is suitable for studying the drug effect on these particularly difficult-to-treat populations.
This study demonstrated strong bactericidal effects of DLM monotherapy; at 4 weeks, the effects were similar to those of the RHZ group or the LEtZA group, and bacteria were undetectable after 8 weeks of treatment. One possible explanation for the exceptionally good efficacy in this model is the high distribution of DLM in guinea pig lung tissue, which is about 3-fold higher than that in mice (see Data Set S3 in the supplemental material) (10). This also provoked concerns about the carryover problem of DLM in lung homogenates because of its relatively low MIC. We evaluated the lung concentration of DLM during the PK study. At 48 h after administration, the DLM remaining in the lungs of the guinea pigs was detected at a concentration of about 0.28 μg/g. According to the PK parameters and the maximum mean lung weight of the animals treated in this study (Data Set S3), the carryover of DLM in lung homogenates was calculated to be below its MIC against the M. tuberculosis Kurono strain after 72 h (the time at which the animals were sacrificed after the last administration). We therefore believe that DLM remaining in lung homogenates did not suppress bacterial growth.
We conducted another study with DLM at 10 mg/kg and compared the results to the effects with INH (25 mg/kg) and RIF (25 mg/kg). As shown in Data Set S4, all three drugs reduced the bacterial load to similar degrees in guinea pig lungs after 4 weeks of treatment. However, histological results indicate that INH had little effect on TB bacilli in the necrotic lesions (Data Set S5C) after 4 weeks of treatment. DLM at the lower dose (10 mg/kg) demonstrated only modest killing of bacilli in the hypoxic regions (Data Set S5), suggesting that DLM at this lower dose could not efficiently eradicate the bacteria inside hypoxic lesions of lung granulomas. We therefore raised the dose of DLM to a human-equivalent dose of 100 mg/kg in our regimen study. DLM at this dose demonstrated a stronger bactericidal activity than it did at the 10-mg/kg dose, with the number of lung CFU declining by about 3 log10 CFU after 4 weeks of treatment, suggesting a dose-dependent effect of this agent in the guinea pig model.
Ahmad et al. (31) suggested that the human-equivalent dose of INH in guinea pigs was 60 mg/kg based on clinical data from healthy people or nontuberculosis patients and that the bactericidal activity exhibited by INH (with only a 10-fold reduction of bacilli in the guinea pig lungs) was a little weaker than that in our result (Data Set S4) (38). Based on the clinical data from TB patients (Table 1), our PK study suggested that the human-equivalent dose of INH is 25 mg/kg, similar to the dose of 30 mg/kg used by other reports (15, 39). In addition, the outcome for INH was similar to that in the report from Hoff et al. (a 1.71-log reduction compared with an ∼1.6- to 1.7-log reduction) (39). Similar differences in the outcomes in guinea pigs were also found for RIF when our data at a dose of 25 mg/kg were compared with those in a report of RIF at a dose of 50 mg/kg (40). The reason for different results among groups is unknown, but the methodological differences, such as bacterial strains, breeding conditions, and the time of initiation of the treatment, could be considerable. Although there were many differences between the two groups, it is worth mentioning that RIF also exhibited a dose-dependent effect in our laboratory (data not shown).
We also demonstrated good efficacy in the RHZ group: no viable bacilli were detected after 8 weeks of treatment. This is in contrast to the efficacy of RHZ in mice, which require a much longer treatment (10). The better efficacy of the D and RHZ regimens in guinea pigs may be due to the lower bacterial load in this animal model at the initiation of treatment. In our laboratory, the lung bacterial load in mice could reach 7 log10 CFU (10), whereas in guinea pigs, the bacterial load was about 5 log10 CFU prior to treatment (Fig. 2 and Table 2). One group (41) reported that an RHZ regimen (RIF at 50 mg/kg, INH 60 at mg/kg, PZA at 300 mg/kg), even administered twice weekly, exhibited very strong bactericidal activity in guinea pigs and that the animal lungs were culture negative after 8 weeks of treatment. This outcome was much better than a clinically acceptable outcome, the outcome of another report (39), and our study result. We consider that this was due mainly to the comparatively higher dose and the species-specific accumulation of RIF in the guinea pigs, which resulted in the main outcome of the combination treatment because INH did not exhibit a dose-dependent effect in guinea pigs (data not shown).
Replacing INH with DLM in the RHZ first-line drug regimen significantly accelerated the eradication of bacilli in the guinea pig, possibly due to DLM's ability to kill bacilli in the hypoxic region within the granuloma. This was confirmed by the reduction of the number of bacilli in the DRZ group versus the RHZ group, revealed by the diminished auramine staining in the DRZ group. Importantly, adding DLM to an MDR-TB drug regimen (LEtZA) also sped the clearance of bacilli in the guinea pig lungs. In fact, adding DLM shortened the eradication from 8 weeks to 4 weeks of treatment with both of these regimens.
We did not observe any obvious side effects in the DLM-treated group or in the regimens that included DLM during the period of the study, which indicated the safety of DLM in this animal model. We also did not observe obvious side effects (such as the gastrointestinal toxicity reported in some papers) in the RHZ group at the indicated doses (15, 32). Examining the differences between our study and those reporting gastrointestinal toxicity, we found that the lack of any obvious toxicity was considered to be due to the lower dose of RIF (25 mg/kg versus 50 or 100 mg/kg). In addition, we believe that the toxicity observed in some studies was associated mainly with the specific accumulation of RIF in the guinea pigs following administration five times per week at higher dosages than ours. The results of our PK studies and another PK report (20) support this view.
Several limitations with the interpretations of our results exist. First, the sample size in our study was limited. Second, although we observed hypoxic lesions within the granuloma, we did not have direct evidence that there are indeed persisters in these regions. We could make this link based only on the general knowledge that hypoxia is a main contributor to inducing persisters. Third, we did not perform relapse studies to determine whether lungs that were sterilized by treatment were indeed cleared of all TB bacilli. Future studies on the rate of relapses are warranted.
In conclusion, we demonstrated that DLM exhibits potent bactericidal effect against M. tuberculosis in both the replicating and the dormant condition in vitro. In guinea pigs infected with M. tuberculosis, DLM effectively killed bacilli in the hypoxic lesions within the granuloma. Adding DLM to current TB regimens may improve treatment outcomes, as demonstrated in recent clinical trials with pulmonary MDR-TB patients. DLM may therefore be an important drug for consideration when constructing new regimens to shorten TB treatment duration.
MATERIALS AND METHODS
Bacterial strains.
M. tuberculosis strain Kurono (ATCC 35812) was purchased from the American Type Culture Collection (ATCC). M. bovis BCG Tokyo was obtained from the Laboratory of Culture Collection, Institute of Medical Science, University of Tokyo. All bacterial strains were grown to logarithmic phase in supplemented Middlebrook 7H9 broth (Becton Dickinson) and then stored at −80°C for further use.
Antibiotics.
Metronidazole (MTZ), isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), ethionamide (ETO), and amikacin (AMK) were purchased from Sigma Chemical Co., Ltd. Levofloxacin (LVX) was purchased from Tokyo Chemical Industry Co., Ltd. DLM was synthesized by Otsuka Pharmaceutical Co., Ltd. Antibiotics were dissolved and diluted with dimethyl sulfoxide (DMSO) in in vitro assays and were suspended in a 5% gum arabic solution in in vivo experiments, except for AMK, which was dissolved in a sterile physiological saline solution.
Aerobic and dormant bactericidal assay.
For the aerobic bactericidal assay, M. bovis BCG Tokyo bacteria from frozen stock were precultured for 1 day in supplemented Middlebrook 7H9 broth with 0.05% Tween 80 at 37°C. DMSO solutions of the antibiotics were added to the cultures to reach the final concentrations shown in Fig. 1A. INH and MTZ were used as positive controls for killing aerobic and anaerobic bacilli, respectively (16). The bacteria were treated with antibiotics at 37°C for 7 days and then harvested by centrifugation at 10,000 rpm for 5 min and washed with sterile distilled water (DW) to remove the antibiotics remaining in the culture broths. The harvested bacterial pellets were resuspended in 1 ml of sterile DW and 10-fold serially diluted. The bacterial dilutions or suspensions were spread on Middlebrook 7H11 agar plates (Becton Dickinson). Plates were incubated for about 2 weeks at 37°C before the numbers of colonies were counted.
We used a modified Wayne model (7) to induce dormant bacilli for the anaerobic bactericidal assay. Briefly, M. bovis BCG Tokyo bacteria from frozen stock were thawed at room temperature and precultured for 7 days in supplemented Dubos broth (Becton Dickinson) with 0.05% Tween 80 at 37°C and then initiated with Wayne culture, an oxygen-limited culture condition, for 21 days at 37°C. The bacterial cultures were then transferred to an anaerobic MIP-1025 incubator (Sanyo) for another 7 days at 37°C before the antibiotic challenge under the same condition. The final concentrations are shown in Fig. 1B. The bacteria were harvested after 7 days of treatment by centrifugation at 10,000 rpm for 5 min and washed with sterile DW to remove the antibiotics remaining in the culture broths. The harvested bacterial pellets were resuspended in 1 ml of sterile DW, 10-fold serially diluted, and spread on Middlebrook 7H11 agar plates for plate CFU counts. The plates were incubated for about 4 weeks at 37°C before the numbers of colonies were counted.
Experimental animals.
This study was carried out in strict accordance with the recommendations in the Guidelines for Animal Care and Use (44), which were approved by the Animal Care and Use Committee of Otsuka Pharmaceutical Co., Ltd.
Four-week-old female Hartley guinea pigs with body weights ranging from 230 to 320 g (purchased from Japan SLC, Inc.) were housed in CGP-2 cages (three animals/cage; Clea Japan, Inc.) and given at least 3 days to acclimate to the housing facility. Animals were of specific-pathogen-free (SPF) grade and housed with free access to LRC4 (Oriental Yeast Co., Ltd.) and water in our biosafety level II (BSL2) and BSL3 animal laboratories in which environmental controls were set to the following conditions: a temperature of 23°C ± 2°C, humidity of 60% ± 10%, and a 12-h light-dark cycle (light period, 7 a.m. to 7 p.m.). Animals were randomized to each group after determination of body weight in each experiment by the “Infectious Disease LA System,” an in-house product.
Plasma and lung concentrations of antibiotics.
Separate groups of three guinea pigs were given a single oral dose of 10 or 100 mg/kg DLM, 25 mg/kg INH, 150 mg/kg PZA, 50 mg/kg LVX, or 50 mg/kg ETO. Animals in the RIF group were given a single oral dose of 25 mg/kg daily for 5 days. The guinea pigs were sacrificed under isoflurane anesthetization for sample collections at the following time points after the final antibiotic dosing: 1 h, 2 h, 4 h, 8 h, 16 h, 24 h, and 48 h for DLM; 15 min, 30 min, 1 h, 2 h, 4 h, 8 h, and 24 h for INH, PZA and RIF; and 5 min, 15 min, 30 min, 1 h, 2 h, 4 h, 8 h, and 24 h for LVX and ETO. Heparinized blood samples were centrifuged (3,000 rpm for 10 min at 4°C) to obtain the plasma. For the DLM group, the animals were bled for euthanasia and the lungs were collected after blood sampling. The lung samples were rinsed gently with ice-cold physiological saline and immediately homogenized after addition of a 4-fold (vol/wt) volume of ice-cold methanol physiological saline buffer (3:1, vol/vol). All plasma and lung samples were frozen and stored below −60°C until analysis. Except for RIF samples, samples were analyzed by a Nanospace SI-2 high-performance liquid chromatography (HPLC) system (Shiseido Co., Ltd.), with an API4000 tandem mass spectrometry (MS/MS) detector (AB SCIEX) equipped with an electrospray ionization (ESI) source and an EPC10W switching valve (Valco Instruments Co. Inc.). RIF plasma samples, mixed with an antioxidant, 10% l-(+)-ascorbic acid (Sigma) aqueous solution at 100:1 (vol/vol), were analyzed using an LC-20A HPLC system (Shimadzu Corp.). For the following pharmacokinetic (PK) parameters of the oral antibiotics, we determined the mean concentrations per sampling time point in uninfected animals with WinNonlin Professional software (version 6.1): Cmax, peak (maximum) concentration of the compound; tmax, time to peak (maximum) concentration; AUCt, area under the concentration-time curve calculated to the last observable concentration at time t; and t1/2, terminal-phase elimination half-life.
Infection of guinea pigs with M. tuberculosis and antibiotic treatments.
The M. tuberculosis Kurono strain has routinely been used in animal studies at our institute. Bacteria from frozen stock were thawed at room temperature, and the inocula were prepared by dilution of the cell suspension with a sterile physiological-saline solution (Otsuka). The guinea pigs were anesthetized by the intramuscular administration of a xylazine solution (a 2% Serakutaru [Bayer Yakuhin]-sterile physiological-saline solution at 3:17 [vol/vol]) and then infected by an intratracheal inoculation with 0.2 ml of the inocula. The infection dose per animal was 6.04 × 102 CFU. Chemotherapy was initiated 4 weeks after infection (day 0). The dosing frequency was daily (5 days per week) for 4 or 8 weeks. The guinea pigs received one of the following regimens: a vehicle control regimen, D (DLM alone), RHZ (RIF-INH-PZA), DRZ (DLM-RIF-PZA), LEtZA (LVX-ETO-PZA-AMK), or DLEtZA (DLM-LVX-ETO-PZA-AMK). The antibiotic mixtures were prepared daily and orally administered, except for AMK, which was dissolved in sterile physiological-saline solution and administered subcutaneously. Regimens that included RIF were dosed by administering RIF 1 h after administration of other drugs to limit drug-drug interactions (42). The following doses were used: 100 mg/kg for DLM, 25 mg/kg for INH and RIF, 150 mg/kg for PZA and AMK, and 50 mg/kg for LVX and ETO. These doses achieved clinically appropriate plasma levels for each drug, as shown in Table 1.
Study endpoints.
Subgroups of animals (3 for each time point) were sacrificed on day 0 or after 4 or 8 weeks of treatment (72 h after the last administration except for day 0). The hypoxia marker pimonidazole hydrochloride (NPI Inc.) was intraperitoneally administered 1.5 h before necropsy at a dose of 60 mg/kg for hypoxic staining of the lung lesions (14). To obtain the lung CFU, all of the lung lobes except for the right-upper lobe were harvested and homogenized by a homogenizer with 5 ml of sterilized DW. Lung homogenates or their 10-fold serial dilutions (with sterile DW) were plated on Middlebrook 7H11 agar plates containing 200 units/ml of polymyxin B, 100 mg/liter of carbenicillin, 10 mg/liter of amphotericin B, and 20 mg/liter of trimethoprim (43). For groups with a lung CFU burden of 0 (indicating that the infection had been eradicated), all of the freezer-stored (−80°C) lung homogenates from the same samples were replated on a sterile square BioAssay dish (Nunc) with 100 ml of Middlebrook 7H11 agar containing the same concentrations of antibiotics as described above to confirm eradication after 4 weeks of incubation.
Histology.
The right upper lung lobe was fixed immediately in 10% formalin and then embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E) and auramine or immunostained with the antipimonidazole antibody by the method of Lenaerts et al. (14). Bacilli were located specifically by visual inspection of three random fields of the primary granuloma. The histopathological analysis was performed at a different institute (Biopathology Institute Co., Ltd.), and the observers were blind to the treatment groups.
Statistical analysis.
Log-transformed values of CFU counts per milliliter and CFU counts per animal were compared for in vitro and in vivo studies, respectively, using the SAS system (SAS Institute Japan; R.8.1). The significance level of the test was set at 5%. Statistically significant differences between the compound-treated groups and the vehicle control group were determined by Dunnett's test alone.
Supplementary Material
ACKNOWLEDGMENTS
We thank Yongge Liu, Zuzanna Huebschmann, Masanori Kawasaki, Yoshiko Himeda, Suguru Hatanaka, Hiraku Hagita, and Chie Kawasome at Otsuka Pharmaceutical, who gave valuable advice and technical support during the studies.
We report working as scientists for Otsuka Pharmaceutical Co., Ltd., the originator and owner of delamanid and sole financial supporter of the studies. None of the authors has or expects to have stock options.
All authors actively participated in the studies related to delamanid described in this article. M. Matsumoto proposed this study, including the aim and procedures, decided the design and procedures for the study in detail, and discussed them with H. Hashizume and X. Chen. The in vitro study was conducted by X. Chen and T. Tomishige, and the pharmacokinetic study was performed by X. Chen, H. Hashizume, M. Matsuba, M. Fujiwara, R. Kitamoto, E. Hanaki, and Y. Ohba. Evaluation of delamanid in a guinea pig model of TB infection was conducted by X. Chen, H. Hashizume, I. Nakamura, and M. Matsuba. The data analysis was conducted by M. Matsumoto and X. Chen. The manuscript was written mainly by X. Chen and revised by M. Matsumoto.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02402-16.
REFERENCES
- 1.World Health Organization. 2016. Global tuberculosis report 2016. Document WHO/HTM/TB/2016.13 World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/global_report/en/. [Google Scholar]
- 2.Sacchettini JC, Rubin EJ, Freundlich JS. 2008. Drugs versus bugs: in pursuit of the persistent predator Mycobacterium tuberculosis. Nat Rev Microbiol 6:41–52. doi: 10.1038/nrmicro1816. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. 2014. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. Document WHO/HTM/TB/2014.11 World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 4.World Health Organization. 2016. WHO treatment guidelines for drug-resistant tuberculosis, 2016 update. Document WHO/HTM/TB/2016.04 World Health Organization, Geneva, Switzerland. [Google Scholar]
- 5.Mitchison D, Davies G. 2012. The chemotherapy of tuberculosis: past, present and future. Int J Tuberc Lung Dis 16:724–732. doi: 10.5588/ijtld.12.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franzblau SG, DeGroote MA, Cho SH, Andries K, Nuermberger E, Orme IM, Mdluli K, Angulo-Barturen I, Dick T, Dartois V, Lenaerts AJ. 2012. Comprehensive analysis of methods used for the evaluation of compounds against Mycobacterium tuberculosis. Tuberculosis (Edinb) 92:453–488. doi: 10.1016/j.tube.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Wayne LG, Hayes LG. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect Immun 64:2062–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol 43:717–731. doi: 10.1046/j.1365-2958.2002.02779.x. [DOI] [PubMed] [Google Scholar]
- 9.Deb C, Lee C-M, Dubey VS, Daniel J, Abomoelak B, Sirakova TD, Pawar S, Rogers L, Kolattukudy PE. 2009. A novel in vitro multiple-stress dormancy model for Mycobacterium tuberculosis generates a lipid-loaded, drug-tolerant, dormant pathogen. PLoS One 4:e6077. doi: 10.1371/journal.pone.0006077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto M, Hashizume H, Tomishige T, Kawasaki M, Tsubouchi H, Sasaki H, Shimokawa Y, Komatsu M. 2006. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med 3:e466. doi: 10.1371/journal.pmed.0030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn JL. 2006. Lessons from experimental Mycobacterium tuberculosis infections. Microbes Infect 8:1179–1188. doi: 10.1016/j.micinf.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 12.McMurray DN. 2001. Disease model: pulmonary tuberculosis. Trends Mol Med 7:135–137. doi: 10.1016/S1471-4914(00)01901-8. [DOI] [PubMed] [Google Scholar]
- 13.Via LE, Lin PL, Ray SM, Carrillo J, Allen SS, Eum SY, Taylor K, Klein E, Manjunatha U, Gonzales J, Lee EG, Park SK, Raleigh JA, Cho SN, McMurray DV, Flynn JL, Barry CE. 2008. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun 76:2333–2340. doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenaerts AJ, Hoff D, Aly S, Ehlers S, Andries K, Cantarero L, Orme IM, Basaraba RJ. 2007. Location of persisting mycobacteria in a guinea pig model of tuberculosis revealed by r207910. Antimicrob Agents Chemother 51:3338–3345. doi: 10.1128/AAC.00276-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ordway DJ, Shanley CA, Caraway ML, Orme EA, Bucy DS, Hascall-Dove L, Henao-Tamayo M, Harton MR, Shang S, Ackart D, Kraft SL, Lenaerts AJ, Basaraba RJ, Orme IM. 2010. Evaluation of standard chemotherapy in the guinea pig model of tuberculosis. Antimicrob Agents Chemother 54:1820–1833. doi: 10.1128/AAC.01521-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wayne LG. 1994. Dormancy of Mycobacterium tuberculosis and latency of disease. Eur J Clin Microbiol Infect Dis 13:908–914. doi: 10.1007/BF02111491. [DOI] [PubMed] [Google Scholar]
- 17.Diacon AH, Dawson R, Hanekom M, Narunsky K, Venter A, Hittel N, Geiter LJ, Wells CD, Paccaly AJ, Donald PR. 2011. Early bactericidal activity of delamanid (OPC-67683) in smear-positive pulmonary tuberculosis patients. Int J Tuberc Lung Dis 15:949–954. doi: 10.5588/ijtld.10.0616. [DOI] [PubMed] [Google Scholar]
- 18.Gler MT, Skripconoka V, Sanchez-Garavito E, Xiao H, Cabrera-Rivero JL, Vargas-Vasquez DE, Gao M, Awad M, Park SK, Shim TS, Suh GY, Danilovits M, Ogata H, Kurve A, Chang J, Suzuki K, Tupasi T, Koh WJ, Seaworth B, Geiter LJ, Wells CD. 2012. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med 366:2151–2160. doi: 10.1056/NEJMoa1112433. [DOI] [PubMed] [Google Scholar]
- 19.Skripconoka V, Danilovits M, Pehme L, Tomson T, Skenders G, Kummik T, Cirule A, Leimane V, Kurve A, Levina K, Geiter LJ, Manissero D, Wells CD. 2013. Delamanid improves outcomes and reduces mortality in multidrug-resistant tuberculosis. Eur Respir J 41:1393–1400. doi: 10.1183/09031936.00125812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dutta NK, Alsultan A, Peloquin CA, Karakousis PC. 2013. Preliminary pharmacokinetic study of repeated doses of rifampin and rifapentine in guinea pigs. Antimicrob Agents Chemother 57:1535–1537. doi: 10.1128/AAC.01933-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veziris N, Ibrahim M, Lounis N, Andries K, Jarlier V. 2011. Sterilizing activity of second-line regimens containing TMC207 in a murine model of tuberculosis. PLoS One 6:e17556. doi: 10.1371/journal.pone.0017556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aly S, Wagner K, Keller C, Malm S, Malzan A, Brandau S, Bange FC, Ehlers S. 2006. Oxygen status of lung granulomas in Mycobacterium tuberculosis-infected mice. J Pathol 210:298–305. doi: 10.1002/path.2055. [DOI] [PubMed] [Google Scholar]
- 23.Stinson K, Kurepina N, Venter A, Fujiwara M, Kawasaki M, Timm J, Shashkina E, Kreiswirth BN, Liu Y, Matsumoto M, Geiter L. 2016. MIC of delamanid (OPC-67683) against Mycobacterium tuberculosis clinical isolates and a proposed critical concentration. Antimicrob Agents Chemother 60:3316–3322. doi: 10.1128/AAC.03014-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boon C, Li R, Qi R, Dick T. 2001. Proteins of Mycobacterium bovis BCG induced in the Wayne dormancy model. J Bacteriol 183:2672–2676. doi: 10.1128/JB.183.8.2672-2676.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boon C, Dick T. 2002. Mycobacterium bovis BCG response regulator essential for hypoxic dormancy. J Bacteriol 184:6760–6767. doi: 10.1128/JB.184.24.6760-6767.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boon C, Dick T. 2012. How Mycobacterium tuberculosis goes to sleep: the dormancy survival regulator DosR a decade later. Future Microbiol 7:513–518. doi: 10.2217/fmb.12.14. [DOI] [PubMed] [Google Scholar]
- 27.Rosenkrands I, Slayden RA, Crawford J, Aagaard C, Barry CE, Andersen P. 2002. Hypoxic response of Mycobacterium tuberculosis studied by metabolic labeling and proteome analysis of cellular and extracellular proteins. J Bacteriol 184:3485–3491. doi: 10.1128/JB.184.13.3485-3491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park H-D, Guinn KM, Harrell MI, Liao R, Voskuil MI, Tompa M, Schoolnik GK, Sherman DR. 2003. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol Microbiol 48:833–843. doi: 10.1046/j.1365-2958.2003.03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim A, Eleuterio M, Hutter B, Murugasu-Oei B, Dick T. 1999. Oxygen depletion-induced dormancy in Mycobacterium bovis BCG. J Bacteriol 181:2252–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wayne LG, Sramek HA. 1994. Metronidazole is bactericidal to dormant cells of Mycobacterium tuberculosis. Antimicrob Agents Chemother 38:2054–2058. doi: 10.1128/AAC.38.9.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmad Z, Klinkenberg LG, Pinn ML, Fraig MM, Peloquin CA, Bishai WR, Nuermberger EL, Grosset JH, Karakousis PC. 2009. Biphasic kill curve of isoniazid reveals the presence of drug-tolerant, not drug-resistant, Mycobacterium tuberculosis in the guinea pig. J Infect Dis 200:1136–1143. doi: 10.1086/605605. [DOI] [PubMed] [Google Scholar]
- 32.Ahmad Z, Nuermberger EL, Tasneen R, Pinn ML, Williams KN, Peloquin CA, Grosset JH, Karakousis PC. 2010. Comparison of the “Denver regimen” against acute tuberculosis in the mouse and guinea pig. J Antimicrob Chemother 65:729–734. doi: 10.1093/jac/dkq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsumiya T, Yamato K, Ryu C. 1985. Studies on the blood level of RFP and new administration method of RFP in relation to other anti-tuberculous drugs. Kekkaku 60:483–494. (In Japanese.) [PubMed] [Google Scholar]
- 34.Israili ZH, Rogers CM, el-Attar H. 1987. Pharmacokinetics of antituberculosis drugs in patients. J Clin Pharmacol 27:78–83. doi: 10.1177/009127008702700113. [DOI] [PubMed] [Google Scholar]
- 35.Tappero JW, Bradford WZ, Agerton TB, Hopewell P, Reingold AL, Lockman S, Oyewo A, Talbot EA, Kenyon TA, Moeti TL, Moffat HJ, Peloquin CA. 2005. Serum concentrations of antimycobacterial drugs in patients with pulmonary tuberculosis in Botswana. Clin Infect Dis 41:461–499. doi: 10.1086/431984. [DOI] [PubMed] [Google Scholar]
- 36.Peloquin CA, Hadad DJ, Molino LPD, Palaci M, Boom WH, Dietze R, Johnson JL. 2008. Population pharmacokinetics of levofloxacin, gatifloxacin, and moxifloxacin in adults with pulmonary tuberculosis. Antimicrob Agents Chemother 52:852–857. doi: 10.1128/AAC.01036-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu M, Namdar R, Stambaugh JJ, Starke JR, Bulpitt AE, Berning SE, Peloquin CA. 2002. Population pharmacokinetics of ethionamide in patients with tuberculosis. Tuberculosis (Edinb) 82:91–96. doi: 10.1054/tube.2002.0330. [DOI] [PubMed] [Google Scholar]
- 38.Dutta NK, Pinn ML, Zhao M, Rudek MA, Karakousis PC. 2013. Thioridazine lacks bactericidal activity in an animal model of extracellular tuberculosis. J Antimicrob Chemother 68:1327–1330. doi: 10.1093/jac/dkt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoff DR, Caraway ML, Brooks EJ, Driver ER, Ryan GJ, Peloquin CA, Orme IM, Basaraba RJ, Lenaerts AJ. 2008. Metronidazole lacks antibacterial activity in guinea pigs infected with Mycobacterium tuberculosis. Antimicrob Agents Chemother 52:4137–4140. doi: 10.1128/AAC.00196-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dutta NK, Illei PB, Peloquin CA, Pinn ML, Mdluli KE, Nuermberger EL, Grosset JH, Karakousis PC. 2012. Rifapentine is not more active than rifampin against chronic tuberculosis in guinea pigs. Antimicrob Agents Chemother 56:3726–3731. doi: 10.1128/AAC.00500-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmad Z, Fraig MM, Pinn ML, Tyagi S, Nuermberger EL, Grosset JH, Karakousis PC. 2011. Effectiveness of tuberculosis chemotherapy correlates with resistance to Mycobacterium tuberculosis infection in animal models. J Antimicrob Chemother 66:1560–1566. doi: 10.1093/jac/dkr188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grosset J, Truffot-Pernot C, Lacroix C, Ji B. 1992. Antagonism between isoniazid and the combination pyrazinamide-rifampin against tuberculosis infection in mice. Antimicrob Agents Chemother 36:548–551. doi: 10.1128/AAC.36.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchison DA, Allen BW, Carrol L, Dickinson JM, Aber VR. 1972. A selective oleic acid albumin agar medium for tubercle bacilli. J Med Microbiol 5:165–175. doi: 10.1099/00222615-5-2-165. [DOI] [PubMed] [Google Scholar]
- 44.Science Council of Japan 2006. Guidelines for proper conduct of animal experiments. Science Council of Japan http://www.scj.go.jp/en/animal.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.