ABSTRACT
Penicillin-binding protein 4 (PBP4), a nonessential, low-molecular-weight penicillin-binding protein of Staphylococcus aureus, has been implicated in low-level resistance to β-lactam antibiotics, although the mechanism is unknown. Mutations in PBP4 and its promoter were identified in a laboratory-generated mutant strain, CRB, which expresses high-level resistance to β-lactams, including resistance to the new-generation cephalosporins active against methicillin-resistant strains of S. aureus. These mutations did not appreciably alter the β-lactam antibiotic binding affinity of purified recombinant mutant PBP4 compared to that of wild-type PBP4. Compared to the susceptible parent strain, COLnex, the CRB strain produces a highly cross-linked cell wall peptidoglycan, indicative of increased transpeptidase activity. The pbp4 promoter mutation of CRB was associated with greatly increased amounts of PBP4 in membranes compared to those in the COLnex parent. Replacement of the native promoter of COLnex with the mutant promoter of CRB resulted in increased amounts of PBP4 in membranes and a highly cross-linked cell wall. PBP4 can be repurposed to provide essential transpeptidase activity in vivo and confer high-level resistance to β-lactam antibiotics, such as ceftobiprole and ceftaroline.
KEYWORDS: Staphylococcus aureus, penicillin-binding protein 4, β-lactam antibiotics, PBP4, β-lactams, ceftaroline, ceftobiprole, drug resistance mechanisms, mechanisms of resistance, penicillin-binding proteins
INTRODUCTION
Staphylococcus aureus is an invasive human pathogen that causes community and hospital-acquired infections, resulting in increased hospitalization and mortality (1). Methicillin-resistant S. aureus (MRSA) strains exhibit broad class resistance to β-lactams, mediated by the low binding affinity of penicillin-binding protein 2a (PBP2a), encoded by mecA (1) or mecC (2). However, we have shown that resistance to β-lactams can also occur independently of PBP2a (3). Such resistance is associated with mutations in penicillin-binding protein 4 (PBP4) (4). PBP4 is a nonessential 45-kDa enzyme with both transpeptidase and carboxypeptidase activities (5). PBP4 functions in conjunction with multiple PBPs in cell wall biosynthesis (6). Overexpression of PBP4 has been associated with low-level methicillin resistance (7–10).
Ceftobiprole and ceftaroline are members of a new class of cephalosporins active against S. aureus, including methicillin-resistant strains, due to their ability to form stable covalent inhibitory complexes with PBPs, including PBP2a (11). Both antibiotics have completed phase III clinical trials, and ceftaroline is approved by the U.S. Food and Drug Administration (FDA) for the treatment of community-acquired bacterial pneumonia and acute bacterial skin infections. Ceftobiprole has also been approved by the European Medicines Agency and is currently being marketed in Europe. Although it is not approved in the United States, the drug has received the qualified infectious disease product designation from the U.S. FDA. Resistance to both of these drugs has been generated in vitro and has been reported clinically and in surveillance studies worldwide, often in association with mutations in mecA (4, 12–17). We previously reported that passage of a mecA-negative strain of S. aureus, COLnex, passaged in ceftobiprole selected for a highly resistant mutant strain, CRB (4, 12, 18). As previously described, COLnex is a staphylococcal cassette chromosome mec element (SCCmec) excision strain derived from COLn, a non-USA300-lineage laboratory strain (19). Whole-genome sequencing revealed that this mutant contained two substitution mutations, E183A and F241R, near the active site of PBP4 (4, 12). CRB also has mutations in a cyclic-di-AMP phosphodiesterase and an efflux pump, GdpP (N182K) and AcrB (I960V), respectively (4). Their roles in β-lactam resistance is not well characterized. We have previously identified mutations directly upstream of the pbp4 gene in strains passaged in ceftaroline and ceftobiprole, but the CRB strain was not tested in those studies (20). Mutations in the promoter region of pbp4 have also been reported to result in overexpression of PBP4 and increased methicillin resistance (9). In the current studies, we investigated the role of mutations in the pbp4 structural gene and/or its promoter of strain CRB (PCRB), a high-level resistant mutant of COLnex generated by in vitro passage in ceftobiprole.
RESULTS
Increased production of PBP4 mediates resistance to ceftobiprole.
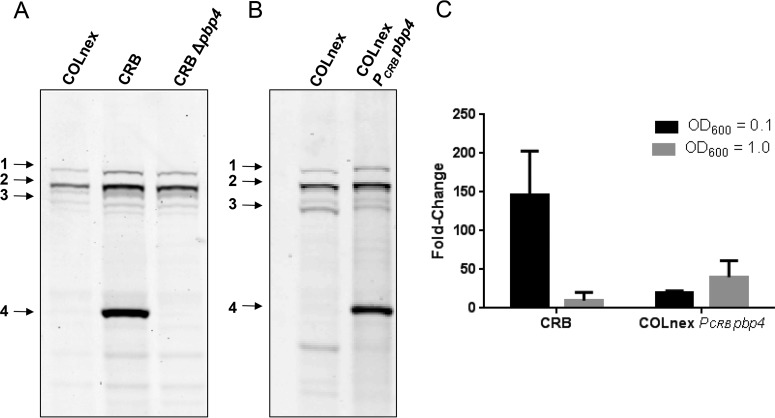
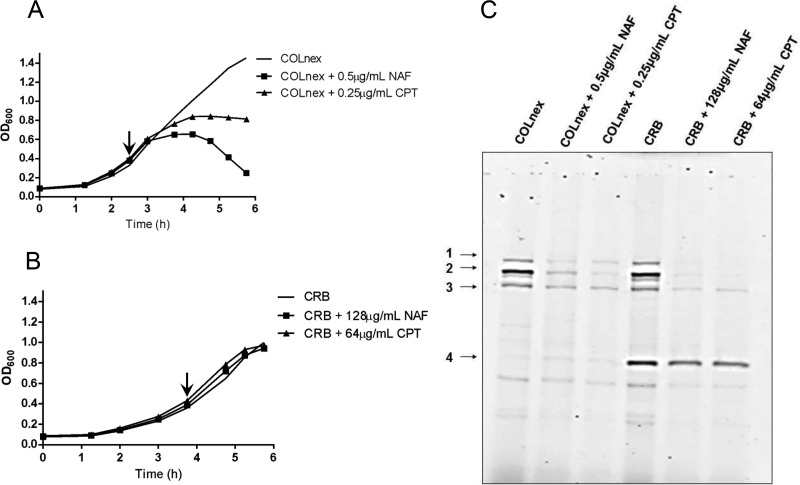
We have previously shown that passage of the COLnex strain in ceftobiprole (generating strain CRB) resulted in resistance to both ceftobiprole and ceftaroline, with the MICs being 128 μg/ml and 64 μg/ml, respectively (Table 1), and that resistance was associated with two amino acid substitutions, E183A and F241R, in PBP4 (3, 4). Resistance was also associated with a markedly increased amount of PBP4 in whole-cell lysates and membrane proteins of CRB compared with the amount in COLnex (Fig. 1A; see also Fig. S1A to C in the supplemental material), in which PBP4 was poorly visualized in Bocillin-FL assays. This correlated with a 145-fold increase in the level of expression of the pbp4 gene in CRB compared to that in COLnex for cells harvested at an optical density at 600 nm (OD600) of 0.1 and an approximately 10-fold increase for cells harvested at an OD600 of 1.0 (Fig. 1C). The in-frame deletion of pbp4 in the CRB strain abolished Bocillin-FL labeling of PBP4 (Fig. 1A), and the resultant mutant, CRB Δpbp4, was susceptible to a wide range of β-lactams (MICs, ≤0.25 μg/ml) (Table 1).
TABLE 1.
MICs of bacterial strainsa
| Strain | Characteristics | MIC (μg/ml) |
Reference(s) or source | ||||
|---|---|---|---|---|---|---|---|
| NAF | AMP | BPR | CFZ | CPT | |||
| COLn | Laboratory S. aureus strain | 256 | 16 | 2 | >256 | 1 | 4, 12 |
| COLnex | COLn with SCCmec excision | 0.5 | 0.5 | 1 | 1 | 0.25 | 4, 12 |
| CRB | COLnex passaged in BPR | 128 | 256 | 128 | >256 | 64 | 4, this study |
| CRB Δpbp4 | pbp4 deletion mutant of CRB | 0.25 | 0.25 | <0.25 | 0.25 | 0.25 | This study |
| COLnex PCRB pbp4 | COLnex with CRB promoter | 8 | 8 | 4 | 64 | 32 | This study |
NAF, nafcillin; AMP, ampicillin; BPR, ceftobiprole; CFZ, cefazolin; CPT, ceftaroline.
FIG 1.
PBP4 amounts and pbp4 transcription in the CRB and COLnex PCRB pbp4 strains. (A) PBPs in Bocillin-FL-labeled whole-cell lysates of parental strain COLnex, mutant CRB, and the CRB Δpbp4 strain. PBPs 1 to 4 are indicated in the left margin. (B) PBPs in Bocillin-FL-labeled whole-cell lysates of COLnex and the COLnex PCRB pbp4 strain with the CRB promoter mutation. (C) S. aureus cells were collected at an OD600 of 0.1 (∼108 CFU/ml) and an OD600 of 1.0 (∼109 CFU/ml). Shown is the fold increase in the level of pbp4 expression relative to that in the COLnex parental strain. Data are from four independent experiments with CRB and two independent experiments with COLnex PCRB pbp4. Each sample was run in duplicate.
We have previously reported the presence of mutations in the pbp4 promoter region of ceftaroline- and ceftobiprole-resistant mutants selected by passage in these antibiotics (20). Sequence analysis of the pbp4 promoter region of CRB revealed a 36-bp duplication (ATTTATATGATAGAATATTTCTATTGCATTTTTTGT) at position −290 bp upstream of the pbp4 gene. To determine the role that promoter mutations play in β-lactam resistance in the CRB strain, the native promoter of COLnex was replaced by the mutant CRB promoter upstream of wild-type pbp4 to create COLnex PCRB pbp4. COLnex PCRB pbp4 had a marked increase in the level of PBP4 protein expression in whole-cell lysates compared to that for the COLnex parent strain, with the amount being similar to that present in CRB (Fig. 1B), which correlated with the 20-fold increase in the level of expression of pbp4 for cells harvested at an OD600 of 0.1 and approximately 40-fold for cells harvested at an OD600 of 1.0 (Fig. 1C). This promoter mutation produced an increase in the MICs of nafcillin and ceftobiprole from 0.5 and 1 μg/ml, respectively, for COLnex to 8 and 4 μg/ml, respectively, for COLnex PCRB pbp4 and an increase in the ceftaroline MIC from 0.25 to 32 μg/ml.
Binding affinity of wild-type and mutant PBP4 proteins.
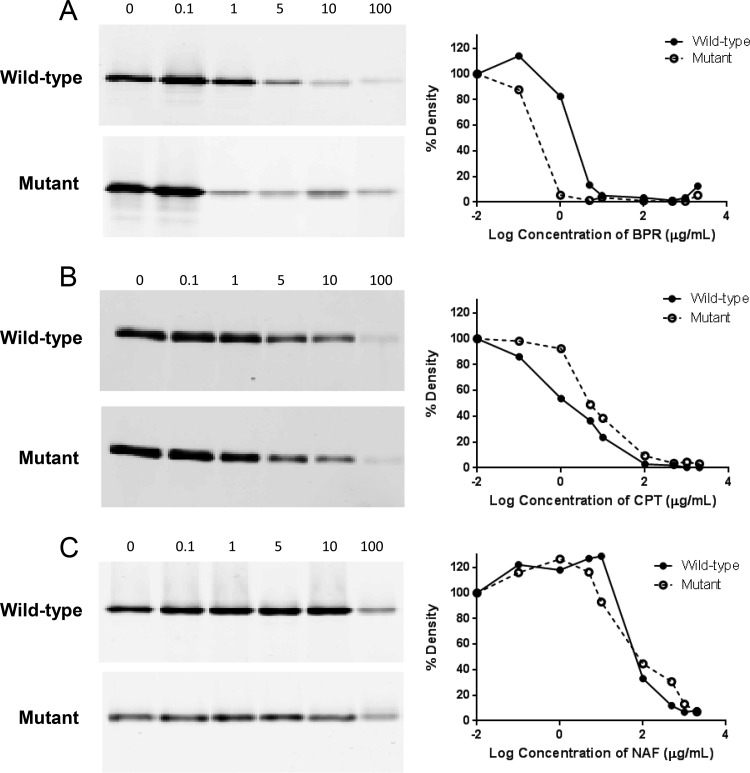
The effect of mutations on the PBP4 binding affinity was determined in competition assays using crude membrane preparations and recombinant wild-type or mutant PBP4 protein. There appeared to be a reduced affinity of PBP4 binding to ceftobiprole, ceftaroline, and nafcillin in membrane preparations of the mutant compared with those of the wild type (Fig. S1A to C). However, marked differences in the amounts of PBP4 in membranes due to low-level expression of wild-type PBP4 in COLnex relative to the level expression of mutant PBP4 of CRB precluded accurate measurement of the amount of Bocillin-FL-labeled PBP4 in membrane preparations of COLnex. Competition assays with similar amounts of recombinant wild-type or mutant PBP4 protein showed a modestly increased affinity of ceftobiprole binding to mutant PBP4 compared to that to the wild-type strain, with 50% saturation occurring at approximately 1 and 5 μg/ml, respectively (Fig. 2A). The binding affinities for nafcillin and ceftaroline were similar for the mutant and wild-type PBP4 proteins (Fig. 2B and C). Thus, PBP4-mediated resistance was not attributable to a lower binding affinity of the mutant protein compared to that of the wild-type protein.
FIG 2.
Antibiotic binding to wild-type and mutant recombinant PBP4. Recombinant PBP4 samples were incubated with 0 to 100 μg/ml (fluorescent gels on the left) and 0 to 2,000 μg/ml (percent densities in the graphs on the right) of ceftobiprole (BPR) (A), ceftaroline (CPT) (B), or nafcillin (NAF) (C) for 15 min at 37°C and then labeled with Bocillin-FL. Band density was measured with a Typhoon Trio variable-mode imager and analyzed with ImageQuant TL software. Percent density is relative to that for the no-antibiotic control (0 μg/ml). Data are representative of those from one of two independent experiments.
Altered cell wall composition and increased peptidoglycan cross-linking in CRB and the COLnex PCRB pbp4 mutant.
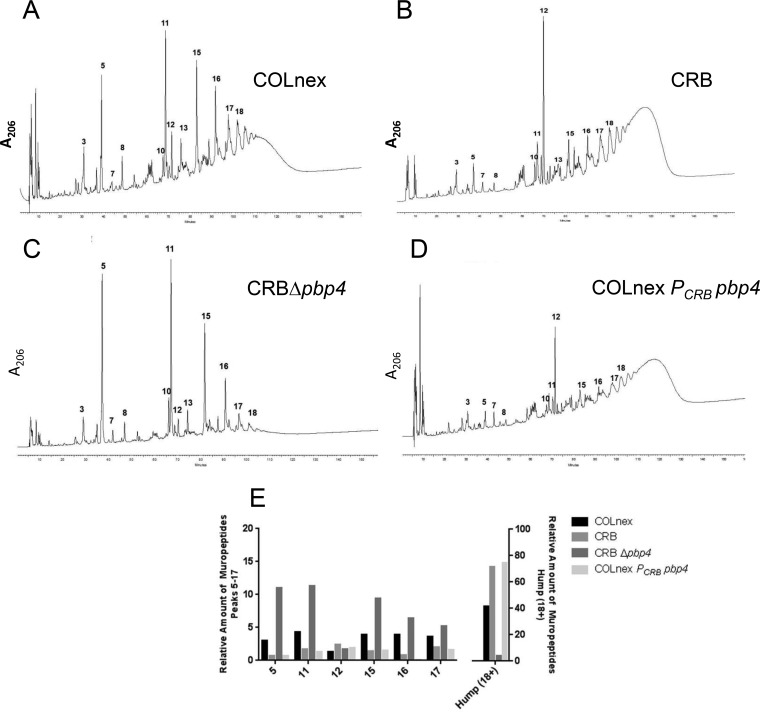
PBP4 has both carboxypeptidase and transpeptidase activities, but it has been demonstrated that transpeptidase activity likely dominates in vivo (5, 6). Overexpression of PBP4, whether it was the wild-type PBP4 in COLnex or the mutant PBP4 in CRB, was associated with resistance, but the binding affinity was not changed. Therefore, we hypothesized that an overall increase in the transpeptidase activity of PBP4 likely accounted for resistance. To test this, cell wall peptidoglycan was isolated from the parent and mutant CRB strains, and the muropeptide compositions were analyzed by high-performance liquid chromatography (HPLC) to quantitate the amounts of cross-linked oligomeric muropeptides, the hallmark signature of cell wall transpeptidase activity. The HPLC profiles showed a dramatic increase in the amount of highly cross-linked oligomeric muropeptides (referred to as the hump) in the CRB mutant strain compared to that in the parent strain, COLnex (Fig. 3A, B, and E), indicative of increased transpeptidase activity (6). Deletion of PBP4 in the CRB strain (generating strain CRB Δpbp4) resulted in the loss of the highly cross-linked peptidoglycan components (Fig. 3C and E). COLnex PCRB pbp4, the promoter mutant expressing wild-type PBP4, had a muropeptide composition similar to that of the CRB strain, indicating that overexpression of PBP4 increased transpeptidase activity (Fig. 3D and E).
FIG 3.
Changes in the peptidoglycan compositions of S. aureus CRB mutant strains. (A to D) Peptidoglycans were prepared, and muropeptide patterns were analyzed by HPLC for the COLnex parental strain (A), mutant CRB (B), CRB in which pbp4 was deleted (CRB Δpbp4) (C), and the COLnex PCRB pbp4 strain with the CRB promoter mutation (D). Peaks 5, 11, 15, 16, and 17 represent the mono-, di-, tri-, tetra-, and pentameric muropeptide derivatives, respectively. Peaks 18+ (the hump) contain highly cross-linked oligomeric components. (E) Quantitative differences in the peptidoglycan compositions of COLnex, CRB, and their derivatives. The bars show the relative amounts of muropeptides calculated from the UV absorbance of the peaks in relation to the total amount of muropeptides. The relative amounts of muropeptides for peaks 5 to 17 and 18+ (the hump) are depicted on the left and right axes, respectively.
In addition to the increase in the amount of highly cross-linked oligomeric muropeptides, the relative amount of muropeptide 12 was also dramatically increased in the CRB mutant strain and in COLnex PCRB pbp4. The structure of muropeptide 12 is such that it cannot be further polymerized into oligomeric components (21, 22). In strains CRB and COLnex PCRB pbp4 with highly expressed PBP4, all mono-, di-, and trimuropeptides (except muropeptide 12) appeared to be used to form the highly polymeric cell wall peptidoglycan (the hump), with an increase in the relative amount of peptide 12.
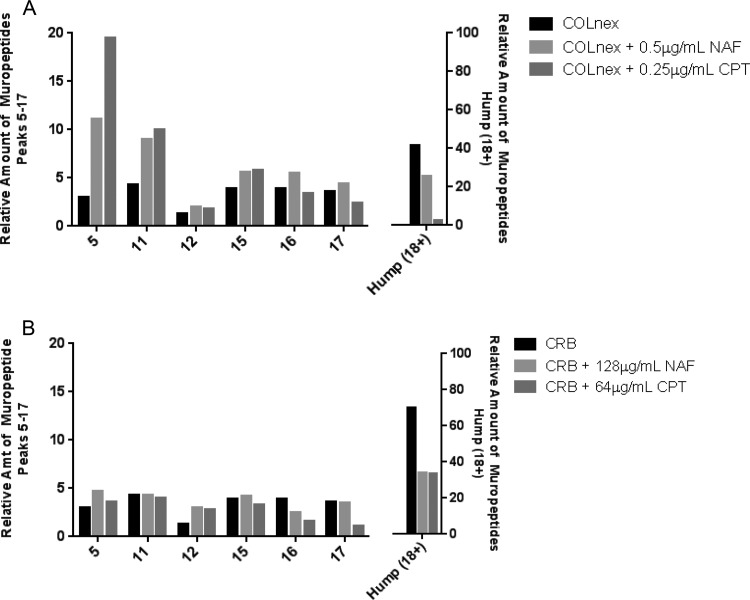
Growth of the COLnex strain in the presence of nafcillin or ceftaroline (0.5 and 0.25 μg/ml, respectively) eliminated the highly polymerized cell wall components and resulted in accumulation of the monomeric muropeptide (peak 5) and muropeptides 11 to 17 (oligomeric cell wall precursors) (Fig. 4A and 5A; see also Fig. S2). Qualitatively similar effects on cell wall composition patterns were observed in strain CRB grown in the presence of nafcillin at 128 μg/ml or ceftaroline at 64 μg/ml but were much attenuated compared to those seen in COLnex (Fig. 4B and 5B; see also Fig. S2). These concentrations of nafcillin or ceftaroline had little effect on the growth of CRB (Fig. 5B), and significant amounts of unbound PBP4 were detectable by the Bocillin-FL assays in the CRB strain (Fig. 5C).
FIG 4.
Effect of antibiotics on peptidoglycan composition. Peptidoglycans were prepared, and muropeptide patterns were analyzed by HPLC for the COLnex parental strain grown with nafcillin (NAF) at 0.5 μg/ml or ceftaroline (CPT) at 0.25 μg/ml) (A) and mutant CRB grown with nafcillin at 128 μg/ml or ceftaroline at 64 μg/ml (B). Peaks 5, 11, 15, 16, and 17 represent the mono-, di-, tri-, tetra-, and pentameric muropeptide derivatives, respectively. Peaks 18+ (the hump) contain highly cross-linked oligomeric components. The bars show the relative amounts of muropeptides calculated from the UV absorbance of the peaks. The relative amounts of muropeptides for peaks 5 to 17 and 18+ (the hump) are depicted on the left and right axes, respectively.
FIG 5.
PBP4 protein amounts in strains grown in the presence of antibiotic. (A and B) COLnex (A) and CRB (B) were grown to an OD600 of 0.4 (arrow), and then no drug, nafcillin (NAF) at 0.5 μg/ml (COLnex) or 128 μg/ml (CRB), or ceftaroline (CPT) at 0.25 μg/ml (COLnex) or 64 μg/ml (CRB) was added. (C) PBPs in Bocillin-FL-labeled whole-cell lysates were prepared from cells, all of which were collected at the same time, at an OD600 of 1.0 for the COLnex strain or the CRB strain. PBPs 1 to 4 are indicated in the left margin. Data are representative of those from one of two independent experiments.
DISCUSSION
Severe infections due to MRSA and the emergence of resistance to β-lactams, including new-generation cephalosporins, emphasize the importance of exploring the mechanisms of antibiotic resistance. The molecular and biochemical bases of PBP4-mediated resistance have not previously been explored. We show that overexpression of mutant PBP4 in the CRB strain is associated with high-level resistance (4, 12) and that deletion of the pbp4 gene restores full susceptibility to a variety of β-lactams. This supports previous work with a ceftobiprole- and ceftaroline-resistant mecA-negative S. aureus USA300 strain (12) and underscores the potential role of PBP4 in resistance to β-lactam antibiotics.
PBP4 is a low-molecular-weight penicillin-binding protein that has a dual function as a transpeptidase and carboxypeptidase to cross-link the oligomeric muropeptide components (the hump) during cell wall biosynthesis (6). Although PBP4 is considered to be nonessential for cell wall biosynthesis, studies have suggested that PBP2, PBP2a, and PBP4 work in concert to synthesize the cell wall and are associated with β-lactam resistance (6, 7). PBP4 overexpression was shown to result in greater cross-linking of the peptidoglycan (9). Our work supports the findings of these previous studies, as PBP4 is highly expressed in CRB (Fig. 1A and C). Overexpression of PBPs drives an overall gain of transpeptidase activity, as evidenced by the presence of a highly cross-linked cell wall product (Fig. 3B and E) and elimination of highly cross-linked muropeptides upon deletion of pbp4. Interestingly, when CRB was incubated with β-lactams at their MICs, unbound PBP4 was still readily detectable and a highly cross-linked cell wall continued to be produced (Fig. 4B and 5C). These data would support a previous hypothesis from our group (7) that PBP4 may be remodeled to a more efficient transpeptidase that can substitute for one or more of the other high-molecular-weight PBPs which are bound to β-lactams (7).
Mutations in the promoter region of PBP4 have been linked to increased methicillin resistance (9). When the mutant promoter of CRB was introduced into a strain with wild-type PBP4 (COLnex PCRB pbp4), the result was reduced β-lactam susceptibility. PBP4 expression increased as well, driving a gain of transpeptidase activity and the production of highly cross-linked cell wall peptidoglycan (Fig. 1B, 1C, 3D, and 3E). These data indicate that the promoter plays a role in resistance, although it cannot account for the full measure of resistance present in CRB. Differences in pbp4 expression between CRB and COLnex PCRB pbp4, which could account in part for the higher MICs for the former than the latter, may reflect differences in the growth rates of the mutants relative to each other and COLnex and may also be influenced by the other mutations that are present in CRB but not COLnex PCRB pbp4. Other mutations present in CRB include two substitution mutations, E183A and F241R in PBP; a mutation in GdpP (N182K), a cyclic-di-AMP phosphodiesterase; and a mutation in AcrB (I960V), an efflux pump (4). Deletion mutation of AcrB in CRB had no effect on its resistance (unpublished data). The mutations in GdpP and PBP4 could account for the higher levels of resistance in CRB than COLnex PCRB pbp4, and their roles are under investigation. With respect to mutations in the PBP4 structural gene, experiments to determine whether these produce changes in binding kinetics not detected by the competition assays, enhance substrate binding and cross-linking, or both are in progress.
These results together further emphasize the role that PBP4 can play in the emergence of high-level β-lactam resistance. Future studies to characterize the quantitative transpeptidase or carboxypeptidase activity of PBP4 will enhance understanding of the role of this enigmatic penicillin-binding protein in cell wall biosynthesis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
We have previously characterized the COLnex parental strain and the mutant CRB strain used in this study (4, 12). All strains of S. aureus were routinely cultured in tryptic soy broth (TSB) at 37°C with aeration.
Allelic replacement.
Deletion of pbp4 in the CRB strain (generating strain CRB Δpbp4) was carried out using a pKOR1-based allelic replacement procedure (23). Briefly, 1-kb up- and downstream regions of the pbp4 gene were PCR amplified using COLnex genomic DNA as a template and published PCR primer pairs (3). The PCR fragments were fused together by spliced overlap PCR and subsequently cloned into the vector pKOR1 as previously described (23). The resultant vector was transformed into the CRB strain. Following this, a standard allelic replacement procedure was performed (23). The fidelity of the pbp4 deletion was verified by analytical PCR and Sanger sequencing.
Genomic DNA extraction and pbp4 promoter sequencing for the CRB strain were performed as previously described (20). A COLnex strain bearing the mutant CRB promoter (strain COLnex PCRB pbp4) was created. PCR products were generated with primer pairs P1/P2 and P3/P4 and COLnex genomic DNA as the template (see Table S1 in the supplemental material). The resultant PCR products were ligated by splice overlap PCR, restriction digested with the NotI and KpnI enzymes, and ligated to the predigested pIMAY vector. The recombinant plasmid was transformed into the COLnex strain, and allelic replacement of the promoter was carried out as previously described (24). The fidelity of the mutant was verified by Sanger sequencing.
MICs and growth curves.
MICs were determined in triplicate by the microdilution method, according to CLSI guidelines (25), using cation-adjusted Mueller-Hinton broth (CAMHB). The MIC was recorded as the lowest concentration of drug preventing visible growth.
Growth curves were performed with aeration at 37°C by inoculation of 10 ml of TSB with a 1:100 dilution of an overnight culture. Antibiotic was added when the OD600 was 0.4.
Antibiotics.
Ceftobiprole (provided by Johnson and Johnson Pharmaceutical Research and Development), ceftaroline (provided by Forest Labs), and nafcillin, ampicillin, and cefazolin (all from Sigma-Aldrich) stocks were prepared daily. Ceftobiprole and ceftaroline were dissolved in dimethyl sulfoxide, and nafcillin, ampicillin, and cefazolin were dissolved in water.
Real-time PCR (qPCR) for pbp4.
For quantitative PCR (qPCR), overnight cultures of S. aureus were diluted 1:100 in TSB and bacteria were harvested during early to mid-log phase (OD600 = 0.1, ∼108 CFU/ml) or late log phase (OD600 = 0.1, ∼109 CFU/ml). Samples were stored in a commercial RNA stabilization reagent (RNAlater; Ambion) at −20°C for subsequent analysis.
Total RNA was extracted and cleared of contaminating genomic DNA (with an RNeasy minikit [Qiagen] and Turbo DNA-free DNase [Ambion]). RNA quantity and quality were assessed by absorption spectrometry and gel electrophoresis, respectively. RNA (1 μg) was reverse transcribed using a high-capacity RNA-to-cDNA kit (Applied Biosystems) according to the manufacturer's instructions. qPCR was performed using SYBR PCR Mastermix in an ABI 7500 system (Applied Biosystems) according to the manufacturer's instructions using qPCR primers for pbp4 or the housekeeping gene rrsA (Table S1). A dissociation curve was run to verify product integrity. Data are expressed as the fold change in expression compared to that by the parental strain.
Cloning, expression, and purification of wild-type and modified PBP4.
For the cloning and expression of wild-type and mutant PBP4, an Escherichia coli expression system (pET-15b; Invitrogen) was used to produce functionally active recombinant PBP4 as previously described with the following changes, indicated below (26). Plasmids coding for wild-type and CRB PBP4 were transformed into E. coli BL21(DE3) and were grown in Luria-Bertani broth (supplemented with 100 μg/ml ampicillin) at 37°C up to an OD600 of 0.5 to 0.8. Cells were then cooled at 4°C for at least 30 min before the addition of isopropyl β-d-1-thiogalactopyranoside to a final concentration of 0.1 mM. Protein was expressed overnight at 17°C with shaking before the cells were harvested by centrifugation and stored at −80°C. Cells from 4.5 liters of culture were resuspended in buffer A (20 mM Tris, pH 7.5, 500 mM NaCl, 20 mM imidazole) containing DNase I (14 μg/ml; Roche) and a tablet containing EDTA-free protease inhibitor cocktail (Roche). Cells were lysed at 4°C with an homogenizer (Avestin). The cell lysate was centrifuged for 45 min at 45,000 rpm in a type 70 Ti rotor (Beckman Coulter) at 4°C. The supernatant was collected and filtered (pore size, 0.45 μm) before being loaded onto a 1-ml HisTrap HP (GE Lifesciences) nickel affinity column equilibrated in buffer A. The column was washed with at least 10 column volumes of buffer A before the protein of interest was eluted from the column with a gradient of from 0 to 50% buffer B (20 mM Tris, pH 7.5, 500 mM NaCl, 1.0 M imidazole) over 40 min at a flow rate of 1 ml/min. The recovered protein was dialyzed overnight in buffer A at 4°C, and 1 μl bovine α-thrombin (Hematologic Technologies Inc.) was added per ml of collected protein to cleave the polyhistidine tag. The dialyzed protein was again run through a 1-ml HisTrap HP column, and the flowthrough was collected and concentrated on a 30-kDa-molecular-mass-cutoff Centricon membrane (Amicon) before being loaded onto a Superdex 75 column (GE Life Sciences) equilibrated in buffer C (20 mM MES [morpholineethanesulfonic acid], pH 6, 300 mM NaCl). Fractions were analyzed via sodium dodecyl sulfate (SDS)-PAGE, and the fractions containing high-purity PBP4 were collected and concentrated to 25 to 30 mg/ml, as determined by measurement of the absorbance at 280 nm. The protein was cooled in liquid nitrogen before storage at −80°C.
Whole-cell and membrane protein isolation and penicillin-binding protein competition assays.
PBPs were detected by fluorography of whole-cell proteins or membrane proteins fluorescently labeled with Bocillin-FL (Thermo Fisher Scientific), as previously described, with modifications (27–31). Briefly, overnight cultures of S. aureus were diluted 1:100 in TSB, and bacterial cells were harvested during late log phase (OD600 = 1.0). Cells were collected by centrifugation and then resuspended in 1/100 the original volume of PBS. For whole-cell preparations, 25 μl of concentrated whole cells was labeled with 10 mM Bocillin-FL at 35°C for 30 min and then lysed with 50 μg/ml lysostaphin at 37°C for 1 h. For membrane protein preparations, cells were mechanically disrupted using a FastPrep-24 disrupter (6.0 m/s for 40 s; MP Biomedical) in the presence of 50 μg/ml lysostaphin. The supernatant was centrifuged at 26,000 × g to pellet the membrane proteins. Membrane preparations (200 μg of protein) were labeled with 10 mM Bocillin-FL at 35°C for 30 min. Following labeling of either whole cells or membranes, an equal volume of sample buffer was added and the samples were boiled for 5 min.
For antibiotic competition assays, membrane preparations (200 μg) or purified recombinant PBP4 samples (250 μg) were incubated with increasing concentration of antibiotics at 37°C for 15 min. Next, the samples were incubated with 10 mM Bocillin-FL at 35°C for 30 min. The reaction was terminated by addition of sample buffer and boiling.
The protein concentration was determined using a NanoDrop spectrophotometer (Thermo Scientific), and 200 μg of total cellular protein or 25 μg of membrane protein was run on 10% SDS-polyacrylamide gels. The bands were visualized by a Typhoon Trio variable-mode imager (GE Healthcare) using Cy3/Cy5 filters. The relative band density was determined using ImageQuant TL software.
Preparation of peptidoglycan and analysis by HPLC.
Preparation of peptidoglycan was performed as described previously (21, 22). Strains were grown overnight in TSB at 37°C with aeration. The overnight culture was then back diluted into 700 ml of TSB containing the antibiotic to an OD620 of 0.01 and incubated at 37°C with aeration. When the OD620 was 0.5 to 0.7, the cells were harvested by centrifugation for 10 min at 16,000 × g (4°C) and subsequently transferred into 4% boiling SDS. The cells were boiled for 30 min, and the pellet was collected by centrifugation for 10 min at 30,000 × g, followed by washing with water until no more SDS could be detected. The crude cell wall was disrupted with glass beads (diameter, 0.2 μm; acid washed; Sigma) on a Vortex mixer at maximal speed at 4°C for 15 min. The suspension was centrifuged at 2,000 × g, and the crude cell wall was collected by centrifugation at 40,000 × g for 15 min. The pellet was treated with DNase I (10 μg/ml; Sigma), RNase A (50 μg/ml; Sigma) in 50 mM Tris-HCl, pH 7.5, supplemented with 20 mM MgSO4 and incubated for 2 h at 37°C. Next, the suspension was treated with trypsin (100 μg/ml; Worthington) in the presence of 10 mM CaCl2 for 16 h at 37°C. The enzymes were inactivated by boiling in 1% SDS for 15 min. The crude cell walls were collected by centrifugation and washed two times with water and once with 8 M LiCl, followed by a wash with 100 mM EDTA. After two washes with water followed by a wash with acetone, the broken cells were resuspended in water and lyophilized. In order to remove the teichoic acid, 5 mg of cell walls was stirred in 2 ml of hydrofluoric acid (49%) for 48 h at 4°C. The peptidoglycan was recovered by centrifugation at 50,000 × g for 45 min, washed three times with water, and then stored in 0.05% sodium azide at 4°C.
Preparation and analysis of muropeptides.
Muropeptides were prepared as described by Glauner (32). Peptidoglycan (1 mg/ml) was digested in 12.5 mM phosphate buffer (pH 5.5), 0.02% sodium azide with 5 μg/ml of muramidase of Streptomyces globisporus (Sigma) at 37°C for 16 h. The sample was boiled for 5 min and centrifuged (Eppendorf centrifuge, top speed). The supernatant was mixed with an equal volume of 0.5 M borate buffer (pH 9.0) and reduced with sodium borohydride for 15 min at room temperature. The pH of this solution was adjusted to 2 with orthophosphoric acid. Samples were stored at −20°C. Separation of muropeptides was performed by HPLC with a Shimadzu LC-10A system as described before (21). The column was eluted at a flow rate of 0.5 ml/min with a linear gradient starting at 5 min after injection of 5% (vol/vol) methanol in 100 mM NaH2PO4 (pH 2.5), 0.00025% azide to 30% (vol/vol) methanol in 100 mM NaH2PO4 (pH 2.5) in 150 min. The column temperature was 52°C. The eluted compounds were detected by determination of the absorption at 206 nm.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by NIH grant R01-AI100291 (to H.F.C.) and operating funds from the Canadian Institutes of Health Research and the Howard Hughes Medical Institute International Scholar program (to N.C.J.S.). J.A.N.A. is a CIHR Vanier Scholar, and N.C.J.S. is a Tier 1 Canada Research Chair. Partial support for this study was provided by U.S. Public Health Service Award 2 R01 AI457838-15 (to A.T.). Personnel (T.M.D.C.) were sponsored by CNPq, Brazil.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02727-16.
REFERENCES
- 1.Chambers HF, Deleo FR. 2009. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol 7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ito T, Hiramatsu K, Tomasz A, de Lencastre H, Perreten V, Holden MT, Coleman DC, Goering R, Giffard PM, Skov RL, Zhang K, Westh H, O'Brien F, Tenover FC, Oliveira DC, Boyle-Vavra S, Laurent F, Kearns AM, Kreiswirth B, Ko KS, Grundmann H, Sollid JE, John JF Jr, Daum R, Soderquist B, Buist G. 2012. Guidelines for reporting novel mecA gene homologues. Antimicrob Agents Chemother 56:4997–4999. doi: 10.1128/AAC.01199-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan LC, Gilbert A, Basuino L, da Costa TM, Hamilton SM, Dos Santos KR, Chambers HF, Chatterjee SS. 2016. PBP 4 mediates high-level resistance to new-generation cephalosporins in Staphylococcus aureus. Antimicrob Agents Chemother 60:3934–3941. doi: 10.1128/AAC.00358-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee R, Gretes M, Harlem C, Basuino L, Chambers HF. 2010. A mecA-negative strain of methicillin-resistant Staphylococcus aureus with high-level beta-lactam resistance contains mutations in three genes. Antimicrob Agents Chemother 54:4900–4902. doi: 10.1128/AAC.00594-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiao Y, Lebar MD, Schirner K, Schaefer K, Tsukamoto H, Kahne D, Walker S. 2014. Detection of lipid-linked peptidoglycan precursors by exploiting an unexpected transpeptidase reaction. J Am Chem Soc 136:14678–14681. doi: 10.1021/ja508147s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leski TA, Tomasz A. 2005. Role of penicillin-binding protein 2 (PBP2) in the antibiotic susceptibility and cell wall cross-linking of Staphylococcus aureus: evidence for the cooperative functioning of PBP2, PBP4, and PBP2A. J Bacteriol 187:1815–1824. doi: 10.1128/JB.187.5.1815-1824.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambers HF, Sachdeva MJ, Hackbarth CJ. 1994. Kinetics of penicillin binding to penicillin-binding proteins of Staphylococcus aureus. Biochem J 301(Pt 1):139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henze UU, Berger-Bachi B. 1995. Staphylococcus aureus penicillin-binding protein 4 and intrinsic beta-lactam resistance. Antimicrob Agents Chemother 39:2415–2422. doi: 10.1128/AAC.39.11.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henze UU, Roos M, Berger-Bachi B. 1996. Effects of penicillin-binding protein 4 overproduction in Staphylococcus aureus. Microb Drug Resist 2:193–199. doi: 10.1089/mdr.1996.2.193. [DOI] [PubMed] [Google Scholar]
- 10.Memmi G, Filipe SR, Pinho MG, Fu Z, Cheung A. 2008. Staphylococcus aureus PBP4 is essential for beta-lactam resistance in community-acquired methicillin-resistant strains. Antimicrob Agents Chemother 52:3955–3966. doi: 10.1128/AAC.00049-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otero LH, Rojas-Altuve A, Llarrull LI, Carrasco-Lopez C, Kumarasiri M, Lastochkin E, Fishovitz J, Dawley M, Hesek D, Lee M, Johnson JW, Fisher JF, Chang M, Mobashery S, Hermoso JA. 2013. How allosteric control of Staphylococcus aureus penicillin binding protein 2a enables methicillin resistance and physiological function. Proc Natl Acad Sci U S A 110:16808–16813. doi: 10.1073/pnas.1300118110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan LC, Basuino L, Diep B, Hamilton S, Chatterjee SS, Chambers HF. 2015. Ceftobiprole- and ceftaroline-resistant methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 59:2960–2963. doi: 10.1128/AAC.05004-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez R, Paz LI, Rosato RR, Rosato AE. 2014. Ceftaroline is active against heteroresistant methicillin-resistant Staphylococcus aureus clinical strains despite associated mutational mechanisms and intermediate levels of resistance. Antimicrob Agents Chemother 58:5736–5746. doi: 10.1128/AAC.03019-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelley WL, Jousselin A, Barras C, Lelong E, Renzoni A. 2015. Missense mutations in PBP2A affecting ceftaroline susceptibility detected in epidemic hospital-acquired methicillin-resistant Staphylococcus aureus clonotypes ST228 and ST247 in western Switzerland archived since 1998. Antimicrob Agents Chemother 59:1922–1930. doi: 10.1128/AAC.04068-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long SW, Olsen RJ, Mehta SC, Palzkill T, Cernoch PL, Perez KK, Musick WL, Rosato AE, Musser JM. 2014. PBP2a mutations causing high-level ceftaroline resistance in clinical methicillin-resistant Staphylococcus aureus isolates. Antimicrob Agents Chemother 58:6668–6674. doi: 10.1128/AAC.03622-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karlowsky JA, Biedenbach DJ, Bouchillon SK, Iaconis JP, Reiszner E, Sahm DF. 2016. In vitro activity of ceftaroline against bacterial pathogens isolated from skin and soft tissue infections in Europe, Russia and Turkey in 2012: results from the Assessing Worldwide Antimicrobial Resistance Evaluation (AWARE) surveillance programme. J Antimicrob Chemother 71:162–169. doi: 10.1093/jac/dkv311. [DOI] [PubMed] [Google Scholar]
- 17.Biedenbach DJ, Alm RA, Lahiri SD, Reiszner E, Hoban DJ, Sahm DF, Bouchillon SK, Ambler JE. 2015. In vitro activity of ceftaroline against Staphylococcus aureus isolated in 2012 from Asia-Pacific countries as part of the AWARE surveillance program. Antimicrob Agents Chemother 60:343–347. doi: 10.1128/AAC.01867-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banerjee R, Gretes M, Basuino L, Strynadka N, Chambers HF. 2008. In vitro selection and characterization of ceftobiprole-resistant methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 52:2089–2096. doi: 10.1128/AAC.01403-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katayama Y, Zhang HZ, Hong D, Chambers HF. 2003. Jumping the barrier to beta-lactam resistance in Staphylococcus aureus. J Bacteriol 185:5465–5472. doi: 10.1128/JB.185.18.5465-5472.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greninger AL, Chatterjee SS, Chan LC, Hamilton SM, Chambers HF, Chiu CY. 2016. Whole-genome sequencing of methicillin-resistant Staphylococcus aureus resistant to fifth-generation cephalosporins reveals potential non-mecA mechanisms of resistance. PLoS One 11:e0149541. doi: 10.1371/journal.pone.0149541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Jonge BL, Chang YS, Gage D, Tomasz A. 1992. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain. The role of penicillin binding protein 2A. J Biol Chem 267:11248–11254. [PubMed] [Google Scholar]
- 22.Boneca IG, Xu N, Gage DA, de Jonge BL, Tomasz A. 1997. Structural characterization of an abnormally cross-linked muropeptide dimer that is accumulated in the peptidoglycan of methicillin- and cefotaxime-resistant mutants of Staphylococcus aureus. J Biol Chem 272:29053–29059. doi: 10.1074/jbc.272.46.29053. [DOI] [PubMed] [Google Scholar]
- 23.Bae T, Schneewind O. 2006. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55:58–63. doi: 10.1016/j.plasmid.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Monk IR, Shah IM, Xu M, Tan MW, Foster TJ. 2012. Transforming the untransformable: application of direct transformation to manipulate genetically Staphylococcus aureus and Staphylococcus epidermidis. mBio 3:e00277-11. doi: 10.1128/mBio.00277-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, ninth edition. Document M07-A09. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 26.Navratna V, Nadig S, Sood V, Prasad K, Arakere G, Gopal B. 2010. Molecular basis for the role of Staphylococcus aureus penicillin binding protein 4 in antimicrobial resistance. J Bacteriol 192:134–144. doi: 10.1128/JB.00822-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chambers HF, Sachdeva M. 1990. Binding of beta-lactam antibiotics to penicillin-binding proteins in methicillin-resistant Staphylococcus aureus. J Infect Dis 161:1170–1176. doi: 10.1093/infdis/161.6.1170. [DOI] [PubMed] [Google Scholar]
- 28.Chambers HF, Sachdeva M, Kennedy S. 1990. Binding affinity for penicillin-binding protein 2a correlates with in vivo activity of beta-lactam antibiotics against methicillin-resistant Staphylococcus aureus. J Infect Dis 162:705–710. doi: 10.1093/infdis/162.3.705. [DOI] [PubMed] [Google Scholar]
- 29.Moisan H, Pruneau M, Malouin F. 2010. Binding of ceftaroline to penicillin-binding proteins of Staphylococcus aureus and Streptococcus pneumoniae. J Antimicrob Chemother 65:713–716. doi: 10.1093/jac/dkp503. [DOI] [PubMed] [Google Scholar]
- 30.Zhao G, Meier TI, Kahl SD, Gee KR, Blaszczak LC. 1999. BOCILLIN FL, a sensitive and commercially available reagent for detection of penicillin-binding proteins. Antimicrob Agents Chemother 43:1124–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davies TA, Page MG, Shang W, Andrew T, Kania M, Bush K. 2007. Binding of ceftobiprole and comparators to the penicillin-binding proteins of Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pneumoniae. Antimicrob Agents Chemother 51:2621–2624. doi: 10.1128/AAC.00029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glauner B. 1988. Separation and quantification of muropeptides with high-performance liquid chromatography. Anal Biochem 172:451–464. doi: 10.1016/0003-2697(88)90468-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.