ABSTRACT
Lascufloxacin exhibited a broad spectrum of activity against various clinical isolates. Furthermore, lascufloxacin showed the most potent activity against Gram-positive bacteria among the quinolones tested and incomplete cross-resistance against existing quinolone-resistant strains. Enzymatic analysis indicated that lascufloxacin had potent inhibitory activity against both wild-type and mutated target enzymes. These results suggest that lascufloxacin may be useful in treating infections caused by various pathogens, including quinolone-resistant strains.
KEYWORDS: antimicrobial activity, antimicrobial agents, quinolones
TEXT
Recently, the emergence of strains resistant to commercially available antibacterial drugs, including β-lactams, macrolides, tetracyclines, and aminoglycosides, has become a clinical problem around the world. Quinolones are also widely used for treatment of various infectious diseases, and the development of quinolone resistance in various bacteria has become a clinical concern. For example, a large proportion of methicillin-resistant Staphylococcus aureus (MRSA) isolates showed resistance to quinolones (1, 2). For Streptococcus pneumoniae, a major causative organism of respiratory tract infections, the acquisition of resistance to quinolones has been reported in various areas of the world, including Southeast Asia (3). Therefore, the development of more-potent agents against resistant Gram-positive bacteria is required in preparation for the emergence of resistant strains.
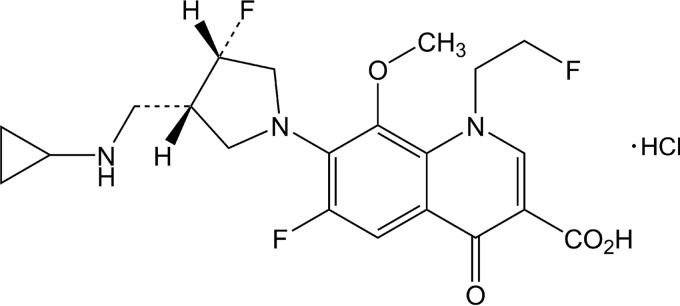
Lascufloxacin is a novel fluoroquinolone antibacterial agent newly developed in Japan (Fig. 1). This study was performed to determine the antibacterial activities of lascufloxacin against various clinical isolates. In addition, the activities of lascufloxacin against sequentially selected quinolone-resistant S. aureus, S. pneumoniae, and Escherichia coli were evaluated, and the inhibitory activities of lascufloxacin against target enzymes of wild-type and quinolone-resistant S. aureus were determined. (This work was presented in part at the Interscience Conference on Antimicrobial Agents and Chemotherapy/International Congress of Chemotherapy and Infection, San Diego, CA, 2015 [4].)
FIG 1.
Structure of lascufloxacin.
Lascufloxacin and garenoxacin were synthesized by Kyorin Pharmaceutical Co., Ltd. (Tokyo, Japan). Other drugs were purchased from Eiken Chemical Co., Ltd. (Tokyo, Japan), Sigma-Aldrich (St. Louis, MO), LKT Laboratories, Inc. (St. Paul, MN), or Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Various clinical strains were isolated from patients in Japanese hospitals from 2013 to 2015. Most of the clinical strains, except for Enterococcus faecalis and methicillin-susceptible S. aureus (MSSA), were limited to one isolate per patient; in some cases, two different bacterial strains, i.e., E. faecalis and MSSA, were isolated from one patient. Clinical strains of S. aureus MS5935, MS5952, MR5867, and MR6009 and their stepwise quinolone-resistant mutant strains were described previously (5). The quinolone-resistant S. pneumoniae isolates selected from strain IID553 were described previously (6). The quinolone-resistant E. coli GF4-3, CP4-1, and SP4-1 isolates were selected from K-12 strain KL-16 with gatifloxacin, ciprofloxacin, and sparfloxacin, respectively. The MICs against various strains other than Mycoplasma pneumoniae were determined by agar or broth dilution methods as described in the Clinical and Laboratory Standards Institute guidelines (7). The MICs against M. pneumoniae were determined by the broth microdilution method according to Yamaguchi et al. (8), with some modifications.
The antibacterial activities of lascufloxacin and reference compounds against clinical isolates (600 strains of 14 bacterial species in total) of Gram-positive and Gram-negative bacteria are shown in Table 1. Lascufloxacin exhibited the most potent activities against Gram-positive pathogens, such as MSSA, MRSA, Staphylococcus epidermidis, E. faecalis, Streptococcus pyogenes, Streptococcus agalactiae, and penicillin-susceptible and penicillin-resistant S. pneumoniae, among the quinolones tested. Lascufloxacin showed more-potent activities against S. epidermidis and E. faecalis than any of the other agents tested. The MIC90 against MRSA was 2 μg/ml. The activity of lascufloxacin against MRSA was almost the same as those of linezolid and vancomycin, and it was 32- to >64-fold superior to those of levofloxacin, garenoxacin, and ciprofloxacin.
TABLE 1.
In vitro antibacterial activities of lascufloxacin against various clinical isolates
| Organisma (no. of isolates) | Drug | MIC (μg/ml) |
||
|---|---|---|---|---|
| Range | MIC50 | MIC90 | ||
| MSSA (30) | Lascufloxacin | ≤0.008 to 0.015 | 0.015 | 0.015 |
| Levofloxacin | 0.12 to 0.25 | 0.25 | 0.25 | |
| Garenoxacin | ≤0.008 to 0.03 | 0.015 | 0.03 | |
| Azithromycin | 1 to >64 | 2 | >64 | |
| Ceftriaxone | 2 to 4 | 2 | 4 | |
| Cefcapene | 1 to 2 | 1 | 2 | |
| Meropenem | ≤0.06 to 0.12 | ≤0.06 | ≤0.06 | |
| Oxacillin | 0.12 to 1 | 0.25 | 0.5 | |
| MRSA (100) | Lascufloxacin | 0.03 to 2 | 0.5 | 2 |
| Levofloxacin | 0.25 to >128 | 64 | >128 | |
| Garenoxacin | 0.06 to 64 | 8 | 64 | |
| Ciprofloxacin | 0.5 to >128 | 128 | >128 | |
| Ceftazidime | 16 to >128 | >128 | >128 | |
| Imipenem | 0.06 to >128 | 32 | 128 | |
| Vancomycin | 0.5 to 2 | 1 | 1 | |
| Teicoplanin | 0.5 to 8 | 1 | 4 | |
| Oxacillin | 16 to >128 | >128 | >128 | |
| Arbekacin | 0.25 to 8 | 1 | 2 | |
| Daptomycin | 0.25 to 1 | 0.5 | 0.5 | |
| Linezolid | 1 to 4 | 2 | 2 | |
| S. epidermidis (30) | Lascufloxacin | 0.015 to 0.12 | 0.03 | 0.12 |
| Levofloxacin | 0.12 to 8 | 0.25 | 4 | |
| Garenoxacin | 0.03 to 1 | 0.06 | 1 | |
| Azithromycin | 0.25 to >64 | 0.5 | >64 | |
| Ceftriaxone | 1 to >128 | 4 | 32 | |
| Cefcapene | 0.12 to >128 | 0.5 | 8 | |
| Meropenem | ≤0.06 to 32 | 0.5 | 4 | |
| Oxacillin | ≤0.06 to >128 | 1 | 32 | |
| E. faecalis (30) | Lascufloxacin | 0.06 to 0.5 | 0.06 | 0.12 |
| Levofloxacin | 1 to >16 | 1 | 2 | |
| Garenoxacin | 0.12 to 2 | 0.12 | 0.25 | |
| Azithromycin | 4 to >64 | >64 | >64 | |
| Ceftriaxone | 64 to >128 | >128 | >128 | |
| Cefcapene | 64 to >128 | >128 | >128 | |
| Meropenem | 2 to 8 | 4 | 8 | |
| S. pyogenes (30) | Lascufloxacin | 0.03 to 0.12 | 0.03 | 0.06 |
| Levofloxacin | 0.5 to 2 | 0.5 | 1 | |
| Garenoxacin | 0.03 to 0.12 | 0.06 | 0.12 | |
| Azithromycin | 0.12 to >16 | 0.12 | 16 | |
| Ceftriaxone | 0.015 | 0.015 | 0.015 | |
| Cefcapene | ≤0.008 | ≤0.008 | ≤0.008 | |
| Meropenem | ≤0.008 | ≤0.008 | ≤0.008 | |
| S. agalactiae (30) | Lascufloxacin | 0.06 to 1 | 0.06 | 0.5 |
| Levofloxacin | 0.5 to >16 | 1 | >16 | |
| Garenoxacin | 0.06 to 4 | 0.06 | 4 | |
| Azithromycin | 0.06 to >16 | 0.06 | >16 | |
| Ceftriaxone | 0.06 to 0.25 | 0.06 | 0.12 | |
| Cefcapene | 0.03 to 0.12 | 0.03 | 0.06 | |
| Meropenem | 0.03 to 0.06 | 0.03 | 0.06 | |
| PSSP (30) | Lascufloxacin | 0.03 to 0.06 | 0.06 | 0.06 |
| Levofloxacin | 0.5 to 1 | 1 | 1 | |
| Garenoxacin | 0.03 to 0.06 | 0.06 | 0.06 | |
| Azithromycin | 0.03 to >16 | >16 | >16 | |
| Ceftriaxone | 0.015 to 0.25 | 0.12 | 0.25 | |
| Cefcapene | ≤0.008 to 0.5 | 0.25 | 0.5 | |
| Meropenem | ≤0.008 to 0.015 | ≤0.008 | 0.015 | |
| Benzylpenicillin | 0.015 to 0.06 | 0.03 | 0.06 | |
| PRSP (30) | Lascufloxacin | 0.03 to 0.06 | 0.06 | 0.06 |
| Levofloxacin | 0.5 to 1 | 1 | 1 | |
| Garenoxacin | 0.03 to 0.06 | 0.06 | 0.06 | |
| Azithromycin | 1 to >16 | >16 | >16 | |
| Ceftriaxone | 0.5 to 4 | 1 | 2 | |
| Cefcapene | 0.5 to 4 | 1 | 2 | |
| Meropenem | 0.25 to 1 | 0.5 | 1 | |
| Benzylpenicillin | 2 to 4 | 2 | 4 | |
| E. coli (30) | Lascufloxacin | 0.06 to >16 | 0.25 | >16 |
| Levofloxacin | 0.015 to >16 | 0.06 | 16 | |
| Garenoxacin | 0.015 to >16 | 0.12 | >16 | |
| Azithromycin | 4 to 64 | 8 | 64 | |
| Ceftriaxone | ≤0.06 to >128 | ≤0.06 | >128 | |
| Cefcapene | 0.25 to >128 | 0.5 | 128 | |
| Meropenem | ≤0.06 | ≤0.06 | ≤0.06 | |
| Enterobacter spp. (30) | Lascufloxacin | 0.12 to 0.5 | 0.25 | 0.25 |
| Levofloxacin | 0.03 to 0.06 | 0.06 | 0.06 | |
| Garenoxacin | 0.06 to 0.25 | 0.12 | 0.12 | |
| Azithromycin | 8 to 32 | 16 | 16 | |
| Ceftriaxone | ≤0.06 to 32 | 0.12 | 16 | |
| Cefcapene | 0.5 to 32 | 0.5 | 32 | |
| Meropenem | ≤0.06 to 0.12 | ≤0.06 | ≤0.06 | |
| K. pneumoniae (30) | Lascufloxacin | 0.12 to 0.5 | 0.25 | 0.25 |
| Levofloxacin | 0.03 to 0.12 | 0.06 | 0.06 | |
| Garenoxacin | 0.06 to 0.25 | 0.12 | 0.12 | |
| Azithromycin | 8 to 16 | 16 | 16 | |
| Ceftriaxone | ≤0.06 | ≤0.06 | ≤0.06 | |
| Cefcapene | 0.25 to 1 | 0.5 | 1 | |
| Meropenem | ≤0.06 | ≤0.06 | ≤0.06 | |
| Acinetobacter spp. (30) | Lascufloxacin | 0.06 to 16 | 0.25 | 0.5 |
| Levofloxacin | 0.03 to 8 | 0.06 | 0.25 | |
| Garenoxacin | 0.015 to 8 | 0.03 | 0.12 | |
| Azithromycin | 0.5 to >64 | 1 | 64 | |
| Ceftriaxone | 2 to >128 | 8 | 16 | |
| Cefcapene | 4 to 128 | 16 | 16 | |
| Meropenem | 0.12 to 2 | 0.25 | 0.5 | |
| P. aeruginosa (30) | Lascufloxacin | 1 to >16 | 4 | >16 |
| Levofloxacin | 0.25 to >16 | 0.5 | >16 | |
| Garenoxacin | 0.5 to >16 | 1 | >16 | |
| Azithromycin | 8 to >64 | 64 | >64 | |
| Ceftriaxone | 8 to >128 | 32 | >128 | |
| Cefcapene | 16 to >128 | 32 | >128 | |
| Meropenem | 0.12 to 64 | 0.25 | 8 | |
| M. catarrhalis (30) | Lascufloxacin | 0.06 to 0.12 | 0.06 | 0.06 |
| Levofloxacin | 0.03 | 0.03 | 0.03 | |
| Garenoxacin | ≤0.008 to 0.015 | ≤0.008 | 0.015 | |
| Azithromycin | ≤0.06 to 0.12 | ≤0.06 | ≤0.06 | |
| Ceftriaxone | ≤0.06 to 1 | 0.5 | 1 | |
| Cefcapene | ≤0.06 to 1 | 0.5 | 1 | |
| Meropenem | ≤0.06 | ≤0.06 | ≤0.06 | |
| BLNAS (30) | Lascufloxacin | 0.03 to 0.06 | 0.03 | 0.06 |
| Levofloxacin | 0.015 to 0.03 | 0.015 | 0.03 | |
| Garenoxacin | ≤0.004 to 0.015 | 0.008 | 0.015 | |
| Azithromycin | 0.5 to 4 | 1 | 2 | |
| Ceftriaxone | ≤0.008 to 0.25 | ≤0.008 | 0.25 | |
| Cefcapene | ≤0.008 to 2 | 0.015 | 1 | |
| Meropenem | 0.015 to 0.06 | 0.03 | 0.06 | |
| Ampicillin | 0.12 to 1 | 0.5 | 1 | |
| BLNAR (30) | Lascufloxacin | 0.03 to 0.12 | 0.03 | 0.06 |
| Levofloxacin | 0.015 to 0.06 | 0.015 | 0.03 | |
| Garenoxacin | ≤0.004 to 0.03 | 0.008 | 0.015 | |
| Azithromycin | 0.5 to 4 | 1 | 2 | |
| Ceftriaxone | 0.03 to 0.25 | 0.25 | 0.25 | |
| Cefcapene | 0.12 to 2 | 2 | 2 | |
| Meropenem | 0.03 to 0.5 | 0.25 | 0.25 | |
| Ampicillin | 2 to 8 | 4 | 4 | |
| M. pneumoniae (50) | Lascufloxacin | 0.06 to 0.25 | 0.12 | 0.25 |
| Levofloxacin | 0.25 to 0.5 | 0.5 | 0.5 | |
| Garenoxacin | 0.008 to 0.03 | 0.015 | 0.03 | |
| Clarithromycin | ≤0.001 to >16 | >16 | >16 | |
| Azithromycin | ≤0.001 to 16 | 8 | 16 | |
PSSP and PRSP, penicillin-susceptible and penicillin-resistant S. pneumoniae, respectively; BLNAS and BLNAR, β-lactamase-negative ampicillin-susceptible and ampicillin-resistant strains of Haemophilus influenzae, respectively.
The activities of lascufloxacin against Gram-negative bacteria and M. pneumoniae were similar to those of existing quinolones. In the Gram-negative bacteria, lascufloxacin showed antibacterial activities against Moraxella catarrhalis and β-lactamase-negative ampicillin-susceptible and ampicillin-resistant strains of Haemophilus influenzae, with an MIC90 value of 0.06 μg/ml in all cases. The MIC90 values against Enterobacter spp., Klebsiella pneumoniae, and Acinetobacter spp. were 0.25, 0.25, and 0.5 μg/ml, respectively. The activities of lascufloxacin against E. coli and P. aeruginosa were almost the same as or lower than those of the other quinolones tested. The MIC50 and MIC90 values of lascufloxacin against M. pneumoniae were 0.12 and 0.25 μg/ml, respectively, which were similar to or slightly superior to those of levofloxacin. The emergence of macrolide resistance in M. pneumoniae has been reported (9), and macrolide-resistant isolates were detected in the present study. The MIC90s of clarithromycin and azithromycin were >16 and 16 μg/ml, respectively; on the other hand, lascufloxacin showed potent activity against macrolide-resistant M. pneumoniae clinical isolates.
Table 2 shows the antibacterial activities of lascufloxacin against clinical strains of S. aureus and their sequentially selected quinolone-resistant mutant strains. As the number of mutations of the target enzyme increased, the susceptibility of bacteria against quinolones decreased. However, compared with the other quinolones tested, lascufloxacin showed more potent activities even against third- and fourth-step mutants of S. aureus. The MICs of lascufloxacin against parent strains ranged from 0.008 to 0.015 μg/ml, and those against fourth-step parC, gyrA, parC, and gyrA mutant strains were all 2 μg/ml. A similar increase in MICs through the level of resistance to quinolones was found in other quinolones tested. The MICs of levofloxacin increased from 0.125 to 128 μg/ml, and those of garenoxacin increased from 0.015 or 0.03 μg/ml to 32 or 64 μg/ml. The MIC ratios of lascufloxacin between parent strains and their fourth-step mutant strains were 128 to 256, whereas those of other quinolones tested were 128 to 4,096. The increases in the MICs of lascufloxacin with the acquisition of resistance were smaller than those of levofloxacin and garenoxacin. These results suggest that lascufloxacin showed incomplete cross-resistance against these mutant strains. Small MIC ratios of lascufloxacin between wild-type and quinolone-resistant strains were also observed in S. pneumoniae and E. coli isolates (Table 2). The activities of lascufloxacin against first- and second-step mutant strains of S. pneumoniae were more potent than the activities of other quinolones, and the MICs of lascufloxacin against gyrA and parC double mutants were 0.25 to 0.5 μg/ml.
TABLE 2.
Antibacterial activity of lascufloxacin against quinolone-resistant strains
| Straina | Mutation |
MIC (μg/ml) for: |
||||
|---|---|---|---|---|---|---|
| GyrA | ParC | Lascufloxacin | Levofloxacin | Garenoxacin | Ciprofloxacin | |
| S. aureus | ||||||
| MS5935 | None | None | 0.015 | 0.125 | 0.015 | 0.25 |
| MS5935 gyrA mutant | Ser84Leu | None | 0.03 | 0.25 | 0.06 | 0.25 |
| MS5935 1st | None | Ser80Phe | 0.03 | 0.5 | 0.03 | 2 |
| MS5935 2nd | Ser84Leu | Ser80Phe | 0.125 | 8 | 2 | 16 |
| MS5935 3rd | Ser84Leu | Ser80Phe, Glu84Lys | 0.5 | 32 | 8 | 32 |
| MS5935 4th | Ser84Leu, Glu88Val | Ser80Phe, Glu84Lys | 2 | 128 | 32 | 32 |
| MS5952 | None | None | 0.015 | 0.125 | 0.03 | 0.25 |
| MS5952 1st | None | Ser80Tyr | 0.03 | 0.5 | 0.06 | 2 |
| MS5952 2nd | Ser84Leu | Ser80Tyr | 0.06 | 2 | 1 | 8 |
| MS5952 3rd | Ser84Leu | Ser80Tyr, Ala116Val | 0.25 | 16 | 0.5 | 32 |
| MR5867 | None | None | 0.015 | 0.125 | 0.015 | 0.25 |
| MR5867 1st | None | Glu84Lys | 0.03 | 0.5 | 0.03 | 2 |
| MR5867 2nd | Ser84Leu | Glu84Lys | 0.125 | 8 | 0.5 | 16 |
| MR5867 3rd | Ser84Leu | Glu84Lys, Ser80Phe | 0.5 | 16 | 4 | 32 |
| MR5867 4th | Ser84Leu, Glu88Lys | Glu84Lys, Ser80Phe | 2 | 128 | 32 | 32 |
| MR6009 | None | None | 0.008 | 0.125 | 0.015 | 0.25 |
| MR6009 1st | None | Ser80Tyr | 0.03 | 0.5 | 0.03 | 2 |
| MR6009 2nd | Glu88Lys | Ser80Tyr | 0.125 | 4 | 0.25 | 8 |
| MR6009 3rd | Glu88Lys | Ser80Tyr, Glu84Lys | 0.25 | 8 | 0.25 | 32 |
| MR6009 4th | Glu88Lys, Ser84Leu | Ser80Tyr, Glu84Lys | 2 | 128 | 64 | 32 |
| S. pneumoniae | ||||||
| IID553 | None | None | 0.06 | 1 | 0.06 | 1 |
| NF9884 | None | Ser79Tyr | 0.125 | 2 | 0.125 | 2 |
| CF9842 | None | Asp83Asn | 0.06 | 2 | 0.125 | 2 |
| SF9863 | Ser81Phe | None | 0.06 | 2 | 0.25 | 2 |
| GF9821 | Ser81Tyr | None | 0.125 | 2 | 0.25 | 2 |
| ST9941 | Ser81Phe | Ser79Phe | 0.5 | 16 | 2 | 64 |
| SN9981 | Ser81Phe | Asp83Tyr | 0.25 | 16 | 1 | 32 |
| E. coli | ||||||
| KL-16 | None | None | 0.125 | 0.03 | 0.03 | 0.008 |
| GF4-3 | Asp87Tyr | None | 0.5 | 0.125 | 0.125 | 0.125 |
| CP4-1 | Asp87Asn | None | 0.5 | 0.25 | 0.125 | 0.125 |
| SP4-1 | Ser83Leu | None | 0.5 | 0.25 | 0.25 | 0.125 |
The mutant strains of S. aureus, S. pneumoniae, and E. coli were sequentially selected from quinolone-susceptible strains.
To evaluate the selectivity of mutant strains, the frequency of resistance of lascufloxacin was determined against S. aureus, S. pneumoniae, and E. coli (Table 3). Bacteria were incubated for ∼70 h on Mueller-Hinton agar (S. aureus and E. coli) or Mueller-Hinton agar containing 5% defibrinated sheep blood (S. pneumoniae) with quinolones at 4, 8, and 16 times the MICs. It was revealed that resistant strains were not selected at all concentrations of lascufloxacin against three bacterial species. The frequencies of lascufloxacin were the same as or lower than those of other quinolones tested. These results and the MIC data shown in Table 2 suggest that the potent activity of lascufloxacin against mutant strains might contribute to the low level of resistance selectivity.
TABLE 3.
Frequencies of appearance of mutant strains by selection with lascufloxacin
| Strain | Drug | MIC (μg/ml) | Frequency at the following multiple of MICa: |
||
|---|---|---|---|---|---|
| 4 × MIC | 8 × MIC | 16 × MIC | |||
| S. aureus ATCC 29213 | Lascufloxacin | 0.008 | <2.1 × 10−9 | <2.1 × 10−9 | <2.1 × 10−9 |
| Levofloxacin | 0.125 | <2.1 × 10−9 | <2.1 × 10−9 | <2.1 × 10−9 | |
| Garenoxacin | 0.015 | 8.2 × 10−9 | <2.1 × 10−9 | <2.1 × 10−9 | |
| Ciprofloxacin | 0.25 | 6.2 × 10−9 | 3.1 × 10−9 | <2.1 × 10−9 | |
| S. pneumoniae ATCC 49619 | Lascufloxacin | 0.06 | <1.1 × 10−8 | <1.1 × 10−8 | <1.1 × 10−8 |
| Levofloxacin | 1 | <1.1 × 10−8 | <1.1 × 10−8 | <1.1 × 10−8 | |
| Garenoxacin | 0.06 | <1.1 × 10−8 | <1.1 × 10−8 | <1.1 × 10−8 | |
| Ciprofloxacin | 1 | 1.1 × 10−8 | <1.1 × 10−8 | <1.1 × 10−8 | |
| E. coli ATCC 25922 | Lascufloxacin | 0.06 | <1.9 × 10−9 | <1.9 × 10−9 | <1.9 × 10−9 |
| Levofloxacin | 0.015 | 3.9 × 10−9 | <1.9 × 10−9 | <1.9 × 10−9 | |
| Garenoxacin | 0.03 | <1.9 × 10−9 | <1.9 × 10−9 | <1.9 × 10−9 | |
| Ciprofloxacin | 0.008 | 1.2 × 10−8 | <1.9 × 10−9 | <1.9 × 10−9 | |
n = 2, average.
To clarify the mechanism underlying the incomplete cross-resistance of lascufloxacin, the inhibitory activities of lascufloxacin against DNA gyrase and topoisomerase IV of wild-type and quinolone-resistant S. aureus were determined (Table 4). The DNA gyrase and topoisomerase IV of S. aureus isolates were cloned and expressed in E. coli, and each subunit was purified (10). Human topoisomerase II was purchased from TopoGen (Buena Vista, CO). The activities of DNA gyrase and topoisomerase IV were determined as described previously (10). The inhibitory activities of quinolones against topoisomerases were assayed by determining the concentrations required to inhibit 50% of the enzyme reaction (IC50). Against human topoisomerase II, the IC50 of lascufloxacin was >2,400 μg/ml, and high selectivity to bacterial topoisomerases was observed. Lascufloxacin showed the most potent inhibitory activity against all target enzymes among the quinolones tested. The ratio of the IC50 against DNA gyrase of the quinolone-resistant strain to that of the wild-type strain was 10 for lascufloxacin, which was the lowest value among the quinolones tested. The smallest IC50 ratio of lascufloxacin was also observed in the topoisomerase IV assay. The ratio of the IC50 of lascufloxacin against mutated topoisomerase IV to that of the wild-type strain was 3.8. Lascufloxacin showed potent inhibitory activities against not only wild-type but also mutated enzymes; therefore, the ratios of the IC50s of lascufloxacin of mutated enzymes to wild-type enzymes were lower than those of the other quinolones. These results support incomplete cross-resistance of the antibacterial activity of lascufloxacin against quinolone-resistant strains.
TABLE 4.
Inhibitory activities of lascufloxacin against DNA gyrase and topoisomerase IV of S. aureus and human topoisomerase II
| Drug | IC50 (μg/ml)a |
IC50 ratio of QR to wild-type strain |
|||||
|---|---|---|---|---|---|---|---|
| Wild-type DNA gyrase | QRb DNA gyrase | Wild-type topoisomerase IV | QRb topoisomerase IV | Human topoisomerase II | DNA gyrase | Topoisomerase IV | |
| Lascufloxacin | 1.7 ± 0.4 | 17 ± 3 | 0.73 ± 0.06 | 2.8 ± 0.3 | >2400 | 10 | 3.8 |
| Levofloxacin | 16 ± 6 | 1300 ± 100 | 2.8 ± 0.9 | 86 ± 19 | 1400 ± 100 | 81 | 31 |
| Garenoxacin | 11 ± 1 | 420 ± 80 | 1.9 ± 0.6 | 27 ± 1 | 750 ± 280 | 38 | 14 |
| Ciprofloxacin | 25 ± 5 | >1200 | 1.8 ± 0.7 | 69 ± 7 | >1100 | >48 | 38 |
n = 3, average ± SD.
QR, quinolone resistant. Mutations of DNA gyrase and topoisomerase IV were GyrA Ser84Leu and ParC Ser80Phe, respectively.
In summary, lascufloxacin exhibited potent antibacterial activity against various pathogens. In particular, lascufloxacin showed the most potent activity against Gram-positive bacteria, such as S. aureus, S. epidermidis, E. faecalis, S. pyogenes, S. agalactiae, and S. pneumoniae, among the quinolones tested. The MIC90s of lascufloxacin against H. influenzae, M. catarrhalis, and M. pneumoniae were 0.06, 0.06, and 0.25, respectively, although the clinical isolates used in this study included various resistant strains. Moreover, lascufloxacin showed potent antibacterial activity against MRSA that was similar to those of linezolid and vancomycin, suggesting the high efficacy of lascufloxacin for severe infectious diseases caused by MRSA. The results presented here suggested that lascufloxacin showed incomplete cross-resistance against existing quinolone-resistant strains and potent antibacterial activities against sequentially selected quinolone-resistant mutant strains. The inhibitory activities of lascufloxacin resulted in only a small decrease against the quinolone-resistant enzymes induced by genetic mutation of the targets, compared with those from the wild-type strain. The greater inhibitory activities of lascufloxacin against mutated target enzymes were likely responsible for the more-potent antibacterial activities against quinolone-resistant bacteria compared to those of the other quinolones examined in this study.
The present study revealed that lascufloxacin showed high selectivity to bacterial topoisomerases, with little activity against the human topoisomerase II (Table 4). In the toxicity studies, lascufloxacin showed similar or decreased class effects of quinolones, such as genotoxicity, articular toxicity, and phototoxicity, than other quinolones in animals. In humans, lascufloxacin was well tolerated in oral single doses up to 800 mg and multiple doses up to 400 mg once daily for 7 days and exhibited appropriate pharmacokinetic (PK) profiles (11). The plasma concentration of lascufloxacin achieved a mean maximum concentration of drug in serum (Cmax) of 0.732 μg/ml and an area under the serum concentration-time curve from time zero to infinity (AUCinf) of 12.7 μg · h · ml−1 and was eliminated with a mean half-life (t1/2) of 16.1 h at the 100-mg oral single dose in healthy subjects. The Cmax and AUC for lascufloxacin increased in an approximately dose-proportional manner up to 800 mg, and the plasma protein binding was 74.0%. Furthermore, a clinical pharmacological study indicated that lascufloxacin showed excellent distribution in the lung; the average ratio of epithelial lining fluid to plasma concentration ranged from 15.0 to 22.4 (12).
Based on its remarkable PK profile and potent antibacterial activity against respiratory pathogens, lascufloxacin may be a promising agent for the treatment of various infectious diseases, including lower respiratory tract infections. Further clinical studies are required to determine the applicability of lascufloxacin.
ACKNOWLEDGMENTS
We are grateful to Misako Nakazawa and Asako Tabuchi for providing MIC data against quinolone-resistant strains.
All authors are employees of Kyorin Pharmaceutical Co., Ltd.
REFERENCES
- 1.Flamm RK, Mendes RE, Hogan PA, Streit JM, Ross JE, Jones RN. 2016. Linezolid surveillance results for the United States (LEADER Surveillance Program 2014). Antimicrob Agents Chemother 60:2273–2280. doi: 10.1128/AAC.02803-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sader HS, Flamm RK, Mendes RE, Farrell DJ, Jones RN. 2016. Antimicrobial activities of ceftaroline and comparator agents against bacterial organisms causing bacteremia in patients with skin and skin structure infections in U.S. medical centers, 2008 to 2014. Antimicrob Agents Chemother 60:2558–2563. doi: 10.1128/AAC.02794-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song JH, Jung SI, Ko KS, Kim NY, Son JS, Chang HH, Ki HK, Oh WS, Suh JY, Peck KR, Lee NY, Yang Y, Lu Q, Chongthaleong A, Chiu CH, Lalitha MK, Perera J, Yee TT, Kumarasinghe G, Jamal F, Kamarulzaman A, Parasakthi N, Van PH, Carlos C, So T, Ng TK, Shibl A. 2004. High prevalence of antimicrobial resistance among clinical Streptococcus pneumoniae isolates in Asia (an ANSORP study). Antimicrob Agents Chemother 48:2101–2107. doi: 10.1128/AAC.48.6.2101-2107.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaguchi Y, Kishii R, Takei M. 2015. AM-1977, a novel fluoroquinolone, possessing potent antibacterial activity against Gram-positive bacteria including quinolone-resistant strains, abstr F1-1191. Abstr 55th Intersci Conf Antimicrob Agents Chemother (ICAAC)-29th Int Congr Chemother Infect (ICC). American Society for Microbiology and International Society of Chemotherapy, San Diego, CA. [Google Scholar]
- 5.Fukuda H, Hori S, Hiramatsu K. 1998. Antibacterial activity of gatifloxacin (AM-1155, CG5501, BMS-206584), a newly developed fluoroquinolone, against sequentially acquired quinolone-resistant mutants and the norA transformant of Staphylococcus aureus. Antimicrob Agents Chemother 42:1917–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuda H, Kishii R, Takei M, Hosaka M. 2001. Contributions of the 8-methoxy group of gatifloxacin to resistance selectivity, target preference, and antibacterial activity against Streptococcus pneumoniae. Antimicrob Agents Chemother 45:1649–1653. doi: 10.1128/AAC.45.6.1649-1653.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—10th ed. CLSI document M07-A10 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 8.Yamaguchi T, Hirakata Y, Izumikawa K, Miyazaki Y, Maesaki S, Tomono K, Yamada Y, Kamihira S, Kohno S. 2000. In vitro activity of telithromycin (HMR3647), a new ketolide, against clinical isolates of Mycoplasma pneumoniae in Japan. Antimicrob Agents Chemother 44:1381–1382. doi: 10.1128/AAC.44.5.1381-1382.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saraya T, Kurai D, Nakagaki K, Sasaki Y, Niwa S, Tsukagoshi H, Nunokawa H, Ohkuma K, Tsujimoto N, Hirao S, Wada H, Ishii H, Nakata K, Kimura H, Kozawa K, Takizawa H, Goto H. 2014. Novel aspects on the pathogenesis of Mycoplasma pneumoniae pneumonia and therapeutic implications. Front Microbiol 5:410. doi: 10.3389/fmicb.2014.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takei M, Fukuda H, Kishii R, Hosaka M. 2001. Target preference of 15 quinolones against Staphylococcus aureus, based on antibacterial activities and target inhibition. Antimicrob Agents Chemother 45:3544–3547. doi: 10.1128/AAC.45.12.3544-3547.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Totsuka K, Odajima M, Nakauchi M, Sesoko S, Nakashima M. 2016. Phase I study to determine the safety and pharmacokinetics (PK) of single and multiple oral doses of lascufloxacin (AM-1977) in healthy subjects, abstr SUNDAY-467. Abstr ASM Microbe 2016. [Google Scholar]
- 12.Furuie H, Yoshida H, Kume K, Owada Y, Yagi M, Mikami H, Nishimura M. 2016. Intrapulmonary pharmacokinetics of lascufloxacin (AM-1977), a new generation fluoroquinolone, in healthy Japanese subjects, abstr SUNDAY-469. Abstr ASM Microbe 2016. [Google Scholar]