Abstract
Background and Aims:
Modified radical mastectomy (MRM) may be associated with severe post-operative pain, leading to chronic pain syndrome. We compared the post-operative analgesic profile of two ultrasound-guided nerve blocks: Paravertebral block (PVB) and serratus plane block (SPB).
Methods:
This double-blind, randomised study was conducted on fifty adult females, scheduled for MRM with axillary dissection. After inducing general anaesthesia with intravenous midazolam 1 mg, fentanyl 1.5 mcg/kg, propofol 1–2 mg/kg and vecuronium 0.1 mg/kg, patients were administered either ultrasound-guided thoracic PVB at T4 (n = 25) or SPB at 5th rib (n = 25) with 20 ml of 0.5% bupivacaine, both as a single level injection. Time to first rescue analgesia and morphine consumption in 4, 6, 24, 48 and 72 h by PCA pump, visual analogue scale score and any adverse effects were recorded. Quantitative variables were compared using the unpaired t-test or the Mann–Whitney U test between the two groups. Qualitative variables were compared using the Chi-square test or Fisher's exact test.
Results:
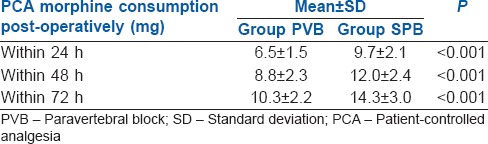
The duration of analgesia (mean ± Standard deviation [SD]) was significantly longer in the PVB group compared to SPB group (346 ± 57 min vs. 245.6 ± 58 min, P < 0.001). The post-operative 24 h morphine consumption (mean ± SD) was significantly higher in the SPB group (9.7 ± 2.1 mg) compared to PVB group (6.5 ± 1.5 mg) (P < 0.001).
Conclusion:
Ultrasound-guided SPB is an alternative analgesic technique to thoracic PVB for MRM although PVB provides a longer duration of analgesia.
Key words: Analgesia, mastectomy, modified radical, pain, post-operative
INTRODUCTION
Modified radical mastectomy (MRM) is a common surgical procedure, accounting for 31% of all breast surgery cases performed.[1] Post-mastectomy pain managed by opioids alone often leads to side effects such as nausea and vomiting. Inadequate control of pain may later develop into chronic pain syndrome (paraesthesias, phantom breast pain and intercostobrachial neuralgia) in 25%–40% of the patients.[2,3] For these reasons, regional analgesic techniques have been advocated for effective pain management.[4,5] Local wound infiltration is safe, but the duration of action is short lived. Intercostal nerve block and interpleural block are effective, but there is a risk of pneumothorax and transient Horner's syndrome.[6,7] In view of the neurological and haemodynamic concerns, thoracic epidural analgesia is not preferred for breast surgeries.[8]
Most recently, thoracic paravertebral block (PVB) has been shown to provide effective analgesia with minimal haemodynamic derangement.[5] Ultrasound-guided interfascial plane blocks such as pectoral nerve (PECS) block type 1 and 2 and serratus plane block (SPB) have also been reported as alternatives, with the advantages of simplicity and ease of performance.[9,10,11]
This study compares the analgesic efficacy of ultrasound-guided PVB and SPB in patients undergoing MRM with axillary dissection. We hypothesise that ultrasound-guided PVB is superior to SPB in analgesic efficacy and duration.
METHODS
This double-blind, randomised, comparative study was conducted, after obtaining approval from the Hospital's Ethics Committee and written informed consent from fifty adult females, who were scheduled for elective MRM. The inclusion criteria were age 18–65 years, weight 40–85 kg and American Society of Anesthesiologists Physical Status I or II. The exclusion criteria were contraindications to nerve block, for example, coagulopathy and local infection at the site of block, local anaesthetic allergy and significant neurological, cardiac, renal, hepatic or respiratory disease and patients planned for breast conservative surgery/simple mastectomy with axillary clearance.
The primary objective of this study was to determine the difference in the duration of analgesia of the two blocks. The secondary objectives were to compare the morphine consumption in 24, 48 and 72 h after surgery, visual analogue scale (VAS) pain scores and morphine-related side effects (nausea/vomiting).
The participants were randomly allocated into two groups of 25 each (PVB and SPB) by a random sequence number generated by the computer kept in sealed envelopes. The sealed envelopes were opened on the day of surgery after induction of anaesthesia, and participants received either PVB (n = 25) or SPB (n = 25) as per the envelope. Both the participants and the attending anaesthesiologist in the operating room were blinded to the type of block (SPB or PVB). The participants were blinded, as the blocks were performed after induction of general anaesthesia. The attending anaesthesiologist was not present in the operating room at the time of block administration, and blocks were performed by a different anaesthesiologist (who was an expert in these blocks).
Before surgery, the participants received education about the VAS pain score (0–100 mm) and the details of the nerve block procedures. After an 8 h fast, the patients were taken into the operation theatre, where an 18-gauge intravenous (IV) cannula was secured and monitors (pulse oximeter, electrocardiography and non-invasive blood pressure) were applied. General anaesthesia was induced with midazolam 1 mg, fentanyl 1.5 mcg/kg and propofol 1–2 mg/kg administered IV, and the trachea was intubated after administering vecuronium bromide 0.1 mg/kg IV for muscle relaxation. The lungs were ventilated to maintain an end-tidal carbon dioxide of 35 mmHg. Anaesthesia was maintained with oxygen, nitrous oxide and 0.8%–1% isoflurane to maintain MAC of 1.0. Paracetamol 1 g was administered IV after induction of anaesthesia. At the end of surgery, ondansetron 4 mg was administered IV, and muscle relaxation was reversed with IV neostigmine 50 mcg/kg and glycopyrrolate 10 mcg/kg. The trachea was extubated, and the patients were transferred to post-anaesthetic care unit (PACU).
Patients in group SPB received serratus anterior plane block and those in group PVB received thoracic PVB. Both these blocks were performed after induction of general anaesthesia. After proper skin sterilisation, all nerve block procedures were performed by an experienced faculty (KG, who has extensive training and practice in ultrasound-guided regional anaesthesia) under ultrasound guidance with a linear probe (8–13MHz) and an ultrasound machine (M-Turbo, SonoSite Inc., USA).
For the ultrasound-guided thoracic PVB, with the patient in the lateral decubitus position and the surgical side uppermost, the probe was placed in a parasagittal plane over the transverse process of T4 and T5 vertebrae, approximately 2.5 cm lateral to the spinous processes. The thoracic paravertebral space was identified as a wedge-shaped hypoechoic space between the superior costotransverse ligament and the pleura. A 22-gauge, 50 mm, echogenic needle was inserted using an out-of-plane approach and advanced from lateral to medial starting from the top side of the probe. During needle advancement, hydrodissection was used to locate the needle tip under ultrasound guidance, until the tip penetrated the superior costotransverse ligament. The block was deemed satisfactory when the pleural membrane was displaced downwards during injection of 20 ml of 0.5% bupivacaine.
For the ultrasound-guided serratus plane block, with the patient lying supine, the ultrasound probe was positioned longitudinally oblique just below the mid-clavicle. After identifying the second rib, the probe was moved caudally and laterally (obliquely), towards the mid-axillary line to identify the 3rd, 4th and 5th ribs, as described by Blanco et al.[10] The final probe position had its cephalad end resting over the anterior axillary line and the caudad end over the posterior axillary line. The fascial plane between the serratus anterior muscle and latissimus dorsi muscle was identified between the 4th and 5th rib in the mid-axillary region. Under ultrasound guidance, a 22-gauge, 50 mm echogenic needle (Stimuplex D; B Braun, Germany) was advanced in-plane to enter this fascial plane in the superoanterior to posteroinferior direction. Once the needle was in proper position, 20 ml of 0.5% bupivacaine was injected superficial to the serratus anterior muscle.
Vital signs (heart rate [HR], systolic, diastolic and mean blood pressure) were recorded immediately before induction of anaesthesia, 10 min after induction, and then every 30 min, until the end of surgery. Bispectral Index (BIS) was used to monitor the depth of anaesthesia and was maintained between 40 and 60 by adjusting the end-tidal concentration of isoflurane. The patient was assumed to have pain, if HR or mean arterial pressure increased >20% from baseline (at the time just before induction), despite adequate anaesthetic depth (BIS – 40–60) and a bolus of 25 mcg fentanyl were administered IV.
In the PACU, patient-controlled analgesia (PCA) pump (PCA-T34 L, Caesarea Medical Electronics, Israel) was attached to the patient intravenously. The pump settings were morphine – 1 mg/ml; bolus dose – 1 mg, lockout interval 10 min and maximum dose 4 mg/h. Pain was assessed by an independent investigator, who was blinded, as he was not aware of the type of block (SPB or PVB) administered to the patient. He used the VAS scale (0 mm = no pain and 100 mm = worst imaginable pain) to assess the pain at 4, 6, 24, 48 and 72 h after surgery and recorded this in a patient diary. The patient was instructed to press the PCA button, whenever pain VAS ≥40 mm. The duration of analgesia was the time from administration of block to the first use of PCA pump by the patient, as recorded by the nurse. Morphine consumption and morphine-related side effects (nausea, vomiting, respiratory depression and itching) were recorded at 4, 6, 24, 48 and 72 h after surgery.
Respiratory depression was defined as respiratory rate <10/min and/or oxygen saturation <90%. Ondansetron was administered IV for nausea/vomiting and diphenhydramine was given IV for itching. Paracetamol 1 g was administered IV every 8 h.
Our preliminary pilot study with ten participants in each group (PVB and SPB) showed that the duration of analgesia (mean ± standard deviation [SD]) of PVB was 35% higher than SPB (PVB, 351 ± 62 min and SPB, 234 ± 61 min). Based on this finding, we estimated the minimum sample size with 90% power of the study and type I error of 0.05 to be 23 patients in each study group. Allowing for dropout of 8% of patients, we calculated a total sample size of 25 patients for each group.
The data were analysed using Statistical Package for the Social Sciences version 21.0 (SPSS Inc. Chicago, Illinois, USA). The categorical variables are presented in numbers and percentage (%), and the continuous variables are presented as mean ± SD. Normality of data was tested by Kolmogorov–Smirnov test. If the normality was rejected, then non-parametric test was used. The quantitative variables were compared using the Unpaired t-test or Mann–Whitney test (when the data sets were not normally distributed) between the two groups. The qualitative variables were compared using the Chi-square test or Fisher's exact test. P < 0.05 was considered statistically significant.
RESULTS
Sixty patients were initially assessed for eligibility for this study, but six participants were excluded due to failure to meet the inclusion criteria and four participants refused to participate [Figure 1]. The demographic data of the two groups (SPB and PVB) were comparable [Table 1]. The intra- and post-operative haemodynamic parameters were also comparable. The duration of analgesia (mean ± SD) was significantly longer in PVB group compared to SPB group (346 ± 57 min vs. 245.6 ± 58 min, P < 0.001). Post-operative VAS scores in the two groups were similar [Figure 2], whereas 24 h morphine consumption was significantly higher in SPB group [9.7 ± 2.1 mg versus 6.5 ± 1.5, P < 0.001, Table 2]. Intra-operative fentanyl requirement was similar in both the groups (134.4 ± 12.9 mcg vs. 133.3 ± 14.4 mcg, P = 0.91). Two patients in each group had nausea and vomiting and were treated with ondansetron IV. None of the patients in either group had any other complication.
Figure 1.
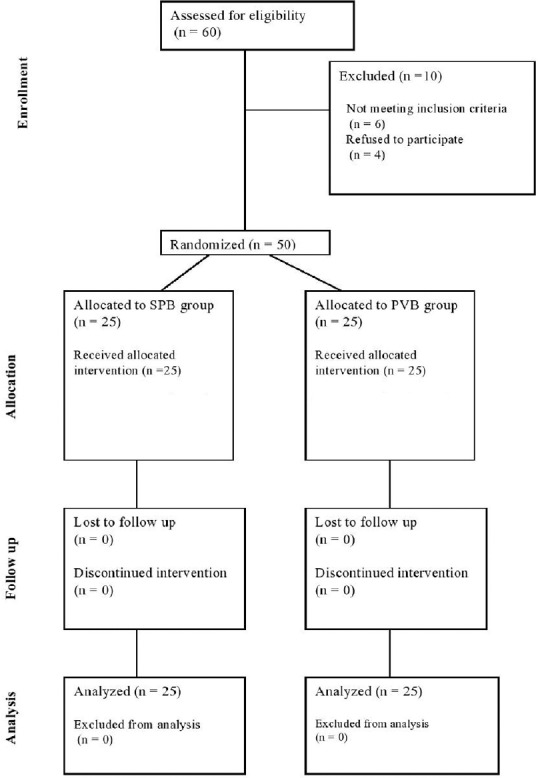
The consort flow diagram
Table 1.
Demographic profile
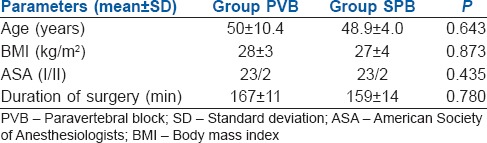
Figure 2.
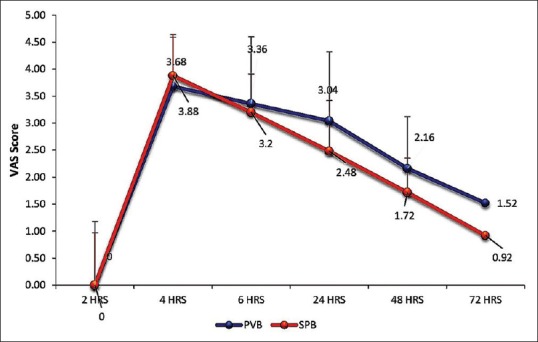
Post-operative mean visual analogue scale scores (p values – 4h-0.38, 6 h-0.46, 24 h-0.09, 48 h-0.19, 72 h-0.52)
Table 2.
Post-operative patient-controlled analgesia morphine consumption
DISCUSSION
This randomised, controlled study compared PVB with SPB for analgesia after MRM with axillary dissection and found that thoracic PVB had a longer duration of analgesia (a difference of 100 min) and 33% lower 24 h morphine consumption 6 h after surgery [Table 2]. Intra-operative fentanyl consumption was low in both study groups, implying good surgical analgesia with both the blocks. The incidence of post-operative nausea and vomiting (PONV) was low, and there was no major complication.
Ultrasound-guided PVB is an excellent analgesic technique for breast surgery because not only does it decrease pain but also it decreases PONV and length of hospital stay.[5,12,13,14,15,16,17] However, the learning curve of ultrasound-guided PVB is rather steep requiring a higher degree of skill. Furthermore, a number of complications have previously been reported with PVB.[12,17] Blanco et al. proposed SPB as an alternative to PVB for surgeries on the anterior and lateral thoracic wall including breast surgeries.[10] SPB is an easy block to learn and perform because the serratus anterior muscle is an easy sonographic landmark to identify for this block.
Our hypothesis of a longer duration of analgesia of PVB in comparison to SPB is based on the mechanism of action of the two blocks. The breast is innervated by anterior and lateral cutaneous branches of the second to sixth thoracic intercostal nerves and supraclavicular nerves.[18] Supraclavicular nerves from the lower fibres of the cervical plexus innervate the upper and lateral portions of the breast and anterior cutaneous branches of intercostal nerves (T2-T4) supply the parasternal region of breast. Similar to the transversus abdominis plane (TAP) block, the mechanism of action of SPB is a blockade of the lateral cutaneous branches of the intercostal nerves (T2–T4 for SPB and T10-L2 for TAP).[19] Because the anterior cutaneous branches of the intercostal nerves and supraclavicular nerves are spared, SPB is expected not to produce complete anaesthesia of the chest wall.[10,11,19] Furthermore, SPB may not achieve adequate somatic and sympathetic blockade in the axillary region, as would be expected with thoracic PVB,[10,11,20] which often affects five somatic and eight sympathetic dermatomes.[20] The local anaesthetic in PVB directly blocks the spinal nerves, extends laterally to block the intercostal nerves, extends medially into the epidural space through the intervertebral foramina and affects the sympathetic chain, leading to profound analgesia.[20,21,22] The local anaesthetic can also spread longitudinally cranially or caudally in PVB. This is supported by Hetta and Rezk[23] who compared SPB and PVB block in patients with MRM and reported adequate sensory blockade over T1–T7 dermatome levels in 100% of the patients after PVB although 40% of the patients had inadequate sensory blockade in the axilla after SPB.[23]
Another study also reported that the duration of analgesia of SPB was significantly shorter compared to PVB (median [range], 6 h [5–7 h] for SPB vs. 11 h [9–13 h] for PVB).[23] In contrast, we found a much shorter duration of analgesia in both our study groups (median [range], SPB 4 h [3–6 h] vs. PVB 5.5 h [4–8 h]). This might be related to the technique of PVB injection, as they[23] performed PVB injections at three different levels (T2, T4 and T6), compared to a single level (T4) PVB injection in our study. The technique of injection varies based on operator's preference balancing the risk of potential complication with multiple level injections against potentially limited local anaesthetic spread with a single level PVB injection. It is interesting to note that four or more intercostal spaces may be anaesthetised by a single level PVB injection,[14] while inconsistent blockade of the chest wall[13] and axillary region has been reported even with multiple level PVB.[15,16]
The volume of local anaesthetic is also likely an important determinant of the extent and duration of analgesia for SPB and thoracic PVB. In another study, a higher volume of bupivacaine was injected in SPB (30 ml vs. 20 ml in our study), and more effective analgesia was reported than our study.[23] Since SPB is a fascial block, a larger volume is expected to promote local anaesthetic spread. In another study, after injection of 15–20 ml of levobupivacaine 0.25% at T4 level for PVB,[24] the duration of analgesia was shorter (137.5 [115–165] min) than in our study (346 ± 57 min), again highlighting the influence of the local anaesthetic injection volume and dose on duration of analgesia.[24,25]
Another study found a higher 24 h post-operative morphine consumption in the SPB group compared to PVB group (a difference of 8 mg), similar to our results.[23] Similar to our study, significantly low pain scores with PVB, lasting up to 24 h have been reported.[24,26] Both SPB and PVB provide excellent post-operative recovery and decrease the opioid requirement, as reflected by post-operative quality recovery scale.[27]
SPB has the option of injecting local anaesthetic either superficial or deep to the serratus anterior muscle. In this study, we injected local anaesthetic superficial to the serratus anterior muscle, as it decreases the risk of pneumothorax and vascular trauma, compared to the deeper approach, where the needle is in the vicinity of pleural membrane. With the injection of local anaesthetic deep to serratus anterior muscle, sensory blockade up to T2 dermatome has been reported.[28,29,30] This approach likely provides a good anterior distribution of the block, which is not achieved with the superficial approach to SPB. SPB should be supplemented with additional analgesic agent during axillary dissection, as sensory loss of T1 is seldom achieved.[28] Similar to our study, a recent study also found a decreased consumption of opioids intraoperatively and postoperatively, decreased PONV and increased duration of analgesia after ambulatory breast cancer surgery.[31]
Our study results add to the limited amount of objective data available today regarding the analgesic profile of these two new blocks after MRM. One of the strengths of our study is the use of a standardised volume and concentration of local anaesthetic in both blocks (20 ml of 0.5% bupivacaine). Hence, our methodology has provided a fair analgesic comparison between groups as compared to other studies which administered unequal volume and/or concentration of local anaesthetic.[23,24] However, our study also has a number of limitations. We could not assess block onset time or sensory dermatomal level because both blocks were performed after induction of general anaesthesia. We performed a single level injection, realising that multiple injection technique may provide more effective analgesia in PVB. We did not insert a catheter to provide continuous analgesia and neither could we comment on clinical safety or the long-term impact (e.g., development of chronic pain) of the two blocks in this small study. We hope that future studies will address the remaining issues, the optimal injection approaches for SPB (superficial or deep to serratus anterior muscle) and the duration of analgesia with and without adjuncts. In future, systematic review and meta-analyses are suggested comparing the post-operative analgesic techniques for MRM.
CONCLUSION
The ultrasound-guided SPB and PVB provide good analgesia post-MRM, but PVB has a superior analgesic profile, with a longer duration of analgesia.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would thank Dr. Mridula Pawar, Professor and Consultant, Department of Anaesthesia, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi, India, for helping us to conduct this study.
REFERENCES
- 1.Poleshuck EL, Katz J, Andrus CH, Hogan LA, Jung BF, Kulick DI, et al. Risk factors for chronic pain following breast cancer surgery: A prospective study. J Pain. 2006;7:626–34. doi: 10.1016/j.jpain.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: A critical review of risk factors and strategies for prevention. J Pain. 2011;12:725–46. doi: 10.1016/j.jpain.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Gärtner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302:1985–92. doi: 10.1001/jama.2009.1568. [DOI] [PubMed] [Google Scholar]
- 4.Marret E, Vigneau A, Salengro A, Noirot A, Bonnet F. Effectiveness of analgesic techniques after breast surgery: A meta-analysis. Ann Fr Anesth Reanim. 2006;25:947–54. doi: 10.1016/j.annfar.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Kairaluoma PM, Bachmann MS, Rosenberg PH, Pere PJ. Preincisional paravertebral block reduces the prevalence of chronic pain after breast surgery. Anesth Analg. 2006;103:703–8. doi: 10.1213/01.ane.0000230603.92574.4e. [DOI] [PubMed] [Google Scholar]
- 6.Shanti CM, Carlin AM, Tyburski JG. Incidence of pneumothorax from intercostal nerve block for analgesia in rib fractures. J Trauma. 2001;51:536–9. doi: 10.1097/00005373-200109000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Dravid RM, Paul RE. Interpleural block – Part 2. Anaesthesia. 2007;62:1143–53. doi: 10.1111/j.1365-2044.2007.05181.x. [DOI] [PubMed] [Google Scholar]
- 8.Doctor JR, Ranganathan P, Divatia JV. Paraplegia following epidural analgesia: A potentially avoidable cause? Saudi J Anaesth. 2014;8:284–6. doi: 10.4103/1658-354X.130751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakraborty A, Khemka R, Datta T. Ultrasound-guided truncal blocks: A new frontier in regional anaesthesia. Indian J Anaesth. 2016;60:703–11. doi: 10.4103/0019-5049.191665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanco R, Parras T, McDonnell JG, Prats-Galino A. Serratus plane block: A novel ultrasound-guided thoracic wall nerve block. Anaesthesia. 2013;68:1107–13. doi: 10.1111/anae.12344. [DOI] [PubMed] [Google Scholar]
- 11.Tighe SQ, Karmakar MK. Serratus plane block: Do we need to learn another technique for thoracic wall blockade? Anaesthesia. 2013;68:1103–6. doi: 10.1111/anae.12423. [DOI] [PubMed] [Google Scholar]
- 12.Coveney E, Weltz CR, Greengrass R, Iglehart JD, Leight GS, Steele SM, et al. Use of paravertebral block anesthesia in the surgical management of breast cancer: Experience in 156 cases. Ann Surg. 1998;227:496–501. doi: 10.1097/00000658-199804000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheema S, Richardson J, McGurgan P. Factors affecting the spread of bupivacaine in the adult thoracic paravertebral space. Anaesthesia. 2003;58:684–7. doi: 10.1046/j.1365-2044.2003.03189_1.x. [DOI] [PubMed] [Google Scholar]
- 14.Eason MJ, Wyatt R. Paravertebral thoracic block-a reappraisal. Anaesthesia. 1979;34:638–42. doi: 10.1111/j.1365-2044.1979.tb06363.x. [DOI] [PubMed] [Google Scholar]
- 15.Bansal P, Saxena KN, Taneja B, Sareen B. A comparative randomized study of paravertebral block versus wound infiltration of bupivacaine in modified radical mastectomy. J Anaesthesiol Clin Pharmacol. 2012;28:76–80. doi: 10.4103/0970-9185.92449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pusch F, Freitag H, Weinstabl C, Obwegeser R, Huber E, Wildling E. Single-injection paravertebral block compared to general anaesthesia in breast surgery. Acta Anaesthesiol Scand. 1999;43:770–4. doi: 10.1034/j.1399-6576.1999.430714.x. [DOI] [PubMed] [Google Scholar]
- 17.Schnabel A, Reichl SU, Kranke P, Pogatzki-Zahn EM, Zahn PK. Efficacy and safety of paravertebral blocks in breast surgery: A meta-analysis of randomized controlled trials. Br J Anaesth. 2010;105:842–52. doi: 10.1093/bja/aeq265. [DOI] [PubMed] [Google Scholar]
- 18.Sarhadi NS, Shaw Dunn J, Lee FD, Soutar DS. An anatomical study of the nerve supply of the breast, including the nipple and areola. Br J Plast Surg. 1996;49:156–64. doi: 10.1016/s0007-1226(96)90218-0. [DOI] [PubMed] [Google Scholar]
- 19.Mayes J, Davison E, Panahi P, Patten D, Eljelani F, Womack J, et al. An anatomical evaluation of the serratus anterior plane block. Anaesthesia. 2016;71:1064–9. doi: 10.1111/anae.13549. [DOI] [PubMed] [Google Scholar]
- 20.Cheema SP, Ilsley D, Richardson J, Sabanathan S. A thermographic study of paravertebral analgesia. Anaesthesia. 1995;50:118–21. doi: 10.1111/j.1365-2044.1995.tb15092.x. [DOI] [PubMed] [Google Scholar]
- 21.Conacher ID. Resin injection of thoracic paravertebral spaces. Br J Anaesth. 1988;61:657–61. doi: 10.1093/bja/61.6.657. [DOI] [PubMed] [Google Scholar]
- 22.Cowie B, McGlade D, Ivanusic J, Barrington MJ. Ultrasound-guided thoracic paravertebral blockade: A cadaveric study. Anesth Analg. 2010;110:1735–9. doi: 10.1213/ANE.0b013e3181dd58b0. [DOI] [PubMed] [Google Scholar]
- 23.Hetta DF, Rezk KM. Pectoralis-serratus interfascial plane block vs. thoracic paravertebral block for unilateral radical mastectomy with axillary evacuation. J Clin Anesth. 2016;34:91–7. doi: 10.1016/j.jclinane.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Wahba SS, Kamal SM. Thoracic paravertebral block versus pectoral nerve block for analgesia after breast surgery. Egypt J Anaesth. 2014;30:129–35. [Google Scholar]
- 25.Kotzé A, Scally A, Howell S. Efficacy and safety of different techniques of paravertebral block for analgesia after thoracotomy: A systematic review and metaregression. Br J Anaesth. 2009;103:626–36. doi: 10.1093/bja/aep272. [DOI] [PubMed] [Google Scholar]
- 26.Klein SM, Bergh A, Steele SM, Georgiade GS, Greengrass RA. Thoracic paravertebral block for breast surgery. Anesth Analg. 2000;90:1402–5. doi: 10.1097/00000539-200006000-00026. [DOI] [PubMed] [Google Scholar]
- 27.Pérez Herrero MA, López Álvarez S, Fadrique Fuentes A, Manzano Lorefice F, Bartolomé Bartolomé C, González de Zárate J. Quality of postoperative recovery after breast surgery. General anaesthesia combined with paravertebral versus serratus-intercostal block. Rev Esp Anestesiol Reanim. 2016;63:564–71. doi: 10.1016/j.redar.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Ohgoshi Y, Yokozuka M, Terajima K. Serratus-intercostal plane block for breast surgery. Masui. 2015;64:610–4. [PubMed] [Google Scholar]
- 29.Diéguez García P, Fajardo Pérez M, López Álvarez S, Alfaro de la Torre P, Pensado Castiñeiras AP. Ultrasound-assisted approach to blocking the intercostal nerves in the mid-axillary line for non-reconstructive breast and axilla surgery. Rev Esp Anestesiol Reanim. 2013;60:365–70. doi: 10.1016/j.redar.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Daga V, Narayanan MK, Dedhia JD, Gaur P, Crick H, Gaur A. Cadaveric feasibility study on the use of ultrasound contrast to assess spread of injectate in the serratus anterior muscle plane. Saudi J Anaesth. 2016;10:198–201. doi: 10.4103/1658-354X.168825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdallah FW, MacLean D, Madjdpour C, Cil T, Bhatia A, Brull R. Pectoralis and serratus fascial plane blocks each provide early analgesic benefits following ambulatory breast cancer surgery: A retrospective propensity-matched cohort study. Anesth Analg. 2017 Mar 21; doi: 10.1213/ANE.0000000000001975. Epub ahead of Print. [DOI] [PubMed] [Google Scholar]


