Abstract
What motivates animal cells to intercalate is a longstanding question that is fundamental to morphogenesis. A basic mode of cell rearrangement involves dynamic multicellular structures called tetrads and rosettes. The contribution of cell-intrinsic and tissue-scale forces to the formation and resolution of these structures remains unclear, especially in vertebrates. Here, we show that Fgfr2 regulates both the formation and resolution of tetrads and rosettes in the mouse embryo, possibly in part by spatially restricting atypical protein kinase C, a negative regulator of non-muscle myosin IIB. We employ micropipette aspiration to show that anisotropic tension is sufficient to rescue the resolution, but not the formation, of tetrads and rosettes in Fgfr2 mutant limb-bud ectoderm. The findings underscore the importance of cell contractility and tissue stress to multicellular vertex formation and resolution, respectively.
Introduction
Epithelial sheets remodel during development due to cell divisions, cell-shape changes, and cell rearrangements (1, 2, 3, 4). An important remodeling mechanism in animals is cell intercalation, in which a limited variety of rearrangements account for a substantial amount of tissue remodeling. Cell divisions and cell-neighbor exchange events are sometimes not concurrent among invertebrates (5), though cell divisions commonly precipitate cell rearrangements in vertebrates (4, 6, 7). Two of the most common types of rearrangement involve multicellular structures among four (tetrads, – T1 exchange) (1) or more (rosettes) (2) cells. Formation of these structures requires actomyosin contraction of selective cell interfaces to form a transient central vertex in both Drosophila (1, 2, 8) and mouse (9, 10), and it is driven upstream by molecular cues that impart spatial information, such as the anteroposterior embryo axis in Drosophila (11, 12). Subsequent resolution of a multicellular vertex may alter the local landscape, and directionally biased resolution of multiples of these processes can morph tissue on a larger scale.
Various morphogenetic cell behaviors are oriented by a combination of cell-intrinsic and cell-extrinsic forces (7, 13, 14, 15, 16, 17, 18, 19, 20, 21). There is evidence in mouse and Drosophila that tissue tension is planar polarized (18, 22) and orients the resolution axis of multicellular vertices (7, 13, 15). In Drosophila, laser ablation experiments have distinguished between 1) local stresses attributable to cell-intrinsic myosin II contractions, which shrink junctions to form tetrads and rosettes and also promote new junction formation during resolution of those structures, and 2) cell-extrinsic tissue-scale stress, which orients the axes of new junctions (2, 13, 23). In mouse limb-bud ectoderm, local and tissue scale forces become parallel as the early mouse limb bud emerges from the flank such that multicellular vertices form and resolve along the same axis. A combination of mesodermal growth and ectodermal convergence at the dorsoventral midline of the emerging limb bud generates tension in the plane of the ectoderm. That tension is initially dorsoventrally biased at limb initiation and becomes proximodistally oriented as the bud emerges (7). Tissue tension is required to orient multicellular vertex resolution along the axis of growth, but it remains unclear whether tension is sufficient to promote or to orient the resolution axes of multicellular vertices.
The resolution of multicellular vertices and the formation of new cell interfaces along the axis of growth requires Pten (24), medial myosin (13), Toll receptors (11), and regulated myosin II phosphorylation (15, 25) in Drosophila. In mouse mutants, molecular control of multicellular vertex resolution has been obscured by pleiotropic effects. Fgfr2 is an important regulator of ectodermal remodeling in the mouse embryo and is essential for growth of the early limb bud (7, 26, 27). However, it is unclear whether the primary problem in Fgfr2 mutants is lack of anisotropic tissue tension or inability to remodel cell-cell junctions. Here, we employ loss- and gain-of-function approaches to show that Fgfr2 promotes cell interface oscillation, which is required for the formation and resolution of tetrads and rosettes. Tissue tension is sufficient to resolve, but not to form, multicellular vertices in mouse ectoderm.
Materials and Methods
Mouse lines
Analysis was performed using the mouse strains CAG::myr-Venus (28), mTmG (Jackson Laboratory, Gt(ROSA)26Sortm4(ACTB_tdTomato,_EGFP)Luo/J), R26R:Venus-Actin (29), floxed Fgfr2 (30), and ectoderm-specific Crect (31). To generate mutant embryos, flox/flox females carrying the appropriate fluorescent reporter were bred to flox/+;Cre males. Both mTmG and Crect were employed in a heterozygous fashion. For all experiments, wild-type (WT) embryos were designated as WT, and Fgfr2f/+;Crect;mTmG embryos were designated as Fgfr2 mutants. All animal experiments were performed in accordance with protocols approved by the Hospital for Sick Children Animal Care Committee.
Live imaging
Embryos were submerged just below the surface in 50% rat serum in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA) in a 35 mm dish with a central coverglass that was surrounded by 4% agarose at 37°C in 5% CO2 (7, 32). Embryos were immobilized using pulled glass needles to pin the head and tail to the agarose and position the initiating limb bud directly against the coverglass. A Quorum Information Technologies (Calgary, Alberta, Canada) spinning-disk confocal microscope at 10× or 20× magnification was used to acquire time-lapse images at 5 min intervals for periods of up to 3 h. GFP fluorophores were excited using a 488 nm argon laser. Confocal images were acquired as z-stacks of xy images in 2- to 5-μm steps. Raw data were processed using Volocity 6.3, then imported into Image J for further analysis.
Quantification of cell behaviors
Cell outlines in confocal images were automatically identified using a plugin in Image J (Automated Multicellular Tissue Analysis) developed by the Advanced Digital Microscopy Core Facility at the Institute for Research in Biomedicine (Barcelona, Spain). After ectodermal cells were segmented in the confocal image sequences for each time point, several cell behaviors were quantified, including the cell elongation axis, cell-division orientation, cell-rearrangement polarity, and cortical actin oscillation. Angles measured from 0° to 179° with respect to the line of reference (which was plotted as 0°) were grouped into bins of 30° and represented in polar plots symmetrically about the horizontal axis. Angles ranging from 0° to 179° were also shown as positive acute angles (0°–90°) to represent the extent of alignment with the line of reference. Acute angles were grouped into five bins of 18° and were represented in histograms. The line of reference was the dorsoventral (DV) limb-bud axis unless otherwise specified. The following describes specifically how each type of cell behavior was measured.
The outline of each cell was fitted into an ellipse, and the longitudinal and transverse axes were calculated automatically in Image J. The angles between each longitudinal axis of the fitted cell ellipses and the line of reference were calculated and referred to as the cell elongation angle. Metaphase-to-telophase transition orientation was defined by a line connecting the centroid of the two daughter cells. The angles between the metaphase-to-telophase transition orientation and the line of reference were measured and referred to as the cell division angle. Multicellular tetrads and rosettes were identified manually in confocal time-lapse sequences. When the central apex of the multicellular structure resolved and vanished, the rearrangement polarity was measured as the angle between the longitudinal axis of the ellipse outlined by each tetrad or rosette and the line of reference.
Oscillation of cortical actin contraction was quantified by manually measuring the length of cell interfaces labeled by Venus-actin in each frame. Interface lengths were normalized against their initial lengths in the first frame of image sequences. Normalized rates of change of interfacial lengths were plotted over time. The amplitude of the oscillations was measured using the standard deviation of the interfacial length for each interface over time. Oscillation of cell interfaces along DV and anteroposterior (AP) axes was analyzed separately to test the possibility of oscillation anisotropy.
Immunofluorescence
Embryonic-day (E) 9.5–10.5 mouse embryos were fixed overnight in 4% paraformaldehyde in phosphate-buffered saline (PBS) followed by three washes in PBS. Embryos were permeabilized in 0.1% Triton X-100 in PBS for 20 min and blocked in 5% normal donkey serum (in 0.05% Triton X-100 in PBS) for 1 h. Embryos were incubated in primary antibody for 5 h at room temperature, followed by incubation overnight at 4°C. Embryos were washed in 0.05% Triton X-100 in PBS (three washes of 15 min each) and then incubated in secondary antibody for 1 h at room temperature in the dark. Images were acquired using a Quorum Information Technologies spinning-disk confocal microscope, and image analysis was performed using Volocity software and Image J.
The antibodies used were atypical protein kinase C (aPKC) (sc-216, Santa Cruz, rabbit, 1:100), Zo 1 (Invitrogen 40–2200, rabbit, 1:200), non-muscle myosin IIB (MYOIIB) (Covance PRB-445P, rabbit, 1:500), and Frizzled 6 (AF1526, R&D Systems, goat, 1:250). All secondary antibodies were purchased from Jackson Immunoresearch and used at 1:1000 dilution.
Atomic force microscopy
The apparent Young’s moduli of early limb-bud ectodermal cells were examined using a commercial atomic force microscope (AFM) (BioScope Catalyst, Bruker, Billerica, MA) under an inverted microscope (Eclipse-Ti, Nikon, Tokyo, Japan). Young’s modulus was incorporated into a finite-element model (FEM) as a material property and was also interpreted as a proxy for the ectodermal cell cortical tension. The detailed experimental approach and data analysis methods follow protocols regarding the use of a colloidal probe, described elsewhere (33). Briefly, colloidal-force probes were custom made by attaching a borosilicate glass microsphere (10 μm diameter; Sigma-Aldrich, St. Louis, MO) to an AFM cantilever (MLCT-D, Bruker/Veeco) using epoxy glue. Force-distance curves were acquired at distal, middle, and proximal regions of limb-bud ectoderm using a consistent 200 pN contact force at an indentation rate of 1 Hz. Because the contact force was relatively small, minimum substratum influence of the underlying mesoderm was assumed, and therefore, no explicit sample thickness correction was performed. At each location of the limb bud, five force curves were acquired with a minimum wait time of 15 s between successive measurements to minimize any history effect (34).
To calculate Young’s modulus from force-distance curves, the Hertz model with Sneddon’s modification was used (35). Young’s modulus was calculated using the following equation for spherical probes:
where F is the loading force, R is the probe radius, E is the Young’s modulus, is Poisson’s ratio, and h is the indentation depth.
The spring constant of AFM cantilevers was calibrated for each experiment using the thermal noise fluctuation method (36). Embryos were immobilized against a 4% agarose gel pad by pulled glass needles in 50% rat serum/50% Dulbecco’s modified Eagle’s medium. AFM measurements were performed within 30 min. of dissection.
Optical projection tomography and limb-bud morphology analysis
E9.5 mouse embryos were harvested and fixed in 4% paraformaldehyde overnight at 4°C. The optical projection tomography (OPT) system was custom built and is fully described elsewhere (37). Three-dimensional (3D) data sets were reconstructed from auto-fluorescence projection images acquired over a 10 min scan time at an isotropic voxel size of 3.85 μm. The 3D surface renderings of OPT data were generated by Amira software, version 5.3.3 (VSGG, Burlington, MA).
Finite-element modeling
FEM was employed to investigate force/stress propagation and deformation of the limb-bud ectodermal sheet under different mechanical forces. All simulations were implemented in ANSYS v14.0 (ANSYS, Canonsburg, PA). 3D models of the ectodermal layer were created according to optical projection tomography images of the limb bud at the 18-somite stage. The length, width, and thickness of the ectodermal layer in the FEM models was 1445 μm (AP axis), 212 μm (DV axis), and 10 μm (proximodistal axis), respectively. The bulging region at the center of the sheet was modeled to be 30 μm tall to resemble the shape of the limb bud.
The mechanical behavior of the ectodermal sheet was modeled as a continuum with homogeneous viscoelastic material properties rather than as individual cells. A generalized Kelvin model (38) was used to account for the tissue viscoelastic behavior. An instantaneous elastic modulus of 0.085 kPa was assigned to limb-bud ectodermal tissue based on AFM indentation data (7). Viscous relaxation of the ectodermal modulus was calculated based on limb-bud compression relaxation data reported previously (38) and was implemented in ANSYS as two-pair Prony relaxation with relative moduli of 0.1 and 0.4 and relaxation time constants of 8 and 45 s, respectively. A Poisson’s ratio of 0.4 was used, as is common for biological material (39).
Micropipette aspiration pressure was modeled as negative pressure exerted on a precise area on the dorsal ectoderm where the micropipette contacted the ectoderm. Boundary conditions were applied to the ectodermal sheet model in which all six degrees of freedom were fixed. A frictionless support underneath the ectodermal sheet was added to resemble the mesodermal support normal to the ectoderm while allowing tangential displacement within the plane.
Ten-node tetrahedral elements were used to discretize all geometries in our model. Since stress and deformation are linearly proportional to the exact applied load, the qualitative characteristics of the stress pattern on the limb bud, such as the direction and ratio of the principal stresses, would be the same at different load magnitudes. Viscoelastic properties according to compression data only represent passive tissue properties, without taking into account the long-term viscous behavior presumably caused by dynamic cell rearrangements or tissue fluidity (40).
Micropipette aspiration
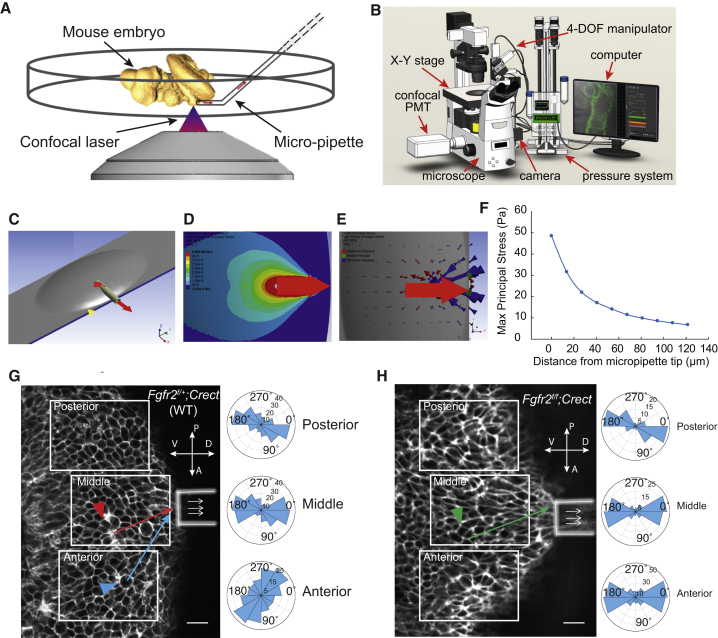
The micropipette aspiration system consists of a confocal microscope with a motorized XY translation stage, a micromanipulator (MX7600, Siskiyou, Yreka, CA) for micropipette positioning, and a custom-built pressure system with a differential pressure transducer (PX409-10WDWU5V, Omega Engineering, Stanford, CT). In experiments, a 35°-bevelled micropipette with an inner diameter of 35 μm was mounted on the micromanipulator and connected to the vacuum pressure system (Fig. S1 C). The embryo was placed down on its flank, immobilized on the petri dish, and imaged with a confocal microscope (Fig. 2 A). The following subtasks were subsequently performed: 1) the system automatically detected the micropipette and moved it to the vicinity of the limb bud (Fig. S2 A); 2) the system detected tissue-micropipette contact before negative pressure was applied (Fig. S2 B); and 3) the system compensated for the 3D drift trajectory of the limb-bud tissue to maintain stable tissue-micropipette contact during the 2 h experimental periods (Fig. S2 C). Micropipette position and aspiration pressure were controlled cooperatively using visual feedback and pressure sensor feedback (Fig. S2, D and E).
Figure 2.
Micropipette aspiration of mouse embryonic ectoderm. (A) Schematic of simultaneous micropipette aspiration and confocal scanning of a mouse embryonic limb bud. (B) The system consists of a micropipette mounted on a micromanipulator, a motorized XY stage, a confocal microscope, a CMOS camera, and a host computer. Pressure was controlled by precisely positioning two water reservoirs. (C) Finite-element simulation of micropipette aspiration. (D) Stress field due to micropipette aspiration. (E) Simulated maximal principal stresses (red arrows) were oriented toward the micropipette opening. (F) Given 0.1 nN/μm2 of aspiration pressure, the graph represents the simulated magnitude of maximal principal stress versus distance from the micropipette opening. (G and H) Micropipette aspiration of limb-bud ectoderm in WT (Fgfr2f/+;Crect) and mutant (Fgfr2f/f;Crect) embryos. Three polar plots represent the cell elongation angles of ectodermal cells corresponding to each of the three white-boxed regions. Tissue was pulled to the aperture, but no cells were lost into the pipette. Red, blue, and green arrowheads identify a rosette, dividing cell and tetrad, respectively, which are shown in further detail in Fig. 3D. The corresponding arrows indicate the direction of applied tissue force. Scale bars represent 10 μm (G and H).
Results
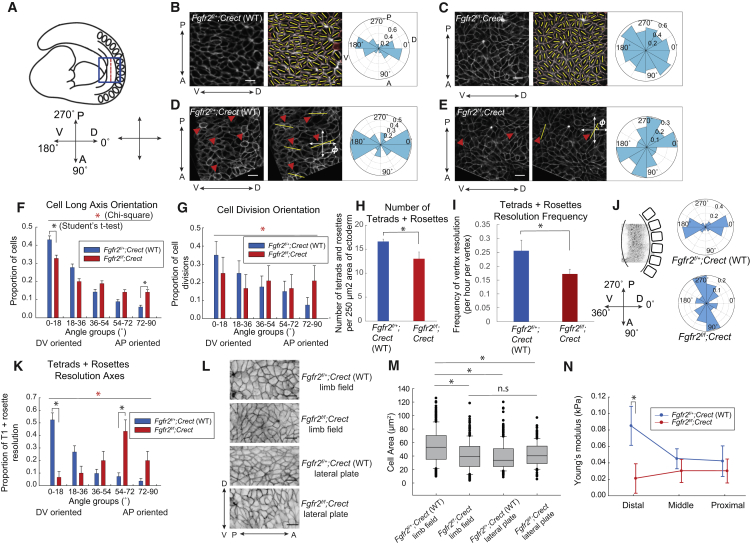
DV-oriented cell behaviors normally shape the ectoderm of the early mouse limb bud (7). (The DV axis of lateral plate ectoderm becomes gradually oriented along the predominant proximodistal axis of growth as the limb bud emerges.) To examine cell behaviors in the absence of a conditional Fgfr2 allele (30), we employed an ectoderm-specific Cre recombinase, Crect (31), together with a transgenic cell membrane reporter (mT/mG) (41). In the limb field of 18- to 22-somite-stage Fgfr2f/f;Crect;mT/mG mouse embryos, ectodermal cells exhibited loss of DV-oriented elongation and division planes, as observed during time-lapse imaging of intact embryos (Fig. 1, A–G; Fig. S1 A). The total number of multicellular vertices per unit area was diminished in the Fgfr2 mutant ectoderm (Fig. 1 H; Fig. S1 B), as was the frequency of multicellular vertex resolution during 3 h imaging sessions (Fig. 1 I; Fig. S1, C and D). Moreover, the usual DV resolution axis of tetrads and rosettes was disoriented in the Fgfr2 mutant ectoderm. Despite proper formation of a transient central vertex, mutant ectodermal rosettes formed (7) and resolved along the AP embryo axis (Fig. 1, J and K; Fig. S1, E; Movies S1 and S2). Therefore, Fgfr2 is required to promote the formation and DV resolution of multicellular vertices. Two cellular processes potentially can explain these observations: Fgfr2 promotes contractility and/or junctional remodeling (13, 15).
Figure 1.
DV-oriented ectodermal cell behaviors and tension are diminished in conditional Fgfr2 mutants. (A) Schematic depiction of the position of a mouse forelimb bud that grows distally out of the plane of the page. The blue box indicates the region of interest for analysis. (B and C) Left: Confocal section of pre-AER (20 som) WT (B) and conditional Fgfr2 mutant (C) limb field ectoderm. Centre: Segmented cells with yellow bars showing cell long axes. Right: Polar plots representing orientation of long axis angles of limb-bud ectodermal cells (n = 3 WT embryos, 150 cells, and 3 Fgfr2 mutant embryos, 160 cells). (D and E) Confocal sections of WT (D) and conditional Fgfr2 mutant (E) limb field ectoderm. Red triangles indicate dividing cells and yellow bars indicate cell division axes. Polar plots represent cell division angles. (F) Distributions of cell long axes with the DV and AP axes represented by 0° and 90°, respectively (n = 5 embryos per condition; 569 cells were examined for the WT versus 699 cells for the Fgfr2 mutant). Distribution was significantly different (p < 0.0001, chi-square test (red asterisk); for the WT versus mutant 0–18° bin, p = 0.0002, Student’s t-test (black asterisk); for the WT versus mutant 72–90° bin, p < 0.0001, Student’s t-test (black asterisk)). (G) Distribution of cell division angles (n = 5 embryos per condition; WT-40 divisions examined versus Fgfr2 mutant-24 divisions examined) was significantly different (p = 0.00014, chi-square test). (H) The total number of tetrads and rosettes per a 250 μm2 area of ectoderm (n = 5 WT, n = 4 Fgfr2 mutant 18- to 22-somite embryos, p = 0.03 by Student’s t-test). (I) Frequency of tetrad and rosette resolution per multicellular vertex in WT and Fgfr2 mutants (n = 5 18- to 22-somite embryos per condition, p = 0.0491, Student’s t-test). (J) Polar plots represent the resolution axes of tetrads and rosettes in WT and conditional Fgfr2 mutant embryos during 2–3 h time-lapse sessions. (K) Distribution of resolution angles among resolving tetrads and rosettes in WT and conditional Fgfr2 mutant limb-bud ectoderm with respect to the DV axis (n = 5 18- to 22-somite embryos per condition, with 82 tetrads and rosettes examined for the WT embryos versus 30 tetrads and rosettes for the Fgfr2 mutant. Distribution was significantly different (p < 0.0001, chi-square test; WT versus mutant for the 0–18° bin, p < 0.0001, Student’s t-test; WT versus mutant for the 54–72° bin, p = 0.0006, Student’s t-test). (L) Confocal section of the apical surface of the WT limb field, the Fgfr2 mutant limb field, the WT lateral plate, and the Fgfr2 mutant lateral plate ectoderm. (M) Box plots demonstrate the distribution of ectodermal cell surface area in the WT limb field (300 cells examined), the Fgfr2 mutant limb field (270 cells examined), the WT lateral plate (274 cells examined), and the Fgfr2 mutant lateral plate (154 cells examined). The average apical cell area in the WT limb field was significantly larger than in the Fgfr2 mutant limb field (p = 0.00423, Student’s t-test), the WT lateral plate (p = 0.00033, Student’s t-test), and the Fgfr2 mutant lateral plate (p < 0.005 by Student’s t-test). The average cell area was not significantly different between the WT lateral plate ectoderm and the Fgfr2 mutant limb field ectoderm (p = 0.612 by Student’s t-test), between the WT lateral plate ectoderm and the Fgfr2 mutant lateral plate ectoderm (p = 0.146 by Student’s t-test), and between the Fgfr2 mutant limb field ectoderm and the Fgfr2 mutant lateral plate ectoderm (p = 0.34 by Student’s t-test). (N) AFM measured the apparent Young’s moduli of the proximal, middle, and distal regions of the WT and conditional Fgfr2 mutant limb buds (n = 5 18- to 22-somite embryos per condition; distal WT versus mutant, p = 0.0204, Student’s t-test). Scale bars represent 10 μm (B–E and K). Error bars indicate the SEM.
Since tissue tension is a key driver of new junction formation (7, 13, 15), we examined this parameter in Fgfr2 mutants. Given that cells exhibit a positive Poisson ratio (39), their apical surface area should increase when tensed along one axis, as is apparent during AP germband extension due to an AP pulling force (surface area decrease is expected when cells are compressed) (18). In the limb-bud ectoderm, we found that the apical surface areas of WT cells where DV stress predominates (7) were larger than those of non-limb lateral plate cells. In contrast, the apical surfaces of Fgfr2f/f;Crect mutant ectodermal cells in the limb field were similar to those in the non-limb lateral plate and smaller than those in the WT limb field (Fig. 1, L and M), suggesting that stress was diminished in mutant limb-bud ectoderm. To measure the distribution of stress in live embryonic tissue, we conducted AFM measurements (7, 42). Fgfr2f/f;Crect mutants lacked a stress gradient that emanates from the prospective apical ectodermal ridge at the distal end of the limb bud (7) (Fig. 1 N). Therefore, loss of a stress differential correlates with diminished frequency and disorientation of cell rearrangements in Fgfr2 mutants.
To test whether anisotropic tissue stress is sufficient to promote new junction formation, we artificially restored an anisotropic stress field in mutants by micropipette aspiration of the ectoderm. In brief, we developed a custom micropipette aspiration system to exert precisely calibrated and stable tension to the ectodermal sheet of intact embryos in situ for an extended period during live confocal imaging (Fig. 2, A and B). The micropipette aspiration system incorporates optical and pressure sensor feedback to enable control of the micropipette position and applied aspiration pressure (see Materials and Methods, Fig. S2). An FEM that incorporated the actual geometry of the 18-somite limb field was built to simulate the ectodermal stress field (Fig. 2 C). Aspiration-induced force was modeled as negative pressure acting on the area covered by the micropipette aperture that was used in experiments (inner diameter, 35 μm). Simulated aspiration at a range of pressures created a radial stress field with a magnitude that decreased away from the micropipette aperture and was directionally biased toward it (Fig. 2, D and E). We determined that with an applied pressure of 0.1 nN/μm2, stress would dissipate from 50 to 10 Pa over a distance of 100 μm from the micropipette aperature (Fig. 2 F). Accordingly, each ectodermal cell would therefore experience 5–10 nN of force, which has been shown by others to be sufficient to elongate cells, recruit myosin, and induce transcription (23, 43, 44).
We applied a constant aspiration pressure of 0.1 nN/μm2 to the limb field of intact 18-somite embryos for 2 h periods and simultaneously performed live, confocal imaging. Within several seconds of initiating aspiration, ectodermal cells in both WT and Fgfr2f/f;Crect mutants became elongated. Cell shapes were changed up to 10 cell diameters away from the micropipette aperture and were oriented in a radial fashion that corresponded to the new stress field (Fig. 2, G and H). Therefore, as expected, tension instantaneously reoriented longitudinal cell axes.
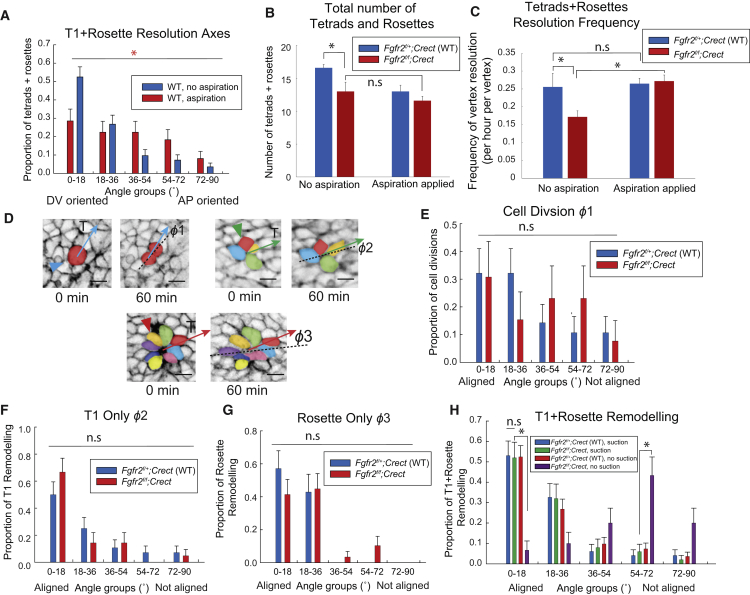
To evaluate tetrad and rosette rearrangements, we first compared WT embryos with or without aspiration using the DV axis as a reference. The native DV orientation of cell rearrangements was significantly diminished by microsuction (Fig. 3 A), suggesting that cells were responding to the ectopic stress field by rotating obliquely toward it at a range of angles, as shown in Fig. 2 G. Micropipette aspiration did not increase the total number of tetrads and rosettes (Fig. 3 B) despite cell stretching (Fig. 2, G and H). Also, the time to resolution of tetrads and rosettes under native (WT, unaspirated) conditions was similar to that under aspiration conditions (in WT and Fgfr2 mutant backgrounds) (Fig. S3 A). Together, these findings suggest that cell elongation and new interface formation are distinct processes. However, aspiration did increase the resolution frequency of existing vertices in Fgfr2f/f;Crect mutants to WT levels (Fig. 3 C). An ectopic stress field is therefore insufficient to promote the formation of multicellular vertices but does restore their resolution frequency.
Figure 3.
Ectopic tension reorients cell rearrangements. (A) Comparative distribution of resolution axes for WT tetrads and rosettes with or without aspiration with respect to the native DV axis (n = 5 18–22 som. embryos per condition; p = 0.0167, chi-square test). (B) The total number of Fgfr2 mutant tetrads and rosettes per 250 μm2 area of ectoderm was not increased by aspiration (n = 5 WT, n = 4 Fgfr2 mutant 18- to 22-somite embryos, p = 0.37 by Student’s t-test). (C) The frequency of tetrad and rosette resolution per vertex was rescued in Fgfr2 mutants (n = 5 18- to 22-somite embryos per condition; mutant before aspiration versus mutant after aspiration, p = 0.0355, Student’s t-test; WT before aspiration versus mutant after aspiration, p = 0.3368. (D) Time series demonstrating 20-somite limb-bud ectodermal cell division, T1 (tetrad), and rosette resolutions. The cell division angle, ϕ1, T1 resolution angle, ϕ2, and rosette resolution angle, ϕ3, were defined with respect to the direction of externally applied tension, as indicated by the arrows. (E) Distribution of cell division angles, ϕ1, with respect to the axis of ectopic stress in WT and Fgfr2 mutant limb-bud ectoderm (n = 5 18- to 22-somite embryos per condition) was not significantly different (p = 0.6723 by the chi-square test). (F) Distribution of tetrad resolution angles, ϕ2, with respect to the axis of ectopic stress. (n = 5 18- to 22-somite embryos per condition, with 28 tetrads examined in the WT and 21 tetrads examined in mutants; p = 0.5588, chi-square test). (G) Distribution of rosette resolution angles, ϕ3, with respect to the axis of ectopic stress (n = 5 18- to 22-somite embryos per condition, with 21 rosettes examined in the WT and 29 rosettes examined in mutants; p = 0.3159, chi-square test). (H) Distribution of resolution angles for tetrads and rosettes combined (ϕ2 and ϕ3) in WT and Fgfr2 mutants after aspiration was not significantly different (n = 5 18- to 22-somite embryos per condition; 49 tetrads and rosettes examined in WT, and 50 tetrads and rosettes were examined in mutants; p = 0.9554, chi-square test). The resolution axes of mutant tetrads and rosettes were aligned with ectopic stress induced by aspiration (for the 0–18° bin, p < 0.0001; for the 54–72° bin, p = 0.0004, by Student’s t-test). Scale bars in (D) represent 10 μm. Error bars indicate SEM.
To precisely quantify how the axes of cell divisions and cell rearrangements respond to extrinsic stress, we employed a new reference axis connecting each target cell to the micropipette aperture. Cell division (ϕ1), T1 (ϕ2), and rosette resolution angles (ϕ3) were measured with respect to this reference axis (Fig. 3 D). The distributions of angle ϕ1 indicated that mutant ectodermal daughter cells were no longer statistically different from those of WT cells (Fig. 3 E), implying that they responded to ectopic stress. However, this effect on mutant cell division was no longer apparent when orientation was categorized as aligned versus misaligned (Fig. S3 B), suggesting that reorientation of cell division may require a higher stress threshold. The resolution axes of tetrads and rosettes became aligned with the imposed axis of stress. Interestingly, this alignment held true to a similar extent for Fgfr2f/f;Crect mutants and WT embryos (Fig. 3, F–H; Fig. S3 C; Movies 3 and 4). These results indicate that ectopic tissue tension is sufficient to reorient multicellular axis resolution in conditional Fgfr2 mutant embryos. They imply that junctional remodeling is intact but force generation is compromised in the absence of Fgfr2.
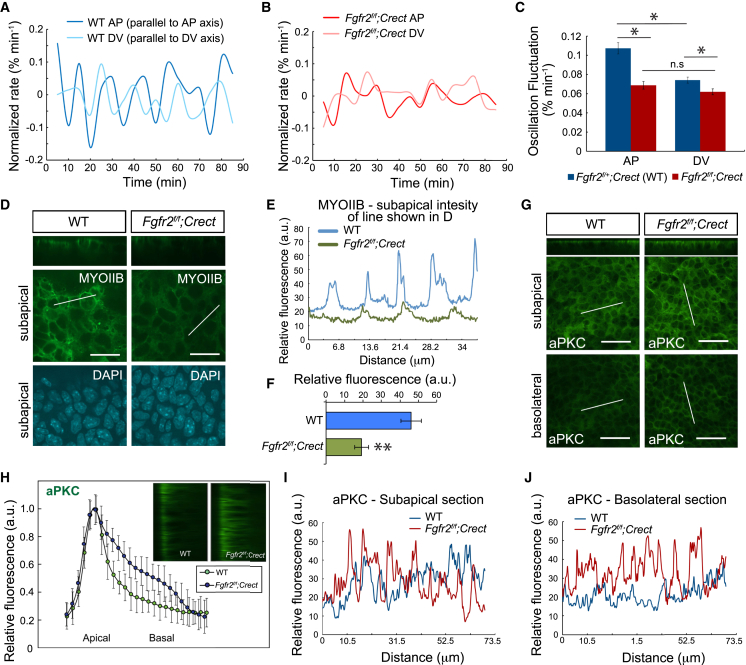
To test whether Fgfr2 regulates force generation, we examined the oscillatory nature of cell interface length changes that are due to actomyosin contractions and are required for new junction formation (13, 15). The rate of change of cell interface lengths was measured in vivo during live imaging experiments. As expected based on the anisotropic stress pattern (7), WT limb-bud ectodermal cells exhibited greater interface length rate of change and amplitude along the DV axis (parallel to AP interface) compared to the AP axis (parallel to DV interface). In contrast, Fgfr2 mutant cells exhibited isotropic rate of change and amplitude (Fig. 4, A–C). Therefore, Fgfr2 is required to promote anisotropic cell interface oscillation that is a consequence of cytoskeletal contraction (but is not necessarily linearly related to the degree of contraction).
Figure 4.
Fgfr2 regulates anisotropic cell interface oscillation. (A and B) Rate of change of interface length from time-lapse videos of WT (Fgfr2f/+;Crect) (A) and conditional Fgfr2 mutant (B) limb-bud ectoderm, normalized to the initial interface length. Shown are representative curves for AP and DV interfaces (parallel to DV and AP axes, respectively). (C) Oscillation amplitude of AP and DV interfaces in WT and conditional Fgfr2 mutant limb-bud ectoderm (n = 40 interfaces for each condition; WT AP versus DV interfaces, p < 0.001; mutant AP versus DV interfaces, p = 0.1807; WT AP interfaces versus mutant AP interfaces, p < 0.001; WT DV interfaces versus mutant DV interfaces, p = 0.0071, Student’s t-test). (D) xy sections at the DV boundary of 21-somite WT and conditional Fgfr2 mutant limb-bud ectoderm showing MYOIIB (green) and DAPI (blue). (E) Fluorescence intensity profiles of subapical MYOIIB in WT and conditional Fgfr2 mutant ectoderm corresponding to the oblique white lines shown on the en-face images in (D). (F) Overall relative fluorescence of three separate 40× subapical fields of view in each of three 20- to 22-somite embryos (p < 0.001). (G) xy sections at the DV boundary of 20-somite WT and conditional Fgfr2 mutant limb-bud ectoderm showing aPKC. Shown are z-sections (top) and subapical and basolateral sections of the same region. (H) Quantification of aPKC intensity from the apical to the basal aspect of WT and conditional Fgfr2 mutant ectoderm. Inset: xz sections showing lateral views of ectoderm with the apical aspect on the left. Intensities were normalized to the maximal level at the apical surface for each condition (n = 3 separate 40× fields in each of three 20- to 22-somite embryos). (I and J) Comparison of aPKC fluorescence intensity at the subapical (I) and basolateral (J) levels of WT and Fgfr2 mutant ectoderm, represented as intensity profiles of the oblique white lines shown on the corresponding en-face images in (G). Scale bars represent 25 μm (D) and 50 μm (G). Error bars indicate the mean ± SE.
To examine how Fgfr2 regulates this process, we studied the distribution of cytoskeletal and cell polarity proteins. We previously showed that planar polarity of cortical actin was diminished in Fgfr2 mutants, although MYOIIB was not polarized (7), nor were select markers of cell polarity FZD6 and ZO1 (Fig. S4). However, quantification revealed that the MyoIIB immunostain intensity was diminished in Fgfr2 mutants (Fig. 4, D–F). Interestingly, we also observed mislocalization of aPKC, which is best known as a component of the Par complex that regulates apicobasal cell polarity. This protein also links apicobasal polarity to planar cell behavior, in part by contributing to the formation and localization of adherens junctions and to the attachment of cortical actomyosin to the cell membrane (45, 46, 47, 48). aPKC was predominantly located at the apical domain of WT ectodermal cells, as expected. In Fgfr2 mutant ectoderm, however, the aPKC expression domain was expanded basolaterally (Fig. 4, G–J). These findings suggest that Fgfr2 promotes MYOIIB-dependent cell interface oscillation and regulates some components of cell polarity.
Discussion
The importance of active versus passive mechanisms of cell intercalation has long been a focus of inquiry. An early computational model of epithelial remodeling assumed that junctional rearrangements accommodate the balance of forces at cell contact sites. Interestingly, stress relaxation in the absence of active junctional remodeling or active cell interdigitation was sufficient to account for cell rearrangements that are observed during Fundulus epiboly (17). Experimentalists deconstructed the phenomenon of widespread cell intercalation to basic units of rearrangement involving tetrads and rosettes (1, 2). These approaches prompted separate consideration of the formation and resolution stages of these multicellular structures, and recent evidence has underscored the importance of cell-extrinsic tissue stress as a spatial cue that orients resolution (7, 13, 15, 18, 22). The data in this study support a model in which Fgfr2 promotes cortical oscillation through MYOIIB, rather than junctional remodeling, to induce the formation of multicellular vertices. FGF signaling is also required to promote MYOIIB-dependent apical constriction that induces rosette-shaped proneuromast formation in the lateral line of zebrafish (49, 50, 51, 52), suggesting that there is a degree of conservation in the morphogenetic role of FGF.
Ectopic tissue tension was sufficient to reorient the resolution, but not rescue the formation, of tetrads and rosettes in Fgfr2 mutants. Our observation that tension can overcome partially depleted MYOIIB in Fgfr2 mutants to resolve multicellular vertices underscores the importance of cell-extrinsic mechanical regulation of polarized cell behaviors. The data imply that formation of tetrads and rosettes requires cell-intrinsic MYOIIB, and we speculate that this is due to greater energy requirement relative to the resolution stage.
Antecorrelation between aPKC and MYOIIB is consistent with the role of aPKC as a kinase that negatively regulates MYOIIB directly (53) and indirectly through Rho-associated kinase (54, 55) and dampens pulsatile actomyosin contractions (46, 56). The basolateral expansion of aPKC seen here is reminiscent of spatial redistribution of this protein during mitosis in Drosophila, which aids assembly of a relatively isotropic metaphase cortex (57). Whether and how basolateral expansion of aPKC actually diminishes subapical MYOIIB in limb-bud ectoderm is not addressed by this study although one possibility is that recruitment of MYOIIB is affected in this context by global cellular levels of aPKC indirectly through Rho-associated kinase. Nonetheless the FGF pathway, a recognized regulator of growth and cell fate, also regulates motor and polarity proteins implying that morphogenesis and pattern formation are closely coordinated.
Mechanisms that form and resolve multicellular vertices may be intertwined. Both actin and myosin accumulate in response to tension (23, 58, 59), and their coordinated contractions likely change the local tensile landscape that feeds back to orient resolution angles. Evidence for a co-dependent relationship includes our observation that cell oscillation is associated with vertex resolution and is reminiscent of the requirement for medial myosin contraction during new junction formation in Drosophila (13, 15). Multicellular vertex formation and resolution often take place at different angles, so it remains unclear how tissue forces might help to coordinate the orientation of these events. Quantitative evaluation of all morphogenetic events that shape a given tissue (60, 61) together (60, 61) with a detailed understanding of how cells sense force cues and translate that information into spatially appropriate junctional rearrangements (62) are steps in the right direction.
Author Contributions
J.W., Y.S., and S.H. designed the experiments and wrote the manuscript. J.W., K.L., H.T., and H.L. performed experimental work. J.W., H.T., H.L., and C.A.S. performed theoretical and image analyses.
Acknowledgments
We thank Rodrigo Fernandez-Gonzalez for critical evaluation of the manuscript. This study was supported by the Canadian Institutes of Health Research, MOP 126115 (S.H.), and by a Canada Research Chair in Micro and Nano Engineering Systems (Y.S.).
Editor: Alexander Dunn.
Footnotes
Four figures and four movies are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(17)30440-X.
Contributor Information
Yu Sun, Email: sun@mie.utoronto.ca.
Sevan Hopyan, Email: sevan.hopyan@sickkids.ca.
Supporting Material
Limb field ectoderm of WT (Fgfr2f/+;Crect;mTmG) 20 somite embryo without micropipette aspiration, oriented as in Fig. 1 A (anterior down, dorsal to the right). A few examples of cell rearrangements are shaded in color, while the remainder of the field is shown for completeness. In the upper right, DV cell division precipitates formation of a tetrad that resolves along the DV axis. In the lower two examples, DV cell division preciptates the formation of rosettes that resolve along the DV axis.
Limb field ectoderm of conditional Fgfr2 mutant (Fgfr2f/f;Crect;mTmG) 20 somite embryo without micropipette aspiration, oriented as in Fig. 1 A (anterior down, dorsal to the right). The upper rosette resolves in an oblique AP/DV axis. Lower down, cell division occurs along the AP axis and precipitates formation of a rosette that begins to resolve along the AP axis.
Limb field ectoderm of WT (Fgfr2f/+;Crect;mTmG) 20 somite embryo during micropipette aspiration, oriented as in Fig. 1A (anterior down, dorsal to the right). Cell divisions and rearrangements occur at oblique DV angles oriented toward the micropipette aperture out of view in the upper left side of the field.
Limb field ectoderm of conditional Fgfr2 mutant (Fgfr2f/f;Crect;mTmG) 20 somite embryo during micropipette aspiration, oriented as in Fig. 1 A (anterior down, dorsal to the right). Rosettes (upper right) and tetrads resolve along the DV axis of artificial tension.
References
- 1.Bertet C., Sulak L., Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- 2.Blankenship J.T., Backovic S.T., Zallen J.A. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev. Cell. 2006;11:459–470. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Irvine K.D., Wieschaus E. Cell intercalation during Drosophila germband extension and its regulation by pair-rule segmentation genes. Development. 1994;120:827–841. doi: 10.1242/dev.120.4.827. [DOI] [PubMed] [Google Scholar]
- 4.Keller R.E. Time-lapse cinemicrographic anaIysis of superficiaI ceII behavior during and prior to gastrulation in Xenopus laevis. J. Morphol. 1978;157:223–248. doi: 10.1002/jmor.1051570209. [DOI] [PubMed] [Google Scholar]
- 5.Gibson M.C., Patel A.B., Perrimon N. The emergence of geometric order in proliferating metazoan epithelia. Nature. 2006;442:1038–1041. doi: 10.1038/nature05014. [DOI] [PubMed] [Google Scholar]
- 6.Firmino J., Rocancourt D., Gros J. Cell division drives epithelial cell rearrangements during gastrulation in chick. Dev. Cell. 2016;36:249–261. doi: 10.1016/j.devcel.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lau K., Tao H., Hopyan S. Anisotropic stress orients remodelling of mammalian limb bud ectoderm. Nat. Cell Biol. 2015;17:569–579. doi: 10.1038/ncb3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rauzi M., Lenne P.F., Lecuit T. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature. 2010;468:1110–1114. doi: 10.1038/nature09566. [DOI] [PubMed] [Google Scholar]
- 9.Lienkamp S.S., Liu K., Walz G. Vertebrate kidney tubules elongate using a planar cell polarity-dependent, rosette-based mechanism of convergent extension. Nat. Genet. 2012;44:1382–1387. doi: 10.1038/ng.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams M., Yen W., Sutherland A. Distinct apical and basolateral mechanisms drive planar cell polarity-dependent convergent extension of the mouse neural plate. Dev. Cell. 2014;29:34–46. doi: 10.1016/j.devcel.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paré A.C., Vichas A., Zallen J.A. A positional Toll receptor code directs convergent extension in Drosophila. Nature. 2014;515:523–527. doi: 10.1038/nature13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zallen J.A., Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev. Cell. 2004;6:343–355. doi: 10.1016/s1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]
- 13.Collinet C., Rauzi M., Lecuit T. Local and tissue-scale forces drive oriented junction growth during tissue extension. Nat. Cell Biol. 2015;17:1247–1258. doi: 10.1038/ncb3226. [DOI] [PubMed] [Google Scholar]
- 14.Legoff L., Rouault H., Lecuit T. A global pattern of mechanical stress polarizes cell divisions and cell shape in the growing Drosophila wing disc. Development. 2013;140:4051–4059. doi: 10.1242/dev.090878. [DOI] [PubMed] [Google Scholar]
- 15.Yu J.C., Fernandez-Gonzalez R. Local mechanical forces promote polarized junctional assembly and axis elongation in Drosophila. eLife. 2016;5:e10757. doi: 10.7554/eLife.10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aigouy B., Farhadifar R., Eaton S. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell. 2010;142:773–786. doi: 10.1016/j.cell.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 17.Weliky M., Oster G. The mechanical basis of cell rearrangement. I. Epithelial morphogenesis during Fundulus epiboly. Development. 1990;109:373–386. doi: 10.1242/dev.109.2.373. [DOI] [PubMed] [Google Scholar]
- 18.Butler L.C., Blanchard G.B., Sanson B. Cell shape changes indicate a role for extrinsic tensile forces in Drosophila germ-band extension. Nat. Cell Biol. 2009;11:859–864. doi: 10.1038/ncb1894. [DOI] [PubMed] [Google Scholar]
- 19.Bosveld F., Bonnet I., Bellaïche Y. Mechanical control of morphogenesis by fat/dachsous/four-jointed planar cell polarity pathway. Science. 2012;336:724–727. doi: 10.1126/science.1221071. [DOI] [PubMed] [Google Scholar]
- 20.Luxenburg C., Heller E., Fuchs E. Wdr1-mediated cell shape dynamics and cortical tension are essential for epidermal planar cell polarity. Nat. Cell Biol. 2015;17:592–604. doi: 10.1038/ncb3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibson W.T., Veldhuis J.H., Gibson M.C. Control of the mitotic cleavage plane by local epithelial topology. Cell. 2011;144:427–438. doi: 10.1016/j.cell.2010.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lye C.M., Blanchard G.B., Sanson B. Mechanical coupling between endoderm invagination and axis extension in Drosophila. PLoS Biol. 2015;13:e1002292. doi: 10.1371/journal.pbio.1002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez-Gonzalez R., Simoes Sde. M., Zallen J.A. Myosin II dynamics are regulated by tension in intercalating cells. Dev. Cell. 2009;17:736–743. doi: 10.1016/j.devcel.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bardet P.L., Guirao B., Bellaïche Y. PTEN controls junction lengthening and stability during cell rearrangement in epithelial tissue. Dev. Cell. 2013;25:534–546. doi: 10.1016/j.devcel.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 25.Kasza K.E., Farrell D.L., Zallen J.A. Spatiotemporal control of epithelial remodeling by regulated myosin phosphorylation. Proc. Natl. Acad. Sci. USA. 2014;111:11732–11737. doi: 10.1073/pnas.1400520111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorivodsky M., Lonai P. 4 Fgfr2/b is required for the morphogenic migration of the limb bud ectoderm. J. Anat. 2002;201:418. [PMC free article] [PubMed] [Google Scholar]
- 27.Xu X., Weinstein M., Deng C. Fibroblast growth factor receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8 and FGF10 is essential for limb induction. Development. 1998;125:753–765. doi: 10.1242/dev.125.4.753. [DOI] [PubMed] [Google Scholar]
- 28.Rhee J.M., Pirity M.K., Hadjantonakis A.K. In vivo imaging and differential localization of lipid-modified GFP-variant fusions in embryonic stem cells and mice. Genesis. 2006;44:202–218. doi: 10.1002/dvg.20203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abe T., Kiyonari H., Fujimori T. Establishment of conditional reporter mouse lines at ROSA26 locus for live cell imaging. Genesis. 2011;49:579–590. doi: 10.1002/dvg.20753. [DOI] [PubMed] [Google Scholar]
- 30.Yu K., Xu J., Ornitz D.M. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130:3063–3074. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
- 31.Reid B.S., Yang H., Williams T. Ectodermal Wnt/β-catenin signaling shapes the mouse face. Dev. Biol. 2011;349:261–269. doi: 10.1016/j.ydbio.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wyngaarden L.A., Vogeli K.M., Hopyan S. Oriented cell motility and division underlie early limb bud morphogenesis. Development. 2010;137:2551–2558. doi: 10.1242/dev.046987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu H., Sun Y., Simmons C.A. Determination of local and global elastic moduli of valve interstitial cells cultured on soft substrates. J. Biomech. 2013;46:1967–1971. doi: 10.1016/j.jbiomech.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Dimitriadis E.K., Horkay F., Chadwick R.S. Determination of elastic moduli of thin layers of soft material using the atomic force microscope. Biophys. J. 2002;82:2798–2810. doi: 10.1016/S0006-3495(02)75620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sneddon I.N. The stress on the boundary of an elastic half-plane in which body forces are acting. Proceedings of the Glasgow Mathematical Association. 1965;7:48–54. [Google Scholar]
- 36.Ohler B. Cantilever spring constant calibration using laser Doppler vibrometry. Rev. Sci. Instrum. 2007;78:063701. doi: 10.1063/1.2743272. [DOI] [PubMed] [Google Scholar]
- 37.Wong M.D., Dazai J., Henkelman R.M. Design and implementation of a custom built optical projection tomography system. PLoS One. 2013;8:e73491. doi: 10.1371/journal.pone.0073491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forgacs G., Foty R.A., Steinberg M.S. Viscoelastic properties of living embryonic tissues: a quantitative study. Biophys. J. 1998;74:2227–2234. doi: 10.1016/S0006-3495(98)77932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trickey W.R., Baaijens F.P., Guilak F. Determination of the Poisson’s ratio of the cell: recovery properties of chondrocytes after release from complete micropipette aspiration. J. Biomech. 2006;39:78–87. doi: 10.1016/j.jbiomech.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 40.He B., Doubrovinski K., Wieschaus E. Apical constriction drives tissue-scale hydrodynamic flow to mediate cell elongation. Nature. 2014;508:392–396. doi: 10.1038/nature13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muzumdar M.D., Tasic B., Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 42.Krieg M., Arboleda-Estudillo Y., Heisenberg C.P. Tensile forces govern germ-layer organization in zebrafish. Nat. Cell Biol. 2008;10:429–436. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- 43.Balaban N.Q., Schwarz U.S., Geiger B. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 44.Campàs O., Mammoto T., Ingber D.E. Quantifying cell-generated mechanical forces within living embryonic tissues. Nat. Methods. 2014;11:183–189. doi: 10.1038/nmeth.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Djiane A., Yogev S., Mlodzik M. The apical determinants aPKC and dPatj regulate Frizzled-dependent planar cell polarity in the Drosophila eye. Cell. 2005;121:621–631. doi: 10.1016/j.cell.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 46.Kishikawa M., Suzuki A., Ohno S. aPKC enables development of zonula adherens by antagonizing centripetal contraction of the circumferential actomyosin cables. J. Cell Sci. 2008;121:2481–2492. doi: 10.1242/jcs.024109. [DOI] [PubMed] [Google Scholar]
- 47.Liu H., Wu Z., Chen L. Atypical PKC, regulated by Rho GTPases and Mek/Erk, phosphorylates Ezrin during eight-cell embryocompaction. Dev. Biol. 2013;375:13–22. doi: 10.1016/j.ydbio.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y.C., Khan Z., Wieschaus E.F. Differential positioning of adherens junctions is associated with initiation of epithelial folding. Nature. 2012;484:390–393. doi: 10.1038/nature10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ernst S., Liu K., Lecaudey V. Shroom3 is required downstream of FGF signalling to mediate proneuromast assembly in zebrafish. Development. 2012;139:4571–4581. doi: 10.1242/dev.083253. [DOI] [PubMed] [Google Scholar]
- 50.Harding M.J., Nechiporuk A.V. Fgfr-Ras-MAPK signaling is required for apical constriction via apical positioning of Rho-associated kinase during mechanosensory organ formation. Development. 2012;139:3130–3135. doi: 10.1242/dev.082271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lecaudey V., Cakan-Akdogan G., Gilmour D. Dynamic Fgf signaling couples morphogenesis and migration in the zebrafish lateral line primordium. Development. 2008;135:2695–2705. doi: 10.1242/dev.025981. [DOI] [PubMed] [Google Scholar]
- 52.Nechiporuk A., Raible D.W. FGF-dependent mechanosensory organ patterning in zebrafish. Science. 2008;320:1774–1777. doi: 10.1126/science.1156547. [DOI] [PubMed] [Google Scholar]
- 53.Even-Faitelson L., Ravid S. PAK1 and aPKCζ regulate myosin II-B phosphorylation: a novel signaling pathway regulating filament assembly. Mol. Biol. Cell. 2006;17:2869–2881. doi: 10.1091/mbc.E05-11-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishiuchi T., Takeichi M. Willin and Par3 cooperatively regulate epithelial apical constriction through aPKC-mediated ROCK phosphorylation. Nat. Cell Biol. 2011;13:860–866. doi: 10.1038/ncb2274. [DOI] [PubMed] [Google Scholar]
- 55.Röper K. Anisotropy of crumbs and aPKC drives myosin cable assembly during tube formation. Dev. Cell. 2012;23:939–953. doi: 10.1016/j.devcel.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.David D.J., Tishkina A., Harris T.J. The PAR complex regulates pulsed actomyosin contractions during amnioserosa apical constriction in Drosophila. Development. 2010;137:1645–1655. doi: 10.1242/dev.044107. [DOI] [PubMed] [Google Scholar]
- 57.Rosa A., Vlassaks E., Baum B. Ect2/Pbl acts via Rho and polarity proteins to direct the assembly of an isotropic actomyosin cortex upon mitotic entry. Dev. Cell. 2015;32:604–616. doi: 10.1016/j.devcel.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galkin V.E., Orlova A., Egelman E.H. Actin filaments as tension sensors. Curr. Biol. 2012;22:R96–R101. doi: 10.1016/j.cub.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pouille P.A., Ahmadi P., Farge E. Mechanical signals trigger myosin II redistribution and mesoderm invagination in Drosophila embryos. Sci. Signal. 2009;2:ra16. doi: 10.1126/scisignal.2000098. [DOI] [PubMed] [Google Scholar]
- 60.Guirao B., Rigaud S.U., Bellaïche Y. Unified quantitative characterization of epithelial tissue development. eLife. 2015;4:4. doi: 10.7554/eLife.08519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Etournay R., Popović M., Eaton S. Interplay of cell dynamics and epithelial tension during morphogenesis of the Drosophila pupal wing. eLife. 2015;4:e07090. doi: 10.7554/eLife.07090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bosveld F., Markova O., Bellaïche Y. Epithelial tricellular junctions act as interphase cell shape sensors to orient mitosis. Nature. 2016;530:495–498. doi: 10.1038/nature16970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Limb field ectoderm of WT (Fgfr2f/+;Crect;mTmG) 20 somite embryo without micropipette aspiration, oriented as in Fig. 1 A (anterior down, dorsal to the right). A few examples of cell rearrangements are shaded in color, while the remainder of the field is shown for completeness. In the upper right, DV cell division precipitates formation of a tetrad that resolves along the DV axis. In the lower two examples, DV cell division preciptates the formation of rosettes that resolve along the DV axis.
Limb field ectoderm of conditional Fgfr2 mutant (Fgfr2f/f;Crect;mTmG) 20 somite embryo without micropipette aspiration, oriented as in Fig. 1 A (anterior down, dorsal to the right). The upper rosette resolves in an oblique AP/DV axis. Lower down, cell division occurs along the AP axis and precipitates formation of a rosette that begins to resolve along the AP axis.
Limb field ectoderm of WT (Fgfr2f/+;Crect;mTmG) 20 somite embryo during micropipette aspiration, oriented as in Fig. 1A (anterior down, dorsal to the right). Cell divisions and rearrangements occur at oblique DV angles oriented toward the micropipette aperture out of view in the upper left side of the field.
Limb field ectoderm of conditional Fgfr2 mutant (Fgfr2f/f;Crect;mTmG) 20 somite embryo during micropipette aspiration, oriented as in Fig. 1 A (anterior down, dorsal to the right). Rosettes (upper right) and tetrads resolve along the DV axis of artificial tension.