Abstract
Expanded access to DNA sequencing now fosters ready detection of site-specific human genome alterations whose actual significance requires in-depth functional study to rule in or out disease-causing mutations. This is a particular concern for genomic sequence differences in glycosyltransferases, whose implications are often difficult to assess. A recent whole-exome sequencing study identifies (c.229 C > T) in the GalNAc-4-ST1 glycosyltransferase (CHST8) as a disease-causing missense R77W mutation yielding the genodermatosis peeling skin syndrome (PSS) when homozygous. Cabral et al. (Genomics. 2012;99:202–208) cite this sequence change as reducing keratinocyte GalNAc-4-ST1 activity, thus decreasing glycosaminoglycan sulfation, as the mechanism for this blistering disorder. Such an identification could point toward potential clinical and/or prenatal diagnosis of a harmful medical condition. However, GalNAc-4-ST1 has minimal activity toward glycosaminoglycans, instead modifying terminal β1,4-linked GalNAc on N- and O-linked oligosaccharides on specific glycoproteins. We find expression, processing and catalytic activity of GalNAc-4-ST1 completely equivalent between wild type and (R77W) sulfotransferases. Moreover, keratinocytes have little or no GalNAc-4-ST1 mRNA, indicating that they do not express GalNAc-4-ST1. In addition, loss-of-function of GalNAc-4-ST1 primarily presents as reproductive system aberrations rather than skin effects. These findings, an allele frequency of 0.004357, and a 10-fold difference in prevalence of CHST8 (c.299 C > T, R77W) across different ethnic groups, suggest that this sequence represents a “passenger” distributed polymorphism, a simple sequence variant form of the enzyme having normal activity, rather than a “driver” disease-causing mutation that accounts for PSS. This study presents an example for guiding biomedical research initiatives, as well as medical and personal/family perspectives, regarding newly-identified genomic sequence differences.
Keywords: CHST8, GalNAc-4-ST1, generalized peeling skin syndrome, glycans, mutation, sulfotransferase
Introduction
The increasing availability and affordability of DNA sequencing now allows the ready detection of site-specific genome alterations in particular proteins in humans at the individual level. Determining the actual significance of codon differences at a given locus is a far more complex task, one that may bear human health as well as societal implications if the differences were initially ascertained because of a clinical presentation. Accurate assessment of protein functional behavior under these circumstances thus is critical. Depending on how debilitating the condition is that prompted pursuit of a genetic basis, or how amenable it is to intervention, patient and family courses of action may range from seeking practical elements of support, to identifying innovative therapies, to pursuing prenatal diagnosis and difficult decisions regarding pregnancy.
The field of glycobiology has generated excellent examples of therapeutic intervention yielding positive outcomes for certain glycosylation-based disorders of genetic origin (Freeze et al. 2014; Hansen et al. 2015). These achievements are rooted in a comprehensive accurate understanding of how oligosaccharide structures are built at the cellular and molecular levels, integrating the genetic basis for glycosylation disorders in terms of genes encoding enzymatic mechanisms that generate such oligosaccharide structures. For example, synthesis of unique sulfated N-linked glycans on pituitary glycoprotein hormones such as luteinizing hormone (LH) and thyroid stimulating hormone (TSH) first requires the action of protein-specific β1,4-N-acetylgalactosaminyl-transferases (β4GalNAcT3 and/or β4GalNAcT4) that generate terminal LacdiNAc sequences (GalNAcβ1,4-GlcNAcβ) on N-linked glycans of certain proteins. The latter are then substrates for sulfate addition by N-acetylgalatosamine-4-sulfotransferase-1 (GalNAc-4-ST1), encoded by the gene CHST8 that is highly expressed in the pituitary gland (Smith and Baenziger 1988, 1990; Baenziger and Green 1991; Okuda et al. 2000; Xia et al. 2000; Hiraoka et al. 2001; Kang et al. 2001; Mi et al. 2008; Fiete et al. 2012a, 2012c).
GalNAc-4-ST1 displays little if any activity toward glycosaminoglycan structures such as terminal β-linked GalNAc in the context of either chondroitin or dermatan (Okuda et al. 2000; Xia et al. 2000). Nonetheless, Cabral et al. (2012) have stated that a mutation in the CHST8 gene accounts for an autosomal recessive genodermatosis, generalized peeling skin syndrome (PSS), in a large consanguineous family. The missence mutation (c.229 C > T) converts the R at residue 77 to W in GaNAc-4-ST1, reportedly reducing expression of GalNAc-4-ST1 and total sulfated glycosaminoglycans (GAG) by keratinocytes transfected with wild type vs. (R77W) mutant GalNAc-4-ST1. However, the R77W mutation is not located in the catalytic domain of GalNAc-4-ST1. This mutation would not be predicted to have any impact on GalNAc-4-ST1 activity. Furthermore, altered levels of GalNAc-4-ST1 activity would in any case be predicted to not have an impact on levels of GAGs but instead to alter modification of preferred substrates, N- and O-linked glycans on glycoproteins such as LH, TSH and adrenocorticotropic hormone, a functional profile that does not match that reported for PSS.
In the interests of clarity we have applied to this problem our chimeric glycosylation substrate probe strategy for defining the parameters of glycosyltransferase activity in vivo and in vitro (Fiete et al. 2012a, 2012c). We have previously utilized chimeric glycoproteins consisting of a secreted form of luciferase (Gaussia luciferase, GLuc), a substrate protein or peptide of interest, and a peptide recognition determinant, to determine the protein-specific modification of N- and O-linked glycans with β1,4-linked GalNAc by the protein-specific transferases β4GalNT3 and β4GalNT4 in vivo and in vitro (Fiete et al. 2012a, 2012c). The fraction of each chimera modified with β1,4-linked GalNAc is quantified by assaying the amount of GLuc activity that is bound by Wisteria floribunda agglutinin (WFA), a lectin that specifically binds glycans terminating with β1,4-linked GalNAc. Modification of terminal β1,4-linked GalNAc with SO4 or sialic acid prevents binding by WFA, making it possible to assay GalNAc-4-ST1 activity based on reduced binding to immobilized WFA. In assessing the properties of the wild type and the c.229 C > T, R77W mutated form of GalNAc-4-ST1 using chimeric glycosylation substrate probes, we now show that neither the processing or the catalytic activity of GalNAc-4-ST1 is altered by the R77W mutation.
Results
Expression of GalNAc-4-ST1 and GalNAc-4-ST1(R > W)
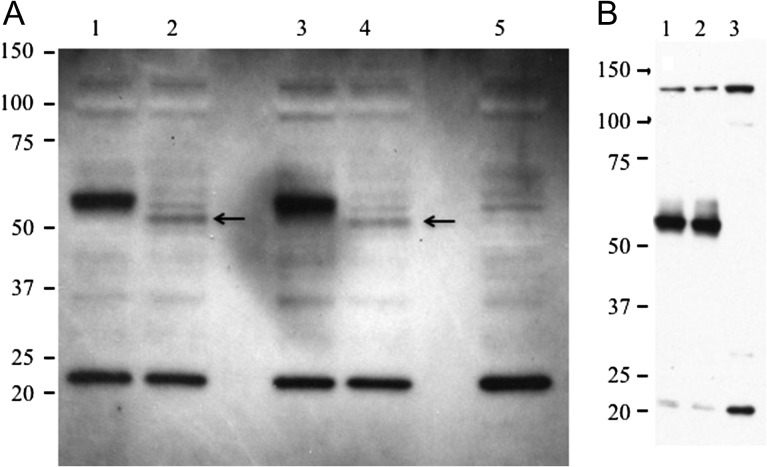
The C > T mutation described by Cabral et al. (2012) was introduced into wild type GalNAc-4-ST1 and GalNAc-4-ST1V5His with a V5His epitope tag added to the carboxyterminus to generate GalNAc-4-ST1(R > W) and GalNAc-4-ST1(R > W)V5His, respectively. Wild type and (R > W) mutant forms were expressed in HEK-293 T and HaCat cells. Equal aliquots of cell extracts were fractionated by SDS-PAGE and analyzed by Western blot using a rabbit polyclonal antibody that we raised to recombinant GalNAc-4-ST1 expressed in bacteria (Figure 1A), and a Sigma Prestige rabbit polyclonal antibody (HPA016004) raised to a peptide from the stem region (Figure 1B). A single band migrating with an Mr of ~55,000 was present for GalNAc-4-ST1 and for GalNAc-4-ST1(R > W). GalNAc-4-ST1V5His and GalNAc-4-ST1(R > W)V5His were also indistinguishable. The epitope tagged forms migrated with a higher Mr reflecting the presence of the epitope tag, which contributes 2558 daltons to the molecular weight (compare panel A lanes 1 and 3 with lanes 2 and 4). Identical results were obtained using the antibody raised to recombinant GalNAc-4-ST1, the Sigma Prestige antibody and the anti-His antibody for the epitope tagged forms (not shown). No differences in the expression or processing of GalNAc-4-ST1 and GalNAc-4-ST1(R > W) were observed. It should be noted that the Sigma Prestige antibody does react with material migrating with an Mr of ~140,000 (Figure 1B).
Fig. 1.
Western blot analysis of GalNAc-4-ST1, GalNAc-4-ST1(R > W), GalNAc-4-ST1-V5His and GalNAc-4-ST1(R > W)V5His expressed in HEK-293T cells. HEK-293T cells were transfected with pcDNA3.1 encoding GalNAc-4-ST1, GalNAc-4-ST1(R > W), GalNAc-4-ST1-V5His, or GalNAc-4-ST1(R > W)V5His. Cells were washed, lysed using SDS-PAGE loading buffer and equal aliquots analyzed by SDS-PAGE. Following electrophoretic transfer to PVDF membranes the GalNAc-4-ST1 was detected by incubation with a rabbit anti-GalNAc-4-ST1 raised to purified recombinant GalNAc-4-ST1 (A) or with Sigma Prestige rabbit polyclonal anti-CHST8 (HPA016004) (B). (A) Lane 1, GalNAc-4-ST1-V5His; lane 2, GalNAc-4-ST1; lane 3, GalNAc-4-ST1(R > W)V5His; and lane 4, GalNAc-4-ST1(R > W). Lane 5, Vector control. (B) Lane 1, GalNAc-4-ST1; lane 2. GalNAc-4-ST1(R > W); and lane 3, vector control. The position of GalNAc-4-ST1 in Lane 2 and GalNAc-4-ST1(R > W) in Lane 4 is indicated by the arrows. The location of molecular weight standards is indicated.
GalNAc-4-ST1 and GalNAc-4-ST1(R > W) both add sulfate to LacdiNAc-containing substrates in vivo
We have shown that GalNAc-4-ST1 transfers SO4 to nonreducing terminal LacdiNAc on certain glycoproteins’ N- and O-linked glycans (Fiete et al. 2012a, 2012c). Although secreted forms of GalNAc-4-ST1 are able to transfer SO4 to chondroitin in vitro, the rate of transfer to glycans terminating with LacDiNAc is 21-fold greater than to chondroitin. Membrane anchored forms of GalNAc-4-ST1 present in cell extracts transfer SO4 to glycans terminating with LacDiNAc at a rate that is >170-fold higher than to chondroitin (Okuda et al. 2000; Xia et al. 2000). Thus, chondroitin represents at best a poor substrate for GalNAc-4-ST1. We have generated glycoprotein substrate probes derived from proteins such as carbonic anhydrase VI (CA) and pregnancy specific glycoprotein 23 (PSG23), each bearing N-linked glycans that are selectively modified with β1,4-linked GalNAc when expressed in the presence of either β4GalNT3 or β4GalNT4. Both of these enzymes recognize specific peptide sequences within these glycan-bearing proteins (Smith and Baenziger 1988, 1990; Fiete et al. 2012a, 2012c), and their enzymatic action produces N-linked glycan substrates for subsequent modification by GalNAc-4-ST1 (Fiete et al. 2012a, 2012c; Mi et al. 2014). For example, N-Linked glycans on the substrate GLuc-alpha-CA1-19 are extensively modified with LacdiNAc when expressed in HEK-293 T cells. WFA binds LacdiNAc-bearing glycans; however, this binding is prevented by the addition of sulfate (Mi et al. 2014), thus providing a means to assess whether glycans are terminally sulfated or not.
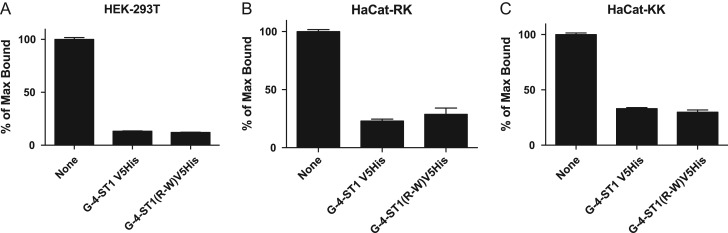
HEK-293 T cells and two clones of HaCat keratinocytes were transfected with GLuc-alpha-CA1-19 alone or in combination with GalNAc-4-ST1V5His or GalNAc-4-ST1(R > W)V5His. In the case of HEK-293 T cells, GalNAc-4-ST1V5His and GalNAc-4-ST1(R > W)V5His reduced the amount of GLuc-alpha-CA1-19 that could be captured by immobilized WFA 10-fold (Figure 2A), indicting that the wild type and mutant forms of GalNAc-4-ST1 were both able to modify the terminal GalNAc with SO4. When expressed in HaCat cells the amount of GLuc-alpha-CA1-19 that could be captured by WFA was between 1/4th and 1/2 of that which could be captured when expressed in HEK-293 T cells. Thus either the level of β4GalNAc transferase activity in HaCat cells is lower than in HEK-293 T cells and/or the LacdiNAc produced is further modified. In either case co-expression of either GalNAc-4-ST1-V5His or GalNAc-4-ST1(R > W)V5His reduced binding by WFA to similar degrees (Figure 2B and C), indicating that both wild-type and mutant forms of GalNAc-4-ST1 were active when expressed in HaCat keratinocytes.
Fig. 2.
GalNAc-4-ST1 and GalNAc-4-ST(R > W) are active when expressed in HEK-293T and HaCat cells. HEK-293T, HaCat-RK and HaCat-KK were each cotransfected with pCMV-GLuc-PSG23-MycHis and either pcDNA3.1 vector, pcDNA3.1-GalNAc-4-ST1 or pcDNA3.1-GalNAc-4-ST1(R > W). Aliquots of medium containing equal light units of Gaussia luciferase activity were incubated with immobilized WFA in 96-well plates. After washing, the amount of luciferase activity bound was determined as described previously (Fiete et al. 2012a). The amount bound in the absence of cotransfected GalNAc-4-ST1 was designated as 100%. The amount of GLuc-PSG23 bound when GalNAc-4-ST1 or GalNAc-4-ST1(R > W) was cotransfected is indicated as a percent of that bound in the absence of GalNAc-4-ST1. Assay were performed in triplicate and the SEM is indicated.
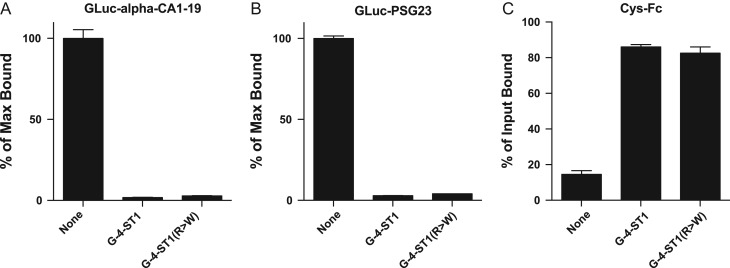
We previously generated CHO cells, CHO/T3, that stably express β4GalNAc-T3 (Miller et al. 2008; Fiete et al. 2012a). Further transfection of these stable transfectants with either GLuc-alpha-CA1-19 (Figure 3A) or GLuc-PSG23 (Figure 3B) yielded secreted glycoproteins in the culture medium with 2 and 7-N-linked glycans, respectively, that were fully modified with unsubstituted LacdiNAc termini (Fiete et al. 2012a, 2012c; Mi et al. 2014). Co-expression of either GalNAc-4-ST1 or GalNAc-4ST1(R > W) in these CHO/T3 cells completely abolished WFA binding, indicating both the wild type and the mutant forms of GalNAc-4-ST1 were able to modify all available LacdiNAc termini with SO4. Since GLuc-PSG23 has between 14 and 21 unsubstituted LacdiNAc termini and a single LacdiNAc terminus is sufficient to mediate binding by WFA, both GalNAc-4-ST1 and GalNAc-4ST1(R > W) must be highly efficient, making it unlikely that overexpression is masking a difference in catalytic activity.
Fig. 3.
GalNAc-4-ST1 and GalNAc-4-ST1(R > W) modify N-glycans bearing LacdiNAc on GLuc-alpha-CA1-19 and GLuc-PSG23 with terminal sulfate. CHO/T3 cells were cotransfected with pCMV-GLuc-PSG23-MycHis or pCMV-GLuc-alpha-CA1-19 and either pcDNA3.1 vector, pcDNA3.1-GalNAc-4-ST1 or pcDNA3.1-GalNAc-4-ST1(R > W). GLuc-alpha bears 2 N-linked glycans and GLuc-PSG23 7 N-linked glycans that can be modified with LAcdiNAc termini. Aliquots of medium containing equal light units of Gaussia luciferase activity were incubated with immobilized WFA (A and B). The amount of GLuc-alpha (A) and GLuc-PSG23 (B) bound in the absence of GalNAc-4-ST1 or GalNAc-4-ST1(R > W) was designated as 100% (None). The amount of GLuc-alpha and GLuc-PSG23 bound when expressed in the presence of GalNAc-4-ST1 or GalNAc-4-ST1(R > W) is presented as a percent of the maximum bound. In Panel C aliquots containing equal light units of GLuc-PSG23 activity were incubated with Cys-Fc immobilized on magnetic beads. The percent of the activity not captured by the Cys-Fc was used to determine the percent of the input bound in the absence of GalNAc-4-ST1 (none) and in the presence of GalNAc-4-ST1 or GalNAc-4-ST1(R > W). Assay were performed in triplicate and error bars designate the SEM.
We have here shown that Cys-Fc, a chimeric protein consisting of the cysteine-rich domain of the macrophage Mannose-Receptor and the constant region of human IgG, binds glycoproteins bearing 2 or more glycans with terminal SO4-4-GalNAcβ1,4GlcNAc (Fiete et al. 1998; Roseman and Baenziger 2000, 2001). In excess of 80% of GLuc-PSG23 secreted into the culture medium of CHO/T3 cells expressing either GalNAc-4-ST1 or GalNAc-4ST1(R > W) (Figure 3C) was bound by immobilized Cys-Fc, confirming that the LacDiNAc moieties had been modified with 4-linked SO4. Thus GalNAc-4-ST1 and GalNAc-4ST1(R > W) are both capable of fully modifying the terminal GalNAc on the N-glycans on GLuc-PSG23 with 4-linked SO4.
GalNAc-4-ST1 mRNA levels in mouse and human keratinocytes
Based on dot blots of multiple human tissues, expression of GalNAc-4-ST1 is significantly higher in pituitary than other tissues (Okuda et al. 2000; Xia et al. 2000); however, human keratinocytes have not been specifically examined for GalNAc-4-ST1 expression. Conflicting results have been reported for the relative expression of GalNAc-4-ST1 and GalNAc-4-ST2 in mouse pituitary using different methods (Okuda et al. 2003; Boregowda et al. 2005). Nonetheless, ablation of GalNAc-4-ST1 results in a loss of luteinizing hormone (LH) glycan sulfation in mouse pituitary. As a consequence the glycans on LH are substituted with sialic acid in place of sulfate; the circulatory half-life of LH is thereby prolonged resulting in elevated levels of estrogen and testosterone in female and male mice, respectively. Resulting phenotypic changes in these mice include precocious sexual maturation, seminal vesicle and uteri enlargement and increased fecundity (Mi et al. 2008). No obvious changes of the dermis were observed.
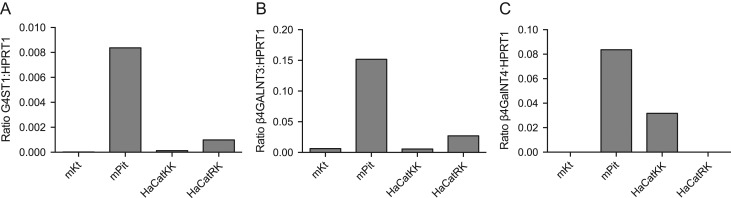
Since β4GalNT3 and β4GalNT4 are the protein-specific β1,4-N-acetylgalactosaminyltransferases that synthesize the LacdiNAc termini on glycoproteins such as LH that are the acceptors for sulfate addition by GalNAc-4-ST1 (Fiete et al. 2012b), we used Taqman to compare GalNAc-4-ST1, β4GalNT3 and β4GalNT4 mRNA levels in primary mouse and human keratinocytes and in two clones of a human keratinocyte cell line HaCat. Figure 4 shows the relative levels of GalNAc-4-ST1, β4GalNT3 and β4GalNT4 message compared to Hprt1, which was used as an endogenous control, for mouse primary keratinocytes, mouse pituitary and human HaCat keratinocytes. Mouse keratinocytes did not have detectable levels of GalNAc-4-ST1, β4GalNT3 or β4GalNT4 message when compared to expression levels in the pituitary (Figure 4). Similarly the human keratinocyte clones HaCat-KK and HaCat-RK expressed at best only low levels of GalNAc-4-ST1. Taqman was also used to compare the Ct values for a second endogenous message, GAPDH and for GalNAc-4-ST1 per 1000 ng of cDNA prepared from HaCat cells and primary cultures of keratinocytes. The Ct values for GAPDH were 16.25 for HaCat and 15.50 for primary human keratinocytes whereas the Ct values for GalNAc-4-ST1 were 33.18 and 32.94, respectively, indicating that GalNAc-4-ST1 is expressed at very low levels or not at all in human keratinocytes.
Fig. 4.
Levels of GalNAc-4-ST1 (CHST8) mRNA are low in mouse and human keratinocytes as compared to mouse pituitary. The steady state levels of GalNAc-4-ST1 (CHST8) (A), β4GalNT3 (B4GALNT3) (B) and β4GalNT4 (B4GALNT4) (C) relative to HPRT1 were determined for mouse primary keratinocytes (mKt), mouse pituitary (mPit), human keratinocyte cells lines HaCatKK and HaCatRK using Taqman as described previously (Mi et al. 2014). Assays were performed in triplicate.
Discussion
The CHST8 (c.299C > T, R77W) mutation, located in the hydrophilic stem region of GalNAc-4-ST1 in close proximity to the transmembrane domain (Xia et al. 2000), has the potential to alter post-translational processing of GalNAc-4-ST1 but is not likely to have a direct impact on the catalytic domain of the transferase. Our studies reveal no difference in the post-translational processing of GalNAc-4-ST1 and GalNAc-4-ST1(R77W) expressed in either HEK-293 cells or in a human keratinocyte cell line HaCat. Furthermore, the amounts of GalNAc-4-ST1 and GalNAc-4-ST1(R77W) that were present in transfected cells were not significantly different. The previously reported altered post-translational processing of the GalNAc-4-ST1(R77W) (Cabral et al. 2012) could not be confirmed in either of these human cell lines.
Analysis by our chimeric glycosylation probe strategy revealed that, when co-expressed with two different substrates bearing N-linked glycans terminating in β1,4-linked GalNAc, both GalNAc-4-ST1 and GalNAc-4-ST1(R77W) reduced binding by WFA to the same extent when expressed in CHO/T3 cells, human embryonic kidney cells and human keratinocytes. Both forms of the sulfotransferase were highly efficient when expressed in cultured cells, since virtually all binding to WFA was abolished, indicating that all LacdiNAc termini were modified. In addition >80% of the chimeric glycoprotein probe GLuc-PSG23, which has 9 N-linked glycans, was captured by immobilize Cys-Fc, which specifically binds terminal GalNAc-4-SO4 (Fiete et al. 1998; Roseman and Baenziger 2000). Thus SO4 was transferred to terminal GalNAc on N-linked glycans to the same extent by both GalNAc-4-ST1 and GalNAc-4-ST1(R77W). GalNAc-4-ST1(R77W) appears to be fully active against well-established LacdiNAc-bearing substrates.
It is unlikely that the GalNAc-4-ST1(R77W) mutation accounts for the recessive PSS as reported by Cabral et al. (2012). There is no evidence that GalNAc-4-ST1 (CHST8) is expressed in skin, and GalNAc-4-ST1 displays little if any activity with chondroitin as a substrate, compared to glycoproteins bearing either N- or O-linked glycans terminating with LacdiNAc (Xia et al. 2000). The specificity of the Sigma Prestige antibody against GalNAc-4-ST1 (CHST8) has not been extensively documented. It is not clear that this antibody has a sufficient titer or specificity to be used for immunohistochemistry, an approach employed by Cabral et al. (2012) in lieu of direct measurements of enzyme activity on well-characterized substrates. It is far more likely that individuals with genuinely altered GalNAc-4-ST1 activity would present with hormonal abnormalities related to reproduction, rather than the observed skin abnormalities.
Cabral et al. (2012) have cited the correlation of the GalNAc-4-ST1(R77W) mutation with recessive PSS as a causative one defining a new genetic determinant of epidermal differentiation, based on its presence in a single large consanguineous Pakistani family and the apparent absence of this mutation in sequences of 200 unrelated healthy controls of Pakistani origin. However, the CHST8 (c.299C > T, R77W) mutation overall is represented in 65 of 12931 alleles deposited on the NHLBI exome variant server as part of the exome sequencing project and is represented in 504 of 115,674 alleles in the exac.broadinstitute.org database. This known sequence change shows a variable degree of prevalence in different ethnic groups in the NHLBI database, ranging from 0.068% among African American alleles (3/4395) to 0.73% of European American alleles (62/8536). The 10-fold difference in frequency among different populations means that 200 control individuals do not represent a sample size sufficient to rule the c.299C > T, R77W sequence in or out as a disease-causing mutation vs. a sequence variant with no clear-cut link to a pathogenic process. Although recessive PSS is cited as asymptomatic (Cabral et al. 2012), not a significant source of morbidity and mortality, it serves as a reminder for the importance of accurately characterizing the genetic bases of enzymatic dysfunctions whose consequences may be far more serious.
Materials and methods
Anti-GalNAc-4-ST1
Recombinant GalNAc-4-ST1 that was expressed in Escherichia coli and purified from inclusion bodies was used to immunize rabbits. Sera containing polyclonal anti-GalNAc-4-ST1 was used without further purification. The Prestige rabbit polyclonal antibody (HPA016004) raised to a peptide from the stem region was purchased from Sigma Chemicals, St. Louis.
Mutagenesis
Overall, 10 cycles of PCR using Klentaq Long and Accurate DNA polymerase (Barnes 1994; Cheng et al. 1994) with the primers 5′-acagagagggtcactTgggacttatccagtgggg-3′ and 5′-ccccactggataagtcccA-3′ were used to introduce the C > T missense mutation into pcDNA3.1-GalNAc-4-ST1 and pcDNA3.1- GalNAc-4-ST1V5His to generate pcDNA3.1- GalNAc-4-ST1(R > W) and pcDNA3.1- GalNAc-4-ST1(R > W)V5His. Introduction of the mutation was confirmed by DNA sequencing.
Constructs
The chimeric glycoprotein substrates GLucα(PLRSKK)CA1-19 and GLuc-CTP-(CA1-19)Myc-His were prepared as expressed as described (Fiete et al. 2012a, 2012c). pCMV-GLuc-PSG23-MycHis encoding a chimeric glycoprotein consisting of Gaussia luciferase followed by Pregnancy Specific Glycoprotein 23 (PSG23) beginning with the Ser at position 35 and followed by the epitope tag MycHis was prepared by ribocloning as described (Fiete et al. 2012a, 2012c)
Assays
Addition of β1,4-linked GalNAc to N- and O-linked glycans on GLuc chimeras expressed in HaCat, HEK-293T, CHO/T3 and CHO cells or subject to in vitro assays was characterized by capture onto 96 well plates coated with WFA, a lectin that binds glycans terminating with GalNAcβ1,4-GlcNAcβ, as described previously (Miller et al. 2008; Fiete et al. 2012a, 2012c). Transfer of SO4 to GalNAcβ1,4-GlcNAcβ by GalNAc-4-ST1 (CHST8) in vivo or in vitro was assessed by quantitating the decrease in binding by WFA. For all Gaussia luciferase assays the input of chimeric glycoprotein with respect to light units of luciferase activity was identical. We previously demonstrated that the R-type lectin domain (Cys-rich domain) present at the carboxyterminus of the macrophage Mannose-receptor (Liu et al. 2000) binds terminal β1,4-linked GalNAc-4-SO4 on N-linked glycans (Fiete et al. 1997, 1998; Roseman and Baenziger 2000, 2001). Therefore we also assessed the presence of terminal SO4-4-GalNAcβ1,4-GlcNAcβ by binding GLuc chimeric glycoproteins bearing modified glycans to Cys-Fc immobilized on Protein-G magnetic beads.
Transfections
pcDNA3.1- and pCMV-GLuc- contructs were expressed in human immortalized keratinocytes, HaCat (Boukamp et al. 1988), HEK-293T (Pear et al. 1993) and β4GT3/CHO Flp-InTM (Miller et al. 2008) grown in serum free medium as described previously (Fiete et al. 2012a).
Conflict of interest statement
None declared.
Funding
National Institutes of Health (grants R01-CA21923 and R01-HD058474 to J.U.B.)
Abbreviation
LH, luteinizing hormone; PSS, peeling skin syndrome; TSH, thyroid stimulating hormone; WFA, Wisteria floribunda agglutinin.
References
- Baenziger JU, Green ED. 1991. Structure, synthesis, and function of the asparagine-linked oligosaccharides on pituitary glycoprotein hormones In: Ginsberg V, Robbins PW, editors. Biology of Carbohydrates. Vol. 3, London, JAI Press Ltd, p. 1–46. [Google Scholar]
- Barnes WM. 1994. PCR amplification of up to 35-kb DNA with high fidelity and high yield from lambda bacteriophage templates. Proc Natl Acad Sci USA 91(6):2216–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boregowda RK, Mi Y, Bu H, Baenziger JU. 2005. Differential expression and enzymatic properties of GalNAc-4-sulfotransferase-1 and GalNAc-4-sulfotransferase-2. Glycobiology. 15(12):1349–1358, doi:cwj024. [pii] 10.1093/glycob/cwj024. [DOI] [PubMed] [Google Scholar]
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 106(3):761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral RM, Kurban M, Wajid M, Shimomura Y, Petukhova L, Christiano AM. 2012. Whole-exome sequencing in a single proband reveals a mutation in the CHST8 gene in autosomal recessive peeling skin syndrome. Genomics. 99(4):202–208, doi:S0888-7543(12)00006-7. [pii] 10.1016/j.ygeno.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Fockler C, Barnes WM, Higuchi R. 1994. Effective amplification of long targets from cloned inserts and human genomic DNA. Proc Natl Acad Sci USA 91(12):5695–5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiete D, Beranek M, Baenziger JU. 2012. a. Molecular basis for protein-specific transfer of N-acetylgalactosamine to N-linked glycans by the glycosyltransferases β1,4-N-acetylgalactosaminyl transferase 3 (β4GalNAc-T3) and β4GalNAc-T4. J Biol Chem. 287(34):29194–29203, doi:M112.371567. [pii] 10.1074/jbc.M112.371567 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiete D, Beranek M, Baenziger JU. 2012. b. Molecular basis for protein-specific transfer of N-acetylgalactosamine to N-linked glycans by the glycosyltransferases β4GalNAc-T3 and β4GalNAc-T4. J Biol Chem. 287:29194–29203, doi:M112.371567. [pii] 10.1074/jbc.M112.371567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiete D, Beranek M, Baenziger JU. 2012. c. Peptide-specific transfer of N-acetylgalactosamine to O-linked glycans by the glycosyltransferases β1,4-N-acetylgalactosaminyl transferase 3 (β4GalNAc-T3) and β4GalNAc-T4. J Biol Chem. 287(34):29204–29212, doi:M112.371880. [pii] 10.1074/jbc.M112.371880 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiete D, Beranek MC, Baenziger JU. 1997. The macrophage/endothelial cell mannose receptor cDNA encodes a protein that binds oligosaccharides terminating with SO4-4-GalNAcβ1,4GlcNAcβ or Man at independent sites. Proc Natl Acad Sci USA. 94(21):11256–11261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiete DJ, Beranek MC, Baenziger JU. 1998. A cysteine-rich domain of the “mannose” receptor mediates GalNAc-4-SO4 binding. Proc Natl Acad Sci USA. 95(5):2089–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze HH, Chong JX, Bamshad MJ, Ng BG. 2014. Solving glycosylation disorders: Fundamental approaches reveal complicated pathways. Am J Hum Genet. 94(2):161–175, doi:10.1016/j.ajhg.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen L, Lind-Thomsen A, Joshi HJ, Pedersen NB, Have CT, Kong Y, Wang S, Sparso T, Grarup N, Vester-Christensen MB, Schjoldager K et al. 2015. A glycogene mutation map for discovery of diseases of glycosylation. Glycobiology. 25(2):211–224, doi:10.1093/glycob/cwu104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka N, Misra A, Belot F, Hindsgaul O, Fukuda M. 2001. Molecular cloning and expression of two distinct human N-acetylgalactosamine 4-O-sulfotransferases that transfer sulfate to GalNAcß14GlcNAcß1R in both N- and O-glycans. Glycobiology. 11:495–504. [DOI] [PubMed] [Google Scholar]
- Kang HG, Evers MR, Xia G, Baenziger JU, Schachner M. 2001. Molecular cloning and expression of an N-acetylgalactosamine-4-O-sulfotransferase that transfers sulfate to terminal and non-terminal β 1,4-linked N-acetylgalactosamine. J Biol Chem. 276(14):10861–10869, doi:10.1074/jbc.M011560200. [doi] M011560200 [pii]. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chirino AJ, Misulovin Z, Leteux C, Feizi T, Nussenzweig MC, Bjorkman PJ. 2000. Crystal structure of the cysteine-rich domain of mannose receptor complexed with a sulfated carbohydrate ligand. J Exp Med. 191(7):1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi Y, Fiete D, Baenziger JU. 2008. Ablation of GalNAc-4-sulfotransferase-1 enhances reproduction by altering the carbohydrate structures of luteinizing hormone in mice. J Clin Invest. 118(5):1815–1824, doi:10.1172/JCI32467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi Y, Lin A, Fiete D, Steirer L, Baenziger JU. 2014. Modulation of mannose and asialoglycoprotein receptor expression determines glycoprotein hormone half-life at critical points in the reproductive cycle. J Biol Chem. 289(17):12157–12167, doi:10.1074/jbc.M113.544973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E, Fiete D, Blake NM, Beranek M, Oates EL, Mi Y, Roseman DS, Baenziger JU. 2008. A necessary and sufficient determinant for protein-selective glycosylation in vivo. J Biol Chem. 283(4):1985–1991, doi:M708160200. [pii] 10.1074/jbc.M708160200 [doi]. [DOI] [PubMed] [Google Scholar]
- Okuda T, Mita S, Yamauchi S, Fukuta M, Nakano H, Sawada T, Habuchi O. 2000. Molecular cloning and characterization of GalNAc 4-sulfotransferase expressed in human pituitary gland. J Biol Chem. 275(51):40605–40613, doi:10.1074/jbc.M007983200. [doi] M007983200 [pii]. [DOI] [PubMed] [Google Scholar]
- Okuda T, Sawada T, Nakano H, Matsubara K, Matsuda Y, Fukuta M, Habuchi O. 2003. Mouse N-acetylgalactosamine 4-sulfotransferases-1 and -2. Molecular cloning, expression, chromosomal mapping and detection of their activity with GalNAcbeta1-4GlcNAcbeta1-octyl. J Biochem. 134(1):111–120. [DOI] [PubMed] [Google Scholar]
- Pear WS, Nolan GP, Scott ML, Baltimore D. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 90(18):8392–8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman DS, Baenziger JU. 2000. Molecular basis of lutropin recognition by the mannose/GalNAc-4-SO4 receptor. Proc Natl Acad Sci USA. 97(18):9949–9954, doi:10.1073/pnas.170184597. 170184597 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman DS, Baenziger JU. 2001. The mannose/N-acetylgalactosamine-4-SO4 receptor displays greater specificity for multivalent than monovalent ligands. J Biol Chem. 276(20):17052–17057, doi:10.1074/jbc.M101027200. M101027200 [pii]. [DOI] [PubMed] [Google Scholar]
- Smith PL, Baenziger JU. 1988. A pituitary N-acetylgalactosamine transferase that specifically recognizes glycoprotein hormones. Science. 242(4880):930–933. [DOI] [PubMed] [Google Scholar]
- Smith PL, Baenziger JU. 1990. Recognition by the glycoprotein hormone-specific N-acetylgalactosaminetransferase is independent of hormone native conformation. Proc Natl Acad Sci USA. 87(18):7275–7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia G, Evers MR, Kang HG, Schachner M, Baenziger JU. 2000. Molecular cloning and expression of the pituitary glycoprotein hormone N-acetylgalactosamine-4-O-sulfotransferase. J Biol Chem. 275(49):38402–38409, doi:10.1074/jbc.M007821200 . [doi] M007821200 [pii]. [DOI] [PubMed] [Google Scholar]