Abstract
There are no commonly used clinical indicators of whether an individual will benefit from cognitive therapy (CT) for depression. A prior study found right ear (left hemisphere) advantage for perceiving dichotic words predicted CT response. This study replicates this finding at a different research center in clinical trials that included clinically representative samples and community therapists. Right-handed individuals with unipolar major depressive disorder who subsequently received 12–14 weeks of CT at the University of Pittsburgh were tested on dichotic fused words and complex tones tests. Responders to CT showed twice the mean right ear advantage in dichotic fused words performance than non-responders. Patients with a right ear advantage greater than the mean for healthy controls had an 81% response rate to CT, whereas those with performance lower than the mean for controls had a 46% response rate. Individuals with a right ear advantage, indicative of strong left hemisphere language dominance, may be better at utilizing cognitive processes and left frontotemporal cortical regions critical for success of CT for depression. Findings at two clinical research centers suggest that verbal dichotic listening may be a clinically disseminative brief, inexpensive and easily automated test prognostic for response to CT across diverse clinical settings.
Keywords: Depression, cognitive therapy, treatment response, dichotic listening, laterality
1. Introduction
Cognitive therapy (CT) is a widely used evidence-based treatment for depression that is effective in 40% to 60% of patients having a major depressive episode (MDE). There are, however, no commonly used prognostic indicators of whether or not a patient will benefit from CT. This could be due, in part, to the continued absence of indicators with essential characteristics that would lend themselves to clinical dissemination, including: 1) usability in clinical settings, i.e., brief, low cost and low tech administration; 2) strong detection of both likely responders and likely non-responders; 3) published replications across sites. There is growing evidence that neuroimaging indices (Siegle et al. 2012; McGrath et al. 2013; Struab et al. 2015; Burkhouse et al., 2016) have some of these features, e.g., have been replicated and have strong signal detection, but they are likely too expensive and complicated for routine clinical use. There are variables that have been linked to CT outcome and are readily assessed in the clinic, e.g., marital status, age, or comorbid personality disorder (Fournier et al., 2008). Also, sensitive and reliable behavioral tests that could be easily administered would be desirable for clinical translation. Self-report measures of cognitive beliefs or attitudes (Rude & Rehm, 1991; Sotsky et al. 1991), or tests estimating intelligence (Haaga et al. 1991; Fournier et al. 2009) have been examined as potential predictors, but conflicting findings have been reported, and performance on neuropsychological tests of premorbid IQ and psychomotor speed was not associated with CT outcome (Kishon et al. 2015).
One promising candidate for such behavioral predictor is suggested by findings of differences in dichotic listening between patients who respond to CT for depression and those who do not (Bruder et al. 1997; Kishon et al. 2014). In dichotic listening tests, different stimuli (words, syllables or tones) are simultaneously presented to the two ears, and difference in reporting items in the right and left ear provides an index of perceptual asymmetry. These tests can be easily administered by computer with few technical requirements and virtually untrained technician, and take only minutes. Healthy adults show a right ear advantage for verbal stimuli indicative of left hemisphere dominance for language processing and a left ear advantage for nonverbal stimuli indicative of right hemisphere dominance for processing tonal or prosodic information (Bryden, 1982). In two studies, individual differences in right ear advantage for verbal dichotic listening were related to response to CT. In the first study (Bruder et al. 1997), depressed patients who responded to 16 weekly CT sessions (n=15) had more than twice the mean right ear advantage than non-responders (n=12) for perceiving dichotic consonant-vowel syllables, but they did not differ in left ear advantage for complex tones. Moreover, there was no significant difference in perceptual asymmetry between placebo responders and non-responders. The greater right ear advantage in CT responders was recently confirmed in depressed patients who were tested on a dichotic fused-words test before receiving 14 weeks of CT (Kishon et al. 2015). In this test, words differing only in the initial consonant (e.g., coat and goat) are simultaneously presented to the right and left ear. The words fuse to form a single percept and subjects indicate the word they heard. The difference in reporting words in the right and left ear yields valid estimates of hemispheric lateralization for speech, as determined by intracarotid amobarbital injections (Zatorre, 1989; Fernandes & Smith, 2000). Patients who responded to CT (n=11) again had more than twice the mean right ear advantage compared to non-responders (n=9).
Why should right ear advantage for verbal dichotic listening, likely reflecting left hemisphere dominance for speech processing (Bryden, 1982; Hugdahl, 2003), be a predictor for efficacy of CT? Increasing left prefrontal activity is frequently used as a treatment for depression (Triggs et al. 2010; Nadeau et al. 2014; Vanderhasselt et al. 2015). Indeed, a peripheral physiological indicator associated with left dorsolateral prefrontal cortex reactivity was found to predict response to CT in a study with the sample examined here (Siegle et al. 2011) Cognitive therapies are highly verbal treatments that involve self-monitoring and reappraisal of negative thoughts, emotions and cognitive distortions, which may be mediated, at least in part, by verbal skills and activation of left prefrontal and temporal cortex (Otto et al. 1987; Price et al. 2013; Silvers et al. 2015). 2011). Although verbal skills involved in CT and dichotic listening may differ, individuals with greater left hemisphere lateralization may be better able to recruit cortical regions critical for the success of CT (Kishon et al. 2014).
The present study examines whether our previously observed finding of greater right ear advantage for CT responders than non-responders in a dichotic fused-words test (Kishon et al. 2015) replicates in a study at a different clinical center. We tested patients having a major depressive disorder (MDD) in clinical trials at the University of Pittsburgh (Siegle et al. 2012) that included clinically representative samples and community therapists, which is of importance before translation to the clinic. In addition, we included a complex-tones test to confirm our prior finding that prediction of CT response is not found for non-verbal dichotic listening (Bruder et al. 1997). This is important for determining whether prediction of CT response is specific to verbal processing or rather reflects a characteristic favoring of left over right hemisphere activation that cuts across verbal and non-verbal dichotic listening. We hypothesized that CT responders would have greater right ear advantage for dichotic words before treatment compared to CT non-responders. We further hypothesized that the mean right ear advantage for healthy controls in our prior study (Kishon et al. 2015) would again provide a meaningful cutoff for predicting clinical response, and patients with nominally above average right ear advantage would respond best to CT. Given evidence of reduced right ear advantage for dichotic words or syllables in patients having an anxiety disorder (Bruder et al. 2004; Bruder et al. 2015), we also examined whether or not comorbidity of depression with anxiety disorders would impact differences in dichotic listening between CT responders and non-responders.
2. Method
2.1 Participants
As shown in Fig. 1 (CONSORT diagram), 74 participants from clinical trials at the Western Psychiatric Institute and Clinic, University of Pittsburgh were recruited in two cohorts. Cohort 1 was an efficacy study (ClinicalTrials.gov: NCT00183664) and cohort 2 (ClinicalTrials.gov: NCT00787501) was a more ecologically representative effectiveness study (see Siegle et al. 2012 for full details). Data from 21 patients (cohort 1; n=9; cohort 2, n=12) were not analyzed because they had no dichotic listening data (n=10), withdrew from the study (n=1) or dropped out before completing at least 7 sessions of CT (n=8), and 2 patients did not correctly report items in one ear in the dichotic listening tasks (see below) and likely had a hearing loss. The dichotic data for the remaining 53 patients were analyzed in this study (Table 1). The 14 patients in cohort 1 met DSM-IV criteria for recurrent major depressive disorder (MDD) based on the Structured Clinical interview for DSM-IV Axis I Disorders—Patient Edition (SCID; First et al. 1995). These patients were treated by the same 3 therapists from the study of Siegle et al. (2006) who continued to receive weekly supervision with taped review from the same master clinician. Therapeutic adherence was deemed adequate based on a random sample of 10 tapes receiving high marks on the Cognitive Therapy Scale (Young & Beck, 1980). The 39 patients in cohort 2 met criteria for recurrent or first episode MDD and were treated by 6 community clinicians (with PhD, MD, Master of education, or social worker degrees) who ranged in CT experience. Therapists received group supervision monthly and as-requested without taped review and therapeutic adherence based on 12 tapes was more variable (see Siegle et al. 2012).
Fig. 1.
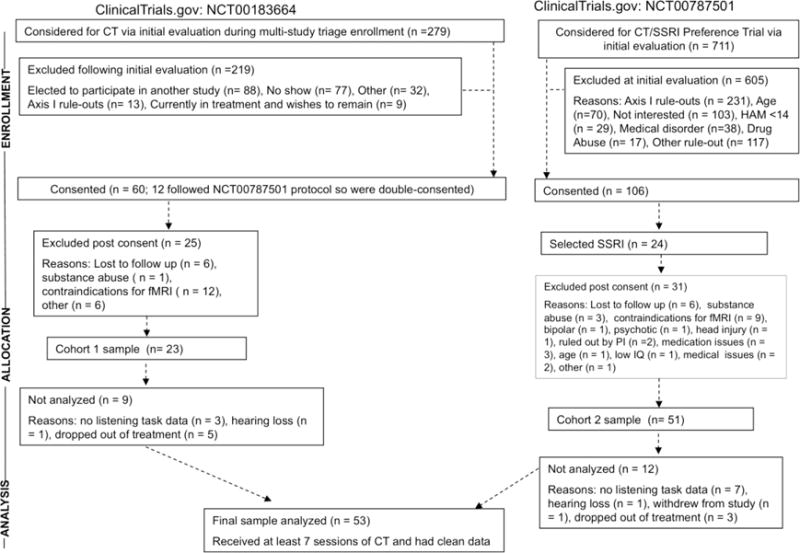
CONSORT diagram for study.
Table 1.
Characteristics of Treatment Responders and Non-Responders.
| Variable | Responders | Non-Responders | Statistics |
|---|---|---|---|
| Gender (men/women) | 8/26 | 6/13 | χ2(1) = 0.40 |
| Age (in years) | |||
| M | 33.9 | 39.9 | t(51) = 2.04* |
| SD | 10.0 | 10.9 | |
| Education (in years) | |||
| M | 15.4 | 15.9 | t(50) = 0.73a |
| SD | 1.9 | 2.7 | |
| Anxiety Disorder (yes/no) | 15/19 | 5/14 | χ2(1) = 1.64 |
| Pre-Treatment HDRS | |||
| M | 21.1 | 20.0 | t(51) = 0.66 |
| SD | 5.2 | 5.7 | |
| Post-Treatment HDRS | |||
| M | 4.5 | 14.0 | t(51) = 8.43*** |
| SD | 4.1 | 3.7 |
HDRS = Hamilton Depression Rating Scale;
Responders: n=34; Non-Responders: n=18;
p<.05;
p<.001
All participants were right handed and reported no health problems or psychoactive drug abuse in the past 6 months and no history of psychosis or manic or hypomanic episodes. None of the patients used antidepressants within 2 weeks of testing (6 weeks if they received fluoxetine) owing to medication naivety or supervised withdrawal from unsuccessful medications. Participants reported no excessive use of alcohol in the 2 weeks before testing and scored in the normal range on a cognitive screen (verbal IQ equivalent ≥85; Bright et al. 2002). All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
2.2 Procedures
After a description of the study, University of Pittsburgh Institutional Review Board approved written informed consent was obtained followed by SCID interview and dichotic listening tests. Depressed participants then received CT: 2 sessions/week for the first 4 weeks followed by 8 weekly sessions for “early treatment responders” (Hamilton Rating Scale for Depression [HRSD, Hamilton, 1960] reduction, <40% at session 9; total 16 sessions) or 2 sessions/week for the first 8 weeks followed by 4 weekly sessions for those without early treatment response (total 20 sessions). CT followed Beck’s (Beck et al. 1979) guidelines (detail in Author Material from Siegle et al, 2012) with weekly HRSD ratings. Final HRSD scores were computed using a non-linear (B-spline) imputation procedure consistent with each patient’s trajectory of HRSD scores during CT, thus avoiding minor reporting changes in the last weeks not associated with true treatment response. Final values were imputed by assessing the expected value from the B-spline regression after the final treatment day. As in our prior study (Kishon et al. 2015), treatment response was defined as 50% or more reduction in final HRSD score.
The dichotic complex-tones and fused-words tests were administered in this fixed order prior to onset of CT. Of the 53 patients, one patient did not receive the words test and 3 patients performed extremely poorly on the tones test (more than 3 SD below the mean for patients) and were not included in the analysis of the data for this test. The tests were administered by computer using an E-prime program and were presented via Sennheiser HD280 headphones at an idiosyncratically determined comfortable sound level.
The complex-tones test (Sidtis, 1981), administered in about 20 minutes, asks participants to compare the pitch of a binaurally presented complex tone with the pitches of a dichotic pair of complex tones presented 1 s earlier. Patients respond Yes when the probe tone is the same as either member of the previous dichotic pair, or respond No when it differs from both. The complex tones are square waves with fundamental frequencies corresponding to eight notes in the octave between C4 and C5. After binaural and dichotic practice trials, participants were tested on 4 blocks of 28 trials in which half of the probe tones matched a member of the dichotic pair and half did not.
The fused-words test (Wexler & Halwes, 1983), administered in about 15 minutes, consists of 15 different single-syllable word pairs in which each member of every pair differs from the other only in the initial consonant (e.g., coat, goat). All words begin with one of 6 stop consonants (b, d, p, t, g, and k) and are natural speech spoken by a male voice. When dichotic word pairs are presented, the members fuse into a single percept. Participants indicated the word they heard via a button press reflecting one of four possible responses, consisting of each member of the dichotic pair and two other words differing from the dichotic stimuli only in the initial consonant. It is a correct response for the right ear if the participant choses the response indicating the word presented to that ear, and vice versa for the left ear. Following monaural and dichotic practice trials, each participant received four 30-item blocks of test trials.
2.3 Data Analyses
The number of correct responses was computed for the right (R) and left (L) ear items in the dichotic word and tone tests. These scores were used to compute a standard asymmetry index, 100 (R−L)/(R+L). The hypothesis that CT responders have greater right ear advantage for words than non-responders was evaluated using a t-test, after determining there was no significant difference in variance of scores between groups, and the same analysis was performed for the tone test. To examine the potential impact of having a comorbid anxiety disorder, a 2 × 2 ANOVA of asymmetry scores in each test was conducted with variables of CT outcome (responder vs. non-responder) and anxiety disorder (present vs. absent).
A binary logistic regression analysis was performed to examine the value of continuous asymmetry scores on the word and tone tests for predicting whether or not a patient will response to CT, when controlling for patient age and pre-treatment severity of depression on the HDRS. A α2 test was also used to compare the response rate of patients with asymmetry scores above versus below the normative cutoff score used in our prior study, i.e., the mean score for 74 healthy participants (see Kishon et al. 2015). Patients with asymmetry scores above normal were predicted to be responders and those below normal were predicted to be non-responders. Sensitivity, specificity, positive predictive value and negative predictive value were also computed.
3. Results
3.1 Patient Characteristics
As indicated in Table 1, the CT responders and non-responders did not differ significantly in gender or education, but non-responders were significantly older (Cohen’s d effect size= .58). Age was not, however, significantly correlated with asymmetry scores on the word (r= .03, p=.82) or tone (r=.13, p=.35) test, and including age as a covariate did not affect the findings reported below. There was no significant difference in the percentage of responders and non-responders who had a comorbid anxiety disorder. Among responders, 44% had an anxiety disorder (10 social phobia, 4 specific phobia, 3 generalized anxiety disorder, 2 panic disorder, 1 anxiety disorder NOS, 1 post-traumatic stress disorder), and among non-responders, 26% had an anxiety disorder (2 panic disorder, 1 social phobia, 1 anxiety disorder NOS, 1 post traumatic stress disorder). There was also no difference between responders and non-responders in pre-treatment HRSD scores. As expected, responders had significantly lower post-treatment depression scores (Cohen’s d=2.46).
3.2 Group Differences in Asymmetry
Responders had a larger right ear advantage than non-responders in the dichotic words test [t(50)=2.42, p=.019], with a moderate effect size (Cohen’s d=.67). As shown in Fig. 2, responders had more than twice the mean right ear advantage for words than non-responders. There was, however, no significant difference between these groups in left ear advantage in the complex tones test [t(48)=1.52, p=.134; Cohen’s d=.48]. Additional analyses were conducted to examine the possible effect of having a comorbid anxiety disorder. A 2 X 2 ANOVA of the asymmetry scores in the words test for responders and non-responders with versus without an anxiety disorder confirmed the greater right ear advantage for words in responders than non-responders [F(1,48)= 4.94, p=.031, ɳ2=.093]. There was no significant effect of having a comorbid anxiety disorder [F(1,48)=0.57, p= .453, ɳ2=.012] and no Outcome X Anxiety Disorder interaction [F(1,48)= 1.39, p=.244, ɳ2=.028]. A parallel ANOVA of the asymmetry scores in the tones test for responders and non-responders with versus without an anxiety disorders did not reveal any significant group differences or interactions [F(1,46) ≤2.10, p≥ .154, ɳ2 ≤.044].
Fig. 2.
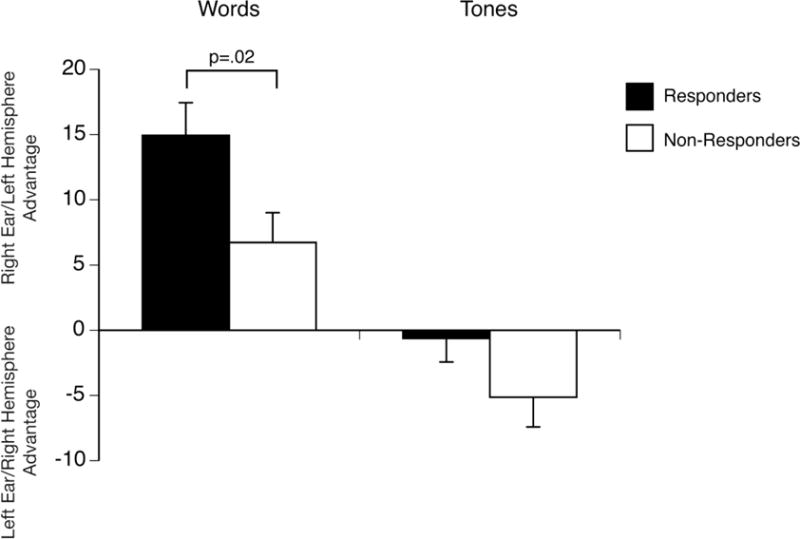
Asymmetry scores for CT responders and non-responders in the dichotic words and complex tones tests (Brackets= standard error of mean).
3.3 Predictions of Treatment Outcome
A binary logistic regression was used to examine the value of continuous asymmetry scores in dichotic words and tones tests for predicting whether or not a patient will response to CBT, when controlling for patient age and pretreatment HDRS. Age was a significant predictor of CT response over a constant in the first step [Wald test (1)=4.65, p=.031]. Asymmetry scores for words entered as a significant predictor of CT response in the next step [Wald test (1)=4.05, P=.044], but pretreatment HDRS and asymmetry scores for tones did not enter into the equation (p≥.19). Using a probability cut-point of .5 with this logistic model, 87% (27 out of 31) were correctly predicted to be responders (sensitivity) and 56% (10 out of 18) were correctly predicted to be non-responders (specificity). Overall, 76% of patients were correctly classified by the logistic regression equation.
The left portion of Fig. 3 shows the distribution of asymmetry scores for each individual in the responder and non-responder groups in the words test and the mean values for each group. The significantly larger right ear advantage for words in responders than non-responders agrees with the findings for a separate sample of patients tested at Columbia University (Kishon et al., 2015), which are shown in the right portion of Fig. 3. The arrow in this figure is the mean right ear advantage for 74 healthy controls in Kishon et al. (2015). As in our prior study, this normative value was used as a cutoff for predicting response to CT, with those having an asymmetry score greater than normal predicted to be responders and those less than normal predicted to be non-responders. Patients in the current study with a right ear advantage greater than normal had a 81% (21 out of 26) response rate to CT, whereas those less than normal had only 46% (12 out of 26) response rate, α2(1)= 6.72, p<.01. This corresponds to a positive predictive value of 81% and negative predictive value of 54%. The percentage of responders with scores greater than normal was 64% (sensitivity) and non-responders less than normal was 74% (specificity). In Kishon et al. study, the positive predictive value was comparable to the current study (89%), but the negative predictive value (73%), sensitivity (73%) and specificity (89%) were somewhat higher.
Fig. 3.
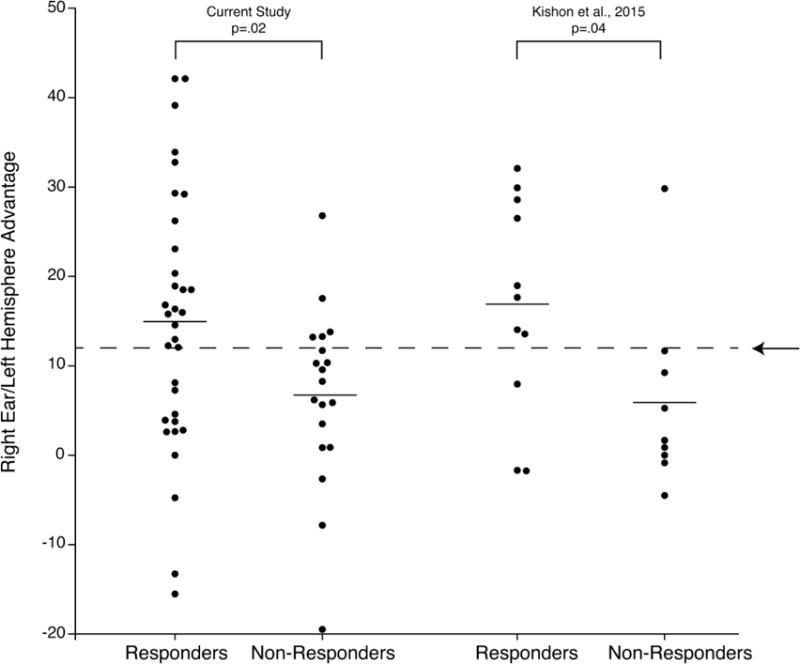
Asymmetry scores in the dichotic words test for individual responders and non-responders and mean value for each group in the current study and in Kishon et al. (2015). The arrow in this figure is the mean right ear advantage for 74 healthy controls in Kishon et al. (2015)
4. Discussion
This study replicates prior findings (Bruder et al. 1997; Kishon et al. 2015) suggesting that greater right ear advantage for verbal dichotic listening is prognostic for clinical response to CT for depression. Importantly, the larger right ear advantage in responders for the dichotic fused words test has now been found at two different clinical research centers, with a moderate to large effect size (Cohen’s d= .67 in current study and d= .99 in Kishon et al. 2015). In both studies, depressed patients with nominally above average right ear advantage had significantly higher response rate (81% and 89%) than those with below average right ear advantage (46% and 27%). The sensitivity and specificity of predictions of CT response were somewhat lower in this study (64% and 74%) than in our prior study (73% and 89%), which may stem from the more clinically representative treatment, in most cases by community clinicians with greater heterogeneity in CT experience, and methodological differences (see below). This does, however, suggest that the value of the dichotic fused words test as a prognostic indicator of response could generalize to standard CT in clinical settings.
4.1 Why Do CT Responders Have Greater Right Ear Advantage For Verbal Dichotic listening?
CT is predicated on reasoning and controlling contrasting automatic thoughts, i.e., streams of simultaneously generated alternate thoughts representing different perspectives. Perhaps the ability to accurately distinguish between two simultaneous streams of information (e.g., as in a dichotic word test), prioritizing the stream associated with executive control, bodes well for success in CT. Executive control, particularly associated with inhibition of negative information processing, has been associated with a left-hemisphere dominant EEG alpha asymmetry (Grimshaw & Carmel, 2014), and relatively greater activity over left than right parietal areas in resting EEG has been associated with greater right ear advantage for dichotic words or syllables (Bruder et al. 2001; Davidson & Hugdahl, 1996). Moreover, techniques for changing EEG alpha power or asymmetry in depressed individuals have been associated with initial increases in executive control functions, such as working memory, when training was directed at parieto-occipital (Escolano et al., 2014) or frontal regions (Choi et al. 2011). Thus, we hypothesize that individuals who respond well to CT are better able to recruit cognitive processes involving left cortical regions critical for the success of CT. Indeed, left prefrontal and temporal activity have been observed in association with CT-related skills, such as reappraisal (Price et al. 2013; Silvers et al. 2015), and extreme endorsement of dysfunctional attitudes, which CT aims to reduce, has been associated with greater activation of left hippocampal, left inferior parietal, and left precuneus regions in MDD patients than healthy controls (Sankar et al. 2015), though the hemispheric specificity of these findings has not been tested. Together, these findings suggest that ability of individuals to access left hemisphere regions, not only during verbal dichotic listening but also during CT, may bode well for successful outcome of treatment.
Larger right ear advantage for dichotic words in responders than non-responders is not specific to treatment with CT but has also been found for antidepressant medications (Bruder et al. 1996; Bruder et al. 2004; Bruder et al. 2007), which suggests an alternative explanation for the association between dichotic listening and treatment response. Strong right ear advantage for verbal dichotic listening may be indicative of a subgroup of patients within the heterogeneous diagnostic category of MDD and this subgroup is especially responsive to CT or antidepressant medications. We have, for instance, hypothesized that patients who respond favorably to a selective serotonin reuptake inhibitor (SSRI) differ from non-responders and healthy controls in having a characteristic favoring of left over right hemisphere activation during dichotic listening, which was supported by the presence of both increased right ear (left hemisphere) advantage for dichotic words and reduced left ear (right hemisphere) advantage for complex tones (Bruder et al. 1996). This was, however, found to be dependent on gender (Bruder et al. 2004) and we did not find a difference in the complex tone performance between CT responders and non-responders in the current study or a prior study (Bruder et al. 1997), which is not consistent with an explanation in terms of characteristic perceptual asymmetry.
It could also be argued that a right ear advantage for words above the average score for healthy controls might reflect an abnormality in CT responders rather than a cognitive advantage. Greater right ear advantage could occur if patients had difficulty perceiving words in the left ear and not from an advantage in the right ear. Asymmetry scores do not distinguish between an affect caused by increased right ear accuracy or decreased left ear accuracy. In the dichotic fused words test, the words simultaneously presented to the right and left ear fuse to form a single percept and subjects only report the word heard. This test does not provide separate measures of accuracy for each ear, but only asymmetry scores. In our initial study (Bruder et al. 1997), we used a dichotic consonant-vowel test that requires subjects to report two syllables, the syllables heard in the right ear and the left ear. CT responders showed greater right ear advantage than non-responders, which was due to their better right ear accuracy than non-responders, whereas there was no difference in left ear accuracy. Given the largely contralateral projections between each ear and hemisphere, the larger right ear accuracy in CT responders supports the hypothesis that greater left hemisphere advantage for verbal processing is associated with favorable response to CT.
4.2 Prognostic Value of Dichotic Words Test
Our findings indicate that having a right ear advantage greater than the normative value is a prognostic indicator of favorable treatment outcome. It should be emphasized, however, that our prior study did not find a difference between placebo responders and non-responders in right ear advantage for dichotic syllables (Bruder et al., 1996), which suggests that the difference between CT responders and non-responders is not merely due to a non-specific placebo effect.
An important question, however, is what to do for those predicted to be non-responders? Patients with weak or no right ear advantage have a more treatment-resistant depression, but may benefit from an intervention that enhances engagement of the left hemisphere (Triggs et al. 2010; Nadeau et al. 2014; Vanderhasselt et al. 2015). For instance, Triggs et al. (2010) found that right cranial rTMS combined with social interaction was effective in treating patients with refractory depression, which they interpreted as being due to enhanced engagement of the left hemisphere. Alternatively, therapies that utilize non-verbal skills involving the right hemisphere, e.g., emotional, musical, or visuospatial processing, may be more effective in people predicted not to respond to standard CT.
Although the dichotic words test by itself may not be of value in predicting differential response to CT and antidepressants, when combined with other predictors to achieve a clinically applicable level of accuracy, it could be useful in advising patients who want to first try psychotherapy as to their likelihood of benefiting from CT. Its high positive predictive value could be particularly helpful in suggesting to individuals their chances of responding to CT. Combining dichotic listening with other predictors may further increase its clinical utility. For instance, tests assessing psychomotor speed (e.g., word fluency or choice RT), which have been found to differ between responders and non-responders to antidepressants (Bruder et al. 2014; Taylor et al. 2006) but not CT (Kishon et al. 2015), when combined with dichotic listening may be a useful package for selecting the strongest treatment option for a given patient. Combining dichotic listening measures with demographic or clinical variables, e.g., age or comorbid disorders (Fournier et al. 2008), might further improve the predictive value.
4.3 Comorbidity of Depressive and Anxiety Disorders
Individuals having an anxiety disorder with or without a comorbid depressive disorder have been found to have smaller right ear advantage for dichotic words or syllables when compared to those without an anxiety disorder (Bruder et al. 2004; Bruder et al. 2015). Given findings of poorer outcome of antidepressant treatment in depressed patients having a comorbid anxiety disorder (Fava et al. 1997), a question arises as to whether the smaller right ear advantage in CT non-responders could be related to comorbid anxiety disorders? This is unlikely because the number of CT non-responders with a comorbid anxiety disorder did not differ from that for responders, and we found no evidence that the difference in right ear advantage between CT responders and non-responders was dependent on having an anxiety disorder. Unlike our prior studies, we did not find a difference in asymmetry between patients with versus without anxiety disorders. It’s not clear why, but the prior studies included patients who agreed to receive treatment with an antidepressant, whereas the current study was in patients who opted to receive treatment with CT. In this regard, we also did not find a significant difference in response rate to CT between patients with vs. without a comorbid anxiety disorder, which has been reported for patients treated with an antidepressant (Fava et al. 1997).
4.4 Limitations and Potential Clinical Applications
A limitation is the absence of a more direct measure of lateralized brain function. Further study using neuroimaging or electrophysiological measures are needed to understand the mechanisms underlying CT response and the relation to left hemisphere language dominance. The dichotic fused words test was, however, found to provide a valid and reliable measure of language lateralization (Wexler & Halwes, 1983; Zatorre, 1989; Fernandes & Smith, 2000). Importantly, it can be quickly and inexpensively administered in clinical settings on any computer without special headphones or other equipment. In the current study, it was administered with a PC and over-the-counter headphones. Moreover, a direct-to-consumer application is possible; for instance, a smartphone application was recently developed for a dichotic consonant-vowel syllable test (Bless et al. 2015). An advantage of this syllable test is that it provides not only a measure of left hemisphere dominance for verbal processing, but also evaluates the impact of attention and cognitive control (Hugdahl et al. 2003). A potential problem with this approach is the lack of calibration of stimuli. This was a limitation of the current study, in that no provision was made to calibrate the output of the headphones and balance the sound level of stimuli in the right and left ear. Although this may have contributed to increased variance of asymmetry scores and somewhat lower effect size when compared to our prior study (Kishon et al. 2015), the positive predictive value remained high and the sensitivity and specificity of predictions of CT response were reasonable.
Our studies have been limited to right-handed individuals and it is therefore unclear that the findings will generalize to left-handers. Although left hemisphere language dominance is the prevailing condition, the incidence of right hemisphere language dominance increases with the degree of left handedness (Knecht et al., 2000). In our prior studies (Bruder et al., 1997; Kishon et al., 2015), we did not find a significant difference between CT responders and non-responders in the strength of handedness as measured by the Edinburgh Inventory (Oldfield, 1971), but the inclusion of only right handers limited the range of scores. A further study including both right and left handed patients is needed to examine the impact of handedness and determine whether inclusion of both dichotic listening and handedness measures could strengthen predictions of CT response.
These limitations notwithstanding, the current study was able to differentiate CT responders and non-responders, suggesting that the dichotic fused-words test is a robust and replicable predictor. In combination with other predictors, dichotic listening could be part of a clinically useful classifier that could help patients in evaluating their treatment options. When combined with other modalities, such as neuroimaging, the mechanisms of the observed differences in treatment response could be elaborated, potentially yielding indications of whether remediation with neurocognitive rehabilitation paradigms would be useful.
Highlights.
Verbal dichotic listening predicts response to cognitive therapy for depression
We replicated this finding at a different research center during clinical trials
Responders had twice the mean right ear advantage for words than non-responders
Predictions of cognitive therapy response have moderate sensitivity and specificity
Verbal dichotic listening may be a clinically disseminative, quick, prognostic test
Acknowledgments
This work was supported by the New York State Office of Mental Health, the National Institute of Mental Health (G.J.S., MH074807, MH082998, MH58356, MH69618), and the Pittsburgh Foundation, Emmerling Fund. We thank Michael Thase, Edward Friedman, Amanda Collier, Jeffrey Borrebach, and the staff of the Mood Disorders Treatment and Research Program as well as the staff of the Program in Cognitive Affective Neuroscience at the Western Psychiatric Institute and Clinic for their work on this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None.
References
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. Guilford Press; New York: 1979. [Google Scholar]
- Bless JJ, Westerhausen R, Von Koss Torkildsen J, Gudmundsen M, Kompus K, Hugdahl K. Laterality across languages: Results from a global dichotic listening study using a smartphone application. Laterality: Asymmetries of Body, Brain Cogn. 2015;20:434–452. doi: 10.1080/1357650X.2014.997245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright P, Jaldow E, Kopelman MD. The National Adult Reading Test as a measure of premorbid intelligence: a comparison with estimates derived from demographic variables. J I Neuropsychol Soc. 2002;8:847–854. doi: 10.1017/s1355617702860131. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Alvarenga J, Abraham K, Skipper J, Warner V, Voyer D, Peterson BS, Weissman MM. Brain laterality, depression and anxiety disorders: New findings for emotional and verbal dichotic listening in individuals at risk for depression. Laterality: Asymmetries of Body, Brain and Cogn. 2015 doi: 10.1080/1357650X.2015.1105247. http://dx.doi.org/10.1080/1357650X.2015.1105247. [DOI] [PMC free article] [PubMed]
- Bruder GE, Alvarenga JE, Alschuler D, Abraham K, Keilp JG, Hellerstein DJ, Stewart JW, McGrath PJ. Neurocognitive predictors of antidepressant clinical response. J Affect Disord. 2014;166:108–114. doi: 10.1016/j.jad.2014.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Otto MW, McGrath PJ, Stewart JW, Fava M, Rosenbaum JF, Quitkin FM. Dichotic listening before and after fluoxetine treatment for major depression: relations of laterality to therapeutic response. Neuropsychopharmacol. 1996;15:171–179. doi: 10.1016/0893-133X(95)00180-L. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Schneier FR, Stewart JW, McGrath PJ, Quitkin F. Left hemisphere dysfunction during verbal dichotic listening tests in patients who have social phobia with or without comorbid depressive disorder. Am J Psychiatry. 2004;161:72–78. doi: 10.1176/appi.ajp.161.1.72. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Stewart JW, Mercier MA, Agosti V, Leite P, Donovan S, Quitkin FM. Outcome of cognitive-behavioral therapy for depression: relation of hemispheric dominance for verbal processing. J Abnorm Psychol. 1997;106:138–144. doi: 10.1037//0021-843x.106.1.138. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Stewart JW, Schaller JD, McGrath PJ. Predicting therapeutic response to secondary treatment with bupropion: dichotic listening tests of functional brain asymmetry. Psychiatry Res. 2007;15:137–143. doi: 10.1016/j.psychres.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Stewart JW, Tenke CE, McGrath PJ, Leite P, Bhattacharya N, Quitkin FM. Electroencephalographic and perceptual asymmetry differences between responders and nonresponders to an SSRI antidepressant. Biol Psychiatry. 2001;49:416–425. doi: 10.1016/s0006-3223(00)01016-7. [DOI] [PubMed] [Google Scholar]
- Bryden MP. Laterality: Functional asymmetry in the intact brain. Academic Press; New York: 1982. [Google Scholar]
- Burkhouse KL, Kujawa A, Kennedy AE, Shankman SA, Langenecker SA, Luan Phan K, Klumpp K. Neural reactivity to reward as a predictor of cognitive behavioral therapy response in anxiety and depression. Depress Anxiety. 2016;33:281–288. doi: 10.1002/da.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SW, Chi SE, Chung SY, Kim JW, Ahn CY, Kim HT. Is alpha wave neurofeedback effective with randomized clinical trials in depression? A pilot study. Neuropsychobiology. 2011;63:43–51. doi: 10.1159/000322290. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Hugdahl K. Baseline asymmetries in brain electrical activity predict dichotic listening performance. Neuropsychology. 1996;10:241–246. [Google Scholar]
- Escolano C, Navarro-Gil M, Garcia-Campayo J, Congedo M, De Ridder D, Minguez J. A controlled study on the cognitive effect of alpha neurofeedback training in patients with major depressive disorder. Front Behav Neurosci. 2014 doi: 10.3389/fnbeh.2014.00296. http://dx.doi.org/10.3389/fnbeh.2014.00296. [DOI] [PMC free article] [PubMed]
- Fava M, Uebelacker LA, Alpert JE, Nierenberg AA, Pava JA, Rosenbaum JF. Major depressive subtypes and treatment response. Biol Psychiatry. 1997;42:568–576. doi: 10.1016/S0006-3223(96)00440-4. [DOI] [PubMed] [Google Scholar]
- Fernandes MA, Smith ML. Comparing the fused dichotic words test and the intracarotid amobarbital procedure in children with epilepsy. Neuropsychologia. 2000;38:1216–228. doi: 10.1016/s0028-3932(00)00035-x. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. The structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition (SCID-I/P, Verson 2) Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- Fournier JC, DeRubeis RJ, Shelton RC, Gallop R, Amsterdam JD, Hollon SD. Antidepressant medications v. cognitive therapy in people with depression with or without personality disorder. Brit J Psychiatry. 2008;192:124–129. doi: 10.1192/bjp.bp.107.037234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier JC, DeRubeis RJ, Shelton RC, Hollon SD, Amsterdam JD, Gallop R. Prediction of response to medication and cognitive therapy in the treatment of moderate to severe depression. J Consult Clin Psychol. 2009;77:775–787. doi: 10.1037/a0015401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimshaw GM, Carmel D. An asymmetric inhibition model of hemispheric differences in emotional processing. Front Psychol. 2014 doi: 10.3389/fpsyg.2014.00489. http://journal.frontiersin.org/article/10.3389/fpsyg.2014.00489. [DOI] [PMC free article] [PubMed]
- Haaga DAF, DeRubeis RJ, Stewart BL, Beck AT. Relationship of intelligence with cognitive therapy outcome. Behav Res Ther. 1991;29:277–281. doi: 10.1016/0005-7967(91)90118-m. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurolog Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl K. Dichotic listening in the study of auditory laterality. In: Hugdahl K, Davidson RJ, editors. The Asymmetrical Brain. MIT Press; Cambridge, Massachusetts: 2003. pp. 441–475. [Google Scholar]
- Hugdahl K, Rund BR, Lund A, Asbjørnsen A, Egeland J, Landrø NI, Roness A, Stordal KI, Sundet K. Attentional and executive dysfunctions in schizophrenia and depression: Evidence from dichotic listening performance. Biol Psychiatry. 2003;53:609–616. doi: 10.1016/s0006-3223(02)01598-6. [DOI] [PubMed] [Google Scholar]
- Kishon R, Abraham K, Alschuler DM, Keilp JG, Stewart JW, McGrath PJ, Bruder GE. Lateralization for speech predicts therapeutic response to cognitive behavioral therapy for depression. Psychiatry Res. 2015;228:606–611. doi: 10.1016/j.psychres.2015.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Dräger B, Deppe M, Bobe L, Lohmann H, Flöel A, Ringelstein EB, Henningsen H. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123:2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- McGrath CL, Kelley ME, Holtzheimer PE, Dunlop BW, Craighead WE, Franco AR, Craddock RC, Mayberg HS. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry. 2013;70:821–829. doi: 10.1001/jamapsychiatry.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau SE, Bowers D, Jones TL, Wu SS, Triggs WJ, Heilman KM. Cognitive effects of treatment of depression with repetitive transcranial magnetic stimulation. Cogn Behav Neurol. 2014;27:77–87. doi: 10.1097/WNN.0000000000000031. [DOI] [PubMed] [Google Scholar]
- Otto MW, Yeo RA, Dougher MJ. Right hemisphere involvement in depression: Toward a neuropsychological theory of negative affective experiences. Biol Psychiatry. 1987;22:1201–215. doi: 10.1016/0006-3223(87)90028-x. [DOI] [PubMed] [Google Scholar]
- Price RB, Paul B, Schneider W, Siegle GJ. Neural correlates of three neurocognitive intervention strategies: A preliminary step towards personalized treatment for psychological disorders. Cogn Ther Res. 2013;37:657–672. doi: 10.1007/s10608-012-9508-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rude SS, Rehm LP. Response to treatments for depression: The role of initial status on targeted cognitive and behavioral skills. Clin Psychol Rev. 1991;11:493–514. [Google Scholar]
- Sankar A, Scott J, Paszkiewicz A, Giampietro VP, Steiner H, Fu CHY. Neural effects of cognitive-behavioral therapy on dysfunctional attitudes in depression. Psychol Med. 2015;45:1425–1433. doi: 10.1017/S0033291714002529. [DOI] [PubMed] [Google Scholar]
- Sidtis JJ. The complex tone test: implications for the assessment of auditory laterality effects. Neuropsychologia. 1981;19:103–112. doi: 10.1016/0028-3932(81)90050-6. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Carter CS, Thase ME. Use of fMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am J Psychiatry. 2006;163:735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Friedman ES, Thompson WS, Thase ME. Remission prognosis for cognitive therapy for recurrent depression using the pupil: utility and neural correlates. Biol Psychiatry. 2011;69:726–733. doi: 10.1016/j.biopsych.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Thompson WK, Collier A, Berman SR, Feldmiller J, Thase ME, Friedman ES. Toward clinically useful neuroimaging in depression treatment: prognostic utility of subgenul cingulate activity for determining depression outcome in cognitive therapy across studies, scanners and patient characteristics. Arch Gen Psychiatry. 2012;69:913–924. doi: 10.1001/archgenpsychiatry.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Weber J, Wager TD, Ochsner KN. Bad and worse: neural systems underlying reappraisal of high- and low-intensity negative emotions. Soc Cogn Affect Neurosci. 2015;10:172–179. doi: 10.1093/scan/nsu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotsky SM, Glass DR, Shea MT, Pilkonis PA, Collins JF, Elkin I, Watkin JT, Imber SD, Leber WR, Moyer J, Oliveri ME. Patient predictors of response to psychotherapy and pharmacotherapy: Findings in the NIMH Treatment for Depression Collaborative Research Program. Am J Psychiatry. 1991;148:997–1008. doi: 10.1176/ajp.148.8.997. [DOI] [PubMed] [Google Scholar]
- Struab J, Plener PL, Sproeber N, Sprenger L, Koelch MG, Groen G, Abler B. Neural correlates of successful psychotherapy of depression in adolescents. J Affect Disord. 2015;183:239–246. doi: 10.1016/j.jad.2015.05.020. [DOI] [PubMed] [Google Scholar]
- Taylor BP, Bruder GE, Stewart JW, McGrath PJ, Halperin J, Ehrlichman H, Quitkin FM. Psychomotor slowing as a predictor of fluoxetine non-response in depressed outpatients. Am J Psychiatry. 2006;163:73–78. doi: 10.1176/appi.ajp.163.1.73. [DOI] [PubMed] [Google Scholar]
- Triggs WJ, Ricciuti N, Ward HE, Cheng J, Bowers D, Goodman WK, Kluger BM, Nadeau SE. Right and left dorsolateral pre-frontal rTMS treatment of refractory depression: A randomized, sham-controlled trial. Psychiatry Res. 2010;178:467–474. doi: 10.1016/j.psychres.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Vanderhasselt MA, De Raedt R, Namur V, Lotufo PA, Bensenor IM, Boggio PS, Brunoni AR. Transcranial electric stimulation and neurocognitive training in clinically depressed patients: a pilot study of the effects on rumination. Prog Neuro-Psychopharmacol Biol Psychiatry. 2015;57:93–99. doi: 10.1016/j.pnpbp.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Halwes T. Increasing the power of dichotic methods. The fused rhymed words test. Neuropsychologia. 1983;21:59–66. doi: 10.1016/0028-3932(83)90100-8. [DOI] [PubMed] [Google Scholar]
- Young J, Beck AT. Cognitive Therapy Scale: Rating Manual. Center for Cognitive Therapy; Philadelphia, PA: 1980. [Google Scholar]
- Zatorre RJ. Perceptual asymmetry on the dichotic fused words test and cerebral speech lateralization determined by the carotid sodium amytal test. Neuropsychologia. 1989;27:1207–1219. doi: 10.1016/0028-3932(89)90033-x. [DOI] [PubMed] [Google Scholar]


