Abstract
Purpose
To examine acute alterations in white matter (WM) diffusion based on diffusion tensor imaging (DTI) in youth with mild traumatic brain injury (mTBI) relative to orthopedic injury (OI) controls.
Methods
A prospective cohort study of 23 patients with mTBI and 20 OI controls ages 11–16 years were recruited from the emergency department (ED). DTI was performed within 96 hours. Voxel based analysis quantified group differences for DTI indices: fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD). The Post Concussion Symptom Scale assessed symptom burden.
Results
Youth with mTBI had significantly higher symptom burdens in the ED and at scanning than controls. The mTBI group had significantly higher levels of FA and AD in several WM regions including the middle temporal gyrus WM, superior temporal gyrus WM, anterior corona radiata, and superior longitudinal fasciculus. The mTBI group had significantly lower levels of MD and/or RD in a few WM regions including the middle frontal gyrus WM and anterior corona radiata. Diffusion alterations correlated poorly with acute symptom burden.
Conclusions
Alterations of diffusivity were detected in spatially heterogeneous WM regions shortly after mTBI in youth. The pattern of alterations may reflect restrictive water diffusion in WM early post-injury.
Keywords: Mild traumatic brain injury, child, diffusion tensor imaging, acute
1. Introduction
The diagnosis of mild traumatic brain injury (mTBI) can be difficult to establish in acute medical settings since it is based on historical details of the injury and nonspecific physical findings [1]. Disruption of axonal integrity caused by tensile forces within the brain is hypothesized to constitute the primary neuropathology of mTBI [2]. This pattern of injury is rarely detected on cranial computed tomography (CT), unless severe. Surrogate measures of axonal injury may help to objectively quantify injury associated with mTBI. The magnetic resonance imaging (MRI) technique of diffusion tensor imaging (DTI) can quantify anisotropic (directionally dependent) water diffusion properties of white matter (WM), which in part reflect axonal integrity. Diffusion follows an ellipsoid pattern with the direction of diffusion commonly measured by three mutually perpendicular eigenvectors and given corresponding eigenvalues. DTI indices derived from these 3 eigenvalues commonly include: (i) fractional anisotropy (FA) – summary measure of the linearity of water with values ranging from 0 (disorganized) to 1 (straight/anisotropic), that depicts the shape of the ellipsoid; (ii) mean diffusivity (MD) – the average magnitude of the three eigenvalues reflective of molecular displacement by diffusion, i.e. the size of the ellipsoid, with larger values reflecting higher degree of diffusion; (iii) radial diffusivity (RD) – the diffusion of water perpendicular to WM fibers, commonly ascribed to cell membrane and myelin properties, and (iv) axial diffusivity (AD) – the diffusion of water parallel to the WM fiber's direction, typically related to axonal changes [3–5]. These are generalized interpretations and the overall pattern of diffusion changes needs to also be taken into account when deriving conclusions. Generally, in healthy myelinated WM tracts, water diffusion tend to parallel the axons corresponding to higher FA values and lower MD values, in contrast to less organized diffusion (i.e. lower FA and higher MD) that occurs in damaged or poorly developed tracts [5]. Higher FA values in WM are associated with an increase in the intracellular to extracellular space (i.e. increased axonal diameter, axonal edema), increased fiber density, and homogeneity of WM fiber direction [5,6].
After traumatic brain injury, water diffusion along and into WM fibers change; however the pattern and timing of these fluctuations are not fully understood. DTI results are influenced by the severity of injury and the timing of image acquisition leading to varying interpretations of results amongst different studies [4]. In the chronic phases of recovery after moderate/severe traumatic brain injury (TBI), investigators have consistently demonstrated regions with reduced directionality of extracellular diffusion (e.g. decreased FA), relative to controls, which has been interpreted as disorganized diffusion due to degenerative changes, axonal degradation, myelin disruption and va-sogenic edema [4,5,7]. As detailed in a recent review, there are contrasting results about the direction of diffusion changes in the “acute” time period (1 day to about 1 week) following mTBI in adults [5]. Some have reported an increased directionality of water diffusion corresponding to increased FA in select regions, suggestive of restricted extracellular diffusion hypothesized to be from cytotoxic edema and resultant axonal swelling [8–10]. This pattern corresponds with the peak in cytotoxic edema suggested by human models within the first 24–48 hours after injury [11]. However, others have failed to find changes [12], or have reported decreased FA [13–18]. For children and adolescents imaged during the “acute” time period post-mTBI, four studies showed an increase in FA in various regions of interest including the corpus callosum, the cingulate bundle, and the fornix [19–22]; however, these were all extracted from the same patient group. Conversely, two studies had null findings [23,24] and there were no reports of decreased FA. Homogeneous patient populations, narrow imaging time frames, larger sample sizes and serial imaging to capture changes in diffusion over time may reconcile disparate results. Improved early diagnostic stratification would facilitate patient selection in future longitudinal observational and interventional studies, and potentially assist in individualized diagnosis and therapy in conjunction with other markers of injury.
The primary objective of this study was to determine if previously healthy uninjured youth with first-time mTBI have identifiable WM alterations as defined by disruption of water diffusion in normal appearing WM acutely after injury. We hypothesized that youth with mTBI would have increased directionality of water flow in normal appearing WM during the initial 24–96 hours following injury as evidenced by higher values of FA compared to youth with orthopedic injuries (OI controls). We postulated that during this acute phase of injury, the mechanistic forces associated with mTBI would more likely trigger cytotoxic, intracellular edema within axons with increased directionality of extracellular water diffusion and resultant increase in FA, as opposed to axotomy, axonal degradation and myelin disruptions that are associated with a decrease in FA on DTI. The secondary objective was to examine the association between acute symptom burden and diffusion parameters in regions that were found to differ between groups on DTI indices.
2. Methods
This was a prospective observational case control study involving youth ages 11-16 years old who presented within 6 hours of an injury to the emergency department (ED) affiliated with a large children's hospital between December 2010 and August 2012. Patients with mTBI were eligible if they had either i. a witnessed blow to the head, ii. injury with acceleration/ deceleration movement of the head, or iii. self-reported injury with evidence of head trauma; a Glasgow Coma Scale (GCS) score of 13 to 15 on presentation; and any one of the following: i. loss of consciousness < 30 minutes, ii. amnesia, or iii. any alteration in mental state at the time of the injury (e.g. agitation, irritability, sleepiness, lethargy, slow to respond, or asking repetitive questions) [25]. Patients with mTBI were excluded if they had more than one minor extracranial injury as defined by an Abbreviated Injury Severity Scale (AIS) ≤ 1 to that region [26]. Youth with OI were recruited as controls to equate the groups for premorbid individual and family characteristics that may predispose a child to injury as well as the acute stresses of emergency treatment [27]. Controls were eligible if they presented with an isolated extremity trauma requiring radiography and an AIS of ≤ 3. Controls were excluded if they had an abnormal neurologic examination, symptoms of concussion, or required immediate surgical care. Additional exclusionary criteria for both groups included an inability to understand English, prior history of concussion, prior traumatic brain injury requiring an ED visit or hospitalization, pre-existing neurologic impairment (stroke, CSF shunt, brain tumor, pre-existing cognitive disorders (seizure disorder, mental retardation), psychological problems, attention deficit disorders, developmental delay, conditions that precluded undergoing MRI or use of a computer, or prescription of a drug that impaired cognition (e.g. narcotic analgesics) that could not be skipped 4 hours prior to follow-up visits. History of exclusionary criteria was based on self-report and medical chart review. Patients who received a T-score of 65 or greater on the Child Behavior Checklist (CBCL) [28] were excluded due to probable preexisting neurocognitive or behavioral impairment.
2.1. Study design
The study was approved by the local Institutional Review Board and registered with ClinicalTrial.gov (NCT01922531). Trained study staff recruited and assented/consented eligible patients/guardians in the ED, usually between 8 am to midnight. They collected demographic and historical information pertaining to the patient, family and injury. The treating ED clinician assigned the AIS for each body region and completed a standard data collection form comprised of variables characterizing the nature of the mTBI that had been previously used in the development of the Pediatric Emergency Care Applied Research Network (PECARN) neuroimaging decision rules for detecting clinically important TBI (ciTBI) in children [1]. Parents completed the following measures: i. CBCL, a 120-item questionnaire measuring child behavior problems and competencies on 3-point Likert scale; and ii. Family Assessment Device-General Function scale (FAD-GF) [29,30], a 12-item questionnaire assessing aspects of family functioning on a 4-level response statement scale. Patients completed the Post Concussion Symptom Survey (PCSS), a 22-item inventory of symptoms associated with concussion graded on 7-point Likert scale (0 none to 6 severe) [31]. All patients underwent routine clinical care. All radiographic images (plain radiographs and CTs) were acquired at the discretion of the treating physician and were not influenced by data acquired by the research team.
Median family income was determined after enrollment to estimate socioeconomic status. This was extracted from the 2011 US census using the patient's principal address and dichotomized at a median income of $50,000 to differentiate middle to higher income families from others. The risk of ciTBI was determined by the research team post-hoc based on physician responses at the time of evaluation to the following 7 variables that constitute the PECARN neuroimaging rule for children ages 2 to 18 years: GCS at the time of evaluation, signs of altered mental status, signs of basilar skull fracture, history of loss of consciousness, vomiting, severity of mechanism of injury, and current headache. The risk of ciTBI based on the rule was used as a proxy measure of severity of injury. As outlined in the rule, mTBI participants without any of the 7 signs or symptoms are considered to be at low risk for ciTBI (< 0.05%). Those with other signs of altered mental status, GCS of 14, or signs of basilar skull fracture are categorized in the high risk category for whom a CT is recommended. Those participants with 1 or more of the above signs or symptoms not including the high risk features comprise the middle risk category for whom a period of observation or a CT are cited as acceptable management options.
MRI data were acquired within 96 hours post injury on Philips Achieva 3T scanner (Philips Medical Systems, Best, The Netherland). At this time, patients completed the PCSS again. The diffusion weighted single shot spin-echo EPI sequence used had the following specifications: TR/TE = 9000/84 ms; FOV = 256 mm × 256 mm; matrix = 128 × 128, in-plane resolution = 2 × 2 mm; slice thickness = 2 mm; 72 slices; SENSE factor = 2. In addition, anatomical T1- and T2- weighted images and susceptibility sensitive (venous BOLD) sequences were acquired. 3D T1-weighted anatomical images were acquired using a MPRAGE sequence with the following specifications: TR/TE = 8.1/3.7 msec; FOV = 256 mm × 256 mm; acquisition matrix = 256 × 256; sagittal in-plane resolution: 1 mm × 1 mm; slice thickness = 1 mm; number of slices = 180. The T2-weighted images were acquired with a TSE sequence with the following specifications: TR/TE = 3000/100 msec; FOV = 240 × 240 mm, acquisition matrix = 240 × 240; axial in-plane resolution: 1 mm × 1 mm; slice thickness = 3 mm; number of slices = 48; 2 averages. Susceptibility sensitive (venous BOLD) sequences were obtained using a three-dimensional FFE sequence with the following parameters: TR/TE = 15/20 msec, FOV = 220 mm × 180 mm; acquisition matrix = 220 × 182; 68 slices; SENSE factor = 2; reconstructed resolution = 1 × 1 × 1 mm, 1 number of signal averages. A total of 3 patients (2 mTBI and 1 OI) were excluded prior to analysis due to mechanical and technical issues with scanner and image acquisition.
Anatomical T1- and T2- weighted images were reviewed for structural abnormalities by two board-certified pediatric neuroradiologists, independent of each other, both of whom were naïve to subject group and clinical presentation. The post-processed susceptibility sensitive images (source maps and 10 mm multi-planar reconstructions) were visually assessed and any abnormal hemorrhagic foci noted.
2.2. Image processing
For the diffusion weighted images, the sequence was applied along 61 non-colinear directions with b-value = 1000 s/mm2 and one non-diffusion weighted volume was acquired as reference b0. Data were corrected for eddy-current and head-motion by aligning all DWI images in the series to the b0 image using rigid body affine registration using FSL Software (FMRIB, Oxford, UK) [32]. To address the concerns about the potential confounding effect of head motion on diffusion measures, the frame-by-frame translational motion (the sudden motion) in the x-, y-, and z- directions and three Euler angles in the frame-by-frame rotational motion were calculated based on the affine registration. No translational motion exceeded 1 voxel in any direction (mTBI: 0.13 ± 0.03 mm, 0.24 ± 0.04 mm, 0.15 ± 0.04 mm in x-, y-, and z-direction, respectively; OI: 0.11 ± 0.06 mm, 0.23 ± 0.06 mm, 0.15 ± 0.07 mm for x-, y- and z-direction, respectively). No rotational motion exceeded 1 degree in any of the three Euler angles (mTBI: 0.13 ± 0.08 degree, 0.15 ± 0.06 degree, and 0.15 ± 0.05 degree for the three angles, respectively; OI control: 0.13 ± 0.11 degree, 0.14 ± 0.06 degree, 0.15 ± 0.06 degree for the three angles, respectively). No statistically significant difference was found in any of the head motion values between the control group and mTBI group. Diffusion tensor was calculated on a voxel-by-voxel basis, and used to derive maps for DTI measures including FA, Mean Diffusivity (MD), Axial and Radial Diffusivity (AD and RD). T1-weighted images were normalized to Montreal Neurologic Institute space using affine transformation without resampling. The Statistical Parametric Mapping analysis package (SPM8; Wellcome Department of Cognitive Neurology, London, UK) was used to segment the whole brain into gray matter, WM and cerebrospinal fluid using the normalized T1-weighed images. The WM segment was used as the mask needed for subsequent group analysis.
2.3. Data analysis
To address the primary aim, it was estimated that a minimum of 20 subjects per group were required to detect a difference in mean FA similar to that found by Wilde and Wu [19,33] using an averaged standard deviation of 0.02 with a α = 0.05 and 85% power. Wilde demonstrated a significant (p < 0.0001) difference in FA values in the corpus callosum in youth with mTBI (mean = 0.464, SD 0.023) versus youth with OI (mean = 0.425, SD 0.031).
Comparisons between patients with mTBI and controls were made using Fishers exact test for categorical variables (sex, race, ethnicity, census tract income, and mechanism of injury) and t-tests for continuous variables (age, weight, body mass index, CBCL, FAD, time from injury to scan, and PCSS). An a priori alpha level of 0.05 was used to evaluate significance for all statistical tests.
A voxel based analysis (VBA) approach was used to quantify the group differences for each DTI measure (FA, MD, AD, and RD) using the Analysis of Functional NeuroImages program [34]. T-tests were used to test differences in diffusion properties between the groups, voxel by voxel. Age and time to scan were used as covariates in the VBA analysis. All significant ROI were generated using the same thresholds: a t = 2.98 score of 2.83, a cluster of nine voxels and voxel-wise p ≤ 0.005. These parameters lead to the desired two-tailed threshold of corrected p < 0.05 using the Monte Carlo simulation based on the method performed by Ledberg et al. The Monte Carlo simulation is commonly used in the second level neuroimaging analysis to estimate statistical significance using cluster statistics and simulation of the characteristics of random noise in the data to minimize false positive findings as a result of multiple comparisons in VBA [35–38].
To explore the association between DTI measures and symptoms, regions of interest (ROI) were constructed for each brain region that showed statistically significant DTI group differences. DTI values (FA, MD, AD, or RD) were calculated for each ROI by averaging the values across the voxels in that corresponding ROI. Using these DTI values, Pearson correlation coefficients were calculated to quantify the association between DTI values and total PCSS in the ED and at the time of scan. This limited ROI approach was used in an effort to reduce the number of comparisons by limiting the area and number of voxels in an effort to show a correlation between areas with diffusion differences and symptom burden. Similarly, student's t-test was used to assess the association between measures of diffusion in the ROIs and the risk of ciTBI. The risk was divided into 2 groups using the risk of ciTBI of > 3.9% that determined the need of a CT in the ED as delineated in the prediction tree in the publication by Kuppermann et al. [1].
3. Results
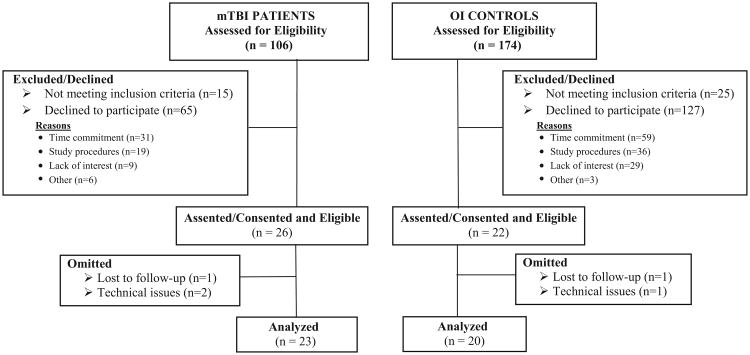
A total of 106 patients with mTBI and 174 OI controls were approached to participate in the study and 24.5% and 12.6% assented/consented, respectively. Details of patient enrollment are displayed in Fig. 1. The final study sample consisted of 23 patients with mTBI and 20 OI controls. There were no statistical differences between mTBI and OI groups with respect to age or gender in the following comparisons: i. those consented/eligible and those excluded/declined; and ii. those in the final sample and those omitted/lost to follow-up. Consented/eligible participants with OI differed significantly from those who were excluded/declined on race (32% white youth excluded/declined versus 69% of nonwhite youth, p < 0.0001). Participants with mTBI omitted/lost to follow-up from final analysis were significantly more likely to be nonwhite than those in the final sample (0% white versus 12% non-white omitted, p = 0.03).
Fig. 1.
Study Flow.
The clinical features of the mTBI and OI groups are reported in Table 1. On arrival to the ED, most patients with mTBI had a normal GCS score of 15, two had a GCS score of 14 that returned to a score of 15 within 2 hours of ED presentation, and none had a GCS of 13. The most common location of impact was the occiput and there was no lateral predilection. Following the PECARN neuroimaging decision rule, 22 of the patients with mTBI received the recommendation to either undergo a CT or a period of observation in the ED for neurological deterioration, with clinicians performing CT scans for five of these patients, all of which were normal. Seven patients with mTBI (30.4%) had one other minor injury (AIS ≤ 1). Two patients with mTBI were admitted to the hospital.
Table 1. Clinical Features of Patients with mTBI and the OI Controls.
| mTBI Clinical Features of Brain Injury | % n = 23 | OI Controls Clinical Features of Orthopedic Injury | % n = 20 |
|---|---|---|---|
| GCS 14 | 8.7 | Fracture | 80.0 |
| LOC | 34.8 | Lower arm | 40.0 |
| Amnesia | 52.2 | Upper arm | 10.0 |
| Other MS changes | 52.2 | Hand | 15.0 |
| Seizure | 4.3 | Lower leg | 15.0 |
| Headache | 82.6 | Contusions | 20.0 |
| Vomiting | 30.4 | Injury severity | |
| Dizziness | 43.5 | AIS 1 | 20.0 |
| Confusion | 39.1 | AIS 2 | 50.0 |
| Palpable skull fracture | 0.0 | AIS 3 | 30.0 |
| Basilar skull fracture | 0.0 | ||
| Other neurologic deficits other than MS changes | 4.3 | ||
| Acting abnormal according to parent | 39.1 | ||
| PECARN Neuroimaging Prediction Tree Percent | |||
| < 0.05% | 4.3 | ||
| 0.6% | 4.3 | ||
| 1.1% | 39.1 | ||
| 3.9% | 52.2 |
Legend: AIS, Abbreviated Injury Severity Scale; GCS, Glasgow Coma Scale; LOC, loss of consciousness; MS, mental status; PECARN, Pediatric Emergency Care Applied Research Network.
As shown in Table 2, patients with mTBI did not differ significantly from controls with respect to age, gender and most other demographics with the exceptions that patients with mTBI were more likely to be white and to have higher median incomes. The most frequent mechanism of injury for both groups was sports and neither group was injured in motor vehicle collisions. Symptom burden on presentation to the ED and at the time of scan are also summarized in Table 2. Due to computer difficulties, one patient with mTBI included in group DTI analysis did not complete the PCSS in the ED and was excluded from corresponding comparative and correlation analyses. The mTBI group had significantly higher symptom burden than the OI group at both time points (p = 0.0009 at ED, p = 0.005 at scan). Both groups had similar pre-morbid behavioral competency (as measured by CBCL) and family functioning (as measured by FAD-GF).
Table 2. Comparison of Patient Characteristics, Time to Scan and Symptom Burden between Patients with mTBI and OI Controls.
| Variable | mTBI Patients n = 23 | OI Controls n = 20 | Significance p-value |
|---|---|---|---|
| Age (years, mean, SD) | 13.2, 1.8 | 12.7, 1.5 | 0.34 |
| Sex (% male) | 91.3 | 75.0 | 0.15 |
| Race (% white) | 73.9 | 30.0 | 0.004 |
| Ethnicity (% non-Hispanic) | 95.7 | 100.0 | 0.35 |
| Census Tract Median Income (% > $50, 000) | 95.7 | 65.0 | 0.01 |
| Weight (kg, mean, sd) | 56.4, 15.7 | 58.0, 11.3 | 0.70 |
| Body Mass Index (mean, SD) | 20.9, 3.5 | 21.9, 3.7 | 0.38 |
| Mechanism of injury† (% sports) | 73.91 | 80.0 | 0.64 |
| Total CBCL (T score, mean, SD) | 45.0, 11.6 | 43.0, 12.9 | 0.58 |
| Total FAD (mean, SD) | 30.0, 1.87 | 29.4, 1.89 | 0.32 |
| Time from injury to scan (hours, mean, SD) | 45.0, 17.6 | 48.2, 21.1 | 0.59 |
| Total PCSS-ED (mean, SD) | 37.8A, 19.6 | 18.3, 15.2 | 0.0009 |
| Total PCSS-Scan (mean, SD) | 25.5,21.7 | 9.9, 10.2 | 0.005 |
Legend:
One patient with mTBI did not complete the PCSS in the ED.
Other mechanisms of injury for the patients with mTBI included other non-motorized wheeled transport (n = 1), fall to ground from standing height (n = 3), fall from elevation (n = 1), and object struck head (n = 1). Other mechanisms of injury for the controls included fall from bike (n = 1), fall from standing height (n = 1), and assault (n = 1); CBCL, Child Behavior Checklist; ED, Emergency Department; FAD, Family Assessment Device; PCSS, Post Concussion Symptom Scale; SD, standard deviation.
The MRI scan was acquired on average two days post-injury in both groups (mTBI: mean 45.0 hours, SD 17.6; OI: mean 48.2 hours, SD 21.1) as displayed in Table 2. On review of the T1- and T2- weighted images from the MRI scans, the neuroradiologists found abnormalities in 5 patients with mTBI and 4 OI controls, none of which necessitated immediate intervention. Findings included punctate WM signal foci (1 patient with mTBI, 1 control), non-punctate signal abnormalities (3 patients with mTBI, 1 control), and small arachnoid cyst (1 control). The etiology of the non-punctate signal abnormalities is unknown, but are similar to those seen in up to 13% of normal children when imaged with high resolution T2 FLAIR sequences [39]. Participants with these findings were included in the subsequent analyses since these findings are presumed to represent small areas of gliosis and not consistent with non-hemorrhagic contusions. Two subjects had Arnold Chiari Type 1 malformations (1 patient with mTBI, 1 control). Due to the possible concern of anatomical heterogeneity introduced by Arnold Chiari Type 1 malformations, subsequent VBA analysis was performed with and without the affected participants. On review of the susceptibility weighted images, there were no hemorrhagic or abnormal susceptibility foci in either the patients with mTBI or controls.
All scans from 23 patients with mTBI and 20 OI controls were used in the group comparison. Significant group differences between the mTBI and the OI groups were found in all four DTI indices as outlined in Fig. 2. Significantly higher levels of FA in the mTBI group relative to OI controls were found in 8 WM regions (p < 0.05, corrected). These included left middle temporal gyrus WM, left superior temporal gyrus WM, left and right anterior corona radiata, right superior longitudinal fasciculus, as well as 3 other WM regions. Significantly higher AD values in the mTBI group relative to the OI controls were found in 4 regions; however, there was one small region in the right superior parietal lobe that had lower AD values. Significantly lower levels of MD were found in 3 regions including the right lateral orbital, left anterior corona radiata and the right middle frontal gyrus in mTBI group relative to OI controls (p < 0.05, corrected). Lower RD values in the mTBI group relative to the OI controls were seen in 11 regions; however, there was one small area in the left corpus callosum that had an increased value. Repeat analysis with the omission of the two children with Arnold Chiari Type 1 malformation revealed no differences.
Fig. 2.
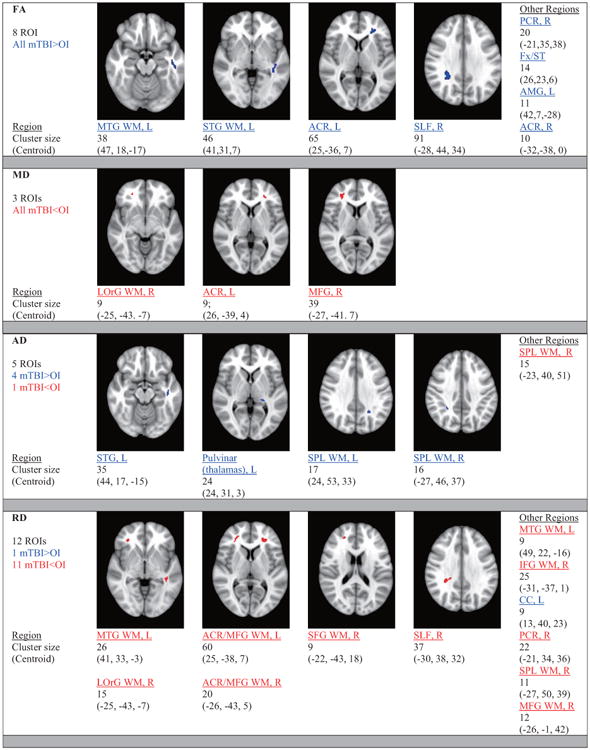
White Matter Regions with Statistically Significant Differences in DTI Indices between Patients with mTBI and OI Controls. All regions: t = 2.98, cluster ≥ 9, corrected p < 0.05. Legend: ACR: anterior corona radiata.
Among patients with mTBI, the majority of the correlation analyses between acute symptom burden, in the ED or at the time of the scan, and DTI values in ROIs with group differences were not statistically significant; however, there were significant inverse correlations between total PCSS in the ED and RD values in the right medial frontal gyrus WM and the left corpus callosum (r = –0.46, p = 0.03 and r = –0.47, p = 0.03, respectively). There were no other statistically significant correlations between DTI indices in other ROIs and total PCSS in the ED or at the time of the scan. Lastly, there was no statistically significant correlation found between risk of ciTBI and the measures of diffusion in any of the ROIs.
4. Discussion
The current findings support our hypothesis that WM alterations can be detected in healthy previously un-injured youth with mTBI using DTI within an average of 2 days post-injury. Youth with mTBI had several spatially heterogeneous regions of WM with higher levels of FA and AD, and regions with lower levels of MD and RD, relative to youth with OI. In total, there were 8 regions demonstrating higher FA, 4 regions with higher AD, 3 regions with lower MD, and 11 regions with lower RD. The findings were not entirely consistent however, with two small regions with lower AD and higher RD. We failed to find areas of microhemorrhages on SWI in any of the youth with mTBI. This study adds another independent cohort of youth to a growing body of literature that demonstrates increased anisotropy in the acute phase [19–22]. The overall pattern of diffusion changes in the majority of the DTI indices in this study implies restricted extracellular diffusion early post-injury. We interpret this pattern of changes as suggestive of cytotoxic edema, (i.e. axonal swelling) occurring at this time [6,40]. The heterogeneity of the diffusion indices altered and the regions affected underscore the complex interplay between injury- and host-related factors and the resultant individual pathophysiologic changes. Acute symptology correlated poorly with diffusion changes suggesting that some aspects of the injury may not be reflected by acute diffusion parameters or symptoms alone.
On a cellular level, it is known that tensile forces lead to stretching and disruption of axonal plasma membranes triggering a cascade of biochemical events including a widespread release of numerous neurotransmitters followed by a deregulated flux of ions with an influx of calcium into the cells [6,40]. This can result in axonal swelling, neurofilament compaction, microtubular disassembly, axonal disconnecion and axotomy [40,41]. In milder injuries, these changes may be fully reversible [42]. Since no microhemorrhages were detected on SWI, we believe the changes in the DTI indices represent changes in the diffusion of water and associated microstructures. It also suggests that our subjects were subjected to relatively mild shear/strain forces and that changes in axonal pathology can occur without detectable changes to the microvasculature [43].
Axonal pathology, as compared to changes in myelin, appears to play a significant role in anisotropic diffusion during this acute phase, particularly in mTBI [44–46]. The increases in FA in 8 regions and the increases in AD in 4 other regions during the acute time period may be due to a more linear and more parallel flow, respectively, of the extracellular water diffusion. The decreases in MD in 3 regions and the decreases in RD in 11 regions may be due to a decrease in overall and perpendicular extracellular water diffusion, respectively. This overall pattern of group differences appears to reflect restricted extracellular due to an alteration in the ratio of intracellular relative to extracellular water. Plausibly, cytotoxic edema and resultant axonal swelling during this acute phase may lead to more compacted axons and less tortuous extracellular water diffusion [7]. On a cellular level, axonal stretching may induce a disturbance in the gated ion channels resulting in intracellular swelling and decreased extracellular water as reflected by the changes in RD [47]. Others have reported similar variations in diffusion changes; however, regions of the brain displaying differences have varied in part due to heterogeneous analytical techniques and regions studied [19–22]. Two areas did not demonstrate this pattern (decreased AD in left superior parietal lobe white matter and increased RD in left corpus callosum). Differences in time intervals from injury to scan, force and site of impact, individual responses to injury, and regional resilience levels may have contributed to some of the variations in the regions injured and the directional patterns of water diffusion seen. Emerging evidence suggests that changes in the indices of WM integrity are also dependent on time since injury [7,21,48]. Wilde et al. demonstrated the complexity of these changes in adults over the first week post-injury after mTBI in terms of individual variations in both magnitude of DTI indices and timing [21].
In total, there were a total number of 26 regions that displayed group differences in a diffusion metric. No evidence of laterality or spatially distinct regions was detected, which is consistent with the lack of focal predilection of the site of impact in this sample and the diffuse nature of mTBI. It is worth noting that when the p-value was liberalized to 0.1, there was considerable overlap in brain regions that demonstrated group differences in FA with regions that had significant changes in the other DTI indices between the patients with mTBI and controls. Regions that demonstrated differences in FA, such as the corona radiata, the superior longitudinal fasciculus, the corpus callosum, and the fornix, contain axons of long-coursing pathways in the midbrain. Disruption of these pathways have been implicated in neurocognitive impairments associated with post-concussion syndrome including attention, working memory and short term memory [49]; however we did not find correlations between changes in these regions and acute symptom burden. Regions that displayed differences in the DTI indices often have functions that have been linked deficits following concussion. For example, the temporal gyri are involved in language functions and verbal memory, the superior longitudinal fasciculi and the superior parietal lobe are involved in working memory, the fornix and the amygdala are involved in memory formation and storage, and the frontal gyri, the anterior corona radiata and the lateral orbital gyrus are involved in executive functioning [6]. Further connectivity analysis may shed light on disruption of these pathways. Emerging DTI analytical techniques that account for patient-by-patient diffusion changes will improve current VBA or traditional ROI analytical techniques which assume that dissimilar patients have spatially overlapping abnormalities [4–6].
Typically, symptom burden is very heavy initially following mTBI and improves with time. Prior efforts to establish a relationship between abnormalities in the WM following TBI of any severity and acute symptomatology or outcomes have resulted in varied success and discordant results [50]. The results from this study only provide limited support for the hypothesis that acute WM alterations are associated with acute symptom burden. A reduced RD, suggestive of cyto-toxic edema, was associated with an increased symptom burden in only two regions – the right medial frontal gyrus and the left corpus callosum. Symptoms are only one aspect of neurocognitive impairment following mTBI. We did not acutely test for deficits in executive functioning, verbal memory, working memory or memory formation that are associated with injury in white matter regions that displayed changes in DTI metrics. If DTI were to be used as a biomarker of injury, it ideally would correlate with severity of neurocognitive impairment and severity of underlying microstructural injury. Clinical decision rules based on common acute signs and symptoms have excellent sensitivity and predictive value to identify children who are at low risk of a ciTBI [1] seen on traditional CT in the ED. Our results do not support the presence of a relationship between risk of ciTBI based on a commonly used neuroimaging decision rule and acute WM changes detected on DTI. These findings further affirm that diffusion changes do not correlate with symptoms. Future studies incorporating simultaneous neurocognitive tests and imaging may help to shed light on the clinical and predictive significance of the diffusion changes found in this study.
This study possesses a number of strengths including a homogeneous sample, a short and narrow interval between injury and imaging, and use of whole brain VBA. The lack of gold standard tests to accurately diagnose the disease and stratify injury severity is a limitation. The low consent rate, primarily because of the complexity of study design, may reduce the generalizability of the results. Despite demographic similarities between the patients with mTBI and controls on most variables, uncontrolled differences in income and race may contribute to variability in the findings. These differences were not controlled for due to the small sample size. Although it is conceivable that there may be differences in cellular structures and response to injury due to race and/or socioeconomic status, there are no reports of such in the literature. The small sample size limits the interpretation of the impact of variations in individual characteristics or mechanism of injury. Only one imaging time frame precludes assessment of longitudinal pattern of changes. In addition, caution must be taken when interpreting results from the analyses examining correlations between ROIs that differed significantly in the mTBI group and PCSS as these findings may be spurious and warrant replication [51]. In DTI studies, spurious findings and inconsistent results may arise from differences in MRI acquisition parameters (such as differences in number of diffusion weighted directions, b-values, image resolution, and scanner field strength) and analytical methods (such as differences in algorithms in registration, smoothing and normalization). In addition, variations in the approach for correcting multiple comparisons may also affect the congruency of significant findings.
5. Conclusion
In summary, the acute alterations in WM diffusion in previously healthy youth with their first mTBI detected in this study may be indicative of restricted extracellular diffusion and appear to be consistent with acute pathophysiological changes of cytotoxic edema. Alterations of anisotropic diffusion based on DTI may serve as an objective biomarker augmenting current diagnostic stratification of mTBI in youth. Translational animal studies elucidating the pathophysiology, as well as longitudinal human studies incorporating premorbid and clinical factors with larger sample sizes of children and teenagers are needed to clarify the role that DTI may play in the field of pediatric mTBI.
Acknowledgments
The authors would like to thank the following people for their contributions: Jeff Bazarian, MD, MPH, (Scientific Advisor, Department of Emergency Medicine, University of Rochester Medical Center); Terri Byczkowski, PhD, MBA, (Scientific Advisor, Division of Pediatric Emergency Medicine, Cincinnati Children's Hospital Medical Center); Richard Hornung, PhD, (Statistical Oversight, Division of Pediatric Emergency Medicine, Cincinnati Children's Hospital Medical Center); Blaise Jones, MD, (Clinical Neuroradiologist, Division of Radiology, Cincinnati Children's Hospital Medical Center); Lynn Mullins, BA, (Data Manager, Division of Pediatric Emergency Medicine, Cincinnati Children's Hospital Medical Center); Brad Kurowski, MD, MS, (Patient Care, Division of Physical Medicine and Rehabilitation, Cincinnati Children's Hospital Medical Center); Richard Ruddy, MD, (Division Chair, Scientific Advisor, Division of Pediatric Emergency Medicine, Cincinnati Children's Hospital Medical Center); Imaging Research Coordinators at the Pediatric Neuroimaging Research Consortium at the Cincinnati Children's Hospital Medical Center (Data Collection); and the Pediatric Emergency Medicine Physicians and Clinical Research Coordinators in the Division of Pediatric Emergency Medicine at Cincinnati Children's Hospital Medical Center (Data Collection, Data Entry).
This study was funded in part by (1) National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2 TR000078 (KL2 RR026315); and (2) Cincinnati Children's Hospital Medical Center Division of Emergency Medicine.
Abbreviations
- AIS
Abbreviated Injury Severity Scale
- AD
axial diffusivity
- CBCL
Child Behavior Checklist
- ciTBI
clinically important TBI
- CT
computed tomography
- DTI
diffusion tensor imaging
- ED
emergency department
- FAD-GF
Family Assessment Device-General Form
- FA
fractional anisotropy
- GCS
Glasgow Coma Scale
- LOC
loss of consciousness
- MRI
magnetic resonance imaging
- MD
mean diffusivity
- MS
mental status
- mTBI
mild traumatic brain injury
- OI
orthopedic injury
- PECARN
Pediatric Emergency Care Applied Research Network
- PCSS
Post Concussion Symptom Survey
- RD
radial diffusivity
- ROI
regions of interest
- SD
standard deviation
- VBA
voxel based analysis
- WM
white matter
Footnotes
Conflict of interest: The authors have no conflict of interest to declare.
References
- 1.Kuppermann N, Holmes JF, Dayan PS, Hoyle JD, Jr, Atabaki SM, Holubkov R, et al. Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. Lancet. 2009;374(9696):1160–70. doi: 10.1016/S0140-6736(09)61558-0. [DOI] [PubMed] [Google Scholar]
- 2.Povlishock JT, Katz DI. Update of neuropathology and neurological recovery after traumatic brain injury. J Head Trauma Rehabil. 2005;20(1):76–94. doi: 10.1097/00001199-200501000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Mori S. Introduction to diffusion tensor imaging. Amsterdam: Oxford: Elsevier; 2007. [Google Scholar]
- 4.Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, Lipton ML. A Decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am J Neuroradiol. 2013;34(11):2064–74. doi: 10.3174/ajnr.A3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shenton ME, Hamoda HM, Schneiderman JS, Bouix S, Pasternak O, Rathi Y, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012;6(2):137–92. doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bigler ED. Neuroimaging biomarkers in mild traumatic brain injury (mTBI) Neuropsychol Rev. 2013;23(3):169–209. doi: 10.1007/s11065-013-9237-2. [DOI] [PubMed] [Google Scholar]
- 7.Kou Z, Wu Z, Tong KA, Holshouser B, Benson RR, Hu J, et al. The role of advanced MR imaging findings as biomarkers of traumatic brain injury. J Head Trauma Rehabil. 2010;25(4):267–82. doi: 10.1097/HTR.0b013e3181e54793. [DOI] [PubMed] [Google Scholar]
- 8.Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neu-rotrauma. 2007;24(9):1447. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- 9.Mayer AR, Ling J, Mannell MV, Gasparovic C, Phillips JP, Doezema D, et al. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology. 2010;74(8):643–50. doi: 10.1212/WNL.0b013e3181d0ccdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayer AR, Ling JM, Yang Z, Pena A, Yeo RA, Klimaj S. Diffusion abnormalities in pediatric mild traumatic brain injury. J Neurosci. 2012;32(50):17961–9. doi: 10.1523/jneurosci.3379-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marmarou A. A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurg Focus. 2007;22(5):E1. doi: 10.3171/foc.2007.22.5.2. [DOI] [PubMed] [Google Scholar]
- 12.Messé A, Caplain S, Paradot G, Garrigue D, Mineo JF, Soto Ares G, et al. Diffusion tensor imaging and white matter lesions at the subacute stage in mild traumatic brain injury with persistent neurobehavioral impairment. Hum Brain Mapp. 32(6):999–1011. doi: 10.1002/hbm.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol. 2002;23(5):794–802. [PMC free article] [PubMed] [Google Scholar]
- 14.Inglese M, Makani S, Johnson G, Cohen BA, Silver JA, Gonen O, et al. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J Neurosurg. 2005;103(2):298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- 15.Miles L, Grossman RI, Johnson G, Babb JS, Diller L, Inglese M. Short-term DTI predictors of cognitive dysfunction in mild traumatic brain injury. Brain Inj. 2008;22(2):115–22. doi: 10.1080/02699050801888816. [DOI] [PubMed] [Google Scholar]
- 16.Kumar R, Gupta RK, Husain M, Chaudhry C, Srivastava A, Saksena S, et al. Comparative evaluation of corpus callosum DTI metrics in acute mild and moderate traumatic brain injury: its correlation with neuropsychometric tests. Brain Inj. 2009;23(7):675–85. doi: 10.1080/02699050903014915. [DOI] [PubMed] [Google Scholar]
- 17.Lipton ML, Gulko E, Zimmerman ME, Friedman BW, Kim M, Gellella E, et al. Diffusion-tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology. 2009;252(3):816–24. doi: 10.1148/radiol.2523081584. [DOI] [PubMed] [Google Scholar]
- 18.Matsushita M, Hosoda K, Naitoh Y, Yamashita H, Kohmura E. Utility of diffusion tensor imaging in the acute stage of mild to moderate traumatic brain injury for detecting white matter lesions and predicting long-term cognitive function in adults. J Neurosurg. 2011;115(1):130–9. doi: 10.3171/2011.2.jns101547. [DOI] [PubMed] [Google Scholar]
- 19.Wilde EA, McCauley SR, Hunter JV, Bigler ED, Chu Z, Wang ZJ, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70(12):948–55. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- 20.Chu Z, Wilde EA, Hunter JV, McCauley SR, Bigler ED, Troyanskaya M, et al. Voxel-based analysis of diffusion tensor imaging in mild traumatic brain injury in adolescents. AJNR Am J Neuroradiol. 2010;31(2):340–6. doi: 10.3174/ajnr.A1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilde EA, McCauley SR, Barnes A, Wu TC, Chu Z, Hunter JV, et al. Serial measurement of memory and diffusion tensor imaging changes within the first week following uncomplicated mild traumatic brain injury. Brain Imaging Behav. 2012;6(2):319–28. doi: 10.1007/s11682-012-9174-3. [DOI] [PubMed] [Google Scholar]
- 22.Yallampalli R, Wilde EA, Bigler ED, McCauley SR, Hanten G, Troyanskaya M, et al. Acute white matter differences in the fornix following mild traumatic brain injury using diffusion tensor imaging. J Neuroimaging. 2013;23(2):224–7. doi: 10.1111/j.1552-6569.2010.00537.x. [DOI] [PubMed] [Google Scholar]
- 23.McAllister TW, Ford JC, Ji S, Beckwith JG, Flashman LA, Paulsen K, et al. Maximum principal strain and strain rate associated with concussion diagnosis correlates with changes in corpus callosum white matter indices. Annals of biomedical engineering. 2012;40(1):127–40. doi: 10.1007/s10439-011-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maugans TA, Farley C, Altaye M, Leach J, Cecil KM. Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics. 2012;129(1):28–37. doi: 10.1542/peds.2011-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kay T, Harrington DE, Adams R, Anderson T, Berrol S, Cicerone K, et al. Report of the Mild Traumatic Brain Injury. Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine. Definition of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8(3):86–7. [Google Scholar]
- 26.Civil ID, Schwab CW. The Abbreviated Injury Scale, 1985 revision: a condensed chart for clinical use. J Trauma. 1988;28(1):87–90. doi: 10.1097/00005373-198801000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Carroll LJ, Cassidy JD, Holm L, Kraus J, Coronado VG. Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;(43 Suppl):113–25. doi: 10.1080/16501960410023877. [DOI] [PubMed] [Google Scholar]
- 28.Achenbach TM, Edelbrock CS. Manual for The Child Behavior Checklist and Revised Child Behavior Profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1983. [Google Scholar]
- 29.Byles J, Byrne C, Boyle MH, Offord DR. Ontario Child Health Study: reliability and validity of the general functioning subscale of the McMaster Family Assessment Device. Fam Process. 1988;27(1):97–104. doi: 10.1111/j.1545-5300.1988.00097.x. [DOI] [PubMed] [Google Scholar]
- 30.Miller IW, Epstein NB, Bishop DS, Keitner GI. The Mc-Master Family Assessment Device: Reliability and Validity. J Marital Fam Ther. 1985;11(4):345–56. [Google Scholar]
- 31.Lovell MR, Iverson GL, Collins MW, Podell K, Johnston KM, Pardini D, et al. Measurement of symptoms following sports-related concussion: reliability and normative data for the post-concussion scale. Appl Neuropsychol. 2006;13(3):166–74. doi: 10.1207/s15324826an1303_4. [DOI] [PubMed] [Google Scholar]
- 32.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM Fsl. Neuroimage. 2012;62(2):782–90. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Wu TC, Wilde EA, Bigler ED, Yallampalli R, McCauley SR, Troyanskaya M, et al. Evaluating the relationship between memory functioning and cingulum bundles in acute mild traumatic brain injury using diffusion tensor imaging. J Neurotrauma. 2010;27(2):303–7. doi: 10.1089/neu.2009.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 35.Yan H, Tian L, Yan J, Sun W, Liu Q, Zhang YB, et al. Functional and anatomical connectivity abnormalities in cognitive division of anterior cingulate cortex in schizophrenia. PLoS One. 2012;7(9):e45659. doi: 10.1371/journal.pone.0045659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ling J, Merideth F, Caprihan A, Pena A, Teshiba T, Mayer AR. Head injury or head motion? Assessment and quantification of motion artifacts in diffusion tensor imaging studies. Hum Brain Mapp. 2012;33(1):50–62. doi: 10.1002/hbm.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ledberg A, Akerman S, Roland PE. Estimation of the probabilities of 3D clusters in functional brain images. Neuroimage. 1998;8(2):113–28. doi: 10.1006/nimg.1998.0336. [DOI] [PubMed] [Google Scholar]
- 38.Schmithorst VJ, Holland SK, Plante E. Diffusion tensor imaging reveals white matter microstructure correlations with auditory processing ability. Ear and hearing. 2011;32(2):156–67. doi: 10.1097/AUD.0b013e3181f7a481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaiser D, Leach J, Vannest J, Schapiro M, Holland S. Unanticipated findings in pediatric neuroimaging research: Prevalence of abnormalities and process for reporting and clinical follow-up. Brain Imaging and Behavior. 2014:1–11. doi: 10.1007/s11682-014-9327-7. [DOI] [PubMed] [Google Scholar]
- 40.Povlishock JT. Pathobiology of traumatically induced axonal injury in animals and man. Ann Emerg Med. 1993;22(6):980–6. doi: 10.1016/s0196-0644(05)82738-6. [DOI] [PubMed] [Google Scholar]
- 41.McGinn MJ, Kelley BJ, Akinyi L, Oli MW, Liu MC, Hayes RL, et al. Biochemical, structural, and biomarker evidence for calpain-mediated cytoskeletal change after diffuse brain injury uncomplicated by contusion. J Neuropathol Exp Neurol. 2009;68(3):241–9. doi: 10.1097/NEN.0b013e3181996bfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tavazzi B, Signoretti S, Lazzarino G, Amorini AM, Delfini R, Cimatti M, et al. Cerebral oxidative stress and depression of energy metabolism correlate with severity of diffuse brain injury in rats. Neurosurg. 2005;56(3):582–9. doi: 10.1227/01.neu.0000156715.04900.e6. [DOI] [PubMed] [Google Scholar]
- 43.Warner MA, Youn TS, Davis T, Chandra A, Marquez de la Plata C, Moore C, et al. Regionally selective atrophy after traumatic axonal injury. Arch Neurol. 2010;67(11):1336–44. doi: 10.1001/archneurol.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dikranian K, Cohen R, Mac Donald C, Pan Y, Brakefield D, Bayly P, et al. Mild traumatic brain injury to the infant mouse causes robust white matter axonal degeneration which precedes apoptotic death of cortical and thalamic neurons. Exp Neurol. 2008;211(2):551–60. doi: 10.1016/j.expneurol.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mac Donald CL, Dikranian K, Bayly P, Holtzman D, Brody D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci. 2007;27(44):11869–76. doi: 10.1523/jneurosci.3647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spain A, Daumas S, Lifshitz J, Rhodes J, Andrews PJ, Horsburgh K, et al. Mild fluid percussion injury in mice produces evolving selective axonal pathology and cognitive deficits relevant to human brain injury. J Neurotrauma. 2010;27(8):1429–38. doi: 10.1089/neu.2010.1288. [DOI] [PubMed] [Google Scholar]
- 47.Rosenblum WI. Cytotoxic edema: Monitoring its magnitude and contribution to brain swelling. J Neuropath Exp Neur. 2007;66(9):771–8. doi: 10.1097/nen.0b013e3181461965. [DOI] [PubMed] [Google Scholar]
- 48.Eierud C, Craddock C, Aulakh M, Fletcher S, King-Casas B, Kuehl D, et al. Spatially and Temporally Resolving the Changes in White Matter in Mild Traumatic Brain Injury. The 19th Annual Meeting of the Organization on Human Brain Mapping; June 16-20; Seattle, WA. 2013. [Google Scholar]
- 49.Bigler ED. Neuropsychology and clinical neuroscience of persistent post-concussive syndrome. J Int Neuropsychol Soc. 2008;14(1):1–22. doi: 10.1017/s135561770808017x. [DOI] [PubMed] [Google Scholar]
- 50.Kinnunen KM, Greenwood R, Powell JH, Leech R, Hawkins PC, Bonnelle V, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134(Pt 2):449–63. doi: 10.1093/brain/awq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12(5):535–40. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]