Abstract
Despite the importance of insulin signaling pathways in human disease, initial concerns that insect physiology and sugar metabolism differ enough from humans that flies would not model human disease hampered research in this area. However, during the past 10–15 years, evidence has accumulated that flies can indeed model various aspects of diabetes and related human disorders. This cluster of diseases impact insulin and insulin signaling pathways, fields which have been discussed in many excellent review articles in recent years. In this chapter, we restrict our focus to specific examples of diabetes-related disease models in Drosophila, discussing the advantages and limitations of these models in light of physiological similarities and differences between insects and mammals. We discuss features of metabolism and sugar regulation that are shared between flies and mammals, and specific Drosophila models for Type 1 and Type 2 diabetes, Metabolic syndrome, and related abnormalities including insulin resistance and heart disease. We conclude that fly models for diabetes and related disorders enhance our ability to identify genes and discern functional interactions that can be exploited for disease intervention.
1. INTRODUCTION
Diabetes and related metabolic disorders are growing health problems worldwide. In the United States alone, incidence of diabetes has increased approximately fourfold between 2008 and 2014 to more than 20 million affected individuals (Centers for Disease Control and Prevention, 2016a). Incidence has been increasing for both adults and children, with differential impact on different ethnic groups. Most severely affected are American Indians and Alaskan Natives, followed by Non-Hispanic Blacks, Hispanics, and Asian Americans. Non-Hispanic Whites show the lowest incidence. Perhaps most alarming in the United States, the CDC SEARCH study noted a 30% rise in diabetic children in recent years (Centers for Disease Control and Prevention, 2016b). Diabetes comprises a set of metabolic disorders defined by increased levels of circulating blood sugar (hyperglycemia) caused by abnormal insulin secretion and/or signaling (American Diabetes Association, 2014). The most common form of childhood diabetes, Type I diabetes (T1D), accounts for 5–10% of all diabetes cases. T1D is an autoimmune disorder that causes destruction of insulin-producing β-cells of the pancreas, resulting in decreased or complete loss of insulin. Among children, the incidence of T1D has increased in recent years (a 20% increase between 2001 and 2009) with the largest rise in Non-Hispanic white children.
Type 2 diabetes (T2D), originally named adult-onset diabetes, accounts for >90% of overall diabetes cases. T2D now affects both children and adults and has a more complex etiology than T1D. It is a disease of insulin resistance, such that hyperglycemia persists despite the presence of high levels of circulating insulin. The incidence of T2D has increased 30% between 2001 and 2009 with American Indians and Alaskan Natives and Non-Hispanic Blacks being disproportionately affected. Correlated, and likely causal in the increasing numbers of individuals with T2D, is the increased incidence of obesity and sedentary lifestyle. It is estimated that approximately 79 million adults (35%) and approximately 13 million children (17%) in the United States are obese—this despite recent widely reported decreases in obesity rates among children this past year. Long-term complications of diabetes include neuropathy, retinopathy, heart disease, and stroke. The cost is measured not only in pain and suffering, but there is also an estimated $245 billion in medical costs and lost wages per year. The differential distribution of disease among ethnic groups points to both environmental and genetic factors influencing the incidence of disease.
Prediabetes, a precursor to T2D, is characterized by insulin resistance and represents a syndrome in its own right. This condition is thought to be frequently undiagnosed, with an estimated 86 million adults (one-third of the US population) affected. Prediabetes can often be controlled by changes in diet and exercise but roughly one quarter of individuals with prediabetes progress to T2D over a 5-year period. A number of other metabolic disorders fall under the general umbrella of diabetes-related disorders. These include gestational diabetes, maturity onset diabetes of the young (MODY) and Metabolic syndrome (MetS) (also known as Syndrome X). Because of their wide prevalence, diabetes and related disorders have been studied and reviewed extensively, as have the roles of insulin signaling pathways in both mammals and Drosophila (for examples, Baker & Thummel, 2007; Das & Dobens, 2015; Garofalo, 2002; Goberdhan & Wilson, 2003; Kahn et al., 2005; Lasko, 2002; Owusu-Ansah & Perrimon, 2014; Padmanabha & Baker, 2014; Tatar, 2004; Wu & Brown, 2006 #141). In this chapter, we have focused on recent studies in which Drosophila models have been developed for specific disease features and types.
2. SUGAR METABOLISM IN DROSOPHILA AND HUMANS
The degree to which any animal can serve as a model for human disease depends upon the degree of similarity among pathways and physiological responses. In some cases, Drosophila can serve as an “in vivo test tube” to test gene function, despite the fact that the physiology differs. For example, the Drosophila eye is an ideal organ to test cellular functions of genes involved in cell growth and viability, even if those genes do not normally function in the fly eye (Bonini & Fortini, 2002; He et al., 2014). Here, we are examining the use of Drosophila as a whole animal model for disease and thus need to ask how conserved are both gene function and physiology. Where do similarities reflect homology and common ancestry, and where are similarities merely superficial? Do signaling pathways end in similar outputs or have signals been co-opted to different downstream processes in different species?
The mechanisms that maintain the balance between stored and circulating forms of energy appear to be largely shared among animals (reviewed in Baker & Thummel, 2007). In mammals, when energy is abundant (after eating), excess glucose is stored as glycogen, primarily in skeletal muscle and the liver. Free fatty acids (FFAs) are stored as triglycerides (TGs) predominantly in adipose tissue but also in the liver (Fig. 1). When energy is scarce (after fasting or exercise), these reserves are mobilized and released for use. Glucose is mobilized first, then FFA. Although skeletal muscle has large reserves of glycogen, the principle organ to mobilize glucose is the liver. The liver can produce glucose in two ways—by breaking down glycogen or by gluconeogenesis from lactate and/or glycerol. Lactate is produced by skeletal muscles when they use glucose, and glycerol is produced by adipocytes when they mobilize the TGs. The principle cells that normally mobilize FFAs are the adipocytes.
Fig. 1.
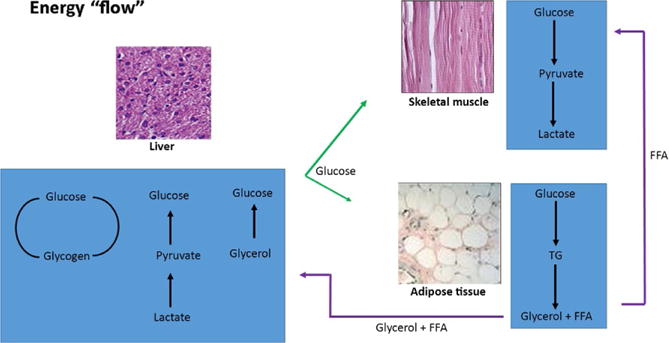
Flow of energy in mammals. Energy is stored as glycogen, primarily in skeletal muscle and the liver. Free fatty acids (FFAs) are stored as triglycerides (TGs) predominantly in adipose tissue. When energy is scarce, these reserves are mobilized and released for use. The principle organ to mobilize glucose is the liver. The liver can produce glucose in two ways—by breaking down glycogen or by gluconeogenesis. Lactate is produced by skeletal muscles when they use glucose, and glycerol is produced by adipocytes when they mobilize the TGs. The principle cells that normally mobilize FFAs are the adipocytes. Images from www.slideshare.net/roger961/adipose-cells-kibbe-sandin-112105 and www.slideshare.net/aravindhravi88/histology-of-liver-by-aravindh-dpi/16.
Mobilization of both glucose and FFA is regulated by the hormones insulin and glucagon, which act in opposition to one another to keep circulating glucose levels stable. When circulating sugar levels rise, insulin is released from the β-cells in the pancreas, triggering glucose uptake and fuel storage (synthesis of glycogen, TGs, and proteins), and repressing secretion of glucagon. When circulating sugar levels drop, glucagon is released from the α-cells of the pancreas triggering the opposite effects on glycogen, TGs, and proteins and repressing insulin secretion.
Circulating glucose levels are sensed by a family of sugar transporters, GLUT proteins, encoded by the SLC2 family of genes (reviewed in Mueckler & Thorens, 2013). While glucose can be taken up into the brain and other tissues by facilitated diffusion via broadly expressed transporters, insulin and insulin-dependent glucose uptake play the major role in homeostatic regulation of circulating sugar levels. Insulin release from the β-cells is exquisitely sensitive to circulating glucose levels, sensed by the glucose transporters GLUT1 and/or GLUT2 at the β-cell surface (reviewed in Joost & Thorens, 2001; Rutter, Pullen, Hodson, & Martinez-Sanchez, 2015). Circulating insulin then promotes the uptake of glucose into peripheral tissues via activation of the insulin-dependent sugar transporter, GLUT4 (Huang & Czech, 2007; Kahn & Cushman, 1986; Lizunov, Matsumoto, Zimmerberg, Cushman, & Frolov, 2005; Lizunov et al., 2012).
Similar processes appear to occur in Drosophila in that insulin-like proteins (Ilps) are released in response to high levels of circulating sugar and a glucagon-like molecule, adipokinetic hormone (AKH) is released in response to low levels of circulating sugar (Colombani et al., 2003; Haselton et al., 2010; Kim & Rulifson, 2004; Lee & Park, 2004). These similarities are discussed in more detail later but we first point out some important differences between the fly and mammalian systems. First, in insects, simple sugars from food are taken up passively from the digestive tract directly into the fat body where they are converted to trehalose, a nonreducing sugar. Trehalose can be stored and/or released into the hemolymph as the primary circulating sugar in insects. Only very low levels of glucose are present insect hemolymph, on the order of one one-hundredth the levels of trehalose (Nation, 2002; Ugrankar et al., 2015; Wyatt & Kale, 1957). Because of this, complications of diabetes that result from the nonspecific glycation of proteins due to the reactivity of high concentrations of glucose, will not be modeled in the fly unless levels of reactive sugars are artificially elevated in this system.
Second, some researchers have questioned the extent to which circulating sugar levels are homeostatically regulated at all in insects (Thompson, Borchardt, & Wang, 2003). Several points suggested that circulating sugars levels might not be homeostatically regulated: In several studies using different insects, levels of circulating sugar were shown to rise with increasing dietary sugar and these sugar levels were shown to reflect metabolic activity of the insect, and to some extent, food source (Abou-Seif et al., 1993; Blatt & Roces, 2001; Maurizio, 1965; Thompson, 1999; Thompson & Redak, 2000; Thompson et al., 2003). Given the fact that high levels of circulating trehalose are likely chemically neutral, it could be argued that there would be little pressure to hormonally regulate them. In addition, insects require the ability to rapidly mobilize energy to fuel flight and much of insect metabolism is thought to be centered around this requirement (Candy, Becker, & Wegener, 1997). This requirement for the immediate availability of large stores of energy—that may be needed to power very long flights—appears to be unique to insects. Finally, the fat body acts as a functional homolog of both the liver and adipocytes in insects suggesting a consolidation of physiological function (Arrese & Soulages, 2010). However, as discussed in the remainder of this chapter, and in contrast to earlier findings and expectations, considerable evidence suggests that flies share with mammals evolutionary ancient mechanisms to regulate sugar homeostasis through conserved and functionally similar pathways.
3. DROSOPHILA MODELS OF TYPE 1 DIABETES
Insulin-like peptides were discovered in Drosophila and other invertebrates in the late 1970s and 1980s (Duve, Thorpe, & Lazarus, 1979; LeRoith, Lesniak, & Roth, 1981; O’Connor & Baxter, 1985; Tager, Markese, Kramer, Speirs, & Childs, 1976), although their roles were not appreciated at the time. Some years later, an insulin-receptor-like protein was purified and the corresponding gene cloned from Drosophila (Nishida, Hata, Nishizuka, Rutter, & Ebina, 1986; Petruzzelli, Herrera, Arenas-Garcia et al., 1986; Petruzzelli, Herrera, Garcia-Arenas, & Rosen, 1986). This receptor (DInR or InR) was shown to function similarly to the mammalian insulin receptor in that it has tyrosine kinase activity and autophosphorylates in response to human insulin, but not other peptide hormones (Fernandez-Almonacid & Rosen, 1987). These studies and many others demonstrated that insulin and insulin-like signaling (IIS) pathways are shared between flies and humans (reviewed in Das & Dobens, 2015; Kannan & Fridell, 2013; Oldham, 2011; Taguchi & White, 2008; Teleman, 2010). Interestingly, insects express a large number of Ilps, from eight in Drosophila to many more in other invertebrates, but only one insulin-like receptor (reviewed in Wu & Brown, 2006). The different Ilps likely play numerous roles in the animal, although to some extent at least, functions in metabolism appear to be redundant (Gronke, Clarke, Broughton, Andrews, & Partridge, 2010). It thus appears that insects have numerous ligands for one receptor, while mammals have receptors with partially redundant functions, but a restricted number of ligands. The cause of this divergence between insect and mammalian lineages remains an unsolved question in the field.
The first indication that Drosophila could serve as a useful model for studying diabetes came from studies of Rulifson, Kim, and Nusse (2002) who showed that ablation of a cluster of brain cells known as insulin-producing cells (IPCs) resulted in animals that display features of T1D (Rulifson et al., 2002). Specifically, these animals displayed an increased level of circulating sugar compared to wild-type controls—the hallmark of diabetes—and this important finding was observed independently by other groups (Broughton et al., 2005; Ikeya, Galic, Belawat, Nairz, & Hafen, 2002). The increase in sugar levels after IPC-ablation was rescued by expression of a Drosophila insulin-like peptide (DILP). The authors went on to propose that IPCs are equivalent to the β-pancreatic islet cells that produce insulin in mammals. In addition to the features of T1D, animals in which IPCs were ablated were small and developmentally delayed, reflecting roles of IIS in both growth and metabolism in insects (Rulifson et al., 2002).
A more recent study examined effects of IPC ablation in adult flies. Similar to results in larvae, circulating sugar levels are higher in these flies than in control animals (Haselton et al., 2010). However, in an elegant set of experiments, Haselton et al. (2010) made use of the fact that adult feeding can be manipulated more readily than larval feeding to perform an Oral Glucose Tolerance Test (OGTT), a test used to diagnose human diabetes. Specifically, animals were fasted, then fed on a glucose solution, and circulating sugar levels were measured over time. Wild-type Drosophila displayed a mammalian-like response with low-circulating sugar levels following the fast, followed by an initial increase after glucose feeding, followed by clearing and return to baseline levels. Ablation of IPCs resulted in higher circulating sugar levels and slower clearance, a response that was abrogated by injection of bovine insulin. This study arguably provides more convincing evidence for flies accurately modeling T1D than those done in larvae, as it made use of a test similar to OGTT, a diagnostic for human diabetes.
Does loss of DILP function explain phenotypes seen for IPC ablation? While IPCs clearly produce DILPs, these cells may have other functions as well that contribute to the “diabetic” phenotypes seen in IPC-ablated larvae and adults. To test this, our lab generated a genomic deletion that simultaneously removed five DILPs (Df[dilp1-5]). This produced animals with “diabetic” symptoms, as well as growth defects and developmental delay similar to those seen for IPC ablation (Zhang et al., 2009). These animals were small (Fig. 2A) and displayed a feature of T1D known as “starvation in the midst of plenty” in that they activated starvation responses even while actively feeding (Fig. 2B–E). Further, Df[dilp1-5] animals had reduced levels of TGs (Fig. 2F), displayed reduced overall metabolic activity (Fig. 2G), and increased levels of circulating sugar (Fig. 2H). Interestingly, circulating sugar levels were slightly but consistently higher in IPC-ablated animals than Df [dilp1-5] animals, suggesting the possibility that additional functions are indeed affected by IPC-ablation. Further study of different combinations of dilp mutants suggested that dilps 2,3 and 5 (Broughton et al., 2005; Ikeya et al., 2002), are primarily responsible for regulation of circulating sugar levels (Gronke et al., 2010), with considerable redundancy among DILPs.
Fig. 2.
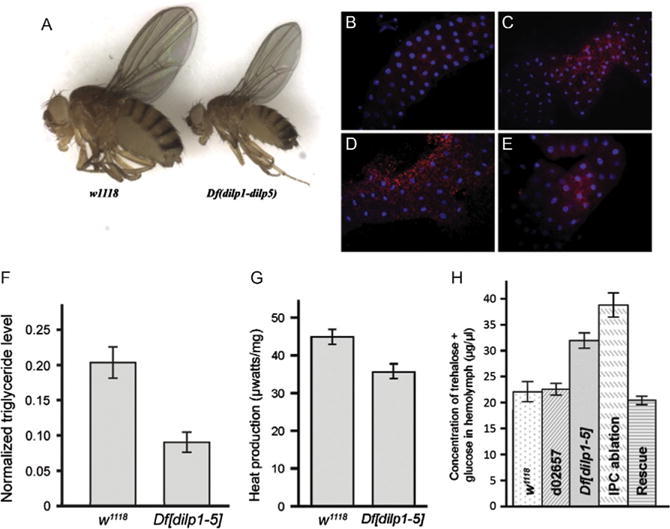
Deletion of dilps 1-5 generates flies with features of T1D. (A) A genomic deletion that removes genes encoding DILPs 1-5 (Df[dilp1-5]) is homozygous viable but flies are developmentally delayed and small. (B–E) Df[dilp1-5] homozygotes induce fat body autophagy while actively eating. Lysotracker staining (red) of dissected fat bodies from third-instar larvae. Hoechst 33342 (blue) reveals nuclei. For B, C, and E, larvae were actively eating, burrowed in the food, and had full guts. Genotypes: (B) Control parental line d02657;(C) Df[dilp1-5]/Df[dilp1-5]; (D) Control parental line d02657;third-instar larva removed from food and starved for 3 h in the presence of water (E) hsGAL4>UASdilp2; Df [dilp1-5]/Df[dilp1-5]. Feeding Df[dilp1-5] homozygotes (C) resembled starved control animals (D). (F) Total body triglyceride levels are lower in Df[dilp1-5] homozygotes. Total body triglyceride level and total body protein from w1118 or Df[dilp1-5] adult male flies were measured; data shows the triglyceride levels normalized to the total protein level. Error bars indicate standard error. (G) Overall metabolic activity was reduced in Df[dilp1-5] homozygotes. (H) Circulating sugar levels are elevated in Df[dilp1-5] homozygotes. Sugar levels (trehalose + glucose) were determined in hemolymph extracted from early third-instar larvae. Levels are indicated for negative controls: w1118 and parental line d02657;for experimental samples: Df[dilp1-5]/Df[dilp1-5], IPC-ablated animals, dilp2-GAL4>UAS-rpr, and rescued hsGAL4>UASdilp2; Df[dilp1-5]/Df[dilp1-5] animals. Larval hemolymph was collected from 10 to 15 animals and pooled for each genotype. The circulating sugar levels of Df[dilp1-5] homozygotes were higher than controls in early and late third-instar larvae (not shown). Levels were lowered by ubiquitous expression of DILP2. Sugar levels were highest in animals in which IPCs had been ablated (dilp2GAL4>UASrpr). Error bars indicate standard error. From Zhang, H., Liu, J., Li, C. R., Momen, B., Kohanski, R. A., & Pick, L. (2009). Deletion of Drosophila insulin-like peptides causes growth defects and metabolic abnormalities. Proceedings of the National Academy of Sciences of the United States of America, 106(46), 19617—19622.
These studies support the notion that T1D can be effectively modeled in the fly because IIS functions in a similar fashion in flies and mammals to regulate circulating sugar levels. However, it is important to note that Df[dilp1-5] homozygous animals, although developmentally delayed and poorly fertile, were viable. In fact they can be maintained in long-term culture under standard lab conditions. This contrasts with lethality seen for mice lacking insulin, which survive to birth but rapidly develop diabetes and die neonatally (Duvillie et al., 1997). This difference between flies and mammals reveals both advantages and disadvantages of the fly system. On one hand, this discrepancy reveals a limitation of this particular fly model, in that lethality seen in mammals is not mimicked. On the other hand, since Df[dilp1-5] flies can be maintained in culture, they provide a model for experimentation, including screens for gene interactions, environmental impacts, and therapeutics.
4. MODELING INSULIN-DEPENDENT SUGAR UPTAKE AND INSULIN RELEASE IN DROSOPHILA
In mammals, insulin mediates sugar uptake by peripheral tissues primarily through activation of the insulin-dependent sugar transporter GLUT4 (Cushman et al., 1998; Dawson, Aviles-Hernandez, Cushman, & Malide, 2001). The “diabetic” fly models discussed earlier suggested that similar mechanisms operate in insects. As a first step to test whether flies have the machinery to mount an insulin-dependent sugar uptake response, our lab generated transgenic flies in which doubly tagged human GLUT4 was expressed in fat cells (Crivat et al., 2013). An HA tag was inserted in the first exofacial loop of GLUT4 to monitor expression at the cell surface (Dawson et al., 2001) and a C-terminal GFP tag was added to monitor transgene expression. We found that fat cells from these transgenic animals responded to mammalian insulin, mobilizing hGLUT4 trafficking, and translocation to the membrane (Fig. 3). This indicates that flies have all the necessary molecules and signals needed to direct mammalian-like vesicular trafficking in response to insulin. Animals that had been sugar-restricted showed an enhanced GLUT4 trafficking response to insulin, suggesting that flies reared on standard food in the laboratory are insulin-resistant (see also below). Thus, Drosophila appear capable of regulating the uptake of circulating sugar in response to hormonal signals in a manner analogous to that used by mammals. Which endogenous sugar transporter(s) function in IIS in the fly and how glucose and trehalose levels are regulated, independently and/or relative to each other, remain to be determined.
Fig. 3.
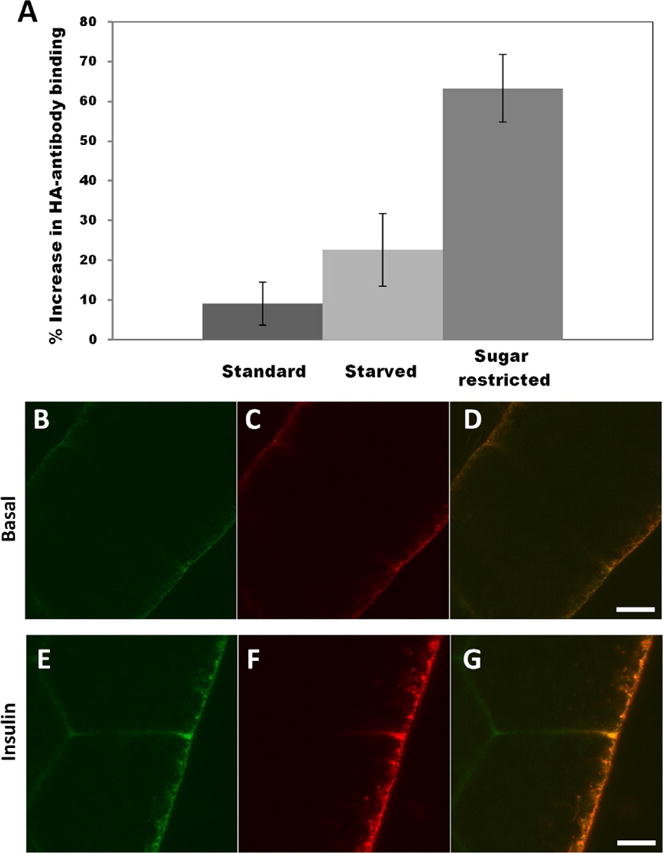
Drosophila harbor the machinery to mediate an insulin-dependent sugar uptake response. (A) Surface exposure of HA-GLUT4 upon insulin stimulation. Fat bodies were collected from larvae reared on different dietary regimes, as indicated. After immunostaining of nonpermeabilized fat body cells, values were determined by measuring Alexa 647 conjugated anti-HA antibody fluorescence and averaging calculations of corrected integrated density. Values are expressed as percent of HA-GLUT4 fluorescence of insulin stimulation over basal conditions. Fat bodies were collected from three animals for each dietary regime. (B–G) Confocal microscopy of HA-GLUT4-GFP expression in fat body cells from animals reared on a sugar-restricted diet in the absence or presence of insulin. (B, C, and D) Basal conditions; (E, F, and G) after addition of 0.1 U/mL insulin. GLUT4 was visualized in nonpermeabilized cells by GFP fluorescence (green B and E) or with anti-HA antibody (red C and F) to monitor membrane translocation. Scale bar is 5 μm. Note increase in GLUT4 at the cell surface (red F). From Crivat, G., Lizunov, V. A, Li, C. R., Stenkula, K. G., Zimmerberg, J., Cushman, S. W., & Pick, L. (2013). Insulin stimulates translocation of human GLUT4 to the membrane in fat bodies of transgenic Drosophila melanogaster. PloS One, 8(11), e77953. doi:10.1371/journal.pone.0077953.
Release of insulin from the β-cells of the pancreas in mammals depends upon glucose sensing by GLUT1 and/or GLUT2 (Joost & Thorens, 2001; Rutter et al., 2015), which triggers glycolysis and the subsequent release of ATP from the mitochondria. The increase in ATP regulates KATP channels in the cell membrane causing the cell to depolarize. Depolarization activates Ca2+ channels and leads to exocytosis of insulin from the β-cells (reviewed in Nassel & Broeck, 2015).
Similar mechanisms appear to trigger the release of insulin from Drosophila IPCs. Studies by Fridell found that IPCs respond to glucose with an influx of Ca2+ and action potentials similar to those seen in mammalian β-cells (Fridell et al., 2009; Kreneisz, Chen, Fridell, & Mulkey, 2010). Furthermore, this response was triggered by a specific KATP channel activator, glibenclamide, implicating KATP channels in the response. Further, GLUT1 appears to play similar roles in flies and mammals. Park et al. (2014) generated a tagged version of DILP2 (ilp2HF) to monitor its secretion (Park et al., 2014). They found a marked increase in ilp2HF-circulating levels upon refeeding after a 24-h fast that is likely a result of glucose sensing by GLUT1 in the IPCs, as IPC-specific knockdown of Glut1 decreased circulating ilp2HF. This investigation also provided additional evidence that ion channels are important for response to glucose in that expression of Kir2.1, a potassium channel that can silence electrical activity of Drosophila neurons and neuroendocrine cells (Kim & Rulifson, 2004) in adult IPCs led to significantly reduced levels of circulating Ilp2HF. Together, these studies suggest that at a mechanistic level, the “diabetic” increase in circulating sugar levels seen in Drosophila models do in fact reflect evolutionarily conserved, hormonally mediated sugar uptake responses.
5. DROSOPHILA AS A MODEL FOR INSULIN RESISTANCE AND TYPE 2 DIABETES
To our knowledge, the first paper to explicitly model T2D in Drosophila did so by testing the role of a high-sugar diet (HSD) on acquisition of insulin resistance (Musselman et al., 2011). In this study, the authors showed that larvae reared from hatching on HSDs have increased levels of circulating sugar, both glucose and trehalose. These results are consistent with findings in honeybees and other insects that hemolymph sugar levels increase in response to HSDs (see above). However, even in nondiabetic humans, circulating sugar levels spike after a high-sugar meal. Typically, a medical diagnosis of insulin resistance involves a glucose clearing or glucose tolerance test in which the ability to clear glucose from the blood after a period of fasting is assessed (see Haselton et al., 2010). This type of test is difficult to perform with Drosophila at larval stages, since larvae eat constantly until the wandering stage when they stop feeding prior to pupation. Thus, the finding that animals continuously feeding on HSDs were hyperglycemic is not sufficient to conclude that they were in a diabetic-like state.
However, the authors provided additional evidence that these animals displayed features of insulin resistance (Musselman et al., 2011). First, rearing on HSD resulted in higher levels of expression of dilp 2,3,5 RNA and circulating DILP2 (as judged with tagged DILP2). Thus, despite higher levels of DILP(s) in circulation, sugar levels remained high—a feature resembling mammalian insulin resistance. Second, they observed decreased levels of phospho-Akt in response to administration of exogenous insulin in flies reared on HSD, suggesting a weakened ability to respond to insulin signaling after chronic levels of high sugar in the diet. Finally, flies reared on HSD had somewhat higher levels of TGs and FFAs as compared to controls fed an isocaloric diet, and the morphology of lipid stores changed, with fewer, larger droplets (Musselman et al., 2013).
Together, these studies showed that flies reared on chronic HSD are hyperglycemic, insulin-resistant and obese, all features of T2D and the precursors thereof. However, attention to detail is necessary when carrying out and interpreting these types of experiments. Musselman et al. (2013) point out that the sugar levels used in the high-sugar feeding experiments are similar to that of a banana, a natural food for these flies. This raises the suggestion that flies are “naturally” diabetic in the wild. Similarly, standard lab conditions may lead to “T2D flies,” as Crivat et al. found that mobilization of a human GLUT4 transgene in response to insulin was enhanced upon sugar-restriction (Crivat et al., 2013). Furthermore, levels of circulating glucose were shown to be influenced by larval density (Ugrankar et al., 2015), an important factor since density may vary in lab conditions in a genotype-dependent fashion, confounding effects of genetic differences. Finally, larvae undergo a natural starvation stage at the end of the third instar—a physiologically “normal” fasting that may result in metabolic changes different from forced fasting at other developmental stages or in mammals. Thus, overall, while it is clear that modeling T2D in flies is possible, such modeling requires extreme caution in controlling for genetic differences, rearing conditions and extrapolating to the human condition. To learn more about modeling T2D in Drosophila, we suggest a recent review on this topic, published while this chapter was in press (Alfa & Kim, 2016).
6. ARE GLUCOSE AND TREHALOSE METABOLISM REGULATED INDEPENDENTLY IN DROSOPHILA?
In most studies that examined levels of circulating sugar described above, total sugars were reported. The standard assay (Rulifson et al., 2002) was to extract hemolymph and incubate it with trehalase to generate glucose, which is then measured spectrophotometrically. The justification for this approach was twofold: First, trehalose levels are up to 100-fold higher in circulation than glucose in insects, and second, trehalose may be broken down to glucose during the extraction process, thus artificially raising estimates of circulating glucose levels. More recent studies have attempted to separately measure each of these circulating sugars.
Pasco and Leopold observed that larvae fed a HSD increased circulating glucose but not trehalose within minutes of high-sugar feeding (Pasco & Leopold, 2012). Larvae reared for longer term on moderately HSD showed increases in both glucose and trehalose by the end of this life stage. Consistent with the findings of Musselman et al. (2013) discussed earlier, larvae reared on HSD displayed features of insulin resistance, including increased levels of circulating DILPs, as well InR expression. Further, isolated fat bodies from these animals exhibited a decreased response to exogenous insulin, suggestive of insulin resistance resulting from long-term feeding on a HSD.
A starker difference in responses seen for glucose and trehalose was observed by Ugrankar et al. (2015) who found an increase in glucose but not trehalose in larvae fed HSD. Circulating glucose levels were also increased in animals carrying mutations in Glut1, chico, and PI3K, while trehalose levels were not affected. These results are consistent with earlier findings in silk moth that trehalose synthesis is inhibited by free trehalose, which also promotes glycogen synthesis. This feedback regulation would result in relatively constant trehalose levels over a range of feeding conditions (Murphy & Wyatt, 1965). However, they appear to differ from previous studies that documented stable changes in trehalose levels in response to loss of DILP function, IPC-ablation, and changes in diet.
What is suggested by Ugrankar et al. (2015) is a more flexible response for glucose than trehalose in Drosophila. Speculating, one could imagine that pathways regulating glucose levels in flies may be those similar to mammalian regulators, while trehalose may be under a regulatory program that is more insect specific. Additionally, circulating glucose may be more tightly regulated than circulating trehalose in insects because it is a reducing sugar that can have negative impact, even though the overall levels of glucose are quite low.
7. DROSOPHILA AS A MODEL FOR OBESITY-RELATED HEART DISEASE
In addition to negative impacts of HSDs, high-fat diets (HFD) are strongly correlated with obesity, heart disease, and T2D in humans (Szendroedi & Roden, 2009; van Herpen & Schrauwen-Hinderling, 2008). HFD caused flies to respond initially (2 days) with increased DILP2 and decreased total glucose (Birse et al., 2010) but, when the HFD was continued for 3 or more days, DILP2 levels dropped, glucose levels rose, and pAKT levels decreased, suggesting that Drosophila respond to HFD with a physiology similar to mammals. Flies also accumulated lipids (specifically TGs) in both the fat body and the midgut in a dose-dependent manner, mimicking major risk factors for T2D and related conditions.
Drosophila are the only invertebrate model organism with a beating heart for which the tools and assays needed to study heart function are available (reviewed in Diop & Bodmer, 2015; Wessells, Fitzgerald, Cypser, Tatar, & Bodmer, 2004). As for IIS, early studies identified genes involved in specification of the Drosophila heart that are shared with vertebrates (Bodmer & Venkatesh, 1998). Furthermore, although Drosophila have a simple tubular heart and an open circulatory system, there seems to be a common physiology with the vertebrate heart. Early studies demonstrated that mutations in ion channels associated with human cardiac arrhythmias also influence heart rate and/or rhythm in Drosophila (see Ganetzky, 2000). More recently, it was shown that chronic high-sugar feeding also led to heart defects in flies (Na et al., 2013) and a HFD increased TG levels and altered contraction patterns, similar to the effects seen in mammals (Birse et al., 2010). In addition, genetically altering metabolic regulators common to both vertebrates and Drosophila led to obesity and related heart defects. The defects caused by a HFD appear to result from deposition of TG in the heart itself, as they were abrogated by genetically altering lipid metabolism in the entire fly, the FB or in the heart (Birse et al., 2010). Together, these findings lead to the somewhat surprising conclusion that Drosophila serve as an ideal model to dissect mechanisms underlying diet-induced heart disease.
8. DROSOPHILA AS A MODEL FOR METABOLIC SYNDROME
Metabolic syndrome (MetS) has been defined by a cluster of clinical presentations that include at least three of five risk factors: Fasting glucose at levels indicative of insulin resistance or even T2D, high blood pressure, elevated TGs, low HDL-cholesterol, and obesity (Alberti, Zimmet, Shaw, & IDF Epidemiology Task Force Consensus Group, 2005). These metabolic abnormalities, also referred to as insulin resistance syndrome, present a high risk of cardiovascular disease. Reed and coworkers have taken a novel approach to identifying the genetic, environmental, and gene × environment (specifically diet, Gene × Diet [G × D]) contributions to features of MetS contributions that are difficult to disentangle in human populations. Using a series of inbred Drosophila lines, they compared effects of diet on traits including weight, TG levels, circulating sugar, and survival. In a comparison of 146 inbred lines obtained from two independent wild populations, they observed that gene and G × D interactions outweighed diet alone in contributing to observed variance, with G × D interactions accounting for ~15% of the variance for the traits most closely resembling those seen in MetS (Reed et al., 2010). They also found that metabolic trait variance increased when flies were reared on a HFD. Interestingly, they found that across genotypes, flies reared on the lowest sugar diets weighed the most, which may reflect a suppression of feeding in high-sugar conditions, as seen in other studies (Lebreton, Witzgall, Olsson, & Becher, 2014; Rovenko et al., 2015).
Reed and coworkers went on to analyze global changes in transcriptome and metabolic profiles in response to varying diets, assessing variations within and across populations at a systems level. Among other findings, these authors identified small groups of candidate genes and metabolites that had not been previously associated with metabolic disorders for further examination. Finally, several studies from these researchers suggested that when populations were exposed to new environments, latent, or cryptic genetic variation was revealed that cannot be observed in long-term stable environments (Gibson & Reed, 2008; Reed et al., 2014, 2010). This may be particularly significant for diseases like MetS, insulin resistance, and T2D, for which dietary changes appear to have had large impacts on disease rates in human populations over a relatively short-time scale (Reed et al., 2014; Williams et al., 2015). Overall, these studies provide a wealth of information on the complexities of metabolic variation in flies that remains to be mined in the future and which will likely have high relevance for human populations.
9. FROM CORRELATION TO CAUSATION: DROSOPHILA AS A MODEL TO STUDY GENE FUNCTION IN METABOLISM
Given the complexity of diabetes and related disorders, genome-wide association studies (GWAS) have been used to identify genetic variants associated with susceptibility to disease, using data from human populations. This is a powerful technique that establishes a correlation between genomic markers and disease risk. However, GWAS alone points to a large number of candidate genes that then need to be evaluated for function. The power of Drosophila genetics makes it an ideal system to screen through numerous candidates to identify causal genes.
Pendse et al. (2013) were among the first to demonstrate that flies can be used to test the function of candidate genes identified in GWAS for diabetes and related disorders, serving as a proof of principle that Drosophila can be used for large-scale screening to narrow down genes associated with SNPs linked to metabolic disease. Starting with 83 orthologs of 71 T2D candidate human genes, they used RNAi knockdown to screen for roles in sugar metabolism based upon larval sucrose tolerance. This screen identified a number of expected candidate genes and further analyzed the role of one of these, dHHEX, which was shown to regulate circulating glucose levels in Drosophila.
Similarly, Park et al. (2014) analyzed loss-of-function mutations in fly orthologs of genes identified as candidates for T2D in human GWAS studies (Park et al., 2014). IPC-specific knockdown of one of these, the fly ortholog of Glis3 (fly Imd) resulted in a decrease in circulating DILP2, similar to the situation in mice where Glis3 regulates insulin expression (Yang, Chang, & Chan, 2013). IPC-specific knockdown of a second gene that had not been characterized in mice, BCL11A, increased circulating DILP2 but not mRNA levels, suggesting a role for this gene in insulin secretion. Although more work remains to be done to determine the mechanisms of action of these genes, they clearly support the notion that flies are a facile model system to study the function of candidate genes. Flies will, of course, be an ideal system to study mechanism of action of these genes as well.
In addition to screening GWAS candidates, flies can also be used as the initial organism to screen for genes potentially involved in metabolic disorders. For example, Pasco and Leopold (2012) revealed a new role for lipocalins in Drosophila metabolism, a class of molecules previously implicated in insulin resistance in mammals. Ugrankar et al. (2015) performed large scale RNAi screens of ~1000 genes and identified >150 that resulted in hyperglycemia. This pool included 76 novel candidates, one of which (CSNK1a1) was tested in mice. Interestingly, loss-of-function mutations of CSNK1a in the adipose tissue of mice caused hyperglycemia, a clear demonstration that genetic screens in Drosophila can be used to identify genes important in mammalian sugar metabolism.
10. CONCLUDING REMARKS
Drosophila have been used to model a large variety of diseases, including both T1D and T2D, as well as related metabolic syndromes. These models reveal that many aspects of sugar metabolism are shared between insects and humans, despite obvious physiological differences. Drosophila bring the advantage of powerful genetic tools that enable gene identification and mechanistic studies. All of the tools have been applied to model diseases of insulin resistance and have identified several new candidate genes for human disease. Future studies with these model systems will build upon these fundamental approaches to identify additional genes and pathways impacting insulin resistance. The use of high-throughput screens and studies of pharmacologic interventions in these fly models have the potential to elucidate mechanisms of drug action and to identify viable therapeutic interventions for human patients.
Acknowledgments
Work on insulin signaling in the Pick lab was supported by the March of Dimes Birth Defects Foundation (1-FY-10-368) and the National Institutes of Health (ROI EY14290).
References
- Abou-Seif MA, Maier V, Fuchs J, Mezger M, Pfeiffer EF, Bounias M. Fluctuations of carbohydrates in haemolymph of honeybee (Apis mellifica) after fasting, feeding and stress. Hormone and Metabolic Research. 1993;25(1):4–8. doi: 10.1055/s-2007-1002034. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet P, Shaw J, IDF Epidemiology Task Force Consensus Group The metabolic syndrome—A new worldwide definition. Lancet. 2005;366(9491):1059–1062. doi: 10.1016/S0140-6736(05)67402-8. http://dx.doi.org/10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- Alfa RW, Kim SK. Using Drosophila to discover mechanisms underlying type 2 diabetes. Disease Models & Mechanisms. 2016;9(4):365–376. doi: 10.1242/dmm.023887. http://dx.doi.org/10.1242/dmm.023887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):581–590. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- Arrese EL, Soulages JL. Insect fat body: Energy, metabolism, and regulation. Annual Review of Entomology. 2010;55:207–225. doi: 10.1146/annurev-ento-112408-085356. http://dx.doi.org/10.1146/annurev-ento-112408-085356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KD, Thummel CS. Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metabolism. 2007;6(4):257–266. doi: 10.1016/j.cmet.2007.09.002. http://dx.doi.org/10.1016/j.cmet.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birse RT, Choi J, Reardon K, Rodriguez J, Graham S, Diop S, Oldham S. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metabolism. 2010;12(5):533–544. doi: 10.1016/j.cmet.2010.09.014. http://dx.doi.org/10.1016/j.cmet.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt J, Roces F. Haemolymph sugar levels in foraging honeybees (Apis mellifera carnica): Dependence on metabolic rate and in vivo measurement of maximal rates of trehalose synthesis. The Journal of Experimental Biology. 2001;204(Pt 15):2709–2716. doi: 10.1242/jeb.204.15.2709. [DOI] [PubMed] [Google Scholar]
- Bodmer R, Venkatesh TV. Heart development in Drosophila and vertebrates: Conservation of molecular mechanisms. Developmental Genetics. 1998;22(3):181–186. doi: 10.1002/(SICI)1520-6408(1998)22:3<181::AID-DVG1>3.0.CO;2-2. http://dx.doi.org/10.1002/(SICI)1520-6408(1998)22:3<181∷AID-DVG1->3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Bonini NM, Fortini ME. Applications of the Drosophila retina to human disease modeling. Results and Problems in Cell Differentiation. 2002;37:257–275. doi: 10.1007/978-3-540-45398-7_15. [DOI] [PubMed] [Google Scholar]
- Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, Driege Y, Partridge L. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(8):3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candy DJ, Becker A, Wegener G. Coordination and integration of metabolism and insect flight. Comparative Biochemistry and Physiology. 1997;117B:497–512. [Google Scholar]
- Centers for Disease Control and Prevention. 2016a http://www.cdc.gov/diabetes.
- Centers for Disease Control and Prevention. 2016b https://www.searchfordiabetes.org/dspHome.cfm. SEARCH.
- Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Leopold P. A nutrient sensor mechanism controls Drosophila growth. Cell. 2003;114(6):739–749. doi: 10.1016/s0092-8674(03)00713-x. [DOI] [PubMed] [Google Scholar]
- Crivat G, Lizunov VA, Li CR, Stenkula KG, Zimmerberg J, Cushman SW, Pick L. Insulin stimulates translocation of human GLUT4 to the membrane in fat bodies of transgenic Drosophila melanogaster. PloS One. 2013;8(11):e77953. doi: 10.1371/journal.pone.0077953. http://dx.doi.org/10.1371/journal.pone.0077953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman SW, Goodyear LJ, Pilch PF, Ralston E, Galbo H, Ploug T, Klip A. Molecular mechanisms involved in GLUT4 translocation in muscle during insulin and contraction stimulation. Advances in Experimental Medicine and Biology. 1998;441:63–71. doi: 10.1007/978-1-4899-1928-1_6. [DOI] [PubMed] [Google Scholar]
- Das R, Dobens LL. Conservation of gene and tissue networks regulating insulin signalling in flies and vertebrates. Biochemical Society Transactions. 2015;43(5):1057–1062. doi: 10.1042/BST20150078. http://dx.doi.org/10.1042/BST20150078. [DOI] [PubMed] [Google Scholar]
- Dawson K, Aviles-Hernandez A, Cushman SW, Malide D. Insulin-regulated trafficking of dual-labeled glucose transporter 4 in primary rat adipose cells. Biochemical and Biophysical Research Communications. 2001;287(2):445–454. doi: 10.1006/bbrc.2001.5620. http://dx.doi.org/10.1006/bbrc.2001.5620. [DOI] [PubMed] [Google Scholar]
- Diop SB, Bodmer R. Gaining insights into diabetic cardiomyopathy from Drosophila. Trends in Endocrinology and Metabolism. 2015;26(11):618–627. doi: 10.1016/j.tem.2015.09.009. http://dx.doi.org/10.1016/j.tem.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duve H, Thorpe A, Lazarus NR. Isolation of material displaying insulin-like immunological biological activity from the brain of the blowfly Calliphora vomitoria. The Biochemical Journal. 1979;184(2):221–227. doi: 10.1042/bj1840221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvillie B, Cordonnier N, Deltour L, Dandoy-Dron F, Itier JM, Monthioux E, Bucchini D. Phenotypic alterations in insulin-deficient mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(10):5137–5140. doi: 10.1073/pnas.94.10.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Almonacid R, Rosen OM. Structure and ligand specificity of the Drosophila melanogaster insulin receptor. Molecular and Cellular Biology. 1987;7:2718–2727. doi: 10.1128/mcb.7.8.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridell YW, Hoh M, Kreneisz O, Hosier S, Chang C, Scantling D, Helfand SL. Increased uncoupling protein (UCP) activity in Drosophila insulin-producing neurons attenuates insulin signaling and extends lifespan. Aging (Albany NY) 2009;1(8):699–713. doi: 10.18632/aging.100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganetzky B. Genetic analysis of ion channel dysfunction in Drosophila. Kidney International. 2000;57(3):766–771. doi: 10.1046/j.1523-1755.2000.00913.x. http://dx.doi.org/10.1046/j.1523-1755.2000.00913.x. [DOI] [PubMed] [Google Scholar]
- Garofalo RS. Genetic analysis of insulin signaling in Drosophila. Trends in Endocrinology and Metabolism. 2002;13(4):156–162. doi: 10.1016/s1043-2760(01)00548-3. [DOI] [PubMed] [Google Scholar]
- Gibson G, Reed LK. Cryptic genetic variation. Current Biology. 2008;18(21):R989–R990. doi: 10.1016/j.cub.2008.08.011. http://dx.doi.org/10.1016/j.cub.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goberdhan DC, Wilson C. The functions of insulin signaling: Size isn’t everything, even in Drosophila. Differentiation. 2003;71(7):375–397. doi: 10.1046/j.1432-0436.2003.7107001.x. [DOI] [PubMed] [Google Scholar]
- Gronke S, Clarke DF, Broughton S, Andrews TD, Partridge L. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genetics. 2010;6(2):e1000857. doi: 10.1371/journal.pgen.1000857. http://dx.doi.org/10.1371/journal.pgen.1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselton A, Sharmin E, Schrader J, Sah M, Poon P, Fridell YW. Partial ablation of adult Drosophila insulin-producing neurons modulates glucose homeostasis and extends life span without insulin resistance. Cell Cycle. 2010;9(15):3063–3071. doi: 10.4161/cc.9.15.12458. http://dx.doi.org/10.4161/cc.9.15.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BZ, Ludwig MZ, Dickerson DA, Barse L, Arun B, Vilhjalmsson BJ, Kreitman M. Effect of genetic variation in a Drosophila model of diabetes-associated misfolded human proinsulin. Genetics. 2014;196(2):557–567. doi: 10.1534/genetics.113.157800. http://dx.doi.org/10.1534/genetics.113.157800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metabolism. 2007;5(4):237–252. doi: 10.1016/j.cmet.2007.03.006. http://dx.doi.org/10.1016/jxmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Current Biology. 2002;12(15):1293–1300. doi: 10.1016/s0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: Nomenclature, sequence characteristics, and potential function of its novel members (review) Molecular Membrane Biology. 2001;18(4):247–256. doi: 10.1080/09687680110090456. [DOI] [PubMed] [Google Scholar]
- Kahn BB, Cushman SW. Cell biology of insulin’s stimulatory action on glucose transport and its perturbation in altered metabolic states. The Annals of the New York Academy of Sciences. 1986;488:356–369. [PubMed] [Google Scholar]
- Kahn RC, King GL, Moses AC, Weir GC, Jacobson AM, Smith RJ. Joslin’s diabetes mellitus. 14th. Boston, MA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- Kannan K, Fridell YW. Functional implications of Drosophila insulin-like peptides in metabolism, aging, and dietary restriction. Frontiers in Physiology. 2013;4:288. doi: 10.3389/fphys.2013.00288. http://dx.doi.org/10.3389/fphys.2013.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Rulifson EJ. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature. 2004;431(7006):316–320. doi: 10.1038/nature02897. [DOI] [PubMed] [Google Scholar]
- Kreneisz O, Chen X, Fridell YW, Mulkey DK. Glucose increases activity and Ca2+ in insulin-producing cells of adult Drosophila. Neuroreport. 2010;21(17):1116–1120. doi: 10.1097/WNR.0b013e3283409200. http://dx.doi.org/10.1097/WNR.0b013e3283409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasko P. Diabetic flies? Using Drosophila melanogaster to understand the causes of monogenic and genetically complex diseases. Clinical Genetics. 2002;62(5):358–367. doi: 10.1034/j.1399-0004.2002.620502.x. [DOI] [PubMed] [Google Scholar]
- Lebreton S, Witzgall P, Olsson M, Becher PG. Dietary glucose regulates yeast consumption in adult Drosophila males. Frontiers in Physiology. 2014;5:504. doi: 10.3389/fphys.2014.00504. http://dx.doi.org/10.3389/fphys.2014.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004;167(1):311–323. doi: 10.1534/genetics.167.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoith D, Lesniak MA, Roth J. Insulin in insects and annelids. Diabetes. 1981;30(1):70–76. doi: 10.2337/diab.30.1.70. [DOI] [PubMed] [Google Scholar]
- Lizunov VA, Matsumoto H, Zimmerberg J, Cushman SW, Frolov VA. Insulin stimulates the halting, tethering, and fusion of mobile GLUT4 vesicles in rat adipose cells. The Journal of Cell Biology. 2005;169(3):481–489. doi: 10.1083/jcb.200412069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizunov VA, Stenkula KG, Lisinski I, Gavrilova O, Yver DR, Chadt A, Cushman SW. Insulin stimulates fusion, but not tethering, of GLUT4 vesicles in skeletal muscle of HA-GLUT4-GFP transgenic mice. American Journal of Physiology Endocrinology and Metabolism. 2012;302(8):E950–E960. doi: 10.1152/ajpendo.00466.2011. http://dx.doi.org/10.1152/ajpendo.00466.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurizio A. Studies on the sugar spectrum of the hemolymph of the honeybee (Apis mellifica L.). I. The blood sugar spectrum of adult bees. Journal of Insect Physiology. 1965;11(6):745–763. doi: 10.1016/0022-1910(65)90155-1. [DOI] [PubMed] [Google Scholar]
- Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Molecular Aspects of Medicine. 2013;34(2–3):121–138. doi: 10.1016/j.mam.2012.07.001. http://dx.doi.org/10.1016/j.mam.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TA, Wyatt GR. The enzymes of glycogen and trehalose synthesis in silk moth fat body. The Journal of Biological Chemistry. 1965;240:1500–1508. [PubMed] [Google Scholar]
- Musselman LP, Fink JL, Narzinski K, Ramachandran PV, Hathiramani SS, Cagan RL, Baranski TJ. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Disease Models & Mechanisms. 2011;4(6):842–849. doi: 10.1242/dmm.007948. http://dx.doi.org/10.1242/dmm.007948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman LP, Fink JL, Ramachandran PV, Patterson BW, Okunade AL, Maier E, Baranski TJ. Role of fat body lipogenesis in protection against the effects of caloric overload in Drosophila. The Journal of Biological Chemistry. 2013;288(12):8028–8042. doi: 10.1074/jbc.M112.371047. http://dx.doi.org/10.1074/jbc.M112.371047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na J, Musselman LP, Pendse J, Baranski TJ, Bodmer R, Ocorr K, Cagan R. A Drosophila model of high sugar diet-induced cardiomyopathy. PLoS Genetics. 2013;9(1):e1003175. doi: 10.1371/journal.pgen.1003175. http://dx.doi.org/10.1371/journal.pgen.1003175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassel DR, Broeck JV. Insulin/IGF signaling in Drosophila and other insects: Factors that regulate production, release and post-release action of the insulin-like peptides. Cellular and Molecular Life Sciences. 2015;73(2):271–290. doi: 10.1007/s00018-015-2063-3. http://dx.doi.org/10.1007/s00018-015-2063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation JL. Insect physiology and biochemistry. Boca Raton: CRC Press LLC; 2002. [Google Scholar]
- Nishida Y, Hata M, Nishizuka Y, Rutter WJ, Ebina Y. Cloning of a Drosophila cDNA encoding a polypeptide similar to the human insulin receptor precursor. Biochemical and Biophysical Research Communications. 1986;141:474–481. doi: 10.1016/s0006-291x(86)80197-8. [DOI] [PubMed] [Google Scholar]
- O’Connor KJ, Baxter D. The demonstration of insulin-like material in the honey bee, Apis mellifera. Comparative Biochemistry and Physiology. 1985;81B:755–760. [Google Scholar]
- Oldham S. Obesity and nutrient sensing TOR pathway in flies and vertebrates: Functional conservation of genetic mechanisms. Trends in Endocrinology and Metabolism. 2011;22(2):45–52. doi: 10.1016/j.tem.2010.11.002. http://dx.doi.org/10.1016/j.tem.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Ansah E, Perrimon N. Modeling metabolic homeostasis and nutrient sensing in Drosophila: Implications for aging and metabolic diseases. Disease Models & Mechanisms. 2014;7(3):343–350. doi: 10.1242/dmm.012989. http://dx.doi.org/10.1242/dmm.012989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabha D, Baker KD. Drosophila gains traction as a repurposed tool to investigate metabolism. Trends in Endocrinology and Metabolism. 2014;25(10):518–527. doi: 10.1016/j.tem.2014.03.011. http://dx.doi.org/10.1016/j.tem.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Park S, Alfa RW, Topper SM, Kim GE, Kockel L, Kim SK. A genetic strategy to measure circulating Drosophila insulin reveals genes regulating insulin production and secretion. PLoS Genetics. 2014;10(8):e1004555. doi: 10.1371/journal.pgen.1004555. http://dx.doi.org/10.1371/journal.pgen.1004555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasco MY, Leopold P. High sugar-induced insulin resistance in Drosophila relies on the lipocalin Neural Lazarillo. PloS One. 2012;7(5):e36583. doi: 10.1371/journal.pone.0036583. http://dx.doi.org/10.1371/journal.pone.0036583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendse J, Ramachandran PV, Na J, Narisu N, Fink JL, Cagan RL, Baranski TJ. A Drosophila functional evaluation of candidates from human genome-wide association studies of type 2 diabetes and related metabolic traits identifies tissue-specific roles for dHHEX. BMC Genomics. 2013;14:136. doi: 10.1186/1471-2164-14-136. http://dx.doi.org/10.1186/1471-2164-14-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzelli L, Herrera R, Arenas-Garcia R, Fernandez R, Birnbaum MJ, Rosen OM. Isolation of a Drosophila genomic sequence homologous to the kinase domain of the human insulin receptor and detection of the phosphorylated Drosophila receptor with an anti-peptide antibody. Proceedings of the National Academy of Sciences of the United States of America. 1986a;83:4710–4714. doi: 10.1073/pnas.83.13.4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzelli L, Herrera R, Garcia-Arenas R, Rosen OM. Acquisition of insulin-dependent protein tyrosine kinase activity during Drosophila embryogenesis. The Journal of Biological Chemistry. 1986b;260:16072–16075. [PubMed] [Google Scholar]
- Reed LK, Lee K, Zhang Z, Rashid L, Poe A, Hsieh B, Gibson G. Systems genomics of metabolic phenotypes in wild-type Drosophila melanogaster. Genetics. 2014;197(2):781–793. doi: 10.1534/genetics.114.163857. http://dx.doi.org/10.1534/genetics.114.163857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LK, Williams S, Springston M, Brown J, Freeman K, DesRoches CE, Gibson G. Genotype-by-diet interactions drive metabolic phenotype variation in Drosophila melanogaster. Genetics. 2010;185(3):1009–1019. doi: 10.1534/genetics.109.113571. http://dx.doi.org/10.1534/genetics.109.113571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovenko BM, Perkhulyn NV, Gospodaryov DV, Sanz A, Lushchak OV, Lushchak VI. High consumption of fructose rather than glucose promotes a diet-induced obese phenotype in Drosophila melanogaster. Comparative Biochemistry and Physiology Part A, Molecular & Integrative Physiology. 2015;180:75–85. doi: 10.1016/j.cbpa.2014.11.008. http://dx.doi.org/10.1016/j.cbpa.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: Growth and diabetic phenotypes. Science. 2002;296(5570):1118–1120. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- Rutter GA, Pullen TJ, Hodson DJ, Martinez-Sanchez A. Pancreatic betacell identity, glucose sensing and the control of insulin secretion. The Biochemical Journal. 2015;466(2):203–218. doi: 10.1042/BJ20141384. http://dx.doi.org/10.1042/BJ20141384. [DOI] [PubMed] [Google Scholar]
- Szendroedi J, Roden M. Ectopic lipids and organ function. Current Opinion in Lipidology. 2009;20(1):50–56. doi: 10.1097/mol.0b013e328321b3a8. [DOI] [PubMed] [Google Scholar]
- Tager HS, Markese J, Kramer KJ, Speirs RD, Childs CN. Glucagon-like and insulin-like hormones of the insect neurosecretory system. The Biochemical Journal. 1976;156(3):515–520. doi: 10.1042/bj1560515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, White MF. Insulin-like signaling, nutrient homeostasis, and life span. Annual Review of Physiology. 2008;70:191–212. doi: 10.1146/annurev.physiol.70.113006.100533. [DOI] [PubMed] [Google Scholar]
- Tatar M. The neuroendocrine regulation of Drosophila aging. Experimental Gerontology. 2004;39(11–12):1745–1750. doi: 10.1016/j.exger.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Teleman AA. Molecular mechanisms of metabolic regulation by insulin in Drosophila. The Biochemical Journal. 2010;425(1):13–26. doi: 10.1042/BJ20091181. http://dx.doi.org/10.1042/BJ20091181. [DOI] [PubMed] [Google Scholar]
- Thompson SN. Blood sugar formation from dietary carbohydrate is facilitated by the pentose phosphate pathway in an insect Manduca sexta Linnaeus. Biochimica et Biophysica Acta. 1999;1472(3):565–575. doi: 10.1016/s0304-4165(99)00163-4. [DOI] [PubMed] [Google Scholar]
- Thompson SN, Borchardt DB, Wang LW. Dietary nutrient levels regulate protein and carbohydrate intake, gluconeogenic/glycolytic flux and blood trehalose level in the insect Manduca sexta L. Journal of Comparative Physiology B. 2003;173(2):149–163. doi: 10.1007/s00360-002-0322-8. [DOI] [PubMed] [Google Scholar]
- Thompson SN, Redak RA. Interactions of dietary protein and carbohydrate determine blood sugar level and regulate nutrient selection in the insect Manduca sexta L. Biochimica et Biophysica Acta. 2000;1523(1):91–102. doi: 10.1016/s0304-4165(00)00102-1. [DOI] [PubMed] [Google Scholar]
- Ugrankar R, Berglund E, Akdemir F, Tran C, Kim MS, Noh J, Graff JM. Drosophila glucome screening identifies Ck1alpha as a regulator of mammalian glucose metabolism. Nature Communications. 2015;6:7102. doi: 10.1038/ncomms8102. http://dx.doi.org/10.1038/ncomms8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Herpen NA, Schrauwen-Hinderling VB. Lipid accumulation in nonadipose tissue and lipotoxicity. Physiology and Behavior. 2008;94(2):231–241. doi: 10.1016/j.physbeh.2007.11.049. http://dx.doi.org/10.1016/j.physbeh.2007.11.049. [DOI] [PubMed] [Google Scholar]
- Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R. Insulin regulation of heart function in aging fruit flies. Nature Genetics. 2004;36(12):1275–1281. doi: 10.1038/ng1476. [DOI] [PubMed] [Google Scholar]
- Williams S, Dew-Budd K, Davis K, Anderson J, Bishop R, Freeman K, Reed LK. Metabolomic and gene expression profiles exhibit modular genetic and dietary structure linking metabolic syndrome phenotypes in Drosophila. G3 (Bethesda, Md) 2015;5(12):2817–2829. doi: 10.1534/g3.115.023564. http://dx.doi.org/10.1534/g3.115.023564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Brown MR. Signaling and function of insulin-like peptides in insects. Annual Review of Entomology. 2006;51:1–24. doi: 10.1146/annurev.ento.51.110104.151011. [DOI] [PubMed] [Google Scholar]
- Wyatt GR, Kale GF. The chemistry of insect hemolymph. II. Trehalose and other carbohydrates. The Journal of General Physiology. 1957;40(6):833–847. doi: 10.1085/jgp.40.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Chang BH, Chan L. Sustained expression of the transcription factor GLIS3 is required for normal beta cell function in adults. EMBO Molecular Medicine. 2013;5(1):92–104. doi: 10.1002/emmm.201201398. http://dx.doi.org/10.1002/emmm.201201398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Liu J, Li CR, Momen B, Kohanski RA, Pick L. Deletion of Drosophila insulin-like peptides causes growth defects and metabolic abnormalities. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(46):19617–19622. doi: 10.1073/pnas.0905083106. [DOI] [PMC free article] [PubMed] [Google Scholar]


