Abstract
Gulf War illness (GWI), which afflicts at least 25% of veterans who served in the 1990–1991 war in the Persian Gulf, is thought to be caused by deployment exposures to various neurotoxicants, including pesticides, anti–nerve gas pills, and low-level nerve agents including sarin/cyclosarin. GWI is a multisymptom disorder characterized by fatigue, joint pain, cognitive problems, and gastrointestinal complaints. The most prominent symptoms of GWI (memory problems, poor attention/concentration, chronic headaches, mood alterations, and impaired sleep) suggest that the disease primarily affects the CNS. Development of urgently needed treatments depends on experimental models appropriate for testing mechanistic hypotheses and for screening therapeutic compounds. Rodent models have been useful thus far, but are limited by their inability to assess the contribution of genetic or epigenetic background to the disease, and because disease-vulnerable proteins and pathways may be different in humans relative to rodents. As of yet, no postmortem tissue from the veterans has become available for research. We are moving forward with a paradigm shift in the study of GWI, which utilizes contemporary stem cell technology to convert somatic cells from Gulf War veterans into pluripotent cell lines that can be differentiated into various cell types, including neurons, glia, muscle, or other relevant cell types. Such cell lines are immortal and will be a resource for GWI researchers to pursue mechanistic hypotheses and therapeutics.
Gulf War illness (GWI) encompasses a constellation of debilitating symptoms experienced by over 25% of the nearly 700,000 US soldiers who served in the 1990–1991 war.1 The symptoms are primarily deficits in CNS functioning, but also include gastrointestinal and musculoskeletal complaints. The CNS symptoms, which include diminished short-term memory, poor attention/concentration, chronic headaches, fatigue, and impaired sleep, are consistent with chronic exposure to neurotoxicants including organophosphate (OP) pesticides and nerve agents.2–4 Gulf War (GW) veterans were exposed to toxicants including OP pesticides, pyridostigmine bromide (PB) anti–nerve gas pills, and low-level sarin nerve agents.1,5 However, a mechanistic explanation for the association of these toxicants to GWI remains undetermined, and there are no current treatments that have substantially improved cognitive functioning or other chronic health problems of veterans with GWI. Urgency is heightened by the fact that environmental pollutants, biological warfare, and terrorism could lead to far greater numbers of patients with diseases with similar etiology to GWI.
As with any human disease, mechanistic studies in the preclinical laboratory have started with animal models, with rodents favored over invertebrate models such as flies or worms. In one early rodent model, rats were exposed to PB, the sarin-surrogate di-isopropyl fluorophosphate (DFP), and the insect repellent N,N-diethyl-meta-toluamide (DEET), and then assessed for behavioral and histologic deficits.6,7 Deficits were noted with or without combining the neurotoxicant exposures with a physical stressor, namely temporary restraint of the animal,8,9 but were heightened with the addition of the physical stressor.9,10 This observation led to the view that chronic GWI results from the combined effects of physical stressors with the toxicants.9 Subsequent studies have used corticosterone to simulate the effects of physical stressors in combination with DFP to create an animal model of chronic GWI.11 Also relevant is the fact that veterans exposed to the same deployment-related conditions may or may not have contracted GWI, suggesting that genetic or epigenetic factors may have contributed to any individual soldier's susceptibility to the illness.12 In fact, recent animal models have shown miRNA and other epigenetic changes in GWI-exposed animal models.13
Despite progress, there has been concern about the continued use of animal models in GWI research by the National Academy of Sciences Institute of Medicine.14 This is because human diseases often involve proteins and pathways that are not well-reflected in rodents, and because animal work cannot appropriately take into account myriad genetic and epigenetic factors relevant to human disease. In light of these issues, there is value in studying human cells, especially if they come directly from GW veterans. Toward this end, the US Department of Defense has funded us to generate a bank of human induced pluripotent stem cells (hiPSC) from the blood of veterans with GWI that can be used for mechanistic studies and to test therapeutic compounds. hiPSC are self-renewing cell lines generated by reprogramming terminally differentiated cells so that they become pluripotent, similar to embryonic stem cells. These cells can then be treated with various growth and patterning factors that induce them to differentiate into different lineages, including various types of neurons and non-neuronal cells of the nervous system, as well as other cell types such as muscle. hiPSC are gaining popularity as models for a number of different human diseases, including neurodegenerative disorders.15
The advantages of hiPSC are that they (1) are human cells, and therefore have the human proteins and pathways that may not be appropriately reflected in animal models; (2) are from the patients themselves, which means that they harbor the array of genetic factors that may contribute to the disease or susceptibility of the disease; (3) may retain some of the epigenetic factors that might also contribute16; (4) can be differentiated into a vast array of cell types and can also be built into complex organoid and tissue ensembles reflective of parts of the body such as the brain17,18; (5) enable rapid and high-throughput experiments and screens of therapeutic compounds; and (6) can be passaged an unlimited number of times so that each stock can be expanded indefinitely, without concern of stocks running out or changing due to too many passages. In addition, results obtained with these cells can be compared against results of parallel studies on an array of neurodegenerative diseases that might share elements of their etiology with GWI.
WHAT DO WE KNOW ABOUT GWI FROM RODENT MODELS AND CLINICAL STUDIES?
Rodent studies of GW-relevant OP neurotoxicants have shown deficits in the axonal transport of proteins and organelles, as well as alterations of microtubules, which form the structural tracks for axonal transport.19–22 It has been reported that rodents exposed to GW-relevant (OP) pesticides and nerve agents display brain microtubules with fewer associated proteins than in a normal situation, leading to reduced microtubule width.19–23 This reduced association of such proteins to the microtubules may be due to modifications in those proteins or to changes in the tubulin backbone of the microtubule. Molecular motor proteins move less efficiently on the microtubules, and the microtubules may be less stable without their normal complement of associated proteins.20,24 Mitochondria are carried down the axon by axonal transport and supply the energy needed for axonal functioning. Several studies have now shown that GW-relevant OP exposures affect multiple facets of axonal transport and mitochondrial dynamics that could lead to GW symptoms of cognitive complaints and fatigue.21,24,25 Alterations in axonal transport mechanisms can result in deficits ranging from delayed information processing and cognitive complaints to the development of neurodegenerative conditions such as those observed in patients with Alzheimer disease and amyotrophic lateral sclerosis.26,27
Chronic low-level OP exposures similar to what GW veterans experienced have been associated with mitochondrial damage as a result of oxidative stress and neuroinflammation.28 One hypothesis of GWI suggests that toxicant exposures caused the chronic symptoms of GWI by directly damaging microtubules or mitochondria in a manner that the neurons and other affected cells are not equipped to self-repair.29,30 A second GWI hypothesis suggests that GW toxicants affected the brain in an additive manner to cause chronic, ongoing neuroinflammation. This chronic neuroinflammation is then thought to negatively affect microtubules and other cellular structures (including mitochondria), pathways, and mechanisms relevant to axonal transport.11,29,30 These 2 hypotheses of GWI, which are not mutually exclusive, remain difficult to assess in current clinical models.
Recent clinical studies comparing brain imaging and cognitive functioning in GW veterans have shown significant evidence of altered CNS functioning in veterans with GWI.5,31–33 However, overlap between individual GWI cases and controls on the outcome measures has precluded their use as objective diagnostic markers. Mechanistic studies have been impeded also by a lack of available postmortem brain tissue.
HUMAN INDUCED NEURONS—BRIDGING THE GAP WITH A PARADIGM SHIFT
Seminal work showed that a cocktail of 4 pluripotency factors in viral expression vectors can effectively reprogram human somatic cells to a pluripotent cell fate within weeks.34 Toward modeling various neurologic disorders, neurons of various types have been generated from hiPSC, such as dopaminergic, spinal motor, cortical glutamatergic, and GABAergic neurons.35 Phenotypes corresponding to human neurodegenerative disorders that have not been reproduced in laboratory animals have, in some instances, been reproduced in hiPSC.36 A major advantage of patient-derived cells is that many diseases cannot be traced to just one gene being mutated, but rather are due to complex interactions of genes, or genes that remain unknown. Other diseases or susceptibility to the disease are not entirely genetically based but may be due at least in part to epigenetic factors. Patient-derived cells preserve the complete genetic composition of the patient and may also preserve some epigenetic modifications.16
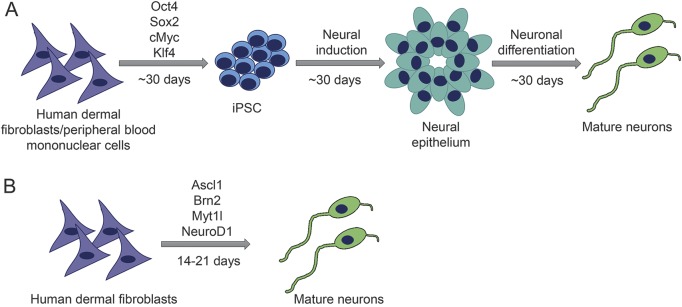
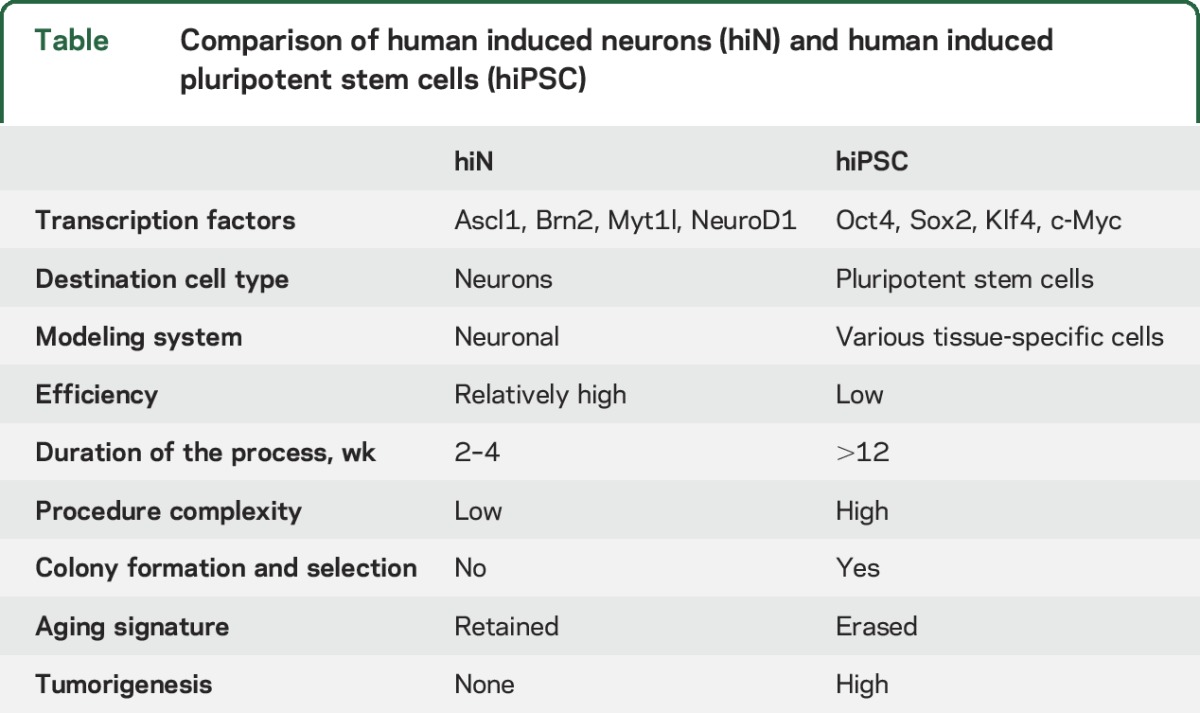
More recently, direct conversion of somatic cells into neurons has been achieved by altering the complement of transcription and growth factors. These directly converted neurons, termed human induced neurons (hiN), have advantages and disadvantages compared to neurons from the hiPSC approach (figure 1 and table). The extensive cloning process used in the hiPSC approach may overwhelm subtle disease phenotypes present in patient cells, and if so, this problem can be avoided with hiN. Also, because hiN bypass the pluripotent state, they preserve more epigenetic factors,37 and also obviate the need for the analysis of multiple colonies from individuals. Recently, age-related transcriptome analyses in hiPSC-derived neurons and hiN from individuals of different ages suggested that certain epigenetic states/signatures, such as those being induced by aging, were erased or reset to the embryonic stage in the case of hiPSC but were largely retained in directly reprogrammed hiN.37 However, current hiN approaches also hold some critical liabilities: for instance, the cells seem to be relatively immature and more vulnerable; only a few neuronal subtypes have been achieved through direct conversion; and they are not expandable, which limits their utilization for any genetic manipulation.
Figure 1. Schematic of neuronal induction using 2 different reprogramming strategies.
(A) Neuronal induction from human induced pluripotent stem cells (iPSC). (B) Direct conversion from somatic cells (e.g., fibroblasts) to mature neurons.
Table.
Comparison of human induced neurons (hiN) and human induced pluripotent stem cells (hiPSC)
We are obtaining peripheral blood from GW veterans with GWI and also from GW veterans without GWI as controls. Mononuclear cells are being isolated from the peripheral blood and are being reprogrammed into hiPSC. These immortalized cell lines are being frozen and stored in a repository to be used for our own studies related to microtubules and axonal transport, and will also be made available for collaborative projects on other potential mechanistic hypotheses and therapeutic strategies across the GWI research community. Although we are prepared to carry out skin biopsy procedures to collect the skin fibroblasts from the recruited veterans for hiN conversion, extra mononuclear cells are also being isolated and frozen from blood for testing of direct conversion methods. With permission from each veteran donor, demographic information is being tallied as well as the particular symptoms experienced by those with GWI.
RECRUITMENT PROCESS AND INFORMED CONSENT
We are currently recruiting blood donors from the 300 study participants from the Gulf War Illness Consortium (GWIC), funded by the US Department of Defense (PI: Kimberly Sullivan). At the Boston GWIC site, 175 study participants are being recruited. The human neuron studies are considered a follow-up to the GWIC study and include one clinic visit for the study participant. A participant is considered eligible if he or she is a 1990–1991 GW veteran who does not have any relevant medical exclusions. GWI cases are determined if the veteran meets the Kansas GWI criteria by endorsing moderately severe or multiple symptoms in 3 of 6 health symptom domains (pain, fatigue, neurologic/cognitive/mood, skin, gastrointestinal, respiratory) on the Kansas questionnaire.38 Veterans are required to have served in the Gulf War for any period between August 1990 and July 1991 and to have participated in the GWI consortium main study. Exclusionary criteria include a history of prior CNS or major psychiatric disorders that may affect cognitive function (e.g., epilepsy, stroke, brain tumor, multiple sclerosis, Parkinson disease, Alzheimer disease, schizophrenia). GW veterans who meet criteria for GWI based on the Kansas GWI case criteria (i.e., cases) or GW veterans who do not meet criteria for GWI based on Kansas criteria (controls) but also do not meet any Kansas exclusion criteria and have participated in the GWIC main study are eligible to participate. GW veterans who have met any of the exclusion criteria for the Kansas GWI case criteria are excluded from this study. Veterans meeting the Kansas criteria but without any symptoms of GWI are taken into the study as controls. The veterans are informed and they agree in writing that their stem cells will become immortalized and used for future treatment and mechanistic studies of GWI.
The number of cell lines that we are funded to generate (4–6 cell lines from veterans with GWI and 4–6 from veterans without GWI) are not sufficient to evaluate the contribution of such factors as race, sex, or genetic background to the disease. For this reason, recruited veterans for the cell lines are primarily male, Caucasian, and age-matched, although future potential funding may allow for an expansion of the number of cell lines from veterans of a broader range of demographic backgrounds. The present number of cell lines is also insufficient to draw strong conclusions on the potential relevance of genetic or epigenetic differences among them. However, clinical data, such as neural imaging, obtained from the same veterans may yield some initial insights into the severity and nature of the disease corresponding to each of the cell lines. Especially notable differences among the cell lines may provide clues that can be pursued in the future with greater numbers of cell lines.
CONTROL SAMPLES
A challenging element to our plan was the decision regarding what to use for control sample hiPSC cells. We considered using cells from veterans of similar age who had not been deployed to the GW. However, epidemiologic studies indicate that comparing deployed ill vs deployed non-ill samples have proven more effective in identifying epidemiologic, cognitive, and biomarker differences between groups.1,5 We decided, for 2 reasons, on cells from veterans who were also deployed to the GW but who did not develop GWI. First, we simply did the best that we could to choose cells as close as possible to the cells from GWI cases. Second, we considered the possibility that the individuals who contracted GWI might be genetically or epigenetically predisposed to contracting the illness from the neurotoxicant exposures, and hence that there may be something to be learned from comparing cells from equally exposed veterans who either did or did not get sick.13 By no means did we think that cells from any human being would be unresponsive to the relevant toxicants, but perhaps the sensitivity would be greater in one group compared to the other, or perhaps the reaction would be qualitatively different. For example, neurons derived from the veterans who contracted the illness might react to the toxicants by hyperphosphorylating tau, a microtubule-related protein that becomes aberrantly phosphorylated in a number of neurodegenerative conditions,36 but the same would not be true of the neurons from the veterans who did not contract the illness. This might be due to a predisposition of the former group to factors that contribute to tauopathies such as Alzheimer disease. Along these lines, there may be a certain threshold of the toxicants that kills any neuron, but below that threshold, the cells of some people can recover while the cells of other people cannot and hence acquire GWI.39 As noted earlier, a greater number of cell lines will most likely be needed to draw strong conclusions, but the current number may provide an initial set of clues.
In the case of GWI, it is not known whether newly differentiated neurons (or other cell types) from blood cells will “have the disease” or essentially start fresh, without the disease. If the former is the case, then we can simply compare cell lines from veterans with GWI against veterans without GWI, with no need to re-expose them to GW toxicants. Such a scenario might be due, for example, to chromosomal or epigenetic damage that affects cells from multiple systems (i.e., hepatic cells, muscle cells, neurons).13 More likely, the newly differentiated neurons will represent the status of the veteran prior to exposure to the toxicants, and the neurons will have to be exposed to GW toxicants (as well as cortisol to model the physical stressors of the war) to mimic the disease phenotype.
EXPERIMENTAL PARADIGM
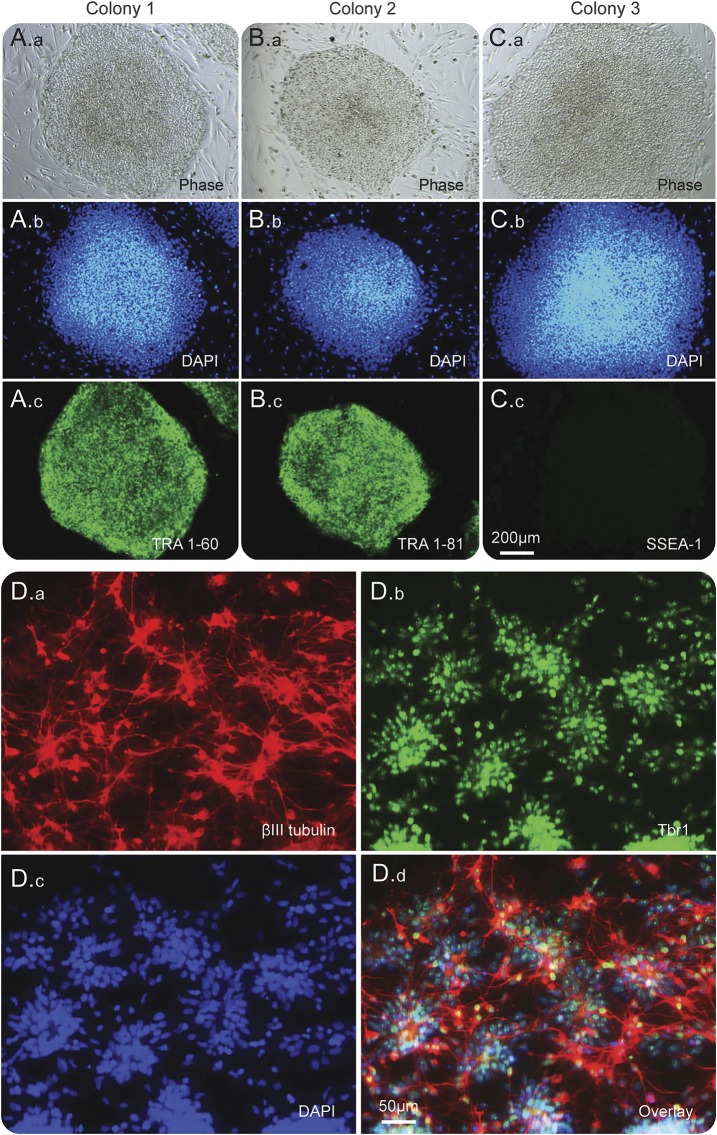
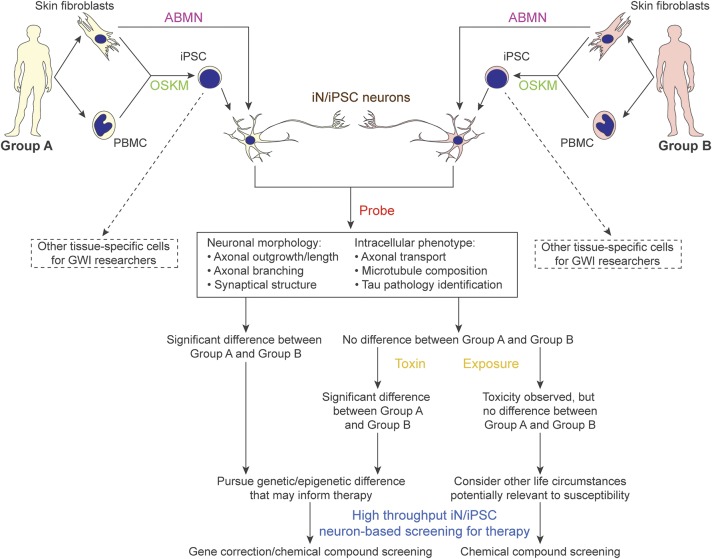
Mononuclear cells from the veterans are being isolated from the peripheral blood and reprogrammed into hiPSC at the Center for Regenerative Medicine at Boston University. Additional peripheral blood mononuclear cells have been stored for potential direct conversion procedures. If necessary, these individuals can be recruited for potential skin biopsies in order to obtain skin fibroblasts for the direct conversion procedures. Our first round of studies will be on cells differentiated into cortical forebrain neurons. Figure 2 shows hiPSC validation and neuronal differentiation from the hiPSC (see figure legend for details). The outline of our experimental strategy is displayed as a flow chart in figure 3. Briefly, neurons from the 2 groups will be compared without the toxicants for differences in such readouts as morphology and axonal transport. Should differences be observed, we will further probe for the possibility of genetic pre-deposition or epigenetic variations in the 2 groups.13 However, should no differences be obtained, the toxicants, such as DFP or PB, will be added into the neuronal cultures from the 2 groups followed by the experimental analyses.
Figure 2. Human induced pluripotent stem cells (hiPSC) validation and neuronal differentiation.
A.a–A.c, B.a–B.c, and C.a–C.c are 3 individual iPSC colonies from 1 of the recruited veterans (1039-2). A.a, B.a, and C.a are phase-contrast images for the 3 individual colonies; A.b, B.b, and C.b are DAPI (nuclear stain) counterstains; colony 1 and 2 are immunostained with pluripotency marker TRA 1-60 (A.c) and TRA 1-80 (B.c). C.c from colony 3 shows that SSEA-1 (only expressed in murine iPSC) is not expressed in the hiPSC; also this indicates that the hiPSC are undifferentiated, since the expression of SSEA-1 is upregulated during hiPSC differentiation. D.a–D.d are the immunostaining validation of the neuronal differentiation of the hiPSC. D.a is the immunostaining for βIII tubulin and D.b is the immunostaining for Tbr1. βIII tubulin is a neuronal specific tubulin whose expression is significantly upregulated in mature neurons; and Tbr1 is a transcription factor which serves as a marker for forebrain cortical glutamatergic neurons. D.c and D.d are DAPI counterstain image and the overlay image.
Figure 3. Schematic flow chart for planned experimental regimen.
Group A, Gulf War (GW) veterans without GW illness (GWI); group B, GW veterans with GWI. The types of assessments that will be done on the cells depend on the hypothesis being pursued. Our initial plan, to probe phenotypes mentioned in the chart, will be to measure lengths of axons after toxicant exposures, as well as to count branches along the axons as normalized per 100 μm, quantify synapses by performing patch-clamp recording to quantify spontaneous activities and by immunostaining for synaptophysin or synapsin, examine axonal transport by performing live-cell imaging using fluorescent markers for various organelles, and examine microtubule structure by immunocytochemistry and Western blotting using antibodies to various tubulin variants and microtubule-associated proteins. We are especially interested in studying tau, a microtubule-associated protein that dissociates from microtubules to form abnormal filaments in a number of human neurodegenerative diseases, a pathologic phenomenon not well reflected in rodent neurons.36 One-way analyses of variance will be used for all the statistic quantitations with n > 50. ABMN = transcription factors Ascl1, Brn2, Myt1l, and NeuroD1; fibs = fibroblasts; iN = induced neurons; iPSC = induced pluripotent stem cells; OSKM = transcription factors Oct4, Sox2, Klf4, and c-Myc; PBMC = peripheral blood mononuclear cells.
A challenge worth mentioning is the difficulty of reproducing the levels or complexity of toxicant exposures in the culture dish to match those experienced by soldiers in the battlefield. For this reason, experiments need to be performed with a range of toxicant concentrations, with informed knowledge of the biochemistry of the particular toxicant. For example, OPs are known to inhibit acetylcholinesterase above a certain threshold concentration, but subthreshold levels are likely more relevant to GWI.21,25,40 Especially when more cell lines become available, attention will be given to variability not only between subject groups but also among individual lines, as line-to-line differences could provide insight into contributing factors to GWI that may be correlated to symptom severity.
A NOTE TO PHYSICIANS
Research on the mechanisms of GWI will allow for the development of novel therapies that will benefit future patients with toxicant exposures similar to GWI. At present, our best chances of helping current patients with GWI is rapid-throughput studies on already available and approved therapeutics. Our cell lines are optimal for this purpose, and we look forward to coordinating with physicians who can rapidly translate our most promising results into urgently needed therapy for the veterans. The best progress can be made through an interactive relationship between scientists working in the laboratory on the human cell lines and physicians working directly with veterans in the clinic.
ACKNOWLEDGMENT
The authors thank the veterans who provided blood samples for the generation of the cell lines.
GLOSSARY
- DFP
di-isopropyl fluorophosphates
- GW
Gulf War
- GWI
Gulf War illness
- GWIC
Gulf War Illness Consortium
- hiN
human induced neurons
- hiPSC
human induced pluripotent stem cells
- OP
organophosphate
- PB
pyridostigmine bromide
AUTHOR CONTRIBUTIONS
Liang Qiang: Stem cell expert who designed the studies and wrote the manuscript. Anand N. Rao: Student conducting primary research on stem cells for Gulf War illness. G. Mostoslavsky: Director of the stem cell facility commissioned to produce the cell lines. Marianne F. James: Scientist in the stem cell facility responsible for the cell lines. Nicole Comfort: Assistant to Dr. Sullivan in working with the veterans to obtain their blood for the production of the cell lines. Kimberly Sullivan: Gulf War illness expert and coordinator of the effort to obtain blood from veterans for the production of the cell lines; contributed to writing manuscript. Peter W. Baas: Leader of the project and wrote the manuscript with Liang Qiang.
STUDY FUNDING
The ideas and work described here are the basis of a grant from the US Department of Defense to P.W. Baas (W81XWH-15-1-0433) and represent an extension of the work of the GWI Consortium (W81XWH-13-2-0072) directed by K.A. Sullivan, also funded by the US Department of Defense. A.N.R. is supported by an F31 Award from the NIH (1F31NS093748-01A1). Additional funding was provided by the Pennsylvania Department of Health CURE program to Drexel University College of Medicine.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.RAC. Gulf War Illness and the Health of Gulf War Veterans: Scientific Findings and Recommendations. Washington, DC: US Government Printing Office; 2008. [Google Scholar]
- 2.Ismail AA, Bodner T, Rohlman D. Neurobehavioral performance among agricultural workers and pesticide applicators: a meta-analytic study. Occup Environ Med 2012;69:457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross SM, McManus I, Harrison V, Mason O. Neurobehavioral problems following low-level exposure to organophosphate pesticides: a systematic and meta-analytic review. Crit Rev Toxicol 2013;43:21–44. [DOI] [PubMed] [Google Scholar]
- 4.Abou-Donia MB, Abou-Donia MM, ElMasry EM, Monro JA, Mulder MF. Autoantibodies to nervous system-specific proteins are elevated in sera of flight crew members: biomarkers for nervous system injury. J Toxicol Environ Health A 2013;76:363–380. [DOI] [PubMed] [Google Scholar]
- 5.White RF, Steele L, O'Callaghan JP, et al. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: effects of toxicant exposures during deployment. Cortex 2016;74:449–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abou-Donia MB. Neurotoxicity resulting from coexposure to pyridostigmine bromide, DEET, and permethrin: implications of Gulf War chemical exposures. J Toxicol Environ Health A 1996;48:35–56. [DOI] [PubMed] [Google Scholar]
- 7.Abou-Donia MB, Wilmarth KR, Abdel-Rahman AA, Jensen KF, Oehme FW, Kurt TL. Increased neurotoxicity following concurrent exposure to pyridostigmine bromide, DEET, and chlorpyrifos. Toxicol Sci 1996;34:201–222. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Rahman A, Abou-Donia S, El-Masry E, Shetty A, Abou-Donia M. Stress and combined exposure to low doses of pyridostigmine bromide, DEET, and permethrin produce neurochemical and neuropathological alterations in cerebral cortex, hippocampus, and cerebellum. J Toxicol Environ Health A 2004;67:163–192. [DOI] [PubMed] [Google Scholar]
- 9.Parihar VK, Hattiangady B, Shuai B, Shetty AK. Mood and memory deficits in a model of Gulf War illness are linked with reduced neurogenesis, partial neuron loss, and mild inflammation in the hippocampus. Neuropsychopharmacology 2013;38:2348–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdullah L, Evans JE, Bishop A, et al. Lipidomic profiling of phosphocholine containing brain lipids in mice with sensorimotor deficits and anxiety-like features after exposure to Gulf War agents. Neuromolecular Med 2012;14:349–361. [DOI] [PubMed] [Google Scholar]
- 11.O'Callaghan JP, Kelly KA, Locker AR, Miller DB, Lasley SM. Corticosterone primes the neuroinflammatory response to DFP in mice: potential animal model of Gulf War illness. J Neurochem 2015;133:708–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steele L, Lockridge O, Gerkovich MM, Cook MR, Sastre A. Butyrylcholinesterase genotype and enzyme activity in relation to Gulf War illness: preliminary evidence of gene-exposure interaction from a case–control study of 1991 Gulf War veterans. Environ Health 2015;14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierce LM, Kurata WE, Matsumoto KW, Clark ME, Farmer DM. Long-term epigenetic alterations in a rat model of Gulf War illness. Neurotoxicology 2016;55:20–32. [DOI] [PubMed] [Google Scholar]
- 14.Medicine Io, National Academies of Sciences E, Medicine. Gulf War and Health: Volume 10: Update of Health Effects of Serving in the Gulf War, 2016. Washington, DC: The National Academies Press; 2016. [Google Scholar]
- 15.Soldner F, Jaenisch R. Medicine: iPSC disease modeling. Science 2012;338:1155–1156. [DOI] [PubMed] [Google Scholar]
- 16.Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature 2010;467:285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi SH, Kim YH, Hebisch M, et al. A three-dimensional human neural cell culture model of Alzheimer's disease. Nature 2014;515:274–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim YH, Choi SH, D'Avanzo C, et al. A 3D human neural cell culture system for modeling Alzheimer's disease. Nat Protoc 2015;10:985–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prendergast MA, Self RL, Smith KJ, et al. Microtubule-associated targets in chlorpyrifos oxon hippocampal neurotoxicity. Neuroscience 2007;146:330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang W, Duysen EG, Hansen H, Shlyakhtenko L, Schopfer LM, Lockridge O. Mice treated with chlorpyrifos or chlorpyrifos oxon have organophosphorylated tubulin in the brain and disrupted microtubule structures, suggesting a role for tubulin in neurotoxicity associated with exposure to organophosphorus agents. Toxicol Sci 2010;115:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terry AV Jr, Stone JD, Buccafusco JJ, Sickles DW, Sood A, Prendergast MA. Repeated exposures to subthreshold doses of chlorpyrifos in rats: hippocampal damage, impaired axonal transport, and deficits in spatial learning. J Pharmacol Exp Ther 2003;305:375–384. [DOI] [PubMed] [Google Scholar]
- 22.Grigoryan H, Lockridge O. Nanoimages show disruption of tubulin polymerization by chlorpyrifos oxon: implications for neurotoxicity. Toxicol Appl Pharmacol 2009;240:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gearhart DA, Sickles DW, Buccafusco JJ, Prendergast MA, Terry AV Jr. Chlorpyrifos, chlorpyrifos-oxon, and diisopropylfluorophosphate inhibit kinesin-dependent microtubule motility. Toxicol Appl Pharmacol 2007;218:20–29. [DOI] [PubMed] [Google Scholar]
- 24.Terry AV Jr, Gearhart DA, Beck WD Jr, et al. Chronic, intermittent exposure to chlorpyrifos in rats: protracted effects on axonal transport, neurotrophin receptors, cholinergic markers, and information processing. J Pharmacol Exp Ther 2007;322:1117–1128. [DOI] [PubMed] [Google Scholar]
- 25.Terry AV Jr, Beck WD, Warner S, Vandenhuerk L, Callahan PM. Chronic impairments in spatial learning and memory in rats previously exposed to chlorpyrfos or diisopropylfluorophosphate. Neurotoxicol Teratol 2012;34:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falnikar A, Baas PW. Critical roles for microtubules in axonal development and disease. Results Probl Cell Differ 2009;48:47–64. [DOI] [PubMed] [Google Scholar]
- 27.Morfini GA, Burns M, Binder LI, et al. Axonal transport defects in neurodegenerative diseases. J Neurosci 2009;29:12776–12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Binukumar B, Bal A, Sunkaria A, Gill KD. Mitochondrial energy metabolism impairment and liver dysfunction following chronic exposure to dichlorvos. Toxicology 2010;270:77–84. [DOI] [PubMed] [Google Scholar]
- 29.Golomb BA. Oxidative stress and mitochondrial injury in chronic multisymptom conditions: from Gulf war illness to autism spectrum disorder. Nature Proc. Epub 2012 Jan 31. [Google Scholar]
- 30.Koslik HJ, Hamilton G, Golomb BA. Mitochondrial dysfunction in Gulf war illness revealed by 31Phosphorus magnetic resonance spectroscopy: a case-control study. PLoS One 2014;9:e92887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chao LL, Abadjian L, Hlavin J, Meyerhoff DJ, Weiner MW. Effects of low-level sarin and cyclosarin exposure and Gulf War illness on brain structure and function: a study at 4T. Neurotoxicology 2011;32:814–822. [DOI] [PubMed] [Google Scholar]
- 32.Heaton KJ, Palumbo CL, Proctor SP, Killiany RJ, Yurgelun-Todd DA, White RF. Quantitative magnetic resonance brain imaging in US army veterans of the 1991 Gulf War potentially exposed to sarin and cyclosarin. Neurotoxicology 2007;28:761–769. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan K, Krengel M, Proctor SP, Devine S, Heeren T, White RF. Cognitive functioning in treatment-seeking Gulf War veterans: pyridostigmine bromide use and PTSD. J Psychopathol Behav Assess 2003;25:95–103. [Google Scholar]
- 34.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861–872. [DOI] [PubMed] [Google Scholar]
- 35.Park IH, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells. Cell 2008;134:877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Israel MA, Yuan SH, Bardy C, et al. Probing sporadic and familial Alzheimer's disease using induced pluripotent stem cells. Nature 2012;482:216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mertens J, Paquola AC, Ku M, et al. Directly reprogrammed human neurons retain aging-associated transcriptomic signatures and reveal age-related nucleocytoplasmic defects. Cell Stem Cell 2015;17:705–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steele L. Prevalence and patterns of Gulf War illness in Kansas veterans: association of symptoms with characteristics of person, place, and time of military service. Am J Epidemiol 2000;152:992–1002. [DOI] [PubMed] [Google Scholar]
- 39.Furlong CE. Genetic variability in the cytochrome P450–paraoxonase 1 (PON1) pathway for detoxication of organophosphorus compounds. J Biochem Mol Toxicol 2007;21:197–205. [DOI] [PubMed] [Google Scholar]
- 40.Gao J, Naughton SX, Wulff H, et al. Diisopropylfluorophosphate impairs the transport of membrane-bound organelles in rat cortical axons. J Pharmacol Exp Ther 2016;356:645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]