Abstract
PURPOSE
To evaluate the efficacy of a dexamethasone intravitreal implant in combination with intravitreal anti-VEGF agents for treatment resistant neovascular age-related macular degeneration (nvAMD).
METHODS
This study was designed as a single-center, retrospective interventional case series. Consecutive patients with treatment-resistant nvAMD underwent simultaneous combined injection of anti-VEGF agent and dexamethasone intravitreal implant. Eighteen patients with mean age of 81.5 years were included. Patients received average of 26.3 anti-VEGF injections before dual therapy, with mean follow up of 8.2 months after dual therapy.
RESULTS
Dual therapy produced a significant mean decrease in CFT (126.3 μm), compared to a mean increase of 29.9 μm when treated with anti-VEGF monotherapy (p=0.0017). Patients also had mean decrease in MCV of −0.85 mm3 with dual therapy compared with anti-VEGF monotherapy (p=0.0014). There was a moderate correlation between the number of prior anti-VEGF injections and the magnitude of anatomic response, suggesting that shorter disease duration may positively influence response to combined treatment. Although there was a slight trend towards improved mean visual acuity after dual therapy, these differences did not reach statistical significance. Nevertheless, with combination treatment, 33% of patients gained one or more lines of vision. Dual therapy resulted in a significantly lower number of required anti-VEGF injections (4.25 vs 5.33) and an increase of the anti-VEGF injection-free interval to 1.41 months from 1.12 months during the 6 months following dual therapy compared to the same interval before dual therapy. Dual therapy was well tolerated; two eyes developed mild IOP elevation effectively managed with topical therapy and one patient developed worsening cataract.
CONCLUSIONS
Combined treatment of anti-VEGF with the dexamethasone intravitreal implant is a viable alternative for treatment-resistant nvAMD, and may reduce treatment burden. Earlier treatment with dual therapy may be beneficial to maximize anatomic and visual outcomes in these patients.
Keywords: Dexamethasone intravitreal implant, Anti-VEGF, Neovascular AMD, Persistent fluid, Treatment resistance
Introduction
Age related macular degeneration (AMD) is a leading cause of vision loss in people aged 50 or older in the United States.1-3 Neovascular AMD (nvAMD) accounts for the majority of the visual impairment in these patients.4 Choroidal neovascularization (CNV) is the hallmark of nvAMD, and results in well-described leakage, exudation and hemorrhage in the macula which can produce rapid vision loss.5 The role of vascular endothelial growth factor (VEGF) in CNV formation and maintenance is well established,6,7 and intravitreal anti-VEGF agents have become the first line treatment and standard of care for nvAMD over the past decade.8-10 Three agents, bevacizumab (Avastin, Genentech, San Francisco, CA, USA), ranibizumab (Lucentis, Genentech, San Francisco, CA, USA) and aflibercept (Eylea / VEGF Trap-Eye, Regeneron, Tarrytown, NY, USA) are in clinical use today. Despite the efficacy of anti-VEGF therapy, a subset of patients do not optimally respond and have persistent subretinal or intraretinal fluid with vision loss despite anti-VEGF monotherapy.11-13 Treatment of these patients is challenging and may include any combination of switching among anti-VEGF agents,14-16 use of photodynamic therapy (PDT)17 or use of intravitreal corticosteroids.18,19
Corticosteroids are an attractive adjuvant for treatment resistant nvAMD because they address the inflammatory component of CNV membrane formation and growth that is not targeted by anti-VEGF agents. Multiple studies have shown that inflammatory elements, including macrophages, play an important role in AMD progression.20-22 Steroids stabilize vascular tight junctions and decrease leakage,23 produce apoptosis of peripheral T-cells24 and eosinophils,25 and may block fibroblast activation and proliferation.26 In addition, glucocorticoids have been shown to directly inhibit VEGF production.27 Dexamethasone is a potent steroid, but its clinical usefulness is limited by its rapid clearance from the vitreous cavity (i.e. three half-lives of <12 hours).28 The dexamethasone intravitreal implant (0.7 mg Ozurdex®, Allergan, Parsippany, NJ) allows steady dexamethasone delivery in the vitreous cavity over a period of up to 6 months via a sustained release injectable biopolymer with dexamethasone integrated with polylacticco-glycolic acid.29, 30 It is approved by the U.S. Food and Drug Administration (FDA) for the treatment of diabetic macular edema (DME), cystoid macular edema associated with retinal vein occlusion and non-infectious uveitis.31 The dexamethasone implant is well suited for treatment of chronic, inflammatory macular diseases.
Recently, Kuppermann and colleagues32 conducted a 6-month, single-masked, multicenter study in which nvAMD patients were randomized to receive either dexamethasone intravitreal implant or sham and 2 intravitreal ranibizumab injections. They showed that the intravitreal dexamethasone implant increased the injection-free interval of anti-VEGF therapy, but did not change visual acuity or retinal thickness outcomes.32 Although their study groups included both patients previously receiving anti-VEGF therapy as well as treatment-naïve nvAMD patients, they did not examine the effect of the dexamethasone intravitreal implant in patients with sub-optimal responses to anti-VEGF monotherapy, which is a likely scenario that would necessitate treatment beyond anti-VEGF agents in the real world.32
Methods
The study herein is a single-center, interventional, retrospective consecutive case series. It was conducted with Institutional Review Board approval and in accordance with the ethical standards of the Helsinki Declaration of 1975, as revised in 2000 and 2008.
Study patient population and inclusion/exclusion criteria
We conducted a retrospective chart review of clinical data of consecutive patients with treatment-resistant nvAMD who underwent dual therapy with anti-VEGF medication and the dexamethasone intravitreal implant concurrently at Associated Retinal Consultants, Royal Oak, Michigan between 2012-2016. To be eligible for the study, patients were required to meet the following inclusion criteria (Table 1): age >50, diagnosis of nvAMD with active subfoveal choroidal neovascularization (as determined by clinical examination, spectral domain optical coherence tomography (SD-OCT), and fluorescein angiography (FA), and best-corrected visual acuity (BCVA) of 20/30-20/800. Patients previously treated with photodynamic therapy or intravitreal triamcinolone acetonide injections were included as long as they received treatment more than 6 months prior to the dual therapy. Patients were required to have received at least two anti-VEGF intravitreal injections that showed treatment resistance and have a minimum of 3 months of follow-up. Treatment resistant nvAMD was defined as: (1) persistent intra- or sub-retinal fluid despite monthly injections, (2) no improvement in central foveal thickness and macular cube volume in response to anti-VEGF injection(s), and (3) new or worsening subretinal hemorrhage despite monthly anti-VEGF injections. The majority of patients had persistent intra-retinal fluid with or without sub-retinal fluid on OCT. The exclusion criteria included prior diagnosis of glaucoma or advanced cupping, history of intraocular pressure (IOP) steroid response to local or systemic steroids, and history of elevated IOP requiring topical therapy or glaucoma surgery. Patients with choroidal neovascularization from any cause other than nvAMD were excluded. Patients were also excluded if they had active diabetic retinopathy, retinal vein occlusion, active ocular infection, aphakia or pseudophakia with an anterior chamber intraocular lens (ACIOL) or scleral-fixated IOL (Table 1).
Table 1.
Study inclusion (A) and exclusion (B) criteria.
| (A) Inclusion criteria | |
| Age | >50 years |
| Active subfoveal CNV (classic or occult) | |
| Anti-VEGF therapy monthly (minimum of 2 injections) | |
| Treatment resistance to monthly anti-VEGF therapy | |
| BCVA | 20/30-20/800 |
| PDT or IVTA more than 6 months before dual therapy | |
| More than 3 months of follow-up | |
| (B) Exclusion criteria | |
| Glaucoma or cup-to-disc ratio >0.8 | |
| History of IOP elevation (>21 mmHg or >10 mmHg from baseline) on prior intraocular, periocular, topical or systemic steroid treatment | |
| Elevated IOP >21 mmHg from any cause with or without topical therapy | |
| History of glaucoma surgery (glaucoma tube or trabeculectomy) for any cause | |
| CNV from condition(s) other than nvAMD | |
| Diabetic retinopathy | |
| Retinal vein occlusion | |
| Active ocular infection at the time of treatment visit | |
| Aphakia or pseudophakia with ACIOL or scleral fixated IOL | |
Intervention
The dual therapy was administered in patients with treatment resistant nvAMD after discussion of the off-label use of the dexamethasone intravitreal implant. All patients were counseled on the risks of potential injection-related complications such as endophthalmitis, cataract progression (if phakic), and IOP elevation. All patients received local anesthesia in the form of sub-conjunctival lidocaine 2% without epinephrine, and were prepped with 5% betadine to the injection sites with the aid of an eyelid speculum. First, the anti-VEGF agent was administered followed by injection of the dexamethasone intravitreal implant using aseptic technique. A sterile cotton-tipped applicator was applied to facilitate closure of the sclerotomy, and the eye was irrigated with balanced salt solution and given a drop of topical antibiotic. All patients received the same anti-VEGF agent they were receiving prior to dual therapy at the time of intravitreal dexamethasone implant injection, and continued to receive anti-VEGF injections of the same agent thereafter at the discretion of treating physician.
For patients who met the inclusion criteria, BCVA and multimodal imaging data (SD-OCT, fundus photography, fundus autofluorescence and FA) were collected and analyzed at every visit (Table 2). In addition, key safety data including IOP changes, worsening or new cataract formation, posterior segment complications, endophthalmitis and any implant-related complications in the course of follow-up were reviewed from patients’ medical records (Table 3).
Table 2.
Demographics and baseline characteristics of patients that met the study inclusion criteria for dual therapy with anti-VEGF therapy and the dexamethasone intravitreal implant.
| Demographics and baseline characteristics | ||
|---|---|---|
| Mean age (years) | 81.5 | |
| Male | 8/18 | 44.44% |
| Female | 10/18 | 55.56% |
| Phakic | 2/18 | 11.11% |
| Pseudophakic | 16/18 | 88.89% |
| Right eye (OD) | 10/ 18 | |
| Left eye (OS) | 8/18 | |
| Mean # prior Anti-VEGFs | 26.3 | (range 2-54) |
| Prior IVTA | 2/18 | 11.11% |
| Prior PDT | 2/18 | 11.11% |
Table 3.
Complications and adverse events during follow-up period after dual therapy with anti-VEGF and dexamethasone intravitreal implant.
| Complications and adverse events (n=18) | ||
|---|---|---|
| N | percentage | |
| IOP elevations | 2 | 11.11% |
| Glaucoma drops | 2 | 11.11% |
| Glaucoma surgery | 0 | 0.00% |
| Pre-existing cataract worsening | 1 | 5.56% |
| New cataract formation | 0 | 0.00% |
| Cataract surgery | 1 | 5.56% |
| Vitreous hemorrhage | 0 | 0.00% |
| Anterior migration of implant | 0 | 0.00% |
| Endophthalmitis | 0 | 0.00% |
Statistics and data analysis
BCVA, central foveal thickness (CFT) and macular cube volume (MCV) data were collected and changes in these parameters were determined at the first visit after dual therapy was begun and at the visit following the last monotherapy anti-VEGF injection prior to the dual therapy. The clinical responses of three index patients to anti-VEGF monotherapy and subsequent dual therapy are summarized with their FA, OCT and BCVA data in Figures 1, 2 and 3. The mean differences in CFT (Figure 4) and MCV (Figure 5) were evaluated for statistical significance using a paired t-test. Snellen visual acuities were converted to logMAR equivalents and their means compared using a twoway ANOVA with multiple comparisons (Figure 6). The correlation using a Pearson coefficient between total number of injections and change in CFT after dual therapy is summarized in Figure 7. The total number of anti-VEGF injections required in the 6 months before and after the dual therapy with the dexamethasone intravitreal implant was determined and the injection free interval computed (Figure 8). All statistical comparisons were performed using GraphPad Prism software (LaJolla, CA). For all comparisons, statistical significance was defined as p<0.05.
Figure 1.
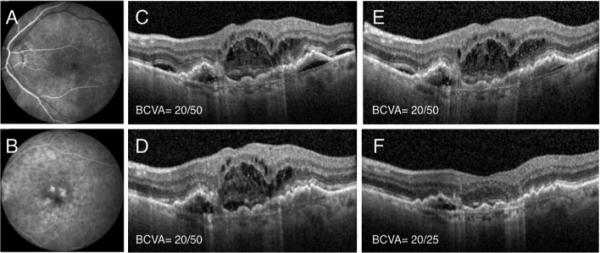
Patient 1 is an 87 year old pseudophakic Caucasian woman with neovascular AMD and active choroidal neovascularization (A, B) who presented with intra- and sub-retinal fluid on OCT (C). She underwent multiple intravitreal ranibizumab and aflibercept injections (>20), but developed persistent fluid despite monthly injections of each and had a BCVA of 20/50 (C, D, E). She underwent dual therapy with intravitreal aflibercept and the dexamethasone intravitreal implant, which resulted in significant improvement of intra- and sub-retinal fluid and improved BCVA to 20/25 (F). She did not have any IOP elevation or injection- related complications.
Figure 2.
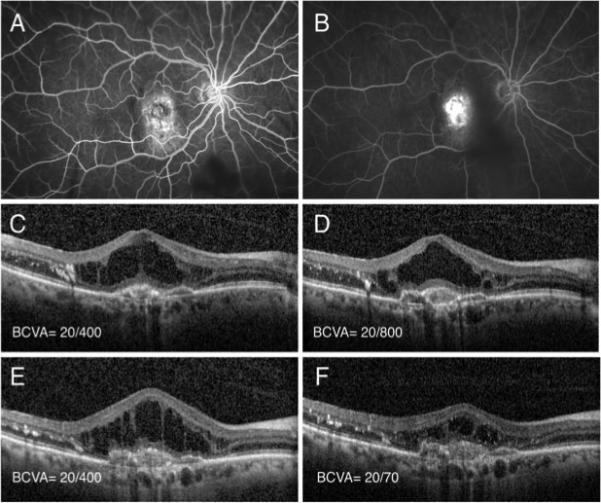
Patient 2 is an 67 year-old phakic Caucasian man with active neovascular AMD with subfoveal choroidal neovascularization (A, B). Patient had significant intra-retinal fluid with exudate on OCT (C) and underwent intravitreal injection of ranibizumab with minimal response (D) after 1 month. He subsequently received another injection of bevacizumab, but with a similar lack of effect (E) after 1 month. At that time, the eye was treated with a combined injection of intravitreal bevacizumab and the dexamethasone intravitreal implant after which there was a significant decrease in intraretinal fluid and improvement of visual acuity to 20/70 after 1 month (F). There was no elevation of IOP or injection-related complications.
Figure 3.
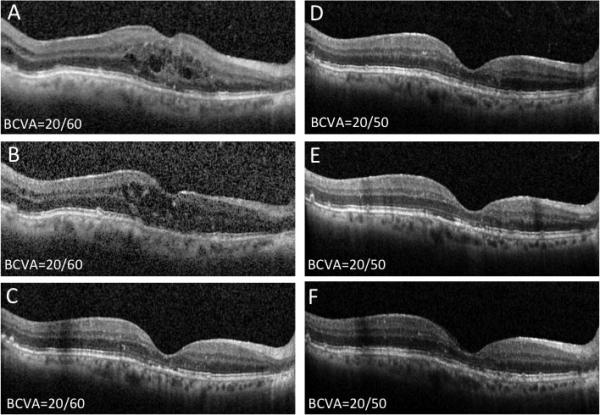
Patient 3 is a 74 year-old pseudophakic man with neovascular AMD and active subfoveal CNV with persistent intra-retinal fluid despite multiple bevacizumab (n=15) and aflibercept (n=15) intravitreal injections. Patient underwent injection of intravitreal ranibizumab (A) with minimal change in fluid appearance on OCT after 1 month (B). Then, combined intravitreal ranibizumab and dexamethasone intravitreal implant were given with complete resolution of the intraretinal fluid (C). As a result, no anti-VEGF treatment was given at this visit and patient continued to do well with observation alone at 1 month (D) and 3 months (E) post-combined treatment without anti-VEGF injections. At that time, additional combined intravitreal ranibizumab and dexamethasone intravitreal implant were given, and patient followed at monthly intervals for 1 year with stable visual acuity without requiring additional anti-VEGF or dual therapy re-treatment (F). The best corrected visual acuity remained stable at 20/50.
Figure 4.
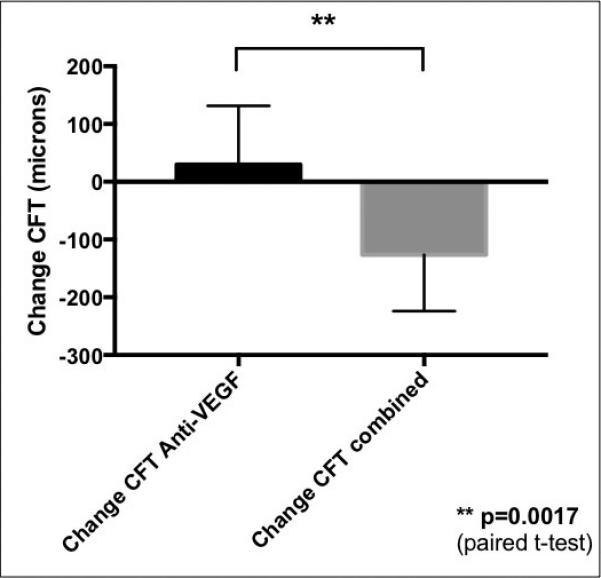
Comparison of mean central foveal thickness (CFT) change in patients with neovascular AMD treated with anti-VEGF agents alone (left, black) and those treated with subsequent combined therapy with dexamethasone intravitreal implant and anti-VEGF agent (grey, right) administered concurrently at the same visit. The mean changes represent treatment effects of the consecutive visits; the last anti- VEGF injection (left graph) and then the subsequent first combined treatment (right graph). The mean difference between the treatments was evaluated using paired t-test with significance level of p<0.05.
Figure 5.
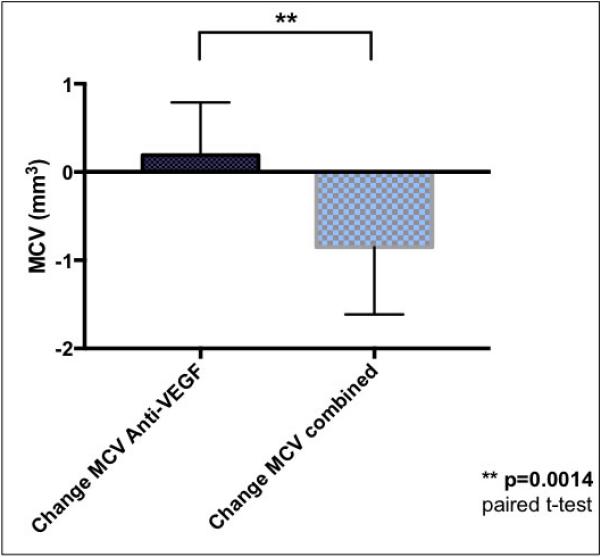
Comparison of mean macular cube volume (MCV) change in patients with neovascular AMD treated with anti-VEGF agents alone (left, black) with those treated with subsequent combined therapy with the dexamethasone intravitreal implant and an anti-VEGF agent (checkered, right) administered concurrently at the same visit. The mean changes represent treatment effects of the consecutive visits; the last anti-VEGF injection (left graph) and the subsequent first combined treatment (right graph). The mean difference between the treatments was evaluated using paired t-test with significance level of p<0.05.
Figure 6.
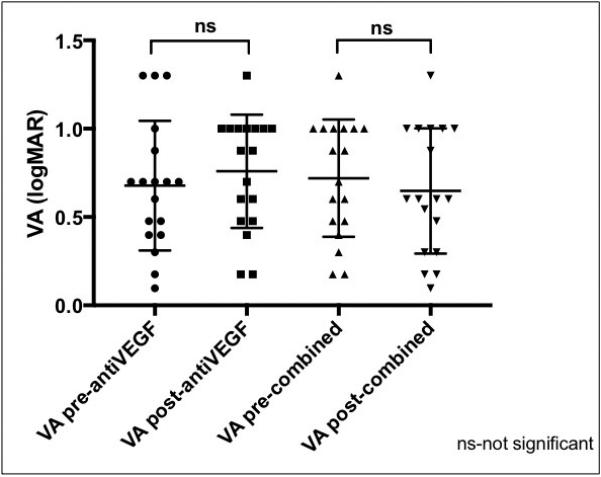
Comparison of best-corrected visual acuities in patients with neovascular AMD treated with anti-VEGF agents alone (left two graphs) and those treated with subsequent combined therapy with the dexamethasone intravitreal implant and an anti-VEGF agent (right two graphs) administered concurrently at the same visit. The mean BCVA represent treatment effects of the consecutive visits; the last anti- VEGF injection (left two plots) and the subsequent first combined treatment (right two plots). The mean differences between the treatments were evaluated using paired ANOVA with multiple comparisons and a significance level of p<0.05 (relevant comparisons shown).
Figure 7.
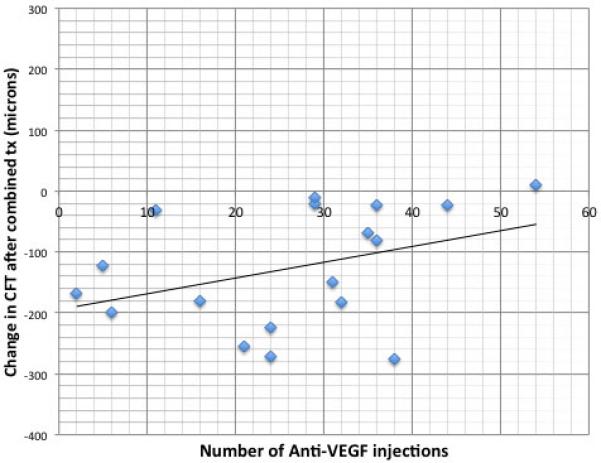
Correlation between the number of prior anti-VEGF injections before dual therapy with the dexamethasone intravitreal implant and an anti-VEGF agent and the change in central foveal thickness after the first combined treatment. A best-fitted linear regression curve and Pearson correlation were determined and demonstrated correlation with Pearson correlation coefficient (r=0.375).
Figure 8.
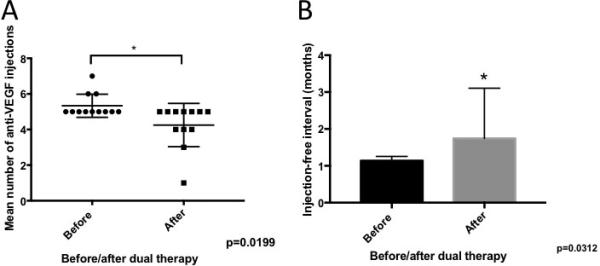
Comparison of the treatment burden before and after dual therapy with the dexamethasone intravitreal implant and anti-VEGF agent. The mean total number of anti-VEGF injections in the 6 months after dual therapy (4.25 injections) was significantly decreased compared to the mean during the 6 months prior to dual therapy (5.33 injections) (p=0.0199). The mean injection-free interval during the 6 months following dual therapy (1.75 months) was significantly longer compared to that seen during the 6 months before dual therapy (1. 13 months) (p=0.0312).
Results
Eighteen eyes of 18 consecutive patients met the inclusion criteria and were included in the study (Table 1). The cohort represented all eyes that received dual therapy. Subjects had a mean age of 81.5 years (range 67–91 years), 8 (44.4%) were male and 10 (55.6%) were female (Table 2) and all were Caucasian. Most eyes were pseudophakic (16 or 88.9%) while only 2 (11.1%) were phakic. Patients underwent a mean of 26.3 anti-VEGF injections (range 2-56) prior to having dual therapy with the dexamethasone intravitreal implant. Prior treatments included injections of bevacizumab, ranibizumab or aflibercept, with 17 (94.4%) patients having received at least two different drugs in their course of prior treatment and one patient having received a series of 6 ranibizumab injections only before dual therapy. Only 2 (11.1%) patients had prior photodynamic therapy and 2 (11.1%) had intravitreal triamcinolone acetonide, all of which occurred at least 6 months before dual therapy.
The mean length of follow-up was 8.2 months (range 4-14.2 months). The dexamethasone intravitreal implant dual therapy was overall well tolerated; the frequency of adverse effects is summarized in Table 3. Transient IOP elevation effectively treated with topical glaucoma drops developed in 2 (11.1%) of patients. None of the patients required glaucoma surgery. One patient with pre-existing cataract developed worsening cataract and became symptomatic. This patient underwent successful cataract extraction without complications. There were no cases of endophthalmitis, vitreous hemorrhage or implant-related complications.
Figures 1, 2 and 3 summarize clinical data from three index patients included in the series. The first is an 87 year-old pseudophakic Caucasian woman with nvAMD and active CNV (Figure 1A, B). The patient received 24 anti-VEGF injections at baseline, but had persistent subretinal fluid on monthly aflibercept therapy (panels C-E demonstrate OCTs following the last three aflibercept injections, each associated with 20/50 vision). Following a single dual therapy treatment, there was significant resolution of intraretinal and subretinal fluid and VA improved to 20/25 (Figure 1F). The second patient is a 67 year-old phakic Caucasian man with recent conversion to nvAMD (Figure 2 A, B) who failed to significantly respond to initial ranibizumab and subsequent bevacizumab intravitreal injections (Figure 2 C-E). However, after one dual therapy treatment with the dexamethasone intravitreal implant combined with bevacizumab, the patient showed significant resolution of intra- and sub-retinal fluid and experienced significant improvement in BCVA to 20/70 from 20/800 (Figure 2F). The third patient is a 74 year-old pseudophakic man with active subfoveal CNV and persistent intra-retinal fluid despite 30 bevacizumab and aflibercept intravitreal injections. He underwent injection of intravitreal ranibizumab with minimal response (Figure 3A, B) before he received a combination treatment of intravitreal ranibizumab and dexamethasone implant. This resulted in complete resolution of the intraretinal fluid (Figure 3C). He was observed monthly without anti-VEGF therapy, without recurrence of fluid (Figure 3D, E), and requiring only a single dual therapy during 12 months of follow up (Figure 3F).
Figures 4 and 5 summarize the mean change in CFT and MCV after dual therapy with the dexamethasone intravitreal implant and anti-VEGF injection compared to anti-VEGF therapy alone. Following dual therapy, there was a significant reduction in mean CFT of 126.3 μm compared to a mean increase in mean CFT of 29.9 μm with anti-VEGF treatment alone. This difference was statistically significant (p=0.0017). Furthermore, as a result of combined treatment there was a statistically significant reduction in MCV of - 0.85 mm3 compared to a mean increase in MCV of 0.19 mm3 with anti-VEGF treatment alone (p=0.0014).
Figure 6 summarizes the visual acuity data. Anti-VEGF therapy alone did not produce significant mean change in logMAR BCVA. Dual therapy with the dexamethasone intravitreal implant resulted in a small trend towards improvement of mean BCVA, but the difference was not statistically significant (p=0.92). Although there was no change in mean BCVA, vision improved by at least one line in 6 (33.3%) of patients as a result of dual therapy.
Patients who had improvement in BCVA as a result of combined treatment had a lower mean number of prior anti-VEGF injections (21.0 vs 28.9), which may suggest that earlier treatment is beneficial. To investigate this, we performed a correlation analysis between the change in CFT and number of anti-VEGF injections as a surrogate marker of baseline nvAMD disease duration. There was mild-moderate correlation between number of prior anti-VEGF injections and the likelihood of CFT reduction in response to dual therapy (Figure 7) with the Pearson correlation coefficient (r=0.375). In addition, dual therapy significantly reduced the number of required anti-VEGF injections after combined injections (Figure 8). The mean total number of injections during the 6 months before dual therapy (5.33) was significantly greater than the total number of injections during the 6 months after dual therapy (4.25) with p=0.0199 (Figure 8A). In addition, the mean treatment-free interval between injections before (1.13 months) was significantly extended to 1.74 months as a result of dual therapy (Figure 8B, p=0.0312).
Discussion
Over the past decade, anti-VEGF therapy has become the standard of care for the treatment of nvAMD. Despite the success of anti-VEGF agents, a significant proportion of eyes with nvAMD will demonstrate partial or no treatment response demonstrated by persistent intraretinal or subretinal fluid. In the Comparison of Age Related Macular Degeneration Treatment Trial (CATT), up to 50% of patients on monthly anti-VEGF therapy had persistent fluid on OCT.10,33 Although the majority of these “incomplete responders” retained good vision with continued anti-VEGF therapy,34 patients with persistent fluid associated with macular exudation, hemorrhage and/or worsening vision despite monthly anti-VEGF therapy can be classified as having treatment-resistant nvAMD and are at risk for vision loss. It can be a challenge for physicians to get complete resolution of the fluid after many injections and a variety of treatment strategies have been employed. These include switching anti-VEGF agents,14,35-37 increasing the injection dose or frequency,38,39 and combining anti-VEGF therapy with PDT40 and/or intravitreal steroids.41,42
Combination therapy with intravitreal steroids may simultaneously address both angiogenic (VEGF-mediated) and inflammatory mechanisms of choroidal neovascularization and its associated intraretinal and subretinal fluid.43,44 Although it was used before in nvAMD eyes because of its high potency,41,42 intravitreal dexamethasone given as a single injection has limited clinical usefulness because of its very short half-life.28 The dexamethasone intravitreal implant, however, offers sustained release of dexamethasone in the vitreous cavity with a clinically meaningful effect of 3-4 months duration. In this study, we report a case-series of consecutive nvAMD patients with treatment resistance to anti-VEGF monotherapy who were then treated with dual therapy of the same anti-VEGF agent plus the off-label use of the dexamethasone intravitreal implant. This is a consecutive series that presents the data from every patient that received dual therapy for persistently active nvAMD. Patients enrolled in the study met strict inclusion criteria and were consistently managed. The patients had a significant decrease in CFT and MCV after a single dual therapy. There was a mild to moderate correlation between CFT change and the number of prior anti-VEGF injections, suggesting that earlier treatment with dual therapy may result in a more significant anatomic response. Although visual improvement of at least one line of BCVA was observed in a third of patients, the mean visual acuity gains did not reach statistical significance. It is possible that earlier treatment with the dexamethasone intravitreal implant, along with continued anti-VEGF treatment may have increased the likelihood of a more significant visual improvement, but this question requires further study with a larger sample size of patients. Dual therapy resulted in a small but significant reduction in the mean number of required injections and resulted in a significant extension in the treatment-free interval. In one patient, we were able to eliminate persistent intra-retinal fluid and get the patient off the anti-VEGF injections completely. This suggests that dual therapy may reduce treatment burden in neovascular AMD as was suggested previously.32 It is also potentially possible that earlier initiation of dual therapy would have shown a greater effect in reducing the subsequent treatment burden also. Unfortunately, the number of patients was too small to adequately comment on differences in response of eyes with intraretinal fluid with a frank cystoid appearance to those that had predominately subretinal fluid and less or no cystoid macular edema as part of the nvAMD complex.
Overall, dual therapy with the intravitreal dexamethasone implant was well tolerated, but it produced the predictable effect of worsening cataract and mild IOP elevation (managed with drops alone) in a small subset of patients, which is consistent with the reported side effects of this agent.45,46 There were no implant-related or same day double injection-related complications.
The limitations of this study include its retrospective design, small sample size and lack of control group. In addition, the patient cohort had a heterogeneous treatment history with varied durations of therapy. This likely reflected the variability of the underlying biology of disease in each patient, including the variability in type and size of the choroidal neovascular lesions. These metrics were not measured as doing so was beyond the intent and scope of our study. Conversely, the study was done at a single-center with relatively homogenous, predominantly Caucasian population, its results may not necessarily be applied to other races, which may respond differently to intravitreal anti-VEGF and/or steroid therapy, and may have higher rates of nvAMD variants such as polypoidal choroidal vasculopathy (PCV). Lastly, the response to the combined treatment is still difficult to predict as there are no widely-available direct measurements of the inflammatory activity in eyes with active CNV lesions. The development of biomarker assays for nvAMD treatment non-responders will be important to help us adequately stratify patients that would benefit most from combined therapy.
The simultaneous use of the dexamethasone intravitreal implant in combination with anti-VEGF therapy for nvAMD may be a safe and effective treatment option for eyes resistant to anti-VEGF monotherapy with persistent intraretinal or subretinal fluid. Earlier treatment with such dual therapy may maximize anatomic and visual outcomes and reduce the treatment burden. We look forward to the continued evaluation of dual therapy as a treatment for treatment-resistant nvAMD through larger, prospective multicenter trials. This will allow us to better understand which patients are the best candidates for such intervention and hopefully lead to earlier intervention. We also look forward to the development of safe, straightforward methods of clinical assessment of inflammatory cytokines in eyes receiving intravitreal injection therapy with the hope that they will correlate with responses to therapy with agents that act through different mechanisms and so treatment paradigms may be more specifically titrated for each individual patient.
Summary statement.
This manuscript summarizes retrospective study of using combined intravitreal dexamethasone implant in combination with anti-VEGF therapy for treatment-resistant neovascular AMD.
Acknowledgements
None
Funding The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Maria A. Woodward was supported by the National Eye Institute, Bethesda, MD (grant K23EY023596-01)
Abbreviations
- VEGF
vascular endothelial growth factor
- CNV
choroidal neovascularization
- nvAMD
neovascular age related macular degeneration
- PDT
photodynamic therapy
- OCT
optical coherence tomography
- FA
fluorescein angiography
- BCVA
best corrected visual acuity
- IOP
intraocular pressure
- CFT
central foveal thickness
- MCV
macular cube volume
- IVTA
intravitreal triamcinolone acetonide
Footnotes
Financial disclosures: Consultant for Alimera (Wolfe), consultants for Allergan (Capone, Hassan, Williams, Wolfe, Yonekawa), consultant for Arctic Dx (Hassan), consultants for Genentech (Hassan, Wolfe), consultant for Novartis (Hassan), consultant for Regeneron (Hassan), National Eye scientific advisory board for Intelligent Retinal Imaging Systems (Woodward). None of the other authors have relevant financial disclosures.
Previous presentations: This manuscript has not been previously presented.
References
- 1.Zambelli-Weiner A, Crews JE, Friedman DS. Disparities in adult vision health in the United States. American journal of ophthalmology. 2012;154:S23–30. e21. doi: 10.1016/j.ajo.2012.03.018. doi:10.1016/j.ajo.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 2.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379:1728–1738. doi: 10.1016/S0140-6736(12)60282-7. doi:10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, et al. Age-related maculopathy in a multiracial United States population: the National Health and Nutrition Examination Survey III. Ophthalmology. 1999;106:1056–1065. doi: 10.1016/S0161-6420(99)90255-5. doi:10.1016/S0161-6420(99)90255-5. [DOI] [PubMed] [Google Scholar]
- 4.Friedman DS, et al. Prevalence of age-related macular degeneration in the United States. Archives of ophthalmology. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. doi:10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 5.Campochiaro PA, Soloway P, Ryan SJ, Miller JW. The pathogenesis of choroidal neovascularization in patients with age-related macular degeneration. Mol Vis. 1999;5:34. [PubMed] [Google Scholar]
- 6.Kwak N, Okamoto N, Wood JM, Campochiaro PA. VEGF is major stimulator in model of choroidal neovascularization. Investigative ophthalmology & visual science. 2000;41:3158–3164. [PubMed] [Google Scholar]
- 7.Grossniklaus HE, Green WR. Choroidal neovascularization. American journal of ophthalmology. 2004;137:496–503. doi: 10.1016/j.ajo.2003.09.042. doi:10.1016/j.ajo.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 8.Brown DM, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009;116:57–65. e55. doi: 10.1016/j.ophtha.2008.10.018. doi:10.1016/j.ophtha.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Rosenfeld PJ, et al. Ranibizumab for neovascular age-related macular degeneration. The New England journal of medicine. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. doi:10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 10.Comparison of Age-related Macular Degeneration Treatments Trials Research, G. et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–1398. doi: 10.1016/j.ophtha.2012.03.053. doi:10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki M, et al. Predictive factors for non-response to intravitreal ranibizumab treatment in age-related macular degeneration. The British journal of ophthalmology. 2014;98:1186–1191. doi: 10.1136/bjophthalmol-2013-304670. doi:10.1136/bjophthalmol-2013-304670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lux A, Llacer H, Heussen FM, Joussen AM. Non-responders to bevacizumab (Avastin) therapy of choroidal neovascular lesions. The British journal of ophthalmology. 2007;91:1318–1322. doi: 10.1136/bjo.2006.113902. doi:10.1136/bjo.2006.113902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krebs I, et al. Non-responders to treatment with antagonists of vascular endothelial growth factor in age-related macular degeneration. The British journal of ophthalmology. 2013;97:1443–1446. doi: 10.1136/bjophthalmol-2013-303513. doi:10.1136/bjophthalmol-2013-303513. [DOI] [PubMed] [Google Scholar]
- 14.Gasperini JL, et al. Bevacizumab and ranibizumab tachyphylaxis in the treatment of choroidal neovascularisation. The British journal of ophthalmology. 2012;96:14–20. doi: 10.1136/bjo.2011.204685. doi:10.1136/bjo.2011.204685. [DOI] [PubMed] [Google Scholar]
- 15.Forooghian F, Cukras C, Meyerle CB, Chew EY, Wong WT. Tachyphylaxis after intravitreal bevacizumab for exudative age-related macular degeneration. Retina. 2009;29:723–731. doi: 10.1097/IAE.0b013e3181a2c1c3. doi:10.1097/IAE.0b013e3181a2c1c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang AA, et al. Intravitreal aflibercept for treatment-resistant neovascular age-related macular degeneration. Ophthalmology. 2014;121:188–192. doi: 10.1016/j.ophtha.2013.08.035. doi:10.1016/j.ophtha.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 17.Dhalla MS, et al. Combined photodynamic therapy with verteporfin and intravitreal bevacizumab for choroidal neovascularization in age-related macular degeneration. Retina. 2006;26:988–993. doi: 10.1097/01.iae.0000247164.70376.91. doi:10.1097/01.iae.0000247164.70376.91. [DOI] [PubMed] [Google Scholar]
- 18.Ahmadieh H, et al. Intravitreal bevacizumab versus combined intravitreal bevacizumab and triamcinolone for neovascular age-related macular degeneration: six-month results of a randomized clinical trial. Retina. 2011;31:1819–1826. doi: 10.1097/IAE.0b013e31820d58f2. doi:10.1097/IAE.0b013e31820d58f2. [DOI] [PubMed] [Google Scholar]
- 19.Forte R, Bonavolonta P, Benayoun Y, Adenis JP, Robert PY. Intravitreal ranibizumab and bevacizumab in combination with full-fluence verteporfin therapy and dexamethasone for exudative age-related macular degeneration. Ophthalmic research. 2011;45:129–134. doi: 10.1159/000318877. doi:10.1159/000318877. [DOI] [PubMed] [Google Scholar]
- 20.Tuo J, Grob S, Zhang K, Chan CC. Genetics of immunological and inflammatory components in age-related macular degeneration. Ocul Immunol Inflamm. 2012;20:27–36. doi: 10.3109/09273948.2011.628432. doi:10.3109/09273948.2011.628432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nozaki M, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2328–2333. doi: 10.1073/pnas.0408835103. doi:10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machalinska A, et al. Elevated plasma levels of C3a complement compound in the exudative form of age-related macular degeneration. Ophthalmic research. 2009;42:54–59. doi: 10.1159/000219686. doi:10.1159/000219686. [DOI] [PubMed] [Google Scholar]
- 23.Felinski EA, Antonetti DA. Glucocorticoid regulation of endothelial cell tight junction gene expression: novel treatments for diabetic retinopathy. Current eye research. 2005;30:949–957. doi: 10.1080/02713680500263598. doi:10.1080/02713680500263598. [DOI] [PubMed] [Google Scholar]
- 24.Migita K, et al. Apoptosis induction in human peripheral blood T lymphocytes by high-dose steroid therapy. Transplantation. 1997;63:583–587. doi: 10.1097/00007890-199702270-00017. [DOI] [PubMed] [Google Scholar]
- 25.Druilhe A, Letuve S, Pretolani M. Glucocorticoid-induced apoptosis in human eosinophils: mechanisms of action. Apoptosis. 2003;8:481–495. doi: 10.1023/a:1025590308147. [DOI] [PubMed] [Google Scholar]
- 26.Spitzer MS, Y. E., Kaczmarek RT, Sierra A, Aisenbrey S, Grisanti S, Bartz-Schmidt KU, Szurman P. Sodium hyaluronate gels as a drug-release system for corticosteroids: release kinetics and antiproliferative potential for glaucoma surgery. Acta Ophthalmol. 2008;86:842–848. doi: 10.1111/j.1755-3768.2007.01149.x. [DOI] [PubMed] [Google Scholar]
- 27.Kompella UB, Bandi N, Ayalasomayajula SP. Subconjunctival nano- and microparticles sustain retinal delivery of budesonide, a corticosteroid capable of inhibiting VEGF expression. Investigative ophthalmology & visual science. 2003;44:1192–1201. doi: 10.1167/iovs.02-0791. [DOI] [PubMed] [Google Scholar]
- 28.Kwak HW, D. A. D. Evaluation of the retinal toxicity and pharmacokinetics of dexamethasone after intravitreal injection. Archives of ophthalmology. 1992;110:259–266. doi: 10.1001/archopht.1992.01080140115038. [DOI] [PubMed] [Google Scholar]
- 29.Chang-Lin JE, A. M., Acheampong AA, Robinson MR, Whitcup SM, Kuppermann BD, Welty D. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. 2011;52:80–86. doi: 10.1167/iovs.10-5285. [DOI] [PubMed] [Google Scholar]
- 30.Cabrera M, Yeh S, Albini TA. Sustained-release corticosteroid options. Journal of ophthalmology. 2014;2014:164692. doi: 10.1155/2014/164692. doi:10.1155/2014/164692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyer DS, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904–1914. doi: 10.1016/j.ophtha.2014.04.024. doi:10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 32.Kuppermann BD, et al. Dexamethasone Intravitreal Implant as Adjunctive Therapy to Ranibizumab in Neovascular Age-Related Macular Degeneration: A Multicenter Randomized Controlled Trial. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2015;234:40–54. doi: 10.1159/000381865. doi:10.1159/000381865. [DOI] [PubMed] [Google Scholar]
- 33.Group CR, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. The New England journal of medicine. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. doi:10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.R., F. B. M. S. a. G.-P Current Strategies for the Management of Treatment-Resistant Neovascular Age-Related Macular Degeneration/. Current Ophthalmology Reports. 2014;2:6–13. [Google Scholar]
- 35.Kent JS, et al. Comparison of outcomes after switching treatment from intravitreal bevacizumab to ranibizumab in neovascular age-related macular degeneration. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2012;47:159–164. doi: 10.1016/j.jcjo.2012.01.003. doi:10.1016/j.jcjo.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Patel KH, et al. Rapid response of retinal pigment epithelial detachments to intravitreal aflibercept in neovascular age-related macular degeneration refractory to bevacizumab and ranibizumab. Eye. 2013;27:663–667. doi: 10.1038/eye.2013.31. quiz 668, doi:10.1038/eye.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yonekawa Y, et al. Conversion to aflibercept for chronic refractory or recurrent neovascular age-related macular degeneration. American journal of ophthalmology. 2013;156:29–35. e22. doi: 10.1016/j.ajo.2013.03.030. doi:10.1016/j.ajo.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 38.Fung AT, et al. Pilot study to evaluate the role of high-dose ranibizumab 2.0 mg in the management of neovascular age-related macular degeneration in patients with persistent/recurrent macular fluid <30 days following treatment with intravitreal anti-VEGF therapy (the LAST Study). Eye. 2012;26:1181–1187. doi: 10.1038/eye.2012.174. doi:10.1038/eye.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown DM, Chen E, Mariani A, Major JC, Jr., Group SS. Super-dose anti-VEGF (SAVE) trial: 2.0 mg intravitreal ranibizumab for recalcitrant neovascular macular degeneration-primary end point. Ophthalmology. 2013;120:349–354. doi: 10.1016/j.ophtha.2012.08.008. doi:10.1016/j.ophtha.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Koh A, et al. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012;32:1453–1464. doi: 10.1097/IAE.0b013e31824f91e8. doi:10.1097/IAE.0b013e31824f91e8. [DOI] [PubMed] [Google Scholar]
- 41.Augustin AJ, Puls S, Offermann I. Triple therapy for choroidal neovascularization due to age-related macular degeneration: verteporfin PDT, bevacizumab, and dexamethasone. Retina. 2007;27:133–140. doi: 10.1097/IAE.0b013e3180323de7. doi:10.1097/IAE.0b013e3180323de7. [DOI] [PubMed] [Google Scholar]
- 42.Ranchod TM, et al. LuceDex: a prospective study comparing ranibizumab plus dexamethasone combination therapy versus ranibizumab monotherapy for neovascular age-related macular degeneration. Retina. 2013;33:1600–1604. doi: 10.1097/IAE.0b013e318285cb71. doi:10.1097/IAE.0b013e318285cb71. [DOI] [PubMed] [Google Scholar]
- 43.Edelman JL, Lutz D, Castro MR. Corticosteroids inhibit VEGF-induced vascular leakage in a rabbit model of blood-retinal and blood-aqueous barrier breakdown. Experimental eye research. 2005;80:249–258. doi: 10.1016/j.exer.2004.09.013. doi:10.1016/j.exer.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 44.Veurink M, Stella C, Tabatabay C, Pournaras CJ, Gurny R. Association of ranibizumab (Lucentis(R)) or bevacizumab (Avastin(R)) with dexamethasone and triamcinolone acetonide: an in vitro stability assessment. Eur J Pharm Biopharm. 2011;78:271–277. doi: 10.1016/j.ejpb.2010.12.018. doi:10.1016/j.ejpb.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 45.Haller JA, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117:1134–1146. e1133. doi: 10.1016/j.ophtha.2010.03.032. doi:10.1016/j.ophtha.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 46.Haller JA, et al. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Archives of ophthalmology. 2010;128:289–296. doi: 10.1001/archophthalmol.2010.21. doi:10.1001/archophthalmol.2010.21. [DOI] [PubMed] [Google Scholar]


