ABSTRACT
Nasopharyngeal carcinoma (NPC) is a highly invasive head-neck cancer derived from the nasopharyngeal epithelium, mainly prevalent in southern China and Southeast Asia. Radiotherapy and adjuvant cisplatin (DDP) chemotherapy are standard administrations applied in the treatment of NPC. However, resistance to chemotherapeutic drugs has recently become more common, resulting in worse treatment outcome for NPC therapy. To elucidate the underlying molecular basis of drug resistance to DDP in NPC cells, we examined the morphocytology, cell motility and molecular changes in DDP-resistant NPC cells with respect to epithelial-mesenchymal transition (EMT) features. We found that EMT is closely associated with DDP-induced drug resistance in NPC cells, as DDP-resistant cells displayed morphological and molecular markers changes consistent with EMT. Wound healing and Transwell Boyden chamber assays revealed an enhanced migration and invasion potential in DDP-resistant NPC cells. Mechanistically, upregulation of NEDD4 was observed to relate to EMT in DDP-resistant cells. More importantly, depletion of NEDD4 in resistant cells led to a partial reversion of EMT phenotypes to MET characteristics. These data suggest that NEDD4 is largely involved in EMT features and chemoresistance of NPC cancer cells. NEDD4 could be a novel therapeutic target to overcome drug resistance in successful administrations of NPC.
KEYWORDS: drug resistance, EMT, Nasopharyngeal carcinoma, NEDD4
Introduction
Nasopharyngeal carcinoma (NPC), a type of head-neck cancer derived from the nasopharyngeal epithelium, is rear but highly invasive. NPC shows a strong geographic and racial preference, which is mainly prevalent in Southern China and Southeast Asia among people of Southern Chinese descent.1,2 It is considered that there were about 60,600 newly diagnosed NPC and estimated 34,100 deaths in China in 2015.3 Radiotherapy and combined with chemotherapy are the main treatment choices, which have helped to achieve good prognoses, especially for early-stage patients with NPC.4 Unfortunately, for advanced NPC, concurrent chemoradiotherapy outcomes have been unsatisfied mainly due to distant metastases and recurrence.5 In many cases, patients are resistant to chemotherapeutic drugs, which lead to failure of chemo-radiotherapy. Therefore, there is a critical need to explore the underlining molecular mechanisms of drug resistance in NPC.
DNA damaging agent cisplatin (DDP) is one of the standard chemotherapeutic drugs that have been commonly used for the treatment of various cancers including NPC.6-8 DDP is clinically used for NPC by inducing tumor cell death. DDP exhibits its cytotoxicity and/or apoptosis-inducer activities via forming DNA adducts or by targeting therapeutically important cancer signaling pathways and molecules.9-11 Increasing evidence, however, indicates that many patients acquired DDP-resistance during cancer chemotherapy. Recent studies have indicated that epithelial mesenchymal transition (EMT) is involved in chemoresistance in human malignancies. EMT is a complex process by which epithelial cells transit to mesenchymal phenotype. A series of marked morphological changes including epithelial cells lose their cell polarity and cell-cell adhesion; subsequently acquire increased migratory and invasive properties as mesenchymal-like cells.12 EMT is essential in the initiation of metastasis for cancer progression.13 There are several transcription factors, such as zinc finger E-box binding homeobox 1 (ZEB 1) and ZEB 2, snail, slug and twist, known to be involved in the regulation of EMT.14 The downregulated epithelial molecular markers (eg E-cadherin and β-catenin) as well as upregulated mesenchymal molecular markers (eg vimentin, fibronectin, and N-cadherin) are also observed during EMT. The activity of matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9), which are associated with the invasive phenotype, is also increased.15,16 Zhang et al. reported that the development of DDP resistance of NPC cells is accompanied by morphological and molecular changes consistent with EMT. Increased metastatic potential was also observed in vitro.17 Recently, it is reported that therapeutically targeting of the CBP/β-catenin signaling pathway effectively reduces the expression of cancer stem-like associated proteins with altered expression of EMT markers, and synergistically suppresses growth of Epstein-Barr virus (EBV)-positive NPC cells by combination with DDP treatment.18 Studies also showed that microRNAs (miRNAs) participate in NPC pathogenesis. For instance, miR-203 has been documented to suppress NPC cell migration, invasion, tumor stemness, and chemotherapy resistance to DDP in vitro and in vivo, by targeting ZEB2 and downstream EMT and tumor stemness signals.19 The miR-10b is upregulated in HNE1/DDP cells, and inhibition of miR-10b expression reversed the EMT phenotype.20 Multiple molecules and various signaling pathways are involved in chemoresistance in human malignancies. However, the mechanisms of DDP-resistant NPC have not been fully elucidated.
Neural precursor cell expressed developmentally downregulated protein 4 (NEDD4) is an E3 protein ligase enzyme, degrades a good deal of membrane proteins, such as ion channels and membrane receptors, through ubiquitination and endocytosis. NEDD4 regulates a diverse range of processes, such as fluid and electrolyte homeostasis,21 neuronal development,22 viral budding,23 and is essential for animal development and survival.24 Accumulating evidence suggests that NEDD4 plays important roles in cancer development. Overexpression of NEDD4 is frequently detected in many types of human cancers including prostate and bladder cancers,25 gastric carcinomas,26 colorectal cancer,27 non-small cell lung carcinomas,28 and breast cancer.29 NEDD4 interacts with many proteins that closely related to tumorigenesis and metastasis.30,31 Tumor suppressor PTEN (phosphatase and tensin homolog depleted on chromosome 10) is inversely correlated with NEDD4 in many human cancer cell lines, such as breast cancer MDA-MB-231 and prostate cancer DU145 cell lines.32 Moreover, impeded NEDD4-mediated Ras degradation underlies Ras-driven tumorigenesis in various types of human cancer cell lines, such as cervical adenocarcinoma HeLa, colorectal adenocarcinoma HT-29, gastric adenocarcinoma BGC-823 and hepatocellular carcinoma HepG2 cells.33 Large tumor suppressor kinase 1 (LATS1), a negative regulator of YAP in the Hippo signaling pathway, directly binds NEDD4 for its ubiquitination and degradation, subsequently leads to the inhibition of Hippo pathway.34 One study showed that haplotype of NEDD4 binding protein 2 is correlated with sporadic NPC.35 Although these studies provide strong evidence for the oncogenic activities of NEDD4 in various human cancers, neither NPC nor DDP-resistant NPC cells have been extensively studied. Thus, in the present study, we established DDP-resistant cells from well-characterized NPC cell lines. We determined whether these DDP-resistant NPC cells have typical EMT-like properties. We further demonstrate whether NEDD4 is involved in DDP-resistance mediated EMT.
Results
Establishment of DDP-resistant NPC cell lines
Both NPC cells, CNE1 and CNE2, were exposed to increasing concentrations of DDP for more than 6 months to establish the DDP-resistant cells. Compared with their parental cells, chemoresistance to DDP was observed in CNE1/DDP and CNE2/DDP cells. As shown in Fig. 1A, 3 μM DDP led to about 50% cell growth inhibition in both CNE1 and CNE2 cells, respectively. CNE1/DDP and CNE2/DDP cells, however, produced resistance to the growth inhibitory properties of 3 μM DDP. Thus, the resistant NPC cells were continuously maintained in RPMI 1600 medium containing 2 μM DDP.
Figure 1.

Cisplatin-resistant cells exhibited EMT phenotype. (A) MTT assay was conducted in parental and CNE/DDP NPC cells. *P < 0.05, **P < 0.01 vs control. (B) Cell morphology was observed by microscopy in parental and CNE/DDP NPC cells. Parental cells displayed an epithelioid appearance, whereas their CNE/DDP cells showed elongated, irregular fibroblastoid morphology. (C) Top panel: Invasion assay was performed to measure the invasive capacity in parental and CNE/DDP NPC cells. Bottom panel: Quantitative results are illustrated for top panel. *P < 0.05 vs control. (D) Cell attachment and attachment assays were assessed in parental and CNE/DDP NPC cells. *P < 0.05, **P < 0.01 vs control.
Figure 3.
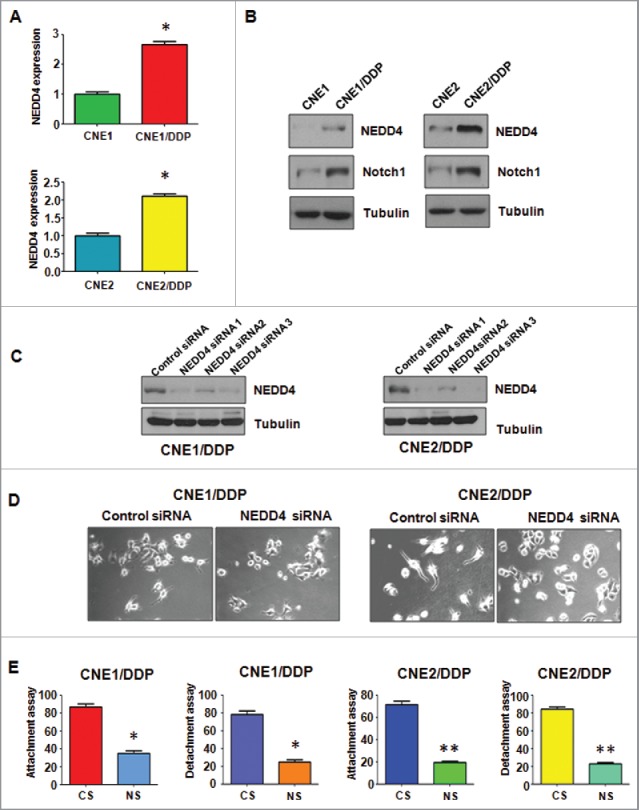
Cisplatin-resistant cells have high expression of NEDD4. (A) Real-time RT-PCR assay was conducted to detect the expression of NEDD4 in parental and CNE/DDP NPC cells. *P < 0.05 vs control. (B) Western blotting analysis was performed to detect the expression of NEDD4 in parental and CNE/DDP NPC cells. (C) Western blotting analysis was performed to detect the efficacy of NEDD4 siRNA transfection. (D) Cell morphology was taken by microscopy in CNE/DDP NPC cells transfected with NEDD4 siRNA. (E) Cell attachment and detachment assays were measured in CNE/DDP NPC cells transfected with NEDD4 siRNA. *P < 0.05, **P < 0.01 vs control siRNA. CS: control siRNA; NS: NEDD4 siRNA.
DDP-resistant NPC cells acquire EMT features and undergo EMT molecular marker changes
As drug-resistant cells often develop EMT phenotype, we examined the morphologic changes of DDP-resistant NPC cells. Consistent with this concept, we found that CNE1/DDP and CNE2/DDP cells developed the EMT phenotype changes. Both DDP-resistant NPC cells exhibited elongated, fibroblastoid morphology, whereas their parental cells displayed a rounded shape (Fig. 1B). We further detected the EMT-associated aggressive characteristics, such as cell attachment, detachment, migration, and invasion, in DDP-resistant NPC cells. The Transwell assay showed that the invasive capacity of CNE1/DDP and CNE2/DDP cells was significantly enhanced compared with parental cells (Fig. 1C). We also observed that DDP-resistant NPC cells have increased ability of attachment and detachment (Fig. 1D). Moreover, DDP-resistant NPC cells acquired enhanced motility activity, detected by wound healing assay (Fig. 2A). We also examined whether DDP-resistant NPC cells undergoes EMT molecular marker changes. The expression of different EMT markers was compared in paired parental and resistant cell lines using Western blotting analysis. As expected, we observed that the expression of epithelial molecules E-cadherin and ZO-1 was significantly decreased in DDP-resistant NPC cells. Meanwhile, the expression of mesenchymal markers, such as Vimentin, N-cadherin and Slug, were extremely increased in resistant cells (Fig. 2). Taken together, these results suggest that DDP-resistant NPC cells acquired EMT characteristics, and the mesenchymal phenotype could be responsible for the DDP resistance in NPC.
Figure 2.
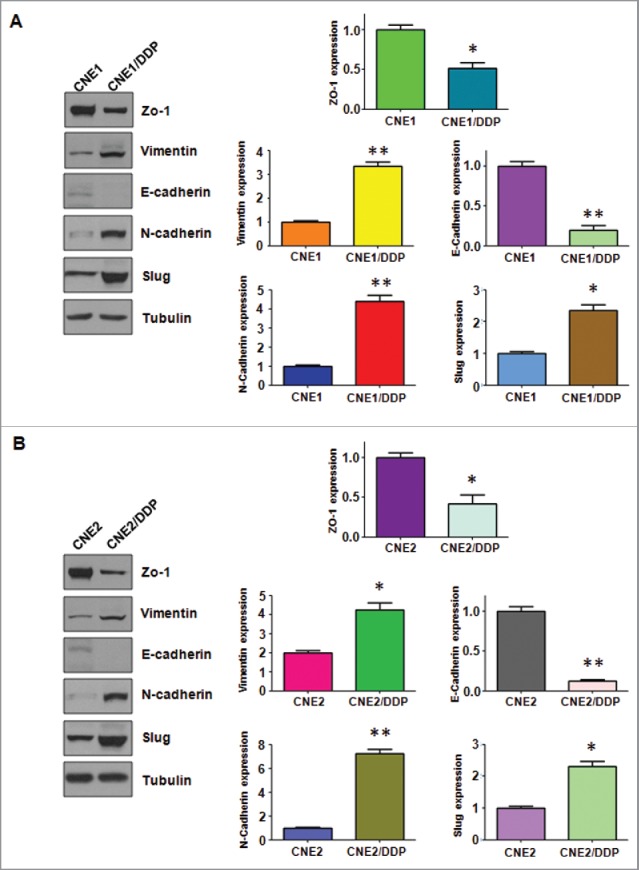
Cisplatin-resistant cells have EMT marker changes. (A) Left panel: Western blotting analysis was used to detect the expression of ZO-1, E-cadherin, N-cadherin, Slug, and Vimentin in CNE/DDP NPC cells. Right panel: Quantitative results are illustrated for panel A. *P < 0.05, **P < 0.01 vs control. (B) Left panel: Western blotting analysis was performed to measure the expression of ZO-1, E-cadherin, N-cadherin, Slug, and Vimentin in CNE/DDP NPC cells. Right Panel: Quantitative results are illustrated for panel (C). *P < 0.05, **P < 0.01 vs control.
Overexpression of NEDD4 was found in DDP-resistant NPC cells
Since accumulating evidence has suggested that NEDD4 plays important roles in cancer development, we further determine whether NEDD4 is involved in EMT in human cancers. We measured the expression of NEDD4 in DDP-resistant NPC cells compared with the parental cells. As shown in Fig. 3A, the expression of NEDD4 mRNA was significantly elevated in both CNE1/DDP and CNE2/DDP cells compared with parental cells using Q-PCR assay. As illustrated in Fig. 3B, NEDD4 protein was also markedly upregulated in DDP-resistant NPC cells, detected by Western blotting. Moreover, the expression of Notch1, one of the important oncoproteins, was measured subsequently. As shown in Fig. 3B, Notch1 was overexpressed in DDP-resistant NPC cells. All of the above findings indicated that the overexpression of NEDD4 could be partially responsible for the acquisition of EMT features in DDP-resistant NPC cells.
Depletion of NEDD4 reverses EMT to MET in DDP-resistant NPC cells
We depleted NEDD4 by siRNA (small interfering RNA) transfection to further investigate whether NEDD4 plays a critical role in DDP-induced EMT. All 3 siRNAs showed valid depletion of NEDD4 in both DDP-resistant NPC cells (Fig. 3C). Subsequently, we used NEDD4 siRNA1 to detect whether depletion of NEDD4 could reverse the EMT features to mesenchymal–epithelial transition (MET) in DDP-resistant NPC cells. As shown in Fig. 3D, depletion of NEDD4 indeed partly reversed EMT to MET phenotype as the NEDD4 siRNA transfected DDP-resistant NPC cells developed round cell-like morphology. Inhibition of cell attachment and detachment capacity was also observed in DDP-resistant NPC cells via NEDD4 siRNA transfection (Fig. 3E).
Suppression of NEDD4 reduces motility and invasion in DDP-resistant NPC cells
We detected the effect of NEDD4 depletion on cell motility and invasion capacities in DDP-resistant NPC cells after NEDD4 siRNA transfection. Transwell assay showed that depletion of NEDD4 significantly inhibited the migration and invasion of DDP-resistant NPC cells (Fig. 4A). Consistently, wound healing assay illustrated that suppression of NEDD4 reduced cell motility in DDP-resistant NPC cells (Fig. 4B). These findings revealed that NEDD4 is critically involved in cell migration and invasion characteristics in DDP-resistant NPC cells.
Figure 4.
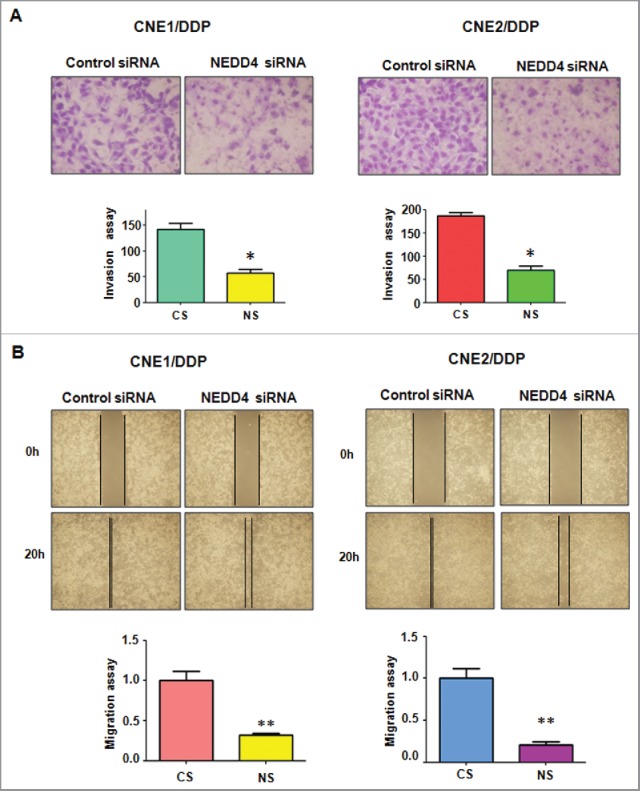
Depletion of NEDD4 inhibits motility and invasion in CNE/DDP NPC cells. (A) Top panel: Invasion assay were performed in CNE/DDP NPC cells transfected with NEDD4 siRNA. Bottom panel: Quantitative results are illustrated for top panel. CS: control siRNA; NS: NEDD4 siRNA. *P < 0.05 vs control. (B) Top panel: Wound healing assays were used to detect the motility in CNE/DDP NPC cells transfected with NEDD4 siRNA. Bottom panel: Quantitative results are illustrated for top panel. *P < 0.05 vs control siRNA.
Suppression of NEDD4 regulates expression of EMT markers
We further determine whether depletion of NEDD4 effects the expression of EMT markers in DDP-resistant NPC cells. We measured the expression of EMT molecules at mRNA and protein levels in DDP-resistant NPC cells transfected with NEDD4 siRNA by Q–PCR and Western blotting analysis, respectively. Our results revealed that the expression of epithelial marker E-cadherin and ZO-1 was markedly elevated in DDP-resistant NPC cells with NEDD4 siRNA transfection. On the contrary, the expression of mesenchymal markers including N-cadherin, Vimentin, and Slug was significantly decreased in DDP-resistant NPC cells after NEDD4 depletion (Figs. 5 and 6A). Taken together, our findings identified that NEDD4 is involved in regulation of EMT in DDP-resistant NPC cells.
Figure 5.
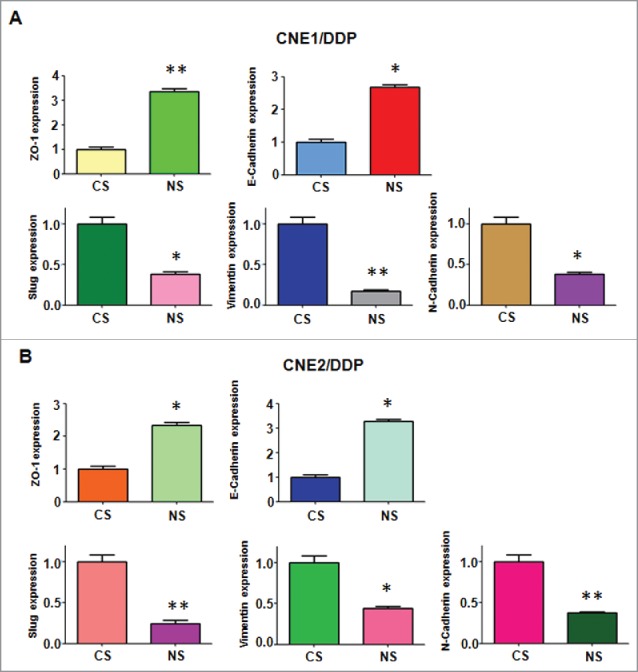
Depletion of NEDD4 regulates mRNA level of EMT markers in CNE/DDP NPC cells. (A) Real-time RT-PCR was performed to quantify mRNA expression of EMT markers in CNE1/DDP NPC cells transfected with NEDD4 siRNA. CS: control siRNA; NS: NEDD4 siRNA. *, P < 0.05, **P < 0.01 compared with control siRNA. (B) Real-time RT-PCR was conducted to measure mRNA level of EMT markers in CNE2/DDP NPC cells after NEDD4 siRNA transfection. CS: control siRNA; NS: NEDD4 siRNA. *, P < 0.05, **P < 0.01 compared with control siRNA.
Downregulation of NEDD4 enhances DDP-resistant NPC cells to DDP sensitivity
MTT assay was performed to examine whether depletion of NEDD4 enhances DDP-resistant NPC cells to DDP sensitivity. After NEDD4 siRNA transfection, we found that NEDD4 suppression significantly antagonized cell growth inhibition induced by DDP treatment (Fig. 6B). This finding suggested that in DDP-resistant NPC cells with downregulated NEDD4 could be significantly more sensitive to DDP treatment induced cell growth inhibition
Figure 6.
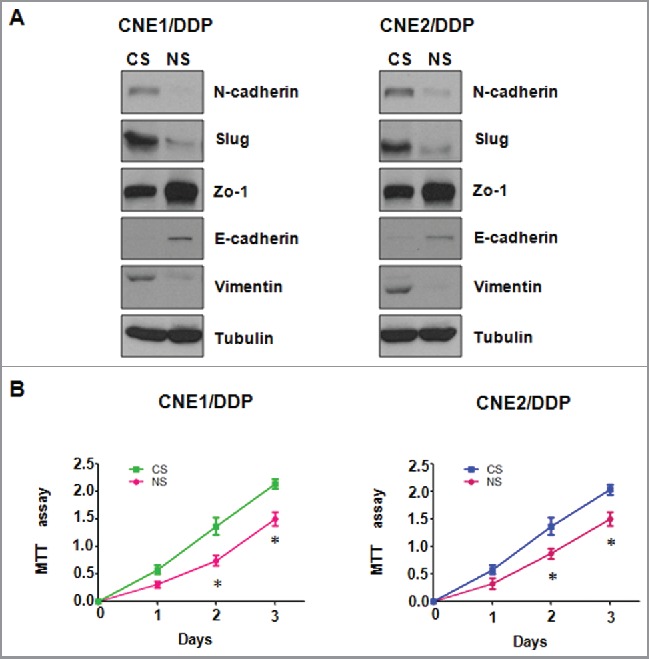
Depletion of NEDD4 regulates protein level of EMT markers in CNE/DDP NPC cells. (A) Western blotting analysis was used to detect the expression of EMT markers in CNE/DDP NPC cells transfected with NEDD4 siRNA. CS: control siRNA; NS: NEDD4 siRNA. (B) Down-regulation of NEDD4 enhanced cisplatin sensitivity to CNE/DDP NPC cells. MTT assay was performed in CNE/DDP NPC cells treated with NEDD4 siRNA. CS: control siRNA; NS: NEDD4 siRNA. *, P < 0.05 compared with control siRNA.
Discussion
Nasopharyngeal carcinoma, consistently associated with Epstein-Barr virus (EBV) latent infection, is an epithelial malignancy and relatively prevalent in Southeast Asia and China.36 NPC is one of the most common types of head and neck cancer and is difficult to accurate detection due to its anatomic location. In conventional clinical therapy, radiotherapy combined with chemotherapy is the primary therapeutic approach in treatment of NPC. The most active and commonly used drug is DDP, especially for the treatment of advanced locoregional recurrence or distant metastasis in NPC patients.37 However, treatment failure rates remain high because many patients develop drug resistance to DDP, either intrinsic or acquired. Hence, there is an urgent need to explore the mechanism of drug resistance in NPC. For this purpose, in the current study, we exploited the underlining molecular mechanism of DDP resistance in NPC cells. Our results showed that DDP-resistant NPC cells developed EMT features and underwent EMT molecular marker changes. Moreover, DDP-resistant NPC cells acquired enhanced increased motility and invasion activities. We further found an overexpression of NEDD4 in DDP-resistant NPC cells. What is noteworthy is that depletion of NEDD4 in resistant cells caused a reversion of EMT phenotypes to MET characteristics. These findings suggested that NEDD4 is involved in EMT features and chemoresistance of NPC cancer cells, and targeting NEDD4 could be a novel choice to overcome drug resistance for successful therapy of NPC patients.
Accumulating data had showed the close association between chemoresistance and the acquisition of EMT-like phenotype of cancer cells. It was reported that chemoresistance to gemcitabine in hepatoma cells induces EMT features with decreased E-cadherin and increased Vimentin, Snail, and Slug.38 Similarly, paclitaxel-resistant breast cancer cells undergo EMT and involve upregulation of S-phase kinase-associated protein 2 (Skp2).39 One study demonstrated that downregulation of Dedicator of cytokinesis 1 (DOCK1) could increase the chemosensitivity in bladder cancer cells via preventing DDP-induced EMT.40 DDP-resistant lung adenocarcinoma cells acquired mesenchymal features and were along with low expression of miR-206 and high migration and invasion abilities.41 In NPC cells, Zhang and the colleagues described that the DDP-resistant cells exhibited morphological and molecular changes consistent with EMT, including the suppression of E-cadherin and β−catenin and the increase of vimentin, fibronectin and MMP-9, Snail, Slug, Twist and ZEB1.17 In line with these reports, our study revealed that DDP-resistant NPC cells develop EMT-like morphological changes with an increased metastatic potential in vitro. We also found the alternation of EMT molecular markers, downregulation of E-cadherin and ZO-1 and upregulation of N-cadherin, Vimentin, and Slug. More importantly, we first demonstrated NEDD4 is closely associated with DDP-induced EMT in NPC cells.
NEDD4–1 comprises a C2-WW(n)-HECT domain architecture and is the leading member of HECT E3 ubiquitin ligase. NEDD4 is frequently overexpressed in a wide range of human tumor types and exerts its oncogenic-like properties through the ubiquitin-mediated degradation of multiple protein substrates.42-44 Protein p34 has been identified to interact with the WW1 domain of NEDD4–1 which enhances NEDD4–1 stability.27 Furthermore, co-expression of p34 and NEDD4 is found responsible for the lowered PTEN levels in colon cancer tissues, suggesting that NEDD4 facilitated tumorigenesis through the p34-dependent PTEN ubiquitination and degradation.27 NEDD4 depletion inhibited cell growth via targeting PTEN in hepatocellular carcinoma.45 NEDD4 also exerts its oncogenic activity by regulating the stability of Mdm2 protein, a RING-type E3 ligase, leading to the suppression of tumor suppressor p53.46 Moreover, it is reported that NEDD4–1 interacts with and enhances Mdm2 poly-ubiquitination through the formation of K63-type polyubiquitin chains.47 Despite these finding proposing that NEDD4 promotes cancer, its role in cancer development appears to be controversial. Recent studies showed that NEDD4 acts as a tumor suppressor rather than an oncoprotein by directly targeting N-Myc and C-Myc oncoproteins for ubiquitin-mediated degradation, and as a result, inhibiting the growth of neuroblastoma and pancreatic cancer.48 The authors further demonstrated that the histone deacetylase SIRT2 (sirtuin 2) represses NEDD4 transcription by deacetylating Lys16 of histone H4 at NEDD4 core promoter, thus enhances expression of N-Myc and c-Myc. Importantly, by the addition of SIRT2 small-molecule inhibitors, NEDD4 gene expression is could be reactivated, leading to reduced N-Myc and c-Myc protein levels, and suppressing tumor cell growth.48 Huang and colleagues revealed that NEDD4 negatively regulates HER3, a member of the epidermal growth factor receptor (EGFR) family, and knockdown of NEDD4 in human prostate and breast cancer cell lines elevates HER3 signaling and cancer cell proliferation in vitro and xenoplant tumor growth in vivo.49 More recently, it is reported that intestinal knockout of Nedd4 enhances growth of Apcmin tumors, suggesting that Nedd4 normally suppresses colonic WNT signaling and growth of colonic tumors.50 One recent study reported that NEDD4 is involved in TGF-β (transforming growth factor−β)-induced EMT in lung cancer cells.51 Here, in this study we found NEDD4 exhibits oncogenic properties in NPC cells, as it facilitates the EMT characters of DDP-resistant cells. Indole-3-carbinol analogs have been found to be potential small molecular inhibitors of NEDD4 in human melanoma cells,52 suggesting that natural compounds could be useful to inhibit NEDD4 in human cancer.
In the present study, for the first time, we showed that DDP-resistant cells underwent EMT at least partly due to overexpression of NEDD4 signaling pathway. We further found that short hairpin RNA knockout of NEDD4 reverses the EMT features to MET and sensitized DDP-resistant cells to DDP, suggesting that repression of NEDD4 could be a promising approach for restoring sensitivity to DDP. Further elucidation of the association between resistance to DDP and NEDD4 overexpression could promote the future development of novel therapeutic strategies. Without a doubt, it is necessary to determine whether NEDD4 is involved in DDP-resistance in NPC mouse models in vivo.
Materials and methods
Cell culture, reagents and antibodies
The human NPC cell lines, CNE1 and CNE2, were cultured in RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100 U/ml), and maintained in a humidified 5% CO2 incubator at 37˚C. DDP and MTT [3-(4,5-dimethythi-azol- 2-yl)-2,5-diphenyl tetrazolium bromide] was purchased from Sigma (St Louis, MO, USA). RPMI-1640 medium, FBS and phosphate-buffered saline (PBS) were purchased from Gibco-BRL (Grand Island, NY, USA). Matrigel was purchased from BD Biosciences (Bedford, MA, USA). Primary antibodies against ZO-1, E-cadherin, N-cadherin, Vimentin, Slug, and Tubulin were bought from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-NEDD4 and anti-Notch1 antibodies were purchased from Abcam (Cambridge, MA, USA). CNE1 and CNE2 cells were exposed to increasing concentrations of DDP for more than 6 months to create DDP-resistant cell lines.
MTT assay
The cells (5×103) were seeded in each well of the 96-well plates for overnight incubation. Then, the cells were treated with different concentrations of DDP for 72h. MTT assay was performed for cell viability analysis as described before.53
Transwell migration and invasion assay
The cell migration and invasion capacities were determined using 24-well inserts with 8mm pores according to the manufacturer protocol. For invasion assay, the Transwell inserts were precoated with Matrigel. Then cells were seeded into an upper-chamber of inserts. RPMI1600 medium with 10% FBS was added to the lower chamber. After the cells were seeded for 20 h, the upper cells of the chambers were removed and the invading cells on the bottom surface cells of the chambers were fixed and dyed with Giemsa solution. The stained invasive cells were photographed under a microscope.
Cell attachment and detachment
For attachment assay, 5 × 104 pretreated cells per well were seeded in 24-well plates. After 1h incubation, removed the unattached cells and counted the attached cells. For cell detachment assay, the cells were seeded and incubated for 24 h. Then the detached cells with 0.05% trypsinization for 3 min were counted. The remaining attached cells were also counted. Data were calculated as a percentage of the attached or detached cells to total cells.
Wound healing assay
The NPC and DDP-resistant cells were seeded into a 6-well plate and incubated till the cells reach to about 90% confluence. Then, the scratch wound was generated by a careful scraping the surface cells of the plates with a pipette tip. After the detached cells were rinsed with PBS, the cells were incubated for 16 h. Photographed the wound healing images at 0 h and 16 h, respectively.
Quantitative real-time RT-PCR (Q-PCR)
Total RNAs were extracted from the cells using Trizol reagent (Invitrogen) and transcribed into cDNA according to the manufacturer's protocol. The mRNA level of NEDD4 and EMT associated markers, including, ZO-1, E-cadherin, N-cadherin, Vimentin, Slug, was performed using SYBR green assay kit (Takara, Dalian, China) and GAPDH level was applied for normalization. The primers and PCR reaction are described previously.39
Transfection
Cells were seeded in 6-well plates and transfected with NEDD4 siRNA (GenePharma, Shanghai, China), or non-targeting control siRNA using Lipofectamine 2000 following the manufacture's instruments.53 After the transfection and incubation, cells were used for further analysis as described in the results section.
Western blotting analysis
After specified treatment, the cells were washed with PBS and harvested using trypsin. Harvested cells were centrifuged and resuspended in RIPA buffer (50m M Tris, 150m M NaCl, 1% TritonX-100, 0.1% sodium dodecyl sulfate, and 1% nadeoxycholate) supplemented with protease inhibitors. Following incubation on ice for 30 min, the homogenate was centrifuged at 12,000 rpm for 30 min at 4˚C. Then, the bicinchoninic acid (BCA) protein assay was performed to determine the protein concentrations. 40 µg of proteins were then separated using 10–15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and subsequently transferred onto membranes. The membranes were blocked with 5% defatted milk and immunoblotted with proper primary antibodies overnight at 4˚C. Thereafter, membranes were washed and probed with secondary antibodies for 1 h at room temperature. The membranes were imaged with gel imaging equipment (Bio-Rad, Hercules, CA, USA). Tubulin was used as the loading control.
Statistical analysis
Data are presented with means ± SEM. Statistical comparisons between different groups were evaluated using GraphPad Prism 4.0 (Graph pad Software, La Jolla, CA, USA). The differences between mean values were analyzed using the 2-tailed Student's t-test. Results are expressed as means ± SD. P-values < 0.05 were considered statistically significant differences.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Razak AR, Siu LL, Liu FF, Ito E, O'Sullivan B, Chan K. Nasopharyngeal carcinoma: the next challenges. Eur J Cancer 2010; 46:1967-78; PMID:20451372; http://dx.doi.org/ 10.1016/j.ejca.2010.04.004 [DOI] [PubMed] [Google Scholar]
- [2].Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet 2005; 365:2041-54; PMID:15950718; http://dx.doi.org/ 10.1016/S0140-6736(05)66698-6 [DOI] [PubMed] [Google Scholar]
- [3].Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66:115-32; http://dx.doi.org/ 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- [4].Chan AT. Current treatment of nasopharyngeal carcinoma. Eur J Cancer 2011; 47 Suppl 3:S302-3; PMID:21943991; http://dx.doi.org/ 10.1016/S0959-8049(11)70179-4 [DOI] [PubMed] [Google Scholar]
- [5].Bensouda Y, Kaikani W, Ahbeddou N, Rahhali R, Jabri M, Mrabti H, Boussen H, Errihani H. Treatment for metastatic nasopharyngeal carcinoma. Eur Ann Otorhinolaryngol Head Neck Dis 2011; 128:79-85; PMID:21177151; http://dx.doi.org/ 10.1016/j.anorl.2010.10.003 [DOI] [PubMed] [Google Scholar]
- [6].Dugbartey GJ, Peppone LJ, de Graaf IA. An integrative view of cisplatin-induced renal and cardiac toxicities: Molecular mechanisms, current treatment challenges and potential protective measures. Toxicology 2016; 371:58-66; PMID:27717837; http://dx.doi.org/ 10.1016/j.tox.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Agbale CM, Cardoso MH, Galyuon IK, Franco OL. Designing metallodrugs with nuclease and protease activity. Metallomics 2016; 8:1159-69; PMID:27714031; http://dx.doi.org/ 10.1039/C6MT00133E [DOI] [PubMed] [Google Scholar]
- [8].Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, Sun Y, Chen XZ, Li JG, Zhu XD, et al.. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 2016; 17:1509-20; PMID:27686945; http://dx.doi.org/ 10.1016/S1470-2045(16)30410-7 [DOI] [PubMed] [Google Scholar]
- [9].Zeng Q, Wang Z, Liu C, Gong Z, Yang L, Jiang L, Ma Z, Qian Y, Yang Y, Kang H, et al.. Knockdown of NFBD1/MDC1 enhances chemosensitivity to cisplatin or 5-fluorouracil in nasopharyngeal carcinoma CNE1 cells. Mol Cell Biochem 2016; 418:137-46; PMID:27334757; http://dx.doi.org/ 10.1007/s11010-016-2739-5 [DOI] [PubMed] [Google Scholar]
- [10].Li Q, Kawamura K, Yamanaka M, Okamoto S, Yang S, Yamauchi S, Fukamachi T, Kobayashi H, Tada Y, Takiguchi Y, et al.. Upregulated p53 expression activates apoptotic pathways in wild-type p53-bearing mesothelioma and enhances cytotoxicity of cisplatin and pemetrexed. Cancer Gene Ther 2012; 19:218-28; PMID:22223137; http://dx.doi.org/ 10.1038/cgt.2011.86 [DOI] [PubMed] [Google Scholar]
- [11].Wang L, Xiang S, Williams KA, Dong H, Bai W, Nicosia SV, Khochbin S, Bepler G, Zhang X. Depletion of HDAC6 enhances cisplatin-induced DNA damage and apoptosis in non-small cell lung cancer cells. PLoS One 2012; 7:e44265; PMID:22957056; http://dx.doi.org/ 10.1371/journal.pone.0044265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, Thompson EW. Epithelial–mesenchymal and mesenchymal–epithelial transitions in carcinoma progression. J Cell Physiol 2007; 213:374-83; PMID:17680632; http://dx.doi.org/ 10.1002/jcp.21223 [DOI] [PubMed] [Google Scholar]
- [13].Wei SC, Fattet L, Yang J. The forces behind EMT and tumor metastasis. Cell Cycle 2015; 14:2387-8; PMID:26083471; http://dx.doi.org/ 10.1080/15384101.2015.1063296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Samatov TR, Tonevitsky AG, Schumacher U. Epithelial-mesenchymal transition: focus on metastatic cascade, alternative splicing, non-coding RNAs and modulating compounds. Mol Cancer 2013; 12:107; PMID:24053443; http://dx.doi.org/ 10.1186/1476-4598-12-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 2006; 7:131-42; PMID:16493418; http://dx.doi.org/ 10.1038/nrm1835 [DOI] [PubMed] [Google Scholar]
- [16].Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139:871-90; PMID:19945376; http://dx.doi.org/ 10.1016/j.cell.2009.11.007 [DOI] [PubMed] [Google Scholar]
- [17].Zhang P, Liu H, Xia F, Zhang QW, Zhang YY, Zhao Q, Chao ZH, Jiang ZW, Jiang CC. Epithelial-mesenchymal transition is necessary for acquired resistance to cisplatin and increases the metastatic potential of nasopharyngeal carcinoma cells. Int J Mol Med 2014; 33:151-9; PMID:24173500 [DOI] [PubMed] [Google Scholar]
- [18].Chan KC, Chan LS, Ip JC, Lo C, Yip TT, Ngan RK, Wong RN, Lo KW, Ng WT, Lee AW, et al.. Therapeutic targeting of CBP/beta-catenin signaling reduces cancer stem-like population and synergistically suppresses growth of EBV-positive nasopharyngeal carcinoma cells with cisplatin. Sci Rep 2015; 5:9979; PMID:25897700; http://dx.doi.org/ 10.1038/srep09979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jiang Q, Zhou Y, Yang H, Li L, Deng X, Cheng C, Xie Y, Luo X, Fang W, Liu Z. A directly negative interaction of miR-203 and ZEB2 modulates tumor stemness and chemotherapy resistance in nasopharyngeal carcinoma. Oncotarget 2016; 7:67288-301; PMID:27589832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang P, Hong H, Sun X, Jiang H, Ma S, Zhao S, Zhang M, Wang Z, Jiang C, Liu H. MicroRNA-10b regulates epithelial-mesenchymal transition by modulating KLF4/Notch1/E-cadherin in cisplatin-resistant nasopharyngeal carcinoma cells. Am J Cancer Res 2016; 6:141-56; PMID:27186392 [PMC free article] [PubMed] [Google Scholar]
- [21].Harvey KF, Dinudom A, Komwatana P, Jolliffe CN, Day ML, Parasivam G, Cook DI, Kumar S. All three WW domains of murine Nedd4 are involved in the regulation of epithelial sodium channels by intracellular Na+. J Biol Chem 1999; 274:12525-30; PMID:10212229; http://dx.doi.org/ 10.1074/jbc.274.18.12525 [DOI] [PubMed] [Google Scholar]
- [22].Donovan P, Poronnik P. Nedd4 and Nedd4-2: ubiquitin ligases at work in the neuron. Int J Biochem Cell Biol 2013; 45:706-10; PMID:23262292; http://dx.doi.org/ 10.1016/j.biocel.2012.12.006 [DOI] [PubMed] [Google Scholar]
- [23].Ikeda M, Ikeda A, Longan LC, Longnecker R. The Epstein-Barr virus latent membrane protein 2A PY motif recruits WW domain-containing ubiquitin-protein ligases. Virology 2000; 268:178-91; PMID:10683340; http://dx.doi.org/ 10.1006/viro.1999.0166 [DOI] [PubMed] [Google Scholar]
- [24].Cao XR, Lill NL, Boase N, Shi PP, Croucher DR, Shan H, Qu J, Sweezer EM, Place T, Kirby PA, et al.. Nedd4 controls animal growth by regulating IGF-1 signaling. Sci Signal 2008; 1:ra5; PMID:18812566; http://dx.doi.org/ 10.1126/scisignal.1160940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang X, Trotman LC, Koppie T, Alimonti A, Chen Z, Gao Z, Wang J, Erdjument-Bromage H, Tempst P, Cordon-Cardo C, et al.. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell 2007; 128:129-39; PMID:17218260; http://dx.doi.org/ 10.1016/j.cell.2006.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sun A, Yu G, Dou X, Yan X, Yang W, Lin Q. Nedd4-1 is an exceptional prognostic biomarker for gastric cardia adenocarcinoma and functionally associated with metastasis. Mol Cancer 2014; 13:248; PMID:25395181; http://dx.doi.org/ 10.1186/1476-4598-13-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hong SW, Moon JH, Kim JS, Shin JS, Jung KA, Lee WK, Jeong SY, Hwang JJ, Lee SJ, Suh YA, et al.. p34 is a novel regulator of the oncogenic behavior of NEDD4-1 and PTEN. Cell Death Differ 2014; 21:146-60; PMID:24141722; http://dx.doi.org/ 10.1038/cdd.2013.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Amodio N, Scrima M, Palaia L, Salman AN, Quintiero A, Franco R, Botti G, Pirozzi P, Rocco G, De Rosa N, et al.. Oncogenic role of the E3 ubiquitin ligase NEDD4-1, a PTEN negative regulator, in non-small-cell lung carcinomas. Am J Pathol 2010; 177:2622-34; PMID:20889565; http://dx.doi.org/ 10.2353/ajpath.2010.091075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jung S, Li C, Jeong D, Lee S, Ohk J, Park M, Han S, Duan J, Kim C, Yang Y, et al.. Oncogenic function of p34SEI-1 via NEDD41mediated PTEN ubiquitination/degradation and activation of the PI3K/AKT pathway. Int J Oncol 2013; 43:1587-95; PMID:23970032 [DOI] [PubMed] [Google Scholar]
- [30].Zou X, Levy-Cohen G, Blank M. Molecular functions of NEDD4 E3 ubiquitin ligases in cancer. Biochim Biophys Acta 2015; 1856:91-106; PMID:26116757 [DOI] [PubMed] [Google Scholar]
- [31].Ye X, Wang L, Shang B, Wang Z, Wei W. NEDD4: a promising target for cancer therapy. Curr Cancer Drug Targets 2014; 14:549-56; PMID:25088038; http://dx.doi.org/ 10.2174/1568009614666140725092430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Liu J, Wan L, Liu P, Inuzuka H, Liu J, Wang Z, Wei W. SCF(beta-TRCP)-mediated degradation of NEDD4 inhibits tumorigenesis through modulating the PTEN/Akt signaling pathway. Oncotarget 2014; 5:1026-37; PMID:24657926; http://dx.doi.org/ 10.18632/oncotarget.1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zeng T, Wang Q, Fu J, Lin Q, Bi J, Ding W, Qiao Y, Zhang S, Zhao W, Lin H, et al.. Impeded Nedd4-1-mediated Ras degradation underlies Ras-driven tumorigenesis. Cell Rep 2014; 7:871-82; PMID:24746824; http://dx.doi.org/ 10.1016/j.celrep.2014.03.045 [DOI] [PubMed] [Google Scholar]
- [34].Salah Z, Cohen S, Itzhaki E, Aqeilan RI. NEDD4 E3 ligase inhibits the activity of the Hippo pathway by targeting LATS1 for degradation. Cell Cycle 2013; 12:3817-23; PMID:24107629; http://dx.doi.org/ 10.4161/cc.26672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zheng MZ, Qin HD, Yu XJ, Zhang RH, Chen LZ, Feng QS, Zeng YX. Haplotype of gene Nedd4 binding protein 2 associated with sporadic nasopharyngeal carcinoma in the Southern Chinese population. J Transl Med 2007; 5:36; PMID:17626640; http://dx.doi.org/ 10.1186/1479-5876-5-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tsao SW, Yip YL, Tsang CM, Pang PS, Lau VM, Zhang G, Lo KW. Etiological factors of nasopharyngeal carcinoma. Oral Oncol 2014; 50:330-8; PMID:24630258; http://dx.doi.org/ 10.1016/j.oraloncology.2014.02.006 [DOI] [PubMed] [Google Scholar]
- [37].Gu MF, Liu LZ, He LJ, Yuan WX, Zhang R, Luo GY, Xu GL, Zhang HM, Yan CX, Li JJ. Sequential chemoradiotherapy with gemcitabine and cisplatin for locoregionally advanced nasopharyngeal carcinoma. Int J Cancer 2013; 132:215-23; PMID:22610788; http://dx.doi.org/ 10.1002/ijc.27638 [DOI] [PubMed] [Google Scholar]
- [38].Wu Q, Wang R, Yang Q, Hou X, Chen S, Hou Y, Chen C, Yang Y, Miele L, Sarkar FH, et al.. Chemoresistance to gemcitabine in hepatoma cells induces epithelial-mesenchymal transition and involves activation of PDGF-D pathway. Oncotarget 2013; 4:1999-2009; PMID:24158561; http://dx.doi.org/ 10.18632/oncotarget.1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yang Q, Huang J, Wu Q, Cai Y, Zhu L, Lu X, Chen S, Chen C, Wang Z. Acquisition of epithelial-mesenchymal transition is associated with Skp2 expression in paclitaxel-resistant breast cancer cells. Br J Cancer 2014; 110:1958-67; PMID:24642627; http://dx.doi.org/ 10.1038/bjc.2014.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chen DJ, Chen W, Jiang H, Yang H, Wang YC, Chen JH. Downregulation of DOCK1 sensitizes bladder cancer cells to cisplatin through preventing epithelial-mesenchymal transition. Drug Des Devel Ther 2016; 10:2845-53; PMID:27660415; http://dx.doi.org/ 10.2147/DDDT.S101998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chen QY, Jiao DM, Wang J, Hu H, Tang X, Chen J, Mou H, Lu W. miR-206 regulates cisplatin resistance and EMT in human lung adenocarcinoma cells partly by targeting MET. Oncotarget 2016; 7:24510-26; PMID:27014910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Liao CJ, Chi HC, Tsai CY, Chen CD, Wu SM, Tseng YH, Lin YH, Chung IH, Chen CY, Lin SL, et al.. A novel small-form NEDD4 regulates cell invasiveness and apoptosis to promote tumor metastasis. Oncotarget 2015; 6:9341-54; PMID:25823820; http://dx.doi.org/ 10.18632/oncotarget.3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhou W, Xu J, Zhao Y, Sun Y. SAG/RBX2 is a novel substrate of NEDD4-1 E3 ubiquitin ligase and mediates NEDD4-1 induced chemosensitization. Oncotarget 2014; 5:6746-55; PMID:25216516; http://dx.doi.org/ 10.18632/oncotarget.2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Li JJ, Wang R, Lama R, Wang X, Floyd ZE, Park EA, Liao FF. Ubiquitin Ligase NEDD4 Regulates PPARgamma Stability and Adipocyte Differentiation in 3T3-L1 Cells. Sci Rep 2016; 6:38550; PMID:27917940; http://dx.doi.org/ 10.1038/srep38550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hang X, Zhu S, Di H, Wu Z, Chu K, Wang J, Xin H, Yu G, Peng H, Miao X, et al.. NEDD4 Depletion Inhibits Hepatocellular Carcinoma Growth via Targeting PTEN. Cell Physiol Biochem 2016; 39:768-79; PMID:27467187; http://dx.doi.org/ 10.1159/000445667 [DOI] [PubMed] [Google Scholar]
- [46].Shadfan M, Lopez-Pajares V, Yuan ZM. MDM2 and MDMX: Alone and together in regulation of p53. Transl Cancer Res 2012; 1:88-9; PMID:23002429 [PMC free article] [PubMed] [Google Scholar]
- [47].Xu C, Fan CD, Wang X. Regulation of Mdm2 protein stability and the p53 response by NEDD4-1 E3 ligase. Oncogene 2015; 34:281-9; PMID:24413081; http://dx.doi.org/ 10.1038/onc.2013.557 [DOI] [PubMed] [Google Scholar]
- [48].Liu PY, Xu N, Malyukova A, Scarlett CJ, Sun YT, Zhang XD, Ling D, Su SP, Nelson C, Chang DK, et al.. The histone deacetylase SIRT2 stabilizes Myc oncoproteins. Cell Death Differ 2013; 20:503-14; PMID:23175188; http://dx.doi.org/ 10.1038/cdd.2012.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Huang Z, Choi BK, Mujoo K, Fan X, Fa M, Mukherjee S, Owiti N, Zhang N, An Z. The E3 ubiquitin ligase NEDD4 negatively regulates HER3/ErbB3 level and signaling. Oncogene 2015; 34:1105-15; PMID:24662824; http://dx.doi.org/ 10.1038/onc.2014.56 [DOI] [PubMed] [Google Scholar]
- [50].Lu C, Thoeni C, Connor A, Kawabe H, Gallinger S, Rotin D. Intestinal knockout of Nedd4 enhances growth of Apcmin tumors. Oncogene 2016; 35:5839-49; PMID:27086928; http://dx.doi.org/ 10.1038/onc.2016.125 [DOI] [PubMed] [Google Scholar]
- [51].Qu MH, Han C, Srivastava AK, Cui T, Zou N, Gao ZQ, Wang QE. miR-93 promotes TGF-beta-induced epithelial-to-mesenchymal transition through downregulation of NEDD4L in lung cancer cells. Tumour Biol 2016; 37:5645-51; PMID:26581907; http://dx.doi.org/ 10.1007/s13277-015-4328-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Quirit JG, Lavrenov SN, Poindexter K, Xu J, Kyauk C, Durkin KA, Aronchik I, Tomasiak T, Solomatin YA, Preobrazhenskaya MN, et al.. Indole-3-carbinol (I3C) analogues are potent small molecule inhibitors of NEDD4-1 ubiquitin ligase activity that disrupt proliferation of human melanoma cells. Biochem Pharmacol 2017; 127:13-27; PMID:27979631; http://dx.doi.org/ 10.1016/j.bcp.2016.12.007 [DOI] [PubMed] [Google Scholar]
- [53].Wang L, Ye X, Cai X, Su J, Ma R, Yin X, Zhou X, Li H, Wang Z. Curcumin suppresses cell growth and invasion and induces apoptosis by down-regulation of Skp2 pathway in glioma cells. Oncotarget 2015; 6:18027-37; PMID:26046466; http://dx.doi.org/ 10.18632/oncotarget.4090 [DOI] [PMC free article] [PubMed] [Google Scholar]


