Abstract
Although the Pseudomonas aeruginosa infection is well known and frequently found in hospitals and nursing care facilities, many cases are also reported outside these boundaries. In general, this pathogen infects debilitated patients either by comorbidities or by any form of immunodeficiency. In cases of respiratory infection, tobacco abuse seems to play an important role as a risk factor. In previously healthy patients, community-acquired pneumonia (CAP) with P. aeruginosa as the etiological agent is extremely rare, and unlike the cases involving immunocompromised or hospitalized patients, the outcome is severe, and is fatal in up to 61.1% of cases. Aerosolized contaminated water or solutions are closely linked to the development of respiratory tract infection. In this setting, metalworking fluids used in factories may be implicated in CAP involving previously healthy people. The authors report the case of a middle-aged man who worked in a metalworking factory and presented a right upper lobar pneumonia with a rapid fatal outcome. P. aeruginosa was cultured from blood and tracheal aspirates. The autopsy findings confirmed a hemorrhagic necrotizing pneumonia with bacteria-invading vasculitis and thrombosis. A culture of the metalworking fluid of the factory was also positive for P. aeruginosa. The pulsed-field gel electrophoresis showed that both strains (blood culture and metalworking fluid) were genetically indistinguishable. The authors highlight the occupational risk for the development of this P. aeruginosa-infection in healthy people.
Keywords: Pseudomonas aeruginosa; Pneumonia, Bacterial; Community-Acquired Infections; Shock, Septic; Autopsy
CASE REPORT
A previously healthy 44-year-old white male presented to our facility complaining of a 1-day history of upper backache. This symptom progressively worsened and was accompanied by a dry cough and fever. Further on the same day, he presented bloody sputum and hemoptysis. The only remarkable feature in his medical history was tabagism of five packs/year for more than 11 years. He did not take any medication and did not report any disease. He worked in a metallurgic factory operating a machine to make bicycle wheel rims.
On admission, the physical examination revealed an ill-looking patient, pale, dehydrated, acyanotic, with peripheral tissue hypoperfusion. Blood pressure was 70/40 mmHg; pulse rate was regular 150 beats per minute; respiratory frequency was 36 breaths per minute; axillary temperature was 35.8°C; capillary glucose was 138 mg/dL; and room air oximetry was 78% (which rose to 88% after O2 supplementation). Cardiac and abdominal examination was unremarkable; whereas the pulmonary examination revealed decreased breath sounds in the right hemi thorax and the presence of bilateral rales, rhonchi, and wheezing. Results of laboratory examinations are summarized in Table 1.
Table 1. Laboratory work-up on admission.
| Exam | Result | RV | Exam | Result | RV |
|---|---|---|---|---|---|
| Hemoglobin |
15.3 |
12.3–15.3 g/dL |
Mg |
1.5 |
1.6–2.6 mg/dL |
| Hematocrit |
46.3 |
36.0–45.0% |
ALT |
39 |
9–36 U/L |
| Leukocytes |
2.88 |
4.4–11.3 × 103/mm3 |
AST |
54 |
10–31 U/L |
| Metamyelocytes |
1 |
0% |
T bil |
0.58 |
0.3–1.2 mg/dL |
| Bands |
20 |
1–5% |
T protein |
6.7 |
6–8 g/dL |
| Segmented |
57 |
45–70% |
Albumine |
3.7 |
3–5 g/dL |
| Eosinophils |
0 |
1–4% |
Lactate |
101.5 |
4.5–19.8 mg/dL |
| Lymphocytes |
20 |
18–40% |
pH |
7.23 |
7.35–7.45 |
| Monocytes |
2 |
2–9% |
pO2 |
55 |
70–100 mmHg |
| Platelets |
86 |
150–400 × 103/mm3 |
pCO2 |
33 |
35–45 mmHg |
| INR |
1.54 |
1.0 |
HCO3 |
14 |
22–26 mEq/L |
| Urea |
68 |
5–25 mg/dL |
BE |
−12.9 |
−3–2.3 mEq/L |
| Creatinine |
1.84 |
0.4–1.3 mg/dL |
Sat O2 |
80 |
95–98% |
| Potassium |
3.1 |
3.5–5.0 mEq/L |
CRP |
159 |
< 5 mg/L |
| Sodium | 142 | 136–146 mEq/L | Anti HIV | negative |
ALT = alanine aminotransferase; AST = aspartate aminotransferase; BE = base excess; CRP = C-reactive protein; INR = international normalization ratio; Mg = magnesium; RV = reference value; T bil = total bilirubin.
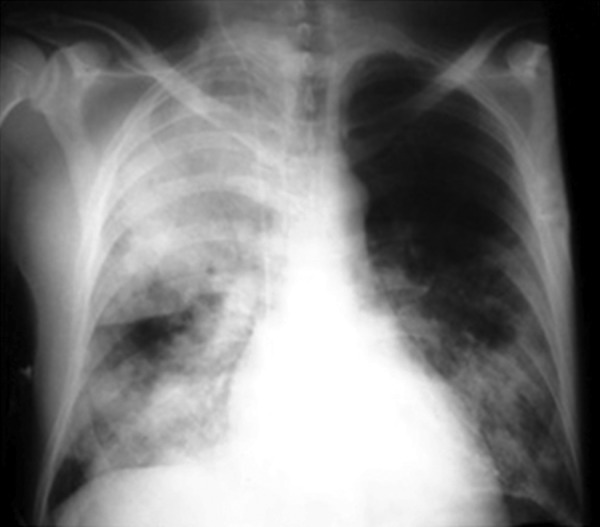
As shown in Figure 1, chest radiography showed a homogeneous consolidation opacity with bronchograms occupying the entire right upper lobe, as well as ill-defined fluffy heterogeneous opacities with air alveolograms in the right and left lower parts compatible with multiple-lobe pneumonia. Edema was absent but livedo reticularis was present in the lower limbs. No computed tomography was performed.
Figure 1. Chest plain radiography showed homogeneous consolidation opacity occupying the entire right upper lobe, as well as ill-defined fluffy heterogeneous opacities in both lower pulmonary fields.
The patient was initially treated with ceftriaxone, clarithromycin, and volume resuscitation followed by a continuous infusion of norepinephrine. His clinical status deteriorated rapidly requiring orotracheal intubation, mechanical ventilatory support, and the addition of vasopressin infusion. Despite all efforts, he died almost 7 hours after hospital admittance.
Blood cultures and culture of the tracheal aspirate yielded the growth of Pseudomonas aeruginosa multi-sensitive. A post-mortem test in pulmonary tissue samples for influenza virus by real-time protein chain reaction was negative for influenza B and A (H1N1).
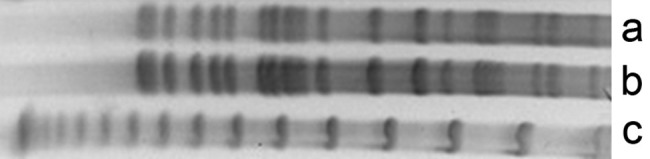
Samples were collected from the metalworking fluid used by the machine operated by the patient, which isolated P. aeruginosa. The strains isolated from the patient’s blood culture and from the metalworking fluid reservoir were typed by pulsed-field gel electrophoresis (PFGE) according to the Ribot et al.1 standardization. Both strains showed PFGE band patterns that were indistinguishable from each other and were considered genetically identical according to the criteria by Tenover et al, (Figure 2).2 This evidence for clonality is best considered relative rather than absolute. The potential for cryptic genetic changes is detectable only by DNA sequencing or other specific analyses.2
Figure 2. Pulsed-field gel electrophoresis patterns of Pseudomonas aeruginosa: Lane a – metalworking fluid reservoir isolate; Lane b – patient’s blood culture isolate; and Lane c – lambda DNA ladder standard. (Enzyme XBAI—Jena Bioscience, 4 hours incubation, initial pulse 5 sec, final pulse 40 sec, time elapsed 21 hours).
AUTOPSY
The external examination findings showed a eutrophic male cadaver with unremarkable ectoscopy except for apparent poor dental care. Microscopic examination detected a mild chronic glossitis with superficial bacterial plaque with predominantly Gram-positive cocci and Gram-negative bacilli.
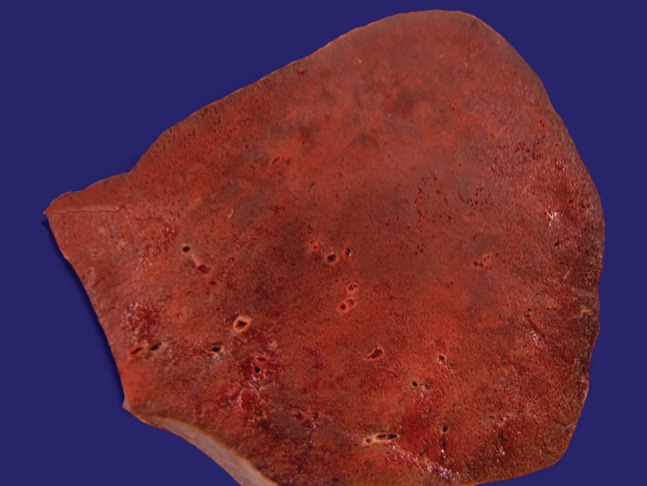
The lungs were heavy, winy-colored, extremely edematous and friable. The right lung weighed 1525 g (mean reference value [mRV]: 450 g) and left lung, 1235 g (mRV: 375 g). Scattered hemorrhagic foci and hemorrhagic condensation involving the entire upper right lobe were present (Figure 3), and a mild fibrinous pleuritis was observed.
Figure 3. Gross examination of the right upper pulmonary lobe showing hemorrhagic infiltration.
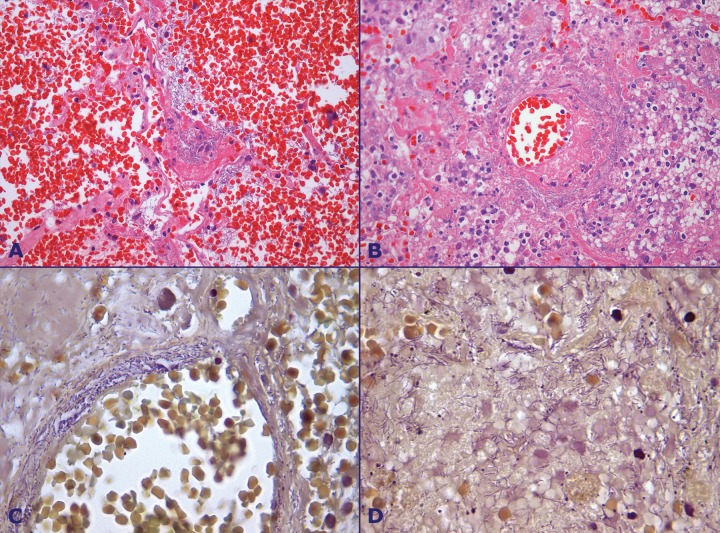
Microscopically, marked pulmonary parenchymal congestion predominated throughout the lungs, with neutrophils, macrophages, and cellular debris filling the alveolar space. Confluent areas of alveolar wall necrosis, areas of liquefactive necrosis, fibrin microthrombi, and hemorrhagic infarction were frequently depicted throughout the lung parenchyma (Figure 4B).
Figure 4. Photomicrography of the lung showing in A - Marked alveolar hemorrhage, and pulmonary parenchyma necrosis (HE, 100X); B - Pulmonary necrosis, polymorphonuclear leukocytes infiltration, vasculitis with thrombus and bacterial colonies in the lung parenchyma and around the vessel wall (HE, 200X); C - Bacterial colonies infiltrating the vessel wall (BH, 1000X); D - Bacterial colonies in the pulmonary parenchyma (BH, 1000X).
Arteriolitis represented by central necrosis surrounded by a hemorrhagic halo and numerous Gram-negative filamentous bacillary forms with the tendency for perivascular aggression was also frequently found (Figures 4C and 4D). Immunohistochemical stainning using an anti- P aeruginosa mouse monoclonal antibody (B11, AbD Serotec, Kidlington, UK, dilution of 1:400 – courtesy by Dr. Keiichi Iwaya, Japan) revealed positive staining of bacilli in the lung samples. Immunostaining was negative in the oral mucosa (tongue sample). Foci of respiratory bronchiolitis were present throughout the preserved lung parenchyma. Anthracosis was present in the lungs and lymph nodes.
The heart was enlarged mainly due to left ventricular hypertrophy and weighted 422 g (mRV: 302 g). The left ventricular wall and the septum measured 2.2 cm (mRV: 1.15 cm) and 2.3 cm (mRV: 1.35 cm), respectively. Although the myocardium and valves were normal, petechiae were present over the epicardium and pericardium. Atherosclerosis was present in coronary arteries (slight), renal arteries (slight), and aorta (moderate).
Grossly, the brain showed slight edema and meningeal congestion. Mild arteriolosclerosis predominated in the basal ganglia, which was compatible with systemic chronic hypertension.
The kidneys showed external granularity and small subcapsular scars. Microscopically acute tubular necrosis was present as well as benign nephrosclerosis and hyaline arteriolosclerosis.
Gastric erosions and hemorrhagic pinhead foci related to septic shock were found in the ileum and sigmoid. The adrenals were friable with interspersed hemorrhagic foci. The spleen and liver were congested. A microscopic infarct related to shock was present in the pancreas.
In summary, autopsy findings were consistent with the diagnosis of hemorrhagic necrotizing pneumonia caused by Gram-negative rod-shaped bacteria and ischemic events due to septic shock. Signs of undiagnosed chronic systemic hypertension were evident in the heart, kidneys, and brain.
DISCUSSION
P. aeruginosa is a Gram-negative non-fermentative rod, which is well known as an opportunistic pathogen in the hospital setting commonly associated with nosocomial and health-care infections.3-5 This pathogen usually infects patients in an immunocompromised state.5 The respiratory tract is the most common site of infection, which is mostly due to mechanical ventilation; it is also associated with the highest mortality rate.6,7
P. aeruginosa is frequently found in soil, water, plants, and moist environments. It has minimal nutritional requirements, grows in distilled water, and tolerates temperatures as high as 45-50°C.6 Community-acquired P. aeruginosa infections more frequently involve the external ear canal8 (swimmer’s ear, and malignant external otitis), the skin (hot tub folliculitis),9 whirlpool-associated urinary tract infection, perinychia,6 the eye (contact lenses keratitis),10 but rarely the lungs. Previous pneumopathy (mainly bronchiectasis and cystic fibrosis), chronic use of corticosteroids, malignancy, previous use of antibiotics, advanced age, alcohol abuse, smoking, nursing-home dwelling, or previous hospitalization are all risk factors for community-acquired pneumonia (CAP) due to Gram-negative bacteria, among which P. aeruginosa and Escherichia coli represent the most commonly isolated microorganisms.11 In 559 hospitalized patients with CAP due to Gram-negative bacteria, Arancibia et al.,11 observed that body temperatures tended to be lower, patients were less likely to refer chills, and preceding symptoms of upper airways infections were less frequent. However, dyspnea, respiratory and renal failures, and shock appeared more significantly on admission.
Although the incidence of P. aeruginosa in CAP ranges between 0.9% and 5% of the cases,12-17 this pathogen is rarer in previously healthy individuals.2,5,18 Hatchette et al.3 described 1 case and found 11 reported cases of P. aeruginosa CAP in healthy patients from 1968 to 2000. However, in 2014, this number was 17, according to Takajo et al.,5 who also described another case. In this subset of patients, unlike the immunocompromised patients, the infection’s clinical course was rapid and aggressive3,5,19 with the mortality rate ranging between 33% and 63.1%,3,5,20 occurring with a median time of 11 hours from admission to death.3 Acute clinical features were characterized by chest pain, dry cough, or bloody sputum/hemoptysis, accompanied by fever and hypotension with a rapid course to septic shock. Although any lung region can be involved, the upper right lobe is more frequently stricken.3 Hemoptysis is frequently observed due to the necrotizing vasculitis, accompanied by the parenchymal necrosis characteristic of the pathologic findings.21 Diagnosis is confirmed by the presence of Gram-negative rods in sputum, and culture positivity for P. aeruginosa, as well as by blood sample cultures since bacteremia is usually frequent (in up to 92% of cases)3; in this scenario, some studies evidenced Gram-negative bacteria invading the blood vessel walls.3,22,23
Environmental and occupational risk factors have been associated with the development of P. aeruginosa CAP. Among the former are the home humidifying devices filled with contaminated water, home whirlpool spas, and hot tub bathing. In these cases, inhalation of the aerosolized water was implicated in the pathogenesis of the pneumonia.4,5,18,24,25 Moreover, tobacco abuse favors and selects the acquisition of Gram-negative bacteria, with emphasis on P. aeruginosa, in the oral cavity in a higher concentration than in non-smokers. The overgrowth of such bacteria in the mouth may also contribute to aspiration and development of pneumonia.26,27
Regarding the occupational risk, Cirigliano and Grippi28 reported a case of P. aeruginosa pneumonia in a healthy young nursing assistant, and Kunimasa et al.29 also reported a case of CAP due to P. aeruginosa in a young man who worked in a nurse care facility. Although, in the latter example, Pseudomonas was isolated in three samples (from a total of nine) collected from this nurse care, using PFGE none of the isolates matched with the strain isolated from the patient. Welders and foundry workers are also considered at occupational risk for P. aeruginosa pneumonia, probably due to dust containing metals.22,30,31 Nevertheless, machine operators using metalworking fluids are also at the same risk.32 Zell et al.33 reported the case of a healthy young man who acquired a P. aeruginosa pneumonia working in a place with aerosolized contaminated metalworking fluid, and mentioned that this CAP had been reported to the industrial injuries insurance companies as an occupational disease. Aqueous oil emulsions used in metal processing are frequently contaminated or colonized by Gram-negative bacteria, where they survive over a period of up to 250 days, and may show very fast proliferation.34 Pseudomonas represents the genus that most frequently colonizes cutting fluids.34 Indeed, P. aeruginosa was isolated from metalworking fluid (cutting fluid) in 17% of 150 examined samples in France,35 and in 48% (of 72 examined samples) in Zagreb (Croatia).34 Favoring this occupational correlation, Harrod et al.36 conducted an experimental model to evaluate the impact of inhaled diesel engine emissions (DEE) in modulating clearance of P. aeruginosa. Their findings concluded that environmental levels of DEE could decrease the clearance of P. aeruginosa and increase lung pathogenicity. In this report, P. aeruginosa was isolated from the metalworking fluid that was collected from the machine operated by the patient. The PGFE from both strains showed the same bacterial DNA-pattern, strongly arguing in favor of this fluid being the source of infection.
The case report herein represents an example of severe acute respiratory syndrome with alveolar hemorrhage. In this setting, influenza pneumonia (H1N1 virus infection), hantavirus infection, pulmonary vasculitis, and leptospirosis could have been considered. However, the chest plain radiography showed a lobar condensation and other foci of occupying space opacity, which raised the suspicion of lobar pneumonia. In our case the diagnosis was confirmed by positeve blood culture, tracheal aspirate culture and positive immunohistochemistry reaction in pulmonary tissue using mouse monoclonal antibody anti- P aerugnosa.5 The clinical history and outcome of our case strictly followed the disastrous and fulminant pattern of P. aeruginosa CAP in a previously healthy person. In this case, we cannot neglect tobacco abuse as a risk factor, but we believe this case relates more to the patient’s occupation. One of the machines operated by the patient uses metalworking fluid, which, unavoidably, is aerosolized. A similar case was reported in the literature,33 reinforcing our theses on the occupational relationship of our case.
Therefore we would like to stress this occupational risk when considering the antimicrobial choice. In these particular cases, Gram-negative pathogens, especially P. aeruginosa, should always be considered. Also, we would like to raise the possibility of a lobectomy in cases where Gram-negative bacilli are detected in pulmonary secretions accompanied by hemoptysis and signs of pulmonary parenchyma necrosis in the scenario of a life-threatening infection. We believe that these considerations may have a positive impact on changing the usual fatal outcome of theses cases.
ACKNOWLEDGEMENT
The authors thank Dr. Keiichi Iwaya (Department of Basic Pathology, National Defense Medical College, Japan) for kindly providing the immunohistochemistry test.
Footnotes
Campos FPF, Felipe-Silva A, Lopes ACFMM, et al. Community-acquired Pseudomonas aeruginosa-pneumonia in a previously healthy man occupationally exposed to metalworking fluids. Autopsy Case Rep [Internet]. 2014;4(3):31-7. http://dx.doi.org/10.4322/acr.2014.026
REFERENCES
- 1.Ribot EM, Fair MA, Gautom R, et al. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog Dis. 2006;3(1):59-67. http://dx.doi.org/ http://dx.doi.org/10.1089/fpd.2006.3.59. PMid: PMid: [DOI] [PubMed] [Google Scholar]
- 2.Tenover FC, Arbeit RD, Goering RV, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33(9):2233-9. PMid: PMid:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hatchette TF, Gupta R, Marrie TJ. Pseudomonas aeruginosa community-acquired pneumonia in previously healthy adults: case report and review of the literature. Clin Infect Dis. 2000;31(6):1349-56. http://dx.doi.org/ http://dx.doi.org/10.1086/317486. PMid: PMid: [DOI] [PubMed] [Google Scholar]
- 4.Huhulescu S, Simon M, Lubnow M, et al. Fatal Pseudomonas aeruginosa pneumonia in a previously healthy woman was most likely associated with a contaminated hot tub. Infection. 2011;39(3):265-9. http://dx.doi.org/ http://dx.doi.org/10.1007/s15010-011-0096-6. PMid: PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takajo D, Iwaya K, Katsurada Y, et al. Community-acquired lobar pneumonia caused by Pseudomonas aeruginosa infection in Japan: a case report with histological and immunohistochemical examination. Pathol Int. 2014;64(5):224-30. http://dx.doi.org/ http://dx.doi.org/10.1111/pin.12162. PMid: PMid: [DOI] [PubMed] [Google Scholar]
- 6.Gerald BP, Ramphal R. Pseudomonas aeruginosa. In: Mandell GL, Bennet JE, Dolin R, editors. Principles and practice of infectious diseases. 7th ed. Philadelphia: Churchill Livingstone, Elsevier; 2010. p. 2835-60. [Google Scholar]
- 7.Fujitani S, Sun HY, Yu VL, Weingarten JA. Pneumonia due to Pseudomonas aeruginosa: part I: epidemiology, clinical diagnosis, and source. Chest. 2011;139(4):909-19. http://dx.doi.org/ http://dx.doi.org/10.1378/chest.10-0166. PMid: PMid: [DOI] [PubMed] [Google Scholar]
- 8.Matar GM, Harakeh HS, Ramlawi F, Khneisser I, Hadi U. Comparative analysis between Pseudomonas aeruginosa genotypes and severity of symptoms in patients with unilateral or bilateral otitis externa. Curr Microbiol. 2001;42(3):190-3. http://dx.doi.org/ http://dx.doi.org/10.1007/s002840010202. PMid: PMid: [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) Pseudomonas dermatitis/folliculitis associated with pools and hot tubs—Colorado and Maine, 1999-2000. MMWR Morb Mortal Wkly Rep. 2000;49(48):1087-91. PMid: PMid:. [PubMed] [Google Scholar]
- 10.Willcox MD. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. Optom Vis Sci. 2007;84(4):273-8. http://dx.doi.org/ http://dx.doi.org/10.1097/OPX.0b013e3180439c3e. PMid: PMid: [DOI] [PubMed] [Google Scholar]
- 11.Arancibia F, Bauer TT, Ewig S, et al. Community-acquired pneumonia due to gram-negative bacteria and pseudomonas aeruginosa: incidence, risk, and prognosis. Arch Intern Med. 2002;162(16):1849-58. http://dx.doi.org/ http://dx.doi.org/10.1001/archinte.162.16.1849. PMid: PMid: [DOI] [PubMed] [Google Scholar]
- 12.Ruiz M, Ewig S, Marcos MA, et al. Etiology of community-acquired pneumonia: impact of age, comorbidity, and severity. Am J Respir Crit Care Med. 1999;160(2):397-405. http://dx.doi.org/ http://dx.doi.org/10.1164/ajrccm.160.2.9808045. PMid: PMid: [DOI] [PubMed] [Google Scholar]
- 13.Fang GD, Fine M, Orloff J, et al. New and emerging etiologies for community-acquired pneumonia with implications for therapy. A prospective multicenter study of 359 cases. Medicine (Baltimore). 1990;69(5):307-16. http://dx.doi.org/ http://dx.doi.org/10.1097/00005792-199009000-00004. PMid: PMid: [DOI] [PubMed] [Google Scholar]
- 14.Neill AM, Martin IR, Weir R, et al. Community acquired pneumonia: aetiology and usefulness of severity criteria on admission. Thorax. 1996;51(10):1010-6. http://dx.doi.org/ http://dx.doi.org/10.1136/thx.51.10.1010. PMid: PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanquer J, Blanquer R, Borrás R, et al. Aetiology of community acquired pneumonia in Valencia, Spain: a multicentre prospective study. Thorax. 1991;46(7):508-11. http://dx.doi.org/ http://dx.doi.org/10.1136/thx.46.7.508. PMid: PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Baum H, Welte T, Marre R, Suttorp N, Ewig S. CAPNETZ Study Group . Community-acquired pneumonia through Enterobacteriaceae and Pseudomonas aeruginosa: Diagnosis, incidence and predictors. Eur Respir J. 2010;35(3):598-605. http://dx.doi.org/ http://dx.doi.org/10.1183/09031936.00091809. PMid: PMid: [DOI] [PubMed] [Google Scholar]
- 17.Kaplan V, Angus DC, Griffin MF, Clermont G, Scott Watson R, Linde-Zwirble WT. Hospitalized community-acquired pneumonia in the elderly: age- and sex-related patterns of care and outcome in the United States. Am J Respir Crit Care Med. 2002;165(6):766-72. http://dx.doi.org/ http://dx.doi.org/10.1164/ajrccm.165.6.2103038. PMid: PMid: [DOI] [PubMed] [Google Scholar]
- 18.Crnich CJ, Gordon B, Andes D. Hot tub-associated necrotizing pneumonia due to Pseudomonas aeruginosa. Clin Infect Dis. 2003;36(3):e55-7. http://dx.doi.org/ http://dx.doi.org/10.1086/345851. PMid: PMid: [DOI] [PubMed] [Google Scholar]
- 19.Kang CI, Kim SH, Park WB, et al. Clinical features and outcome of patients with community-acquired Pseudomonas aeruginosa bacteraemia. Clin Microbiol Infect. 2005;11(5):415-8. http://dx.doi.org/ http://dx.doi.org/10.1111/j.1469-0691.2005.01102.x. PMid: PMid: [DOI] [PubMed] [Google Scholar]
- 20.Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996;275(2):134-41. http://dx.doi.org/ http://dx.doi.org/10.1001/jama.1996.03530260048030. PMid: PMid: [PubMed] [Google Scholar]
- 21.Fetzer AE, Werner AS, Hagstrom JWC. Pathologic features of pseudomonal pneumonia. Am Rev Respir Dis. 1967;96(6):1121-30. PMid: PMid:. [DOI] [PubMed] [Google Scholar]
- 22.Govan J, Reiss-Levy E, Bader L, Schonell M. Pseudomonas pneumonia with bacteraemia. Med J Aust. 1977;1(17):627-8. PMid: PMid:. [DOI] [PubMed] [Google Scholar]
- 23.Henderson A, Kelly W, Wright M.. Fulminant primary Pseudomonas aeruginosa pneumonia and septicaemia in previously well adults. Intensive Care Med. 1992;18(7):430-2. http://dx.doi.org/ http://dx.doi.org/10.1007/BF01694348. PMid: PMid: [DOI] [PubMed] [Google Scholar]
- 24.Rose HD, Franson TR, Sheth NKN, Chusid MJ, Macher AM, Zeirdt CH. Pseudomonas pneumonia associated with use of a home whirlpool spa. JAMA. 1983;250(15):2027-9. http://dx.doi.org/ http://dx.doi.org/10.1001/jama.1983.03340150069030. PMid: PMid: [PubMed] [Google Scholar]
- 25.Harris AA, Goodman L, Levin S. Community-acquired Pseudomonas aeruginosa pneumonia associated with the use of a home humidifier. West J Med. 1984;141(4):521-3. PMid: PMid:. [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar PS, Matthews CR, Joshi V, de Jager M, Aspiras M. Tobacco smoking affects bacterial acquisition and colonization in oral biofilms. Infect Immun. 2011;79(11):4730-8. http://dx.doi.org/ http://dx.doi.org/10.1128/IAI.05371-11. PMid: PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ertel A, Eng R, Smith SM. The differential effect of cigarette smoke on the growth of bacteria found in humans. Chest. 1991;100(3):628-30. http://dx.doi.org/ http://dx.doi.org/10.1378/chest.100.3.628. PMid: PMid: [DOI] [PubMed] [Google Scholar]
- 28.Cirigliano MD, Grippi MA. Overwhelming pneumonia in a healthy young nursing assistant. Hosp Pract (Off Ed). 1994;29(1):31-4. PMid: PMid:. [DOI] [PubMed] [Google Scholar]
- 29.Kunimasa K, Ishida T, Kimura S, et al. Successful treatment of fulminant community-acquired Pseudomonas aeruginosa necrotizing pneumonia in a previously healthy young man. Intern Med. 2012;51(17):2473-8. http://dx.doi.org/ http://dx.doi.org/10.2169/internalmedicine.51.7596. PMid: PMid: [DOI] [PubMed] [Google Scholar]
- 30.Hoogwerf BJ, Khan MY. Community-acquired bacteremic Pseudomonas pneumonia in a health adult. Am Rev Respir Dis. 1981;123(1):132-4. PMid: PMid:. [DOI] [PubMed] [Google Scholar]
- 31.Jayatilleke K, Bandara P, Satharasinghe R.. A case of community acquired pneumonia caused by Pseudomonas aeruginosa. Sri Lanka J Infect Dis. 2012;2:53-4. [Google Scholar]
- 32.Tant CO, Bennett EO. The growth of aerobic bacteria in metal-cutting fluids. Appl Microbiol. 1958;6(6):388-91. PMid: PMid:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zell L, Mack U, Sommerfeld A, Buchter A, Sybrecht GW. Abszedierende Pneumonie durch Pseudomonas aeruginosa als Berufskrankheit bei einem Bohrwerksdreher. Pneumologie. 1999;53(12):620-5. http://dx.doi.org/ http://dx.doi.org/10.1055/s-1999-9052. [DOI] [PubMed] [Google Scholar]
- 34.Jaksić S, Uhitil S, Zivković J. Bacterial pollution of cutting fluids: a risk factor for occupational diseases. Arh Hig Rada Toksikol. 1998;49(3):239-44. PMid: PMid:. [PubMed] [Google Scholar]
- 35.Chazal PM. Pollution of modern metalworking fluids containing biocides by pathogenic bacteria in France. Reexamination of chemical treatments accuracy. Eur J Epidemiol. 1995;11(1):1-7. http://dx.doi.org/ http://dx.doi.org/10.1007/BF01719939. PMid: PMid: [DOI] [PubMed] [Google Scholar]
- 36.Harrod KS, Jaramillo RJ, Berger JA, Gigliotti AP, Seilkop SK, Reed MD. Inhaled diesel engine emissions reduce bacterial clearance and exacerbate lung disease to Pseudomonas aeruginosa infection in vivo. Toxicol Sci. 2005;83(1):155-65. http://dx.doi.org/ http://dx.doi.org/10.1093/toxsci/kfi007. PMid: PMid: [DOI] [PubMed] [Google Scholar]