Abstract
Septic pelvic thrombophlebitis (SPT) is a rare and severe entity, which may occur after abortion, delivery, gynecological diseases, or surgeries. Diagnosis is challenging when no risk factor is clearly present, since clinically, symptoms are non-specific. Nowadays, with the aid of imaging methods, diagnosis has become more achievable, but the treatment still bears some uncertainties. The authors present a fatal case of SPT in a young woman who sought medical care already presenting signs of septic shock, referring fever and non-characteristic abdominal pain. The patient evolved rapidly to multiple organ failure and respiratory distress, which was also due to septic pulmonary embolism. The autopsy confirmed the computed tomographic findings of a right ovarian vein septic thrombophlebitis and multiple septic pulmonary embolization foci. The patient did not present any of the recognized risk factors; neither did she present signs of pelvic inflammatory disease on admission or at autopsy. However, an intrauterine device was present. The authors call attention to this entity in the differential diagnosis of a woman with fever and abdominal pain, as well as for an empiric broad-spectrum antibiotic regimen in these cases.
Keywords: Pelvis, Thrombophlebitis, Infection, Venous Thrombosis, Abdominal Pain
CASE REPORT
A 31-year-old previously healthy Caucasian female was brought to the emergency department with a 5-day history of fever, nausea without vomiting, and epigastric pain. She had self-medicated with antipyretic and antiemetic medication, without relief. She denied any other symptoms such as dysuria, diarrhea, vaginal discharge, or menstrual delay. The patient had had a copper intrauterine device (IUD) for 4 years and reported an ovarian cyst resection 20 years ago.
On admission, she was conscious, afebrile, pale, dehydrated, and slightly icteric. Her blood pressure was 60/30 mmHg; pulse was regular and 124 beats per minute; respiratory rate was 24 breaths per minute; room air oximetry was 93%. The clinical and gynecological examinations were unremarkable except for mild diffuse tenderness of the upper abdomen.
The diagnosis of sepsis was considered; the patient received volume resuscitation, plus ceftriaxone and metronidazole considering the hypothesis of urinary tract or abdominal infection foci. The laboratory workup is shown in Table 1.
Table 1. Laboratory tests on admission.
| Result | RV | Result | RV | ||
|---|---|---|---|---|---|
| Hemoglobin |
8.9 |
12.3-15.3 g/dL |
Magnesium |
2 |
1.6-2.6 mEq/L |
| Hematocrit |
27.4 |
36.0-45.0% |
ALT |
18 |
9-36 U/L |
| Leukocytes |
5.17 |
4.4-11.3 × 103/mm3 |
AST |
50 |
10-31 U/L |
| Metamyelocytes |
1 |
0% |
ALP |
529 |
10-100 U/L |
| Bands |
10 |
1-5% |
γGT |
48 |
2-30 U/L |
| Segmented |
79 |
45-70% |
Amylase |
38 |
20-104 U/L |
| Eosinophils |
0 |
1-4% |
Lipase |
25 |
5-250 U/L |
| Lymphocytes |
9 |
18-40% |
TB/DB |
3.29/2.7 |
0.3-1.2 mg/dL |
| Monocytes |
1 |
2-9% |
T protein |
6.4 |
6-8 g/dL |
| Platelets |
28 |
150-400 × 103/mm3 |
Albumin |
2.4 |
3-5 g/dL |
| INR |
1.28 |
1.0 |
Glucose |
93 |
< 100 mg/dL |
| Urea |
93 |
5-25 mg/dL |
pH |
7.36 |
7.35-7.45 |
| Creatinine |
1.27 |
0.4-1.3 mg/dL |
HCO3 |
20 |
22-26 mEq/L |
| Potassium |
3.7 |
3.5-5.0 mEq/L |
CRP |
328 |
< 5 mg/L |
| Sodium | 132 | 136-146 mEq/L | LDH | 239 | < 251 U/L |
ALP = alkaline phosphatase; ALT = alanine aminotransferase; AST = aspartate aminotransferase; CRP = C-reactive protein; DB = direct bilirubin; γGT = gamma glutamyl transferase; INR = international normalization ratio; LDH = lactate dehydrogenase; RV = reference value; TB = total bilirubin.
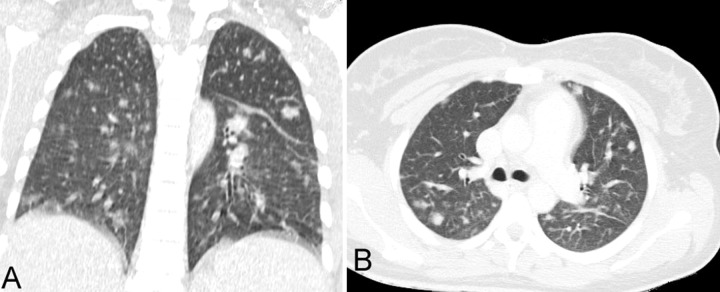
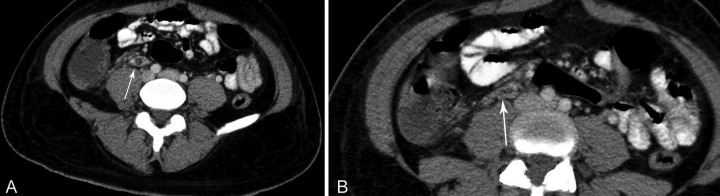
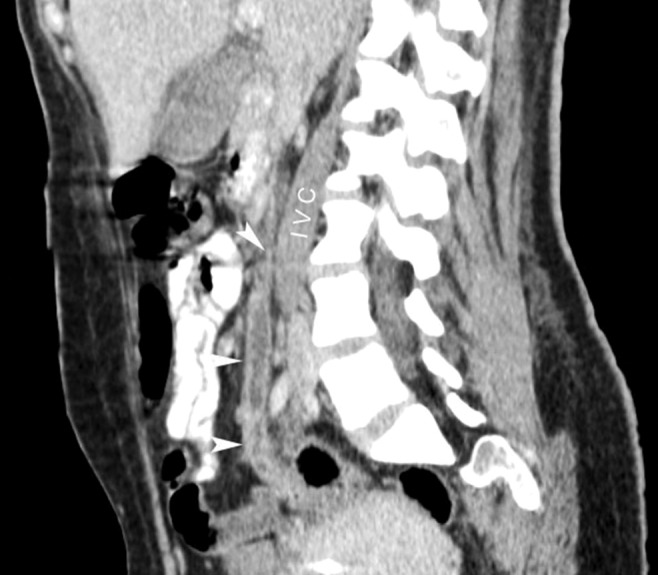
Anti-HIV was negative, ferritin was 568 ng/mL (reference value [RV]: 10-291 ng/mL), and fibrinogen was within the normal range. Iron profile tests were consistent with the diagnosis of iron deficiency anemia. Cerebral spinal fluid and the transthoracic echocardiography were normal. Thoracic plain radiography and abdominal ultrasonography were normal, but the thoracic computed tomography (CT) revealed multiple scattered bilateral nodules consistent with septic emboli (Figure 1); the abdominal CT revealed a filling defect in the right gonadal vein, which was compatible with thrombosis (Figures 2 and 3). The initial blood culture was positive for Gram-negative bacilli, which did not maintain viability for final identification.
Figure 1. Chest computed tomography (CT). A - Coronal reformation; B - Axil plane showing multiple bilateral scattered pulmonary nodules.
Figure 2. A and B - Axial CT of the abdomen showing the right ovarian vein thrombosis (arrow).
Figure 3. Sagittal reformation of abdominal CT showing ovarian vein thrombosis (arrowheads) and the inferior vena cava (IVC).
Within 48 hours of admission, the patient’s clinical status worsened due to hemodynamic and respiratory failure. Although the gynecologic evaluation was considered normal, the IUD was removed. Doxycycline was added to the antibiotic regimen, which was empirically changed to piperacillin/tazobactam and vancomycin. Further blood cultures were positive for multi-sensible Enterococcus faecalis. The patient’s clinical status continued to deteriorate and she subsequently died.
AUTOPSY FINDINGS
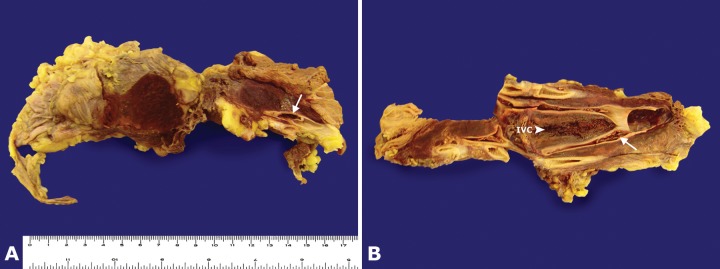
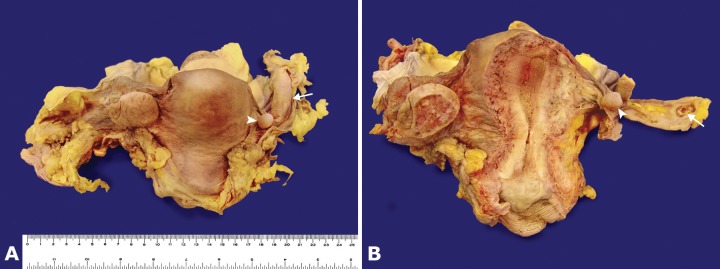
A eutrophic female corpse presenting an old surgical scar in the right iliac fossa was autopsied. The opening of the abdominal cavity evidenced an extensive hemorrhagic area located in the right iliac fossa adjacent to the inferior vena cava, encircling and compressing the right ovarian vein, but sparing the ipsilateral fallopian tube and residual surgical structure (Figure 4A). The cross-cutting and longitudinal sections showed extensive right ovarian phlebothrombosis that extended into the inferior vena cava (Figure 4B). In the right ovary topography, a residual elongated surgical fragment was present (Figure 5A and 5B), which, at the cut surface, showed a hemorrhagic area and a paratubal cyst (Figure 5A and 5B). The contralateral ovary, fallopian tube, uterine corpus, uterine cervix, and vagina were preserved (Figure 5B).
Figure 4. A - Gross images of extensive hemorrhagic area surrounding the thrombotic ovarian vein (arrow); B - Longitudinal sections of the inferior vena cava and adjacent right gonadal vein filled in with thrombus (arrow). IVC = inferior vena cava.
Figure 5. Gross posterior view of the internal genital organs. In A and B, note the paratubal cyst (arrowhead) and the residual surgical structure (arrow).
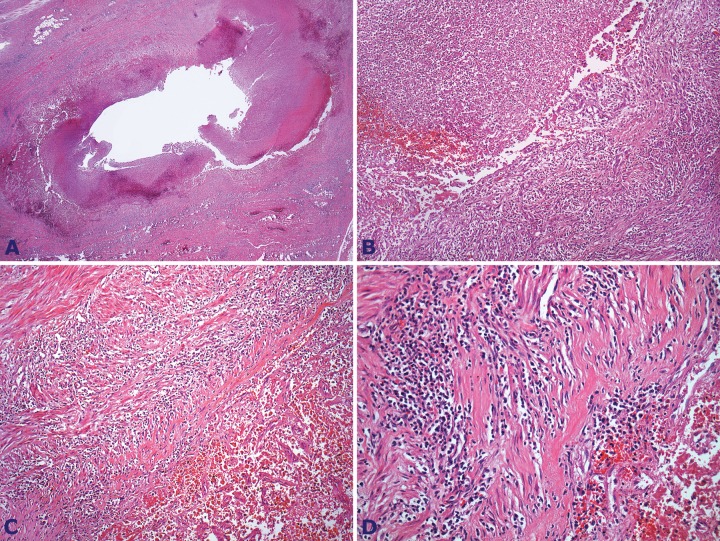
Within the residual surgical fragment, histological examination showed an old hemorrhagic area with hemossiderophages surrounding acute inflammatory infiltrate. The right ovarian vein evidenced thrombophlebitis with vascular intima and media extensive neutrophils infiltration (Figure 6). The remaining adjacent structures—psoas muscle, left ovary and left fallopian tube, uterus (endometrium, endocervix, and ectocervix), and bladder—were free of any inflammatory process.
Figure 6. Photomicrography of the right ovarian vein. A and B - Part of the thrombus consisting of numerous neutrophils (H&E 12.5X and 40X, respectively); C and D – Infiltration of the vascular muscular wall featuring thrombophlebitis (H&E, 100X & 200X, respectively).
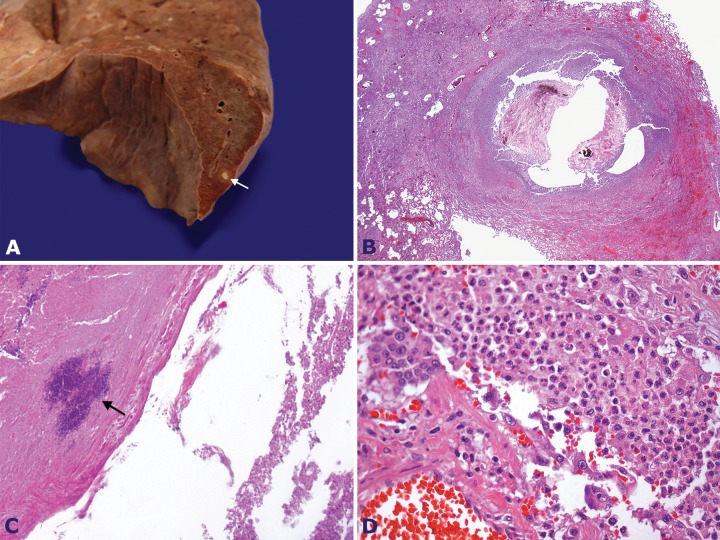
The opening of the chest showed a slight right sero-hemorrhagic pleural effusion and heavy lungs (right lung weight, 795.0 g; mean reference value (mRV): 450 g; and left lung weight, 604.0 g; mRV: 375 g), both with multiple small nodular areas of hemorrhagic and friable appearance. Histological examination showed vascular emboli consisting of neutrophils and fibrin-forming nodular areas of septic pulmonary microinfarctions, abscesses, and bronchopneumonia (Figure 7). The remaining lung parenchyma showed areas of filamentary fibrin deposition in the alveolar septa (hyaline membrane) and edema.
Figure 7. A - Gross finding of the lung showing a yellowish nodule (arrow); B - Photomicrography of the same nodule showing a pulmonary abscess (H&E, 12.5X); C - Photomicrography of the pulmonary abscess content showing bacteria colonies (arrow) (H&E, 100X); D - Photomicrography of the pulmonary parenchyma showing an acute inflammatory infiltrate corresponding to a septic embolization (H&E, 200X).
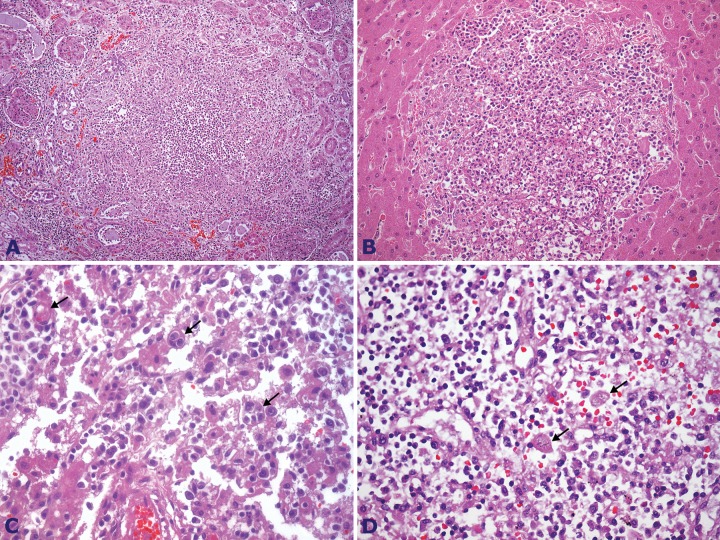
Increased bilateral kidney weight (right 225.0 g, and left 172.0 g; mRV for both kidneys: 313 g) with focal microabscesses formed by septic emboli was observed (Figure 8A), as well as acute tubular necrosis due to the septic shock. The liver weighed 2010 g (mRV: 1225 g), and, like the kidneys, showed focal microabscesses formed by septic emboli (Figure 8B). Also, peri-centrilobular vein hepatocyte ischemic necrosis was evident, due to the shock. The spleen was overweight (277 g; RV: 112 g) with congestion and moderate reactive splenitis. Intra-abdominal and pulmonary hilar lymph nodes were slightly enlarged—one of them with diffuse ischemic necrosis due to septic shock. Both spleen and lymph nodes showed macrophages containing intracytoplasmic erythrocytes and leucocytes (hemophagocytosis) (Figure 8C and 8D). The adrenals, pancreas, gastrointestinal tract, heart, and brain were preserved.
Figure 8. A - Photomicrography of the kidney showing a micro abscess (H&E, 200X); B - Photomicrography of the liver with a micro abscess (H&E, 200X); C and D - Photomicrography of the lymph node showing figures of hemophagocytosis ( arrows) (H&E, 1000X and 400X, respectively).
DISCUSSION
By the end of the 19th century, von Recklinghausen described an entity that later would be known as septic pelvic thrombophlebitis (SPT). In his description, all cases presented ovarian vein thrombosis and pelvic infection.1,2 In the middle of the 20th century, Collins published a great series of what he called suppurative pelvic thrombophlebitis.3 Since then, reports on this entity have been declining.3,4
While SPT strongly predominates the post-delivery period, with an overall incidence of 1:3000 deliveries (incidence for vaginal delivery is 1:9000 compared to 1:800 for cesarean section),5 the association with gynecologic diseases is the minority.1 The latter includes pelvic inflammatory disease, thrombophilia,6 gynecological surgery or procedures (including laparoscopy), underlying malignancy, fibroids, and hormonal stimulation.2 Idiopathic cases are even more rare.7,8
The right ovarian vein is involved in approximately 90% of the cases, most probably because of the longer length of this vein and the incompetence of their valves.9 At the beginning of the 20th century, the average mortality rate was roughly 50%, dropping to 10% in the 1950s, and in 1981 was reported to be 4.4%.1,3,10 Since the very first description until now, mortality has been declining, mostly due to a new therapeutic arsenal, modern approaches, and greater awareness of the entity.
The pathogenesis is thought to involve the Virchow’s triad, with endothelial damage to the pelvic veins caused by an infection, endotoxins, or minor trauma like delivery or surgery, along with a hypercoagulable state and venous stasis, which are both established conditions present during pregnancy and postpartum.1,9
There is a left-to-right flow in venous drainage in this area in the upright position, which can explain a higher prevalence of right ovarian vein thrombosis.10
SPT comprises ovarian vein thrombophlebitis (OVT) and deep septic pelvic thrombophlebitis; although these two types share a common physiopathogeny, they may differ somewhat in their clinical presentation.2 In the case of OVT, the thrombus can progress to the inferior vena cava and the renal vein, and become potentially fatal.11,12
Clinically, SPT is often characterized by a prolonged spiking fever, leukocytosis, and abdominal pain. In the case of post surgery or delivery, fever usually ensues within the first 5 days after the procedure, or may be delayed up to 3 weeks following the event.2 Prolonged fever was found in 20% of patients regardless of the appropriate antibiotic therapy.5 Abdominal pain is characterized to be constant, non-colicky, and may be referred in the flank or the lower abdomen, irradiating to the groin or upper abdomen, and may be accompanied by paralytic ileus.1 Pulmonary emboli used to be more frequent than are currently observed. In the past, the frequency of pulmonary embolization was reported to be found in up to 38% of the cases, but this incidence dropped to 2.7% in recent observations.1 Physical examination can reveal lower abdominal tenderness, like that observed in our patient, but rarely is a palpable mass detected.1,13
In the past, the diagnosis of SPT was made on clinical grounds and surgical findings; in the present day, diagnostic workup involves an imaging study added to a high level of suspicion in those patients with the aforementioned risk factors. CT findings comprise enlargement of the involved vein, enhancement or sharply defined vessel wall, perivascular inflammatory stranding, and a low-density filling defect of the gonadal vessel representing the thrombus.14 Magnetic resonance imaging (MRI) may furnish similar findings and therefore may not show an advantage over CT,9 although a comparative study shows the superiority of MRI over CT.15 However, both methods have limitations when small vessels are involved.1 Ultrasound is much less sensitive in case of obesity and because of the obscuration of the gonadal/ovarian vein by overlying bowel gas besides the dependence on the technique operator. Even with these constraints, the ultrasound method is still used given its safety and availability.14 Blood sample culture is of paramount importance for diagnosis and to guide antibiotic treatment; however, its sensitivity is low.3,4,10
The first treatment approach to SPT was excision of the thrombosed vein. In 1902, Trendelemburg, Freund and Bumn published their first operations for pelvic thrombophlebitis.10 In 1951, Collins et al. proposed the ligature of the inferior vena cava and ovarian veins as the treatment of choice, which showed some survival improvement.3 With the introduction of the imaging techniques and therefore better accuracy on SPT diagnosis, other therapeutics have been proposed. Antibiotic therapy regimens have been studied and published since 1979 when diZerega et al.16 advocated the combination of clindamycin and gentamycin for puerperal infection following cesarean delivery. Afterwards, ampicillin was added to this regimen because of the failure to treat an enterococci infection. Nowadays, treatment is based on broad-spectrum antibiotics depending on the primary source of infection, but Enterobacteriaceae, streptococci, and anaerobes should always be considered. The role of anticoagulation therapy is controversial, and insufficient data prevents its routine recommendation,17 although Garcia et al.1 published a successful experience managing eight cases with antibiotics and anticoagulation. There are also no firm recommendations on the length of treatment, ranging from 2 weeks (in the case of small vessel thrombosis), to 3-6 months when right ovarian vein thrombosis is involved.1 Surgery is indicated, in some cases, after the failure of conservative therapy or whenever the risk of pulmonary embolism is high.9,13
The patient reported herein had a short-term history of fever and non-characteristic abdominal pain, presenting, on admission, signs of septic shock. The gynecologic examination did not find signs of internal genital organ infection, although an IUD was present and almost totally exteriorized. This form of presentation was considered somewhat peculiar, preventing SPT suspicion. However, the results of a pelvic CT were unquestionable in diagnosing right ovarian vein thrombosis, with a thrombus extending quite into the entrance of the inferior vena cava. Furthermore, the blood sample cultures were positive for a Gram-negative bacillus and also for Enterococcus faecalis. The diagnosis of septic ovarian vein thrombosis was concluded and further confirmed by autopsy findings.
Intriguingly, there seemed to be no causative event. Autopsy findings also failed to demonstrate endometritis or salpingitis, but an acute inflammatory infiltrate was present in the residual post-operatory fragment, which enclosed a hematoma. For this reason, we dare suspect that the IUD, or this retained post-surgical hematoma, somehow might have had a role in the development of this case of SPT. If the IUD had been the causative agent, the patient may have presented an inflammatory disease, which was previously treated, but retained a remnant of latent infection. Another possibility is that the residual post-surgical structure was secondarily infected, which triggered the septic OVT.
Without a confirmed cause, we are obliged to consider this case as of idiopathic or unknown origin. Despite the broad-spectrum antibiotic regimen that was prescribed to this patient, initially Enterococcus faecalis was not specifically treated, it is difficult to ascertain if this lack of antimicrobial coverage did have influenced the unfavorable outcome.
Another outstanding finding of this case was the dramatic and severe form of presentation with septic pulmonary embolization, which, although a well-known complication, was unusually found in recent publications.5
Unfortunately, our patient sought medical care in an advanced stage of sepsis, and was already presenting multiple organ failure. The history of ovarian cyst resection and the use of an IUD could have raised the suspicion of infection from a pelvic origin, but the spectrum of the antibiotic regimen adopted at first was insufficient to treat all the possible types of pathogens.
We would like to draw attention to this entity in the differential diagnosis of women presenting acute abdominal pain and fever. We emphasize that diagnostic delay is potentially life threatening, as observed in the case reported herein.
Footnotes
Roepke RML, Campos FPF, Lovisolo SM, Santos EHS. Septic pelvic thrombophlebitis of unknown origin: an ever threatening entity. Autopsy Case Rep [Internet]. 2014;4(3):39-46. http://dx.doi.org/10.4322/acr.2014.027
REFERENCES
- 1.Garcia J, Aboujaoude R, Apuzzio J, Alvarez JR Septic pelvic thrombophlebitis: diagnosis and management. Infect Dis Obstet Gynecol. 2006;2006:15614. http://dx.doi.org/ http://dx.doi.org/10.1155/IDOG/2006/15614. PMid: PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nezhat C, Farhady P, Lemyre M. Septic pelvic thrombophlebitis following laparoscopic hysterectomy. JSLS. 2009;13(1):84-6. PMid: PMid:. [PMC free article] [PubMed] [Google Scholar]
- 3.Collins CG, MacCallum EA, Nelson EW, Weinstein BB, Collins JH. Suppurative pelvic thrombophlebitis. I. Incidence, pathology, and etiology; a study of 70 patients treated by ligation of the inferior vena cava and ovarian vessels. Surgery. 1951;30(2):298-310. PMid: PMid:. [PubMed] [Google Scholar]
- 4.Collins CG. Suppurative pelvic thrombophlebitis. A study of 202 cases in which the disease was treated by ligation of the vena cava and ovarian vein. Am J Obstet Gynecol. 1970;108(5):681-7. PMid: PMid:. [PubMed] [Google Scholar]
- 5.Brown CE, Stettler RW, Twickler D, Cunningham FG. Puerperal septic pelvic thrombophlebitis: incidence and response to heparin therapy. Am J Obstet Gynecol. 1999;181(1):143-8. http://dx.doi.org/ http://dx.doi.org/10.1016/S0002-9378(99)70450-3. PMid: PMid: [DOI] [PubMed] [Google Scholar]
- 6.Salomon O, Apter S, Shaham D, et al. Risk factors associated with postpartum ovarian vein thrombosis. Thromb Haemost. 1999;82(3):1015-9. PMid: PMid:. [PubMed] [Google Scholar]
- 7.Stafford M, Fleming T, Khalil A. Idiopathic ovarian vein thrombosis: a rare cause of pelvic pain - case report and review of literature. Aust N Z J Obstet Gynaecol. 2010;50(3):299-301. http://dx.doi.org/ http://dx.doi.org/10.1111/j.1479-828X.2010.01159.x. PMid: PMid: [DOI] [PubMed] [Google Scholar]
- 8.Murphy CS, Parsa T. Idiopathic ovarian vein thrombosis: a rare cause of abdominal pain. Am J Emerg Med. 2006;24(5):636-7. http://dx.doi.org/ http://dx.doi.org/10.1016/j.ajem.2006.01.004. PMid: PMid: [DOI] [PubMed] [Google Scholar]
- 9.Dessole S, Capobianco G, Arru A, Demurtas P, Ambrosini G. Postpartum ovarian vein thrombosis: an unpredictable event: two case reports and review of the literature. Arch Gynecol Obstet. 2003;267(4):242-6. PMid: PMid:. [DOI] [PubMed] [Google Scholar]
- 10.Dunnihoo DR, Gallaspy JW, Wise RB, Otterson WN. Postpartum ovarian vein thrombophlebitis: a review. Obstet Gynecol Surv. 1991;46(7):415-27. http://dx.doi.org/ http://dx.doi.org/10.1097/00006254-199107000-00002. PMid: PMid: [DOI] [PubMed] [Google Scholar]
- 11.Munsick RA, Gillanders LA. A review of the syndrome of puerperal ovarian vein thrombophlebitis. Obstet Gynecol Surv. 1981;36(2):57-66. http://dx.doi.org/ http://dx.doi.org/10.1097/00006254-198102000-00001. PMid: PMid: [DOI] [PubMed] [Google Scholar]
- 12.Hippach M, Meyberg R, Villena-Heinsen C, et al. Postpartum ovarian vein thrombosis. Clin Exp Obstet Gynecol. 2000;27(1):24-6. PMid: PMid:. [PubMed] [Google Scholar]
- 13.Brown TK, Munsick RA. Puerperal ovarian vein thrombophlebitis: a syndrome. Am J Obstet Gynecol. 1971;109(2):263-73. PMid: PMid:. [DOI] [PubMed] [Google Scholar]
- 14.Sharma P, Abdi S.. Ovarian vein thrombosis. Clin Radiol. 2012;67(9):893-8. http://dx.doi.org/ http://dx.doi.org/10.1016/j.crad.2012.01.013. PMid: PMid: [DOI] [PubMed] [Google Scholar]
- 15.Kubik-Huch RA, Hebisch G, Huch R, Hilfiker P, Debatin JF, Krestin GP. Role of duplex color Doppler ultrasound, computed tomography, and MR angiography in the diagnosis of septic puerperal ovarian vein thrombosis. Abdom Imaging. 1999;24(1):85-91. http://dx.doi.org/ http://dx.doi.org/10.1007/s002619900448. PMid: PMid: [DOI] [PubMed] [Google Scholar]
- 16.diZerega G, Yonekura L, Roy S, Nakamura RM, Ledger WJ. A comparison of clindamycin-gentamicin and penicillin-gentamicin in the treatment of post-cesarean section endomyometritis. Am J Obstet Gynecol. 1979;134(3):238-42. PMid: PMid:. [DOI] [PubMed] [Google Scholar]
- 17.Tsibris AMN. Septic pelvic thrombophlebitis. Boston: Massachusetts General Hospital; 2003 [cited 2010 Sept 2]. Available from: http://www2.massgeneral.org/id/hms/handouts2002/es.pdf