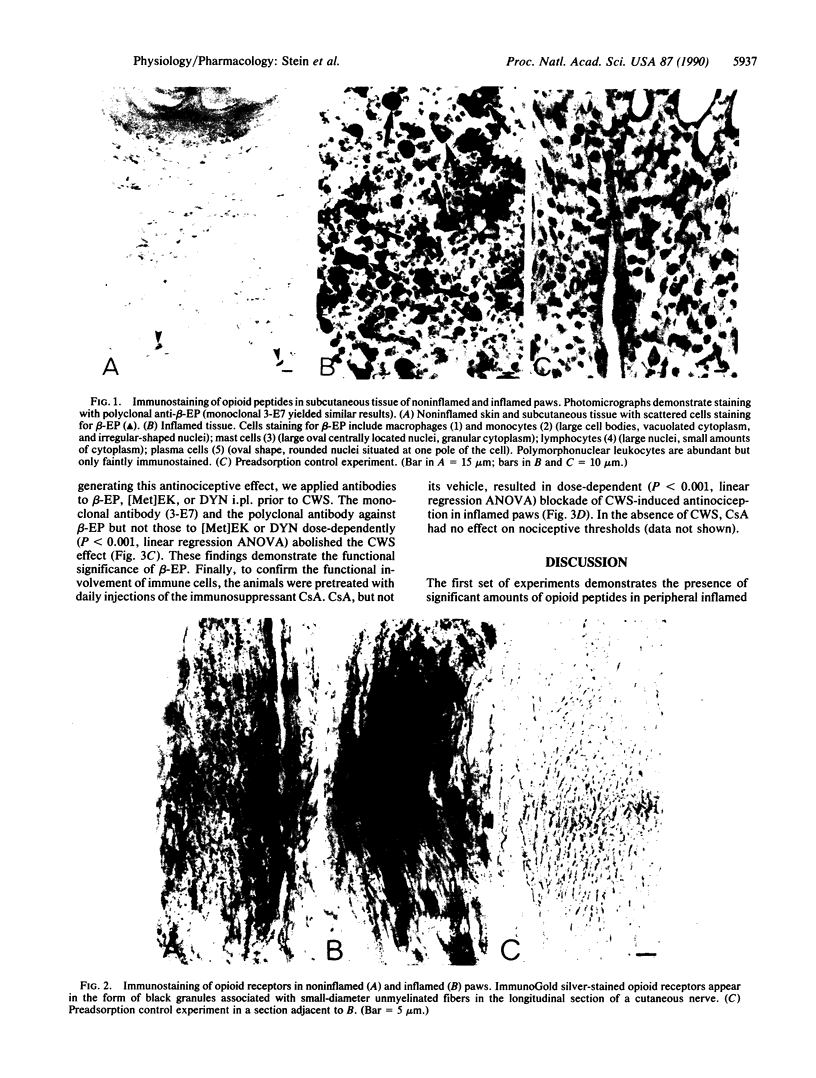
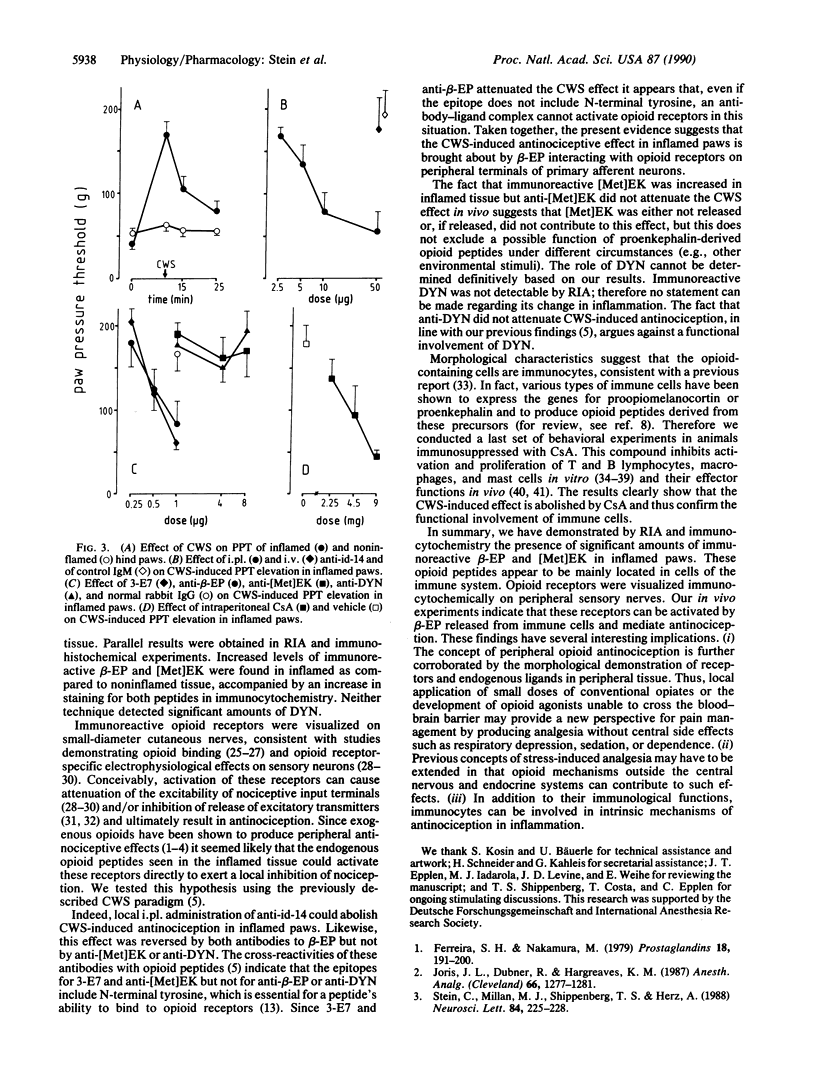
Abstract
Exogenous opioids can produce localized opioid receptor-mediated antinociception in peripheral inflamed tissue. Previous studies show that activation of endogenous opioids by a cold water swim in rats with hind paw inflammation results in a similar local antinociceptive effect but suggest that pituitary-adrenal opioid pools are not directly involved in producing this effect. Here we show increased amounts of opioid peptides in immune cells infiltrating the inflamed tissue. Furthermore, we demonstrate immunoreactive opioid receptors on peripheral terminals of sensory neurons. The local administration of antibodies against opioid peptides or receptors or systemic pretreatment with the immunosuppressant cyclosporine blocks cold water swim-induced antinociception. These findings suggest that antinociception in inflammation can be brought about by endogenous opioids from immune cells interacting with opioid receptors on peripheral sensory nerves.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bommer M., Herz A. Neuropeptides and other secretagogues in bovine chromaffin cells: their effect on opioid peptide metabolism. Neuropeptides. 1989 May-Jun;13(4):243–251. doi: 10.1016/0143-4179(89)90077-2. [DOI] [PubMed] [Google Scholar]
- Bunjes D., Hardt C., Röllinghoff M., Wagner H. Cyclosporin A mediates immunosuppression of primary cytotoxic T cell responses by impairing the release of interleukin 1 and interleukin 2. Eur J Immunol. 1981 Aug;11(8):657–661. doi: 10.1002/eji.1830110812. [DOI] [PubMed] [Google Scholar]
- Chisholm P. M., Bevan D. J. T cell activation in the presence of cyclosporine in three in vivo allograft models. Transplantation. 1988 Aug;46(2 Suppl):80S–85S. doi: 10.1097/00007890-198808001-00015. [DOI] [PubMed] [Google Scholar]
- Cummins A. G., Munro G. H., Ferguson A. Effect of cyclosporin A on rat mucosal mast cells and the associated protease RMCPII. Clin Exp Immunol. 1988 Apr;72(1):136–140. [PMC free article] [PubMed] [Google Scholar]
- Ferreira S. H., Nakamura M. II - Prostaglandin hyperalgesia: the peripheral analgesic activity of morphine, enkephalins and opioid antagonists. Prostaglandins. 1979 Aug;18(2):191–200. doi: 10.1016/0090-6980(79)90104-7. [DOI] [PubMed] [Google Scholar]
- Fields H. L., Emson P. C., Leigh B. K., Gilbert R. F., Iversen L. L. Multiple opiate receptor sites on primary afferent fibres. Nature. 1980 Mar 27;284(5754):351–353. doi: 10.1038/284351a0. [DOI] [PubMed] [Google Scholar]
- Frank G. B. Stereospecific opioid drug receptors on excitable cell membranes. Can J Physiol Pharmacol. 1985 Sep;63(9):1023–1032. doi: 10.1139/y85-168. [DOI] [PubMed] [Google Scholar]
- Gramsch C., Meo T., Riethmüller G., Herz A. Binding characteristics of a monoclonal beta-endorphin antibody recognizing the N-terminus of opioid peptides. J Neurochem. 1983 May;40(5):1220–1226. doi: 10.1111/j.1471-4159.1983.tb13560.x. [DOI] [PubMed] [Google Scholar]
- Gramsch C., Schulz R., Kosin S., Herz A. Monoclonal anti-idiotypic antibodies to opioid receptors. J Biol Chem. 1988 Apr 25;263(12):5853–5859. [PubMed] [Google Scholar]
- Guillemin R., Vargo T., Rossier J., Minick S., Ling N., Rivier C., Vale W., Bloom F. beta-Endorphin and adrenocorticotropin are selected concomitantly by the pituitary gland. Science. 1977 Sep 30;197(4311):1367–1369. doi: 10.1126/science.197601. [DOI] [PubMed] [Google Scholar]
- Hassan A. H., Almeida O. F., Gramsch C., Herz A. Immunocytochemical demonstration of opioid receptors in selected rat brain areas and neuroblastoma x glioma hybrid (NG108-15) cells using a monoclonal anti-idiotypic antibody. Neuroscience. 1989;32(1):269–278. doi: 10.1016/0306-4522(89)90126-7. [DOI] [PubMed] [Google Scholar]
- Herold K. C., Lancki D. W., Moldwin R. L., Fitch F. W. Immunosuppressive effects of cyclosporin A on cloned T cells. J Immunol. 1986 Feb 15;136(4):1315–1321. [PubMed] [Google Scholar]
- Holgate C. S., Jackson P., Cowen P. N., Bird C. C. Immunogold-silver staining: new method of immunostaining with enhanced sensitivity. J Histochem Cytochem. 1983 Jul;31(7):938–944. doi: 10.1177/31.7.6189883. [DOI] [PubMed] [Google Scholar]
- Höllt V. Opioid peptide processing and receptor selectivity. Annu Rev Pharmacol Toxicol. 1986;26:59–77. doi: 10.1146/annurev.pa.26.040186.000423. [DOI] [PubMed] [Google Scholar]
- Kunkl A., Klaus G. G. Selective effects of cyclosporin A on functional B cell subsets in the mouse. J Immunol. 1980 Dec;125(6):2526–2531. [PubMed] [Google Scholar]
- Lamotte C., Pert C. B., Snyder S. H. Opiate receptor binding in primate spinal cord: distribution and changes after dorsal root section. Brain Res. 1976 Aug 13;112(2):407–412. doi: 10.1016/0006-8993(76)90296-1. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Donnerer J. Opioid control of the function of primary afferent substance P fibres. Eur J Pharmacol. 1985 Aug 27;114(3):241–246. doi: 10.1016/0014-2999(85)90365-6. [DOI] [PubMed] [Google Scholar]
- Lolait S. J., Clements J. A., Markwick A. J., Cheng C., McNally M., Smith A. I., Funder J. W. Pro-opiomelanocortin messenger ribonucleic acid and posttranslational processing of beta endorphin in spleen macrophages. J Clin Invest. 1986 Jun;77(6):1776–1779. doi: 10.1172/JCI112501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkovic M., Hunt S. P., Gleave J. R. Localization of opiate and histamine H1-receptors in the primate sensory ganglia and spinal cord. Brain Res. 1982 Jun 10;241(2):197–206. doi: 10.1016/0006-8993(82)91056-3. [DOI] [PubMed] [Google Scholar]
- Palay D. A., Cluff C. W., Wentworth P. A., Ziegler H. K. Cyclosporine inhibits macrophage-mediated antigen presentation. J Immunol. 1986 Jun 15;136(12):4348–4353. [PubMed] [Google Scholar]
- Parsons C. G., Członkowski A., Stein C., Herz A. Peripheral opioid receptors mediating antinociception in inflammation. Activation by endogenous opioids and role of the pituitary-adrenal axis. Pain. 1990 Apr;41(1):81–93. doi: 10.1016/0304-3959(90)91112-V. [DOI] [PubMed] [Google Scholar]
- Pawelec G., Rehbein A., Katrilaka K., Balko I., Busch F. W. Prevention of human helper T-cell clone activation by cyclosporin A also blocks secretion of granulocyte/macrophage colony stimulating activity. Immunopharmacology. 1988 Nov-Dec;16(3):207–216. doi: 10.1016/0162-3109(88)90009-4. [DOI] [PubMed] [Google Scholar]
- Pedersen C., Permin H., Stahl Skov P., Norn S., Svenson M., Mosbech H., Bendtzen K. Inhibitory effect of cyclosporin A on histamine release from human leukocytes and rat mast cells. Allergy. 1985 Feb;40(2):103–107. doi: 10.1111/j.1398-9995.1985.tb02668.x. [DOI] [PubMed] [Google Scholar]
- Przewłocki R., Gramsch C., Pasi A., Herz A. Characterization and localization of immunoreactive dynorphin, alpha-neo-endorphin, Met-enkephalin and substance P in human spinal cord. Brain Res. 1983 Nov 28;280(1):95–103. doi: 10.1016/0006-8993(83)91177-0. [DOI] [PubMed] [Google Scholar]
- Russell N. J., Schaible H. G., Schmidt R. F. Opiates inhibit the discharges of fine afferent units from inflamed knee joint of the cat. Neurosci Lett. 1987 Apr 23;76(1):107–112. doi: 10.1016/0304-3940(87)90201-1. [DOI] [PubMed] [Google Scholar]
- Sibinga N. E., Goldstein A. Opioid peptides and opioid receptors in cells of the immune system. Annu Rev Immunol. 1988;6:219–249. doi: 10.1146/annurev.iy.06.040188.001251. [DOI] [PubMed] [Google Scholar]
- Smith E. M., Morrill A. C., Meyer W. J., 3rd, Blalock J. E. Corticotropin releasing factor induction of leukocyte-derived immunoreactive ACTH and endorphins. 1986 Jun 26-Jul 2Nature. 321(6073):881–882. doi: 10.1038/321881a0. [DOI] [PubMed] [Google Scholar]
- Springall D. R., Hacker G. W., Grimelius L., Polak J. M. The potential of the immunogold-silver staining method for paraffin sections. Histochemistry. 1984;81(6):603–608. doi: 10.1007/BF00489542. [DOI] [PubMed] [Google Scholar]
- Stein C., Gramsch C., Herz A. Intrinsic mechanisms of antinociception in inflammation: local opioid receptors and beta-endorphin. J Neurosci. 1990 Apr;10(4):1292–1298. doi: 10.1523/JNEUROSCI.10-04-01292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C., Millan M. J., Herz A. Unilateral inflammation of the hindpaw in rats as a model of prolonged noxious stimulation: alterations in behavior and nociceptive thresholds. Pharmacol Biochem Behav. 1988 Oct;31(2):445–451. doi: 10.1016/0091-3057(88)90372-3. [DOI] [PubMed] [Google Scholar]
- Stein C., Millan M. J., Shippenberg T. S., Herz A. Peripheral effect of fentanyl upon nociception in inflamed tissue of the rat. Neurosci Lett. 1988 Jan 22;84(2):225–228. doi: 10.1016/0304-3940(88)90412-0. [DOI] [PubMed] [Google Scholar]
- Stein C., Millan M. J., Shippenberg T. S., Peter K., Herz A. Peripheral opioid receptors mediating antinociception in inflammation. Evidence for involvement of mu, delta and kappa receptors. J Pharmacol Exp Ther. 1989 Mar;248(3):1269–1275. [PubMed] [Google Scholar]
- Weihe E., Millan M. J., Höllt V., Nohr D., Herz A. Induction of the gene encoding pro-dynorphin by experimentally induced arthritis enhances staining for dynorphin in the spinal cord of rats. Neuroscience. 1989;31(1):77–95. doi: 10.1016/0306-4522(89)90031-6. [DOI] [PubMed] [Google Scholar]
- Weihe E., Nohr D., Millan M. J., Stein C., Müller S., Gramsch C., Herz A. Peptide neuroanatomy of adjuvant-induced arthritic inflammation in rat. Agents Actions. 1988 Dec;25(3-4):255–259. doi: 10.1007/BF01965027. [DOI] [PubMed] [Google Scholar]
- Werz M. A., Macdonald R. L. Heterogeneous sensitivity of cultured dorsal root ganglion neurones to opioid peptides selective for mu- and delta-opiate receptors. Nature. 1982 Oct 21;299(5885):730–733. doi: 10.1038/299730a0. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L. Substance P release from knee joint afferent terminals: modulation by opioids. Brain Res. 1988 Aug 23;458(2):319–324. doi: 10.1016/0006-8993(88)90474-x. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983 Jun;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Benedik M., Kamb B. J., Abrams J. S., Zurawski S. M., Lee F. D. Activation of mouse T-helper cells induces abundant preproenkephalin mRNA synthesis. Science. 1986 May 9;232(4751):772–775. doi: 10.1126/science.2938259. [DOI] [PubMed] [Google Scholar]