Abstract
Metabolic disease comprises a set of risk factors highly associated with obesity and insulin resistance and is a consequence of central adiposity, hyperglycemia, and dyslipidemia. Furthermore, obesity increases the risk of the development of metabolic disease due to ectopic fat deposition, low-grade inflammation, and systemic energy disorders caused by dysregulated adipose tissue function. Piceatannol is a naturally occurring polyphenolic stilbene found in various fruits and vegetables and has been reported to exhibit anticancer and anti-inflammatory properties. In addition, recently reported beneficial effects of piceatannol on hypercholesterolemia, atherosclerosis, and angiogenesis underscore its therapeutic potential in cardiovascular disease. However, investigation of its role in metabolic disease is still in its infancy. This review intensively summarizes in vitro and in vivo studies supporting the potential therapeutic effects of piceatannol in metabolic disease, including inhibition of adipogenesis and lipid metabolism in adipocytes, and regulation of hyperlipidemia, hyperglycemia, insulin resistance, and fatty acid-induced inflammation and oxidative stress.
Keywords: : adipocytes, adipose tissue, bioavailability, insulin resistance, obesity, resveratrol
Introduction
Metabolic syndrome affects approximately one in every four adults across the globe and as many as 34% of people in the United States.1,2 Insulin resistance, adiposity, dyslipidemia, hypertension, inflammation, and hyperglycemia characterize the syndrome, resulting in increased risk for a number of chronic diseases, including cardiovascular disease (CVD), stroke, type 2 diabetes (T2D), and all-cause mortality.3 In this review, diagnostic criteria for metabolic syndrome will simply be referred to as metabolic disorders as they can occur individually without clinical diagnosis of the condition.
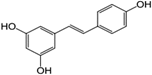
Phytochemicals, plant secondary metabolites produced in response to environmental stress, are putative therapeutic agents in the treatment of metabolic disorders. Resveratrol is a well-studied phytochemical in the treatment of metabolic disorders. This polyphenol has been shown to reverse the adverse effects of a high-calorie diet, protect against diet-induced obesity, and improve overall health in rodents.4,5 Although a meta-analysis of randomized clinical trials revealed that resveratrol lowered fasting insulin, glucose, and insulin resistance, a conclusive physiologically relevant benefit has not been demonstrated in healthy populations.6,7 Limited oral bioavailability is suspected to be one of the main barriers to resveratrol's potency in human studies: resveratrol is extensively metabolized and has an estimated oral bioavailability of less than 1.0%.8 Therefore, identification of more stable resveratrol metabolites and synthetic derivatives with improved bioavailability is emerging as an alternative option to treat metabolic disorders. Piceatannol is identical in structure to resveratrol, with the exception of an additional hydroxyl group at the 3′-carbon (Table 1). Of note, piceatannol has been shown to be more metabolically stable than resveratrol.9 Although reviews of piceatannol's role in cancer, CVD, and other chronic diseases have been conducted, the literature regarding its effect in metabolic disorders is limited.10–13 This review will focus on the biological role of piceatannol in metabolic disorders. We conclude that although in vivo and human studies of piceatannol are currently insufficient to recommend its clinical use, the effect of piceatannol in modulating energy metabolism and inflammation merits further investigation of its use in the treatment and prevention of metabolic disease.
Table 1.
Structure and Physical Properties of Piceatannol and Resveratrol
| Piceatannol | Resveratrol | |
|---|---|---|
| Molecular weight | 244.24 | 228.24 |
| Solubility | 10 g/L in DMSO, 10 g/L in ethanol, 0.5 g/L in water | ≥16 g/L in DMSO, 50 g/L in ethanol, 0.03 g/L in water |
| Structure | 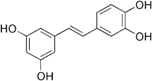 |
 |
Sources of Piceatannol
Dietary sources
Piceatannol is naturally present in a variety of foods typically consumed in the human diet. For example, both red grapes and white grapes contain piceatannol at concentrations of 374 and 43 ng/g, respectively.14 In addition, passion fruit contains a high amount of piceatannol in its seed, detected at a dry weight concentration of 4.8 mg/g.15 Vaccinium berries (blueberries) also contain piceatannol at a dry concentration of 138–422 ng/g.16 As with most secondary plant metabolites, piceatannol is produced in response to stress. UV irradiation, for example, has been shown to increase piceatannol in both peanut callusses and grapes.17,18 Additionally, wine made with UV-irradiated grapes contains 1.5 times as much piceatannol (311 μg/L) as wine made form untreated grapes.18 Fungal stress also increases piceatannol in peanut calluses to levels as high as 6.93 μg/g.19 For further details regarding natural sources of piceatannol, the reader is directed to the extensive review by Piotrowska et al.10
Piceatannol as a resveratrol metabolite
In addition to natural sources, piceatannol is also a metabolite of the well-studied resveratrol. In vitro, piceatannol is formed by CYP450 metabolism of resveratrol, as seen in human lymphoblasts, liver microsomes, and other models.10,20,21 Furthermore, piceatannol has been detected as a resveratrol metabolite in vivo: 5 min following resveratrol administration (75 mg/kg), piceatannol was found in the plasma (5.26 μmol), skin (2.4 nmol/g), and liver (11.5 nmol/g) of athymic mice.22 In addition, after 5 weeks of resveratrol administration in mice, piceatannol was found as a product of phase 1 metabolism in the small intestine.23
Metabolism and Bioavailability of Piceatannol
An understanding of piceatannol's metabolism and bioavailability is necessary to determine physiologically relevant doses and to translate findings from model systems to human applications. Bioactive compounds such as piceatannol must be present in sufficient concentration, stable for an adequate exposure time, and in an active form at the site of putative action to exert their effects.
Piceatannol metabolites
In vivo, piceatannol is metabolized to glucuronidated, methylated, and sulfated metabolites and to unique compounds, as outlined in Table 2. Following intravenous (IV) administration, glucuronidated compounds appeared to be the main piceatannol metabolites. In addition, piceatannol was metabolized by the liver and highly distributed in tissues, as indicated by a high hepatic clearance rate and volume distribution, respectively.24,25 Oral administration of piceatannol provides additional understanding of piceatannol metabolism, as described in the study by Setoguchi et al. using Sprague-Dawley rats. As with the Roupe studies, this group also identified a monoglucuronide as the main piceatannol metabolite. Unlike the other study, novel compounds such as the antiarteriosclerotic and anticancer compound isorhapontigenin, were also detected. Rhapontigenin was also detected in plasma and urine, as were glucuronidated, methylated, and sulfated piceatannol metabolites: piceatannol diglucuronide, piceatannol monoglucuronide, O-methyl piceatannol monoglucuronide, and O-methyl piceatannolmonosulfate.9 In vitro, piceatannol is transformed to sulfated and glucuronidated metabolites, as previously reviewed.10
Table 2.
Piceatannol Metabolism In Vitro and In Vivo
| Model | Route | Detected metabolites | Ref. |
|---|---|---|---|
| Sprague-Dawley rats | Oral | Piceatannol-diglucuronide, piceatannol monoglucuronide, O-methyl piceatannol monoglucuronide,O-methyl piceatannol monosulfate, isorhapontigenin, rhapontigenin | 9 |
| Sprague-Dawley rats | Oral | Glucuronidated piceatannol, O-methylated piceatannol, isorhapontigenin | 33 |
| Sprague-Dawley rats | IV | Glucurondiated metabolites | 24 |
| Sprague-Dawley rats | IV | Glucurondiated metabolites | 25 |
| Rat liver microsomes with uridine diphosphate–glucuronosyltransferase | In vitro | Glucurondiated metabolites | 25 |
| Human liver cytosol | In vitro | Piceatannol disulfate, piceatannol monosulfate (two peaks) | 77 |
| Recombinant sulfotransferase isoforms | In vitro | ||
| Human liver microsomes | In vitro | Piceatannol monoglucuronides (three peaks) | 78 |
| Recombinant UDP-glucuronosyltransferase isoforms | In vitro |
IV, intravenous.
Of note, piceatannol was not detected as a metabolite of resveratrol in vivo, in the study by Setoguchi et al. which differs from previous studies.9,22,23 In contrast to piceatannol, where O-methyl piceatannol conjugates were identified, only conjugates of the resveratrol parent compound were detected. This observation supports the hypothesis that piceatannol's catechol group enables unique metabolism by catechol-O-methyltransferase, which catalyzes methylation of meta-OH functional groups, specifically. Therefore, it is suggested that piceatannol undergoes more complex metabolism than resveratrol, which could affect its properties such as toxicity.9,26,27
Bioavailability, pharmacokinetics, and stability of piceatannol
In addition to understanding the metabolism of bioactive compounds, greater knowledge of phytochemical bioavailability and pharmacokinetics can inform clinical recommendations (Table 3). Following an IV injection of piceatannol (10 mg/kg body weight) in Sprague-Dawley rats, plasma area under curve (AUC) reached 8.48 μg h/mL with a plasma elimination half-life of 4.23 h. Because piceatannol was detected in urine 84 h postdose and displayed a urinary elimination half-life of 19.88 h, the authors hypothesized that actual half-life may be much higher.24,25 Following oral administration, others have found a maximum piceatannol concentration of 8.1 μM at the highest tested dose of 360 μmol/kg. Of note, piceatannol was undetected in plasma after 24 h for any dose in this study.9
Table 3.
In Vivo Bioavailability of Piceatannol
| Model | Route | Dose (μmol/kg) | Modification | AUC (0–8 h) (μmol h/L) | Cmax (μM) | Tmax (min) | t1/2 plasma (h) | t1/2 urine (h) | Cl (L/h kg) | Vd (L/kg) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sprague-Dawley rats | Oral | 90 | — | 4.3 ± 0.3 | 3.3 | 15 | — | — | — | — | 9 |
| 180 | — | 12.3 ± 1.8 | 7.5 | 15 | — | — | — | — | |||
| 360 | — | 20.6 ± 2.2 | 8.1 | 15 | — | — | — | — | |||
| Sprague-Dawley rats | Oral | 180 | — | 9.1 ± 1.0 | 2.5 | 30 | — | — | — | — | 33 |
| 180 | 90 μmol/kg α-cyclodextrin | 10.1 ± 0.6 | 5.3 | 15 | — | — | — | — | |||
| 180 | 180 μmol/kg α-cyclodextrin | 9.0 ± 0.7 | 4.8 | 30 | — | — | — | — | |||
| 180 | 540 μmol/kg α-cyclodextrin | 8.6 ± 0.9 | 5.8 | 15 | — | — | — | — | |||
| Sprague-Dawley rats | IV | 40.94 | — | 34.72 ± 10.15 | — | — | 4.23 ± 1.25 | 19.88 ± 5.66 | 2.13 ± 0.92 | 10.76 ± 2.88 | 24 |
| Sprague-Dawley rats | Oral | 40.94 | Methylated piceatannol Analog in 0.3 M 2-hydroxypropyl-β-cyclodextrin | 9.819 ± 2.91 (12 h) | 2.91 ± 0.89 | 45, 60, or 120 | — | — | — | — | 31 |
| IV | 16.38 | 7.75 ± 0.984 (12 h) | — | — | 5.21 ± 0.33 | — | 1.986 ± 0.234 | 3.396 ± 5.07 |
AUC, area under curve.
Phytochemical stability is a product of both metabolism and pharmacokinetics and provides a useful measure of bioactive potential. Piceatannol, as an intact polyphenol, exhibited stability 2.1–2.6 times higher than resveratrol. Although total polyphenol concentration (intact and metabolites) of resveratrol was higher than that of piceatannol, piceatannol showed greater metabolic stability as calculated as the ratio of intact stilbene to total stilbene (3.7–4.3-fold higher than resveratrol) and as a percentage of intact parent compound in relation to total detected polyphenol (16.3% compared with 5.5% for resveratrol).9 As further support of piceatannol's superior metabolic stability, serum from piceatannol-treated, but not resveratrol-treated, rats maintained the ability to suppress hepatoma cell proliferation, even though both parent polyphenols showed antiproliferative properties.28 Differences in metabolite action and degradation kinetics could partially explain the disparate biological effects of piceatannol and resveratrol treatment. In addition, hepatoma cells treated with rat serum samples collected 2 h postpiceatannol administration showed a greater antiproliferative effect than serum samples collected at 1 h; considering piceatannol's maximum serum concentration (Cmax) of 15 min, this finding could imply that piceatannol metabolites are responsible for some of the anticancer properties.9,28 These observations highlight the need for more studies investigating the biological effects of piceatannol metabolites.
To have clinical relevance, piceatannol must be present at a biologically relevant concentration in humans. Although no human studies of piceatannol pharmacokinetics have been conducted to our knowledge, studies of resveratrol may provide an approximation. After repeated doses of resveratrol (as high as 150 mg) for 2 weeks, a Cmax of 63.8 ng/mL (0.28 μM) and an AUC of 78.9 ng h/mL (0.35 μmol h/L) were observed.29 Even if intact piceatannol was present at a concentration 2.6 times higher than resveratrol, as suggested by the Setoguchi study, the maximum concentration of piceatannol would still be <1.0 μmol, which falls short of the concentration found in majority of in vitro studies. Therefore, there is a need to investigate methods to increase piceatannol exposure, as discussed in the following section.
Strategies to increase piceatannol bioavailability
As piceatannol is metabolized relatively quickly, improving its stability could increase potency. Modification of piceatannol is one strategy to improve its bioavailability. Brents et al. found that the prenylated form of piceatannol (trans-arachidin-1 [tA1]) exhibited slower glucuronidation. Of note, prenylated piceatannol (tA1) showed higher affinity for cannabinoid receptors; therefore, prenylated piceatannol may demonstrate higher biological activity than its parent compound in certain pathways.30 Additionally, methylated piceatannol (trans-3,5,3′,4′-tetramethoxystilbene) demonstrated enhanced oral bioavailability, further demonstrating the potential of piceatannol modification to enhance absorption.31 Delivery method can also enhance piceatannol bioavailability. Messiad et al. demonstrated that β-cyclodextrin dose-dependently increased piceatannol solubility.32 Furthermore, Inagaki et al. found that delivering piceatannol with α-cyclodextrin more than doubled its solubility in vitro. In Sprague-Dawley rats, the use of α-cyclodextrin increased the Cmax of piceatannol from 2.5 to 5.8 μmol; however, this change was not statistically significant. Interestingly, the use of α-cyclodextrin also increased the AUC and Cmax of O-methyl conjugates and recovery of total stilbenes from the small intestine.33
Taken together, these findings suggest that therapeutic concentrations of piceatannol may be achievable in vivo. Although several researchers have evaluated piceatannol levels in blood and serum, data revealing piceatannol concentration in specific tissues are lacking. Future research focusing on piceatannol content in target tissues will provide critical information to understand the potential mode of action. Furthermore, investigations of piceatannol pharmacokinetics in humans are needed.
The Role of Piceatannol in Metabolic Disorders
As delineated in the Introduction section, metabolic syndrome is characterized by adiposity, insulin resistance, hyperglycemia, and inflammation.3 Therefore, the following sections will highlight piceatannol's putative role in addressing each of these risk factors. Table 4 summarizes metabolic targets of piceatannol, whereas a summary of in vivo studies of piceatannol in metabolic disorders is shown in Table 5.
Table 4.
Metabolic Targets of Piceatannol
| Model | Target and effect | Putative role | Ref. |
|---|---|---|---|
| Murine adipocytes (3T3-L1) | Inhibited adipogenesis, blocked mitotic clonal expansion, inhibited insulin signaling due to noncompetitive binding to insulin receptor, lowered lipid accumulation during late stages of differentiation | Antiadipogenic | 36 |
| Human liposarcoma preadipocytes (LiSa-2) | Induced CHOP expression, inhibited adipocyte differentiation as demonstrated by lack of lipid accumulation | Antiadipogenic | 37 |
| Human liposarcoma adipocytes (LiSa-2) | Induced CHOP expression, reduced VEGF production | Antiadipogenic | 37 |
| Mouse (C57BL/6) WAT | Lowered PPARγ and FAS protein expression, increased AMPKα, PPARα, and CPT1-α protein expression and ACC phosphorylation | Antiadipogenic, antilipogenic | 45 |
| Mouse (C57BL/6) liver | Lowered PPARγ, C/EBPα, and FAS protein expression, increased AMPKα and ACC phosphorylation, | Antiadipogenic, antilipogenic | 45 |
| Murine adipocytes (3T3-L1) | Lowered TG accumulation, lipid droplet accumulation | Antilipogenic | 41 |
| Murine adipocytes (3T3-L1) | Lowered TG accumulation | Antilipogenic | 44 |
| HUVECs | Inhibited ATP synthesis | Antilipogenic | 42 |
| Rat brain mitochondrial fractions | Inhibited F0F1-ATPase | Antilipogenic | 79 |
| Recombinant, full-length human holocarboxylase synthase | Inhibited holocarboxylase synthase | Antilipogenic | 80 |
| Rat myoblasts (L6) | Increased glucose uptake, increased AMPK phosphorylation and GLUT4 translocation | Glucose handling improvement | 46 |
| Yeast α-glucosidase | Inhibited α-glucosidase activity | Glucose handling improvement | 81 |
| Murine subcutaneous adipocytes | Impaired insulin-stimulated lipogenesis, inhibited basal and stimulated glycerol and FFA release | Antilipolytic | 43 |
| Primary human osteoarthritic osteoblasts | Inhibited alkaline phosphatase activity, carboxy-terminal propeptide of collagen type 1 production, lowered TGF-β1 expression | Leptin signaling | 48 |
| Human adipose tissue | Lowered H2O2 production, inhibited tyramine- and benzylamine-induced H2O2 production, inhibited [14C]-tyramine oxidation | Antioxidant | 43 |
| Rat liver microsomes | Increased oxygen consumption | Antioxidant | 27 |
| WAT explants from obese Zucker rats | Lowered amine-induced hydrogen peroxide generation | Antioxidant | 44 |
| HUVECs | Increased expression and activity of HO-1 through Nrf2; decreased IL-6, TNF-α, ROS formation, p-65 phosphorylation, NF-κB activation in palmitic acid-induced inflammation; restored IRS-1 phosphorylation, eNOS phosphorylation in palmitic acid-induced inflammation through HO-1 | Anti-inflammatory | 53 |
| Bone marrow-derived macrophages | Restored HO-1 mRNA and protein expression after RANKL inhibition, decreased miR-183 | Anti-inflammatory | 59 |
| Human mammary epithelial cells (MCF10A) | Upregulated HO-1 mRNA and protein expression, decreased Keap1 levels, increased Nrf2 translocation, increased Akt activation | Anti-inflammatory | 61 |
| Rat liver | Increased HO-1 expression and Akt activation, reduced trauma-induced IL-6, ICAM-1, CINC-1, and CINC-3 levels | Anti-inflammatory | 62 |
| Murine neuronal cells (HT22) | Reduced glutamate-induced toxicity and ROS production, increased HO-1 expression and activity, induced Nrf2 activation | Anti-inflammatory, antioxidant | 63 |
| Murine macrophages (RAW264.7) | Decreased LPS-induced TNF-α and IL-1β levels, induced HO-1 expression | Anti-inflammatory | 58 |
| Bovine aortic endothelial cells | Increased HO-1 protein and mRNA expression | Anti-inflammatory | 60 |
| Murine macrophages (RAW264.7) | Decreased LPS-induced NO release and iNOS expression; prevented LPS-induced degradation of κBα, p65 translocation, STAT3 phosphorylation and translocation; increased HO-1 protein and mRNA expression | Anti-inflammatory | 65 |
| Human lymphoma (RAMOS) cells | Inhibited STAT3 and STAT5 phosphorylation | Anti-inflammatory | 67 |
| Human lymphocytes (Jurkat T cells) | Inhibited Jak1 and IFNAR1 tyrosine phosphorylation | Anti-inflammatory | 67 |
| human prostate cancer (DU145) cells | Inhibited STAT3 phosphorylation, IL-6 secretion | Anti-inflammatory | 66 |
| Human astrocytoma (U373) cells | Inhibited LPS-induced IRF3 activation, ISG induction, and TNF-α, ICAM-1, and MCP-1 upregulation | Anti-inflammatory | 74 |
| Murine macrophages (RAW264.7) | Inhibited LPS-induced IL-6 upregulation | Anti-inflammatory | 74 |
| Swiss mice | Prevented LPS-induced septic shock and liver and spleen damage | Anti-inflammatory | 74 |
| HUVECs | Inhibited CT-1-induced IL-6 mRNA and protein expression | Anti-inflammatory | 70 |
| Murine microglia (BV2) cells | Inhibited LPS-induced NO release, PGE2 release, iNOS expression, COX-2 mRNA and protein expression, NF-κB activity, p65 translocation, IL-1β levels, IL-6 levels, and TNF-α levels, | Anti-inflammatory | 71 |
| Peripheral blood mononuclear cell | Lowered OK-432-stimulated (penicillin-killed Streptococcus pyogenes) IL-6 and TNF-α secretion and mRNA expression | Anti-inflammatory | 69 |
| Human peripheral blood mononuclear leukocytes | Inhibited PGE2 levels, Lowered LPS and IFN-γ-stimulated TNF-α, IL-8 levels, and COX-2, TNF-α, IL-8, IL-6, IL-1α mRNA expression | Anti-inflammatory | 72 |
| Human monocytes (mono Mac 6) | Inhibited LPS-stimulated IL-6 levels | Anti-inflammatory | 68 |
| Human bronchial epithelial cells | Inhibited TNF-α-induced ICAM-1 expression and IL-6 release | Anti-inflammatory | 73 |
| Human myeloid cells (KBM-5) | Lowered TNF-α, PMA, LPS, okadic acid, ceramide, and H2O2-induced NF-κB activation; inhibited TNF-α-induced IκBα phosphorylation, IKK activation, and p65 phosphorylation and translocation | Anti-inflammatory | 82 |
| Human lymphocytes (Jurkat T cells) | Lowered TNF-α-induced NF-κB activation | Anti-inflammatory | 82 |
| Epithelial cells (MCF-7, HeLa) | Lowered TNF-α-induced NF-κB activation | Anti-inflammatory | 82 |
| Human monocytic (THP-1) cells | Upregulated SIRT1 mRNA and protein expression | SIRT activation | 83 |
| HeLa cells | Stimulated SIRT1 activity | SIRT activation | 84 |
| Human hepatoma (HepG2) cells | Increased SIRT1 expression, decreased c-Myc, β-catenin, and PHD2 expression | SIRT activation | 85 |
AMPK, 5′ AMP-activated protein kinase; eNOS, endothelial nitric oxide synthase; FFAs, free fatty acids; GLUT4, glucose transporter 4; HO-1, heme oxygenase-1; HUVEC, human umbilical vein endothelial cell; IL, interleukin; IRS-1, insulin receptor substrate-1; MCP-1, monocyte chemotactic protein-1; NF-κB, nuclear factor-κB; Nrf2, nuclear factor erythroid 2-related factor 2; ROS, reactive oxygen species; STAT3, signal transducer and activator of transcription factor-3; TG, triglyceride; TNF-α, tumor necrosis factor-alpha; WAT, white adipose tissue.
Table 5.
In vivo Studies of Piceatannol in Metabolic Disorders
| Model | Dose and route | BW | Food intake | Glucose | Lipids | Insulin | Fat mass | Other | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Male C57BL/6 mice, HFD-induced obesity (60% fat), 12 weeks old | 1, 3, 10, and 30 mg/kg piceatannol, daily oral, 5 weeks | NSD | NSD | Lower serum glucose | — | — | NSD | NSD in leptin | 47 |
| Male db/db mice, 11 weeks old | 50 mg/kg, single oral dose | — | — | Lower serum glucose after 1 h. NSD after 2–4 h | — | — | — | — | 47 |
| Male Zucker rats, 7 weeks old | 15 or 45 mg/kg, daily oral, 6 weeks | NSD | NSD | NSD in serum glucose | Lower NEFA. NSD in TG. Lower serum LDL. NSD in cholesterol, HDL | NSD in serum insulin | NSD | Lower serum leptin. Lowered serum lactate. NSD in urea, adiponectin, or LPS. NSD in gut microbiota phyla | 44 |
| Male db/db mice, 6 weeks old | 50 mg/kg, daily oral, 3 weeks | NSD | NSD | Lower blood glucose after 2 and 3 weeks | — | — | — | — | 46 |
| Male db/db mice, 11 weeks old | 50 mg/kg, single oral dose | — | — | Lower fasting blood glucose levels 1, 3, and 5 h postadministration. Lower blood glucose AUC during a GTT | — | — | — | — | 46 |
| Male Sprague-Dawley rats, 11 weeks old, chow diet | 10, 50, and 100 mg/kg, single intravascular dose | — | — | Lower blood glucose 150 and 180 min postadministration with 100 mg/kg dose only. Lowered blood glucose AUC during a GTT | — | Higher insulinogenic index. NSD in overall insulin secretion | — | NSD in blood glucose concentrations, plasma insulin concentrations, or glucose infusion rate during euglycemic clamp | 49 |
| Male C57BL/6 mice, HFD-induced obesity (45% fat), 6 weeks old | 0.1% or 0.25% piceatannol mixed into diet, 18 weeks | Lower body weight | NSD | Lower glucose | Lower total cholesterol, LDL, HDL, and LDL/HDL ratio. Lowered serum TG | — | Lowered % body fat, retroperitoneal WAT, and perigonadal fat. Decreased adipocyte size | Lowered PPARγ, C/EBPα, and FAS. Increased ACC phosphorylation, PPARα, and CPT1. Increased Firmicutes-to-Bacteroidetes ratio. Increased Lactobacillus | 45 |
ACC, acetyl coa carboxylase; FAS, fatty-acid synthase; GTT, glucose tolerance test; HDL, high-density lipoprotein; HFD, high-fat diet; LDL, low-density lipoprotein; NEFA, non-esterified fatty acids; NSD, no significant difference.
Adipogenesis
Obesity is characterized by excess adipose tissue and is a risk factor for a number of metabolic diseases, including T2D and CVD.34 Visceral adiposity, in particular, is correlated with insulin resistance. Indeed, weight reduction is a primary strategy to counter metabolic syndrome.3 An increase in adipocyte size (hypertrophy) and number (hyperplasia) both contribute to increased fat mass. In obese individuals, more adipocytes are added annually than in lean individuals. Although the number of adipocytes is set primarily in childhood and adolescence, 10% of adipocytes turnover annually.35 Thus, preventing adipogenesis, especially during developmental stages, may be a strategy to combat obesity.
Our group demonstrated that piceatannol inhibited adipogenesis in 3T3-L1 adipocytes. Piceatannol dose-dependently inhibited differentiation and intracellular lipid accumulation in cultured murine 3T3-L1 adipocytes, as shown by both Oil red O staining and by coherent anti-Stokes Raman scattering microscopy, independent of cellular toxicity. The effect of piceatannol on the differentiation process was further confirmed by lower protein and gene expression of key adipogenic markers, such as peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer-binding protein β (C/EBPβ). Furthermore, piceatannol inhibited adipogenesis by targeting mitotic clonal expansion during the early phase of adipocyte differentiation. In addition, piceatannol blocked insulin signaling during differentiation, as seen by inhibited phosphorylation of protein kinase B (Akt), insulin receptor substrate-1 (IRS-1), insulin receptor (IR), ERK (a serine/threonine kinase involved in adipogenesis), and lower phosphatidylinositol 3-kinase (PI3K) and IR kinase activities. Further studies revealed a direct binding of piceatannol to IR in a dose-dependent and ATP-noncompetitive manner. It is possible that piceatannol binds to both IR on the cell surface and to intracellular PI3K, although more research is needed to fully elucidate its effect on insulin signaling.36
In addition to targeting mitotic clonal expansion and impairing insulin signaling, piceatannol may affect adipogenesis through other mechanisms. Huang et al. found that piceatannol treatment induced C/EBP homologous protein (CHOP) expression in both undifferentiated and differentiated LiSa-2 adipocytes; of note, CHOP expression delays C/EBPβ activity.37 Additionally, the immune receptor mediator spleen tyrosine kinase (Syk), a well-established piceatannol target, plays a key role in the early stages of adipocyte differentiation: overexpression of Syk promotes adipogenesis.38,39 It should be noted that for both of these putative pathways, more evidence is needed to establish piceatannol's mechanistic involvement.
Lipid synthesis and accumulation
Piceatannol also affects lipid synthesis and storage, as demonstrated in several in vitro models. Our group found that piceatannol lowered lipid accumulation when added during later stages (days 4–6) of adipocyte differentiation, suggesting a possible inhibitory role of piceatannol in lipogenesis.36 Additionally, inhibition of H+-ATP synthase, a piceatannol target that is highly expressed during adipocyte differentiation, blocked triglyceride (TG) accumulation in 3T3-L1 adipocytes.40–42 However, more evidence is needed to directly link piceatannol-induced H+-ATP synthase inhibition as the mechanism responsible for decreased lipid droplet accumulation, as causal evidence has not been investigated.40 Piceatannol also lowered TG content in 3T3-L1 adipocytes and insulin-stimulated lipogenesis in isolated mouse adipocytes.43,44 Although piceatannol shows several potential antiobesity mechanisms in vitro, caution should be taken to interpret these findings in the context of whole-body metabolism as only limited evidence of the role of piceatannol in obesity in animals and humans is available.
In vivo effects of piceatannol in metabolic disorders
Although piceatannol has demonstrated antiadipogenic and antilipogenic effects in vitro, only one study has shown a significant impact on body weight, while the remainder of in vivo studies showed no significant differences.45 Indeed, orally administered piceatannol in db/db obese mice, obese Zucker rats, or diet-induced obese C57BL/6 mice was ineffective for preventing weight gain, inducing weight loss, or altering adipose mass or food intake in mice.44,46,47 However, a recent study employing a milder obesity model and increased dosage and duration (0.25% in the diet [∼370 mg/kg body weight] for 18 weeks) demonstrated a preventative effect of piceatannol in high-fat diet-induced obesity.45 The same group demonstrated decreased white adipose tissue (WAT) and adipocyte size and no difference in food intake.45 Route of administration, a treatment or prevention model, and dose must all be considered when evaluating piceatannol's role in body weight and adiposity.
Despite limited evidence of an effect on body weight, piceatannol influenced other obesity-related parameters. Decreased low-density lipoprotein (LDL) was observed in both obese Zucker rats and DIO mice.44,45 While no difference in cholesterol or high-density lipoprotein (HDL) was observed in the study by Hijona et al., Tung et al. noted decreased cholesterol and LDL:HDL ratio in piceatannol-treated mice.44,45 Piceatannol also decreased serum-free fatty acids (FFAs) and TGs.44,45 Taken together, these data support a role of piceatannol in altering lipid handling. However, although lowered serum lactate levels were observed, piceatannol failed to alter hepatic TG content, degree of steatosis, or markers of hepatic health (glutamate-pyruvate transaminase and glutamic oxaloacetic transaminase), suggesting that piceatannol was unable to prevent hepatic steatosis.44 Piceatannol has also been shown to lower circulating leptin.44 Although more evidence is required, a few hypotheses may explain piceatannol's impact on this key adipokine: piceatannol could lower leptin indirectly as a result of decreasing adipose mass or directly by inhibiting signal transducer and activator of transcription factor-3 (STAT3), a downstream leptin target.48
Hyperglycemia and insulin resistance
Several studies have demonstrated piceatannol's glucose-lowering properties, thus suggesting a role in glucose handling and insulin signaling.45–47,49 Daily administration of piceatannol lowered fasting glucose levels in both db/db mice and diet-induced obese C57BL/6 mice without affecting body weight or food intake.46,47 Furthermore, acute piceatannol administration lowered serum glucose levels and improved glucose tolerance in various rodent models.46,47,49 Collectively, these data demonstrate that both chronic and acute piceatannol treatments influence glucose handling in obese and healthy rodents. However, the supraphysiological doses used in many of these studies must be considered when translating the evidence. Furthermore, the glucose-lowering effect of piceatannol has not been observed in all studies.44,50 Clearly, more research is needed to ascertain the mechanism of piceatannol involvement in hyperglycemia.
Although influencing insulin secretion or sensitivity is one possible explanation of piceatannol's glucose-lowering properties, current literature suggests that other mechanisms may have a more significant role. Oritani et al. observed that an acute high dose (100 mg/kg body weight) of piceatannol lowered fasting glucose concentration independent of insulin secretion in healthy Sprague-Dawley rats. Furthermore, a subsequent euglycemic clamp study showed no difference in glucose infusion rates, implying that piceatannol did not significantly affect insulin sensitivity.49 Others have also found no difference in serum insulin levels.44 Furthermore, piceatannol stimulated glucose uptake in the absence of insulin in cultured myotubes.46 However, piceatannol significantly increased the insulinogenic index in healthy rats during a glucose tolerance test despite no significant difference in plasma insulin level.49 Timing and physiological relevance of any observed differences must all be taken into consideration when determining piceatannol action in insulin secretion. Indeed, it is possible that piceatannol may lower hyperglycemia through insulin-independent mechanisms.
Piceatannol may affect glucose handling by increasing glucose disposal. Minakawa et al. found that piceatannol dose-dependently promoted glucose uptake in cultured myotubes through activation of 5′ AMP-activated protein kinase (AMPK) and glucose transporter 4 (GLUT4) translocation.46 Of note, AMPK activators may be a useful strategy to treat T2D as AMPK is a known stimulator of GLUT4 translocation and skeletal muscle accounts for a large majority of glucose disposal.51,52 In vivo studies examining piceatannol's effect on AMPK-mediated skeletal muscle glucose uptake are needed to test this hypothesis.
Collectively, the observations that piceatannol lowered fasting glucose in vivo, activated AMPK in myotubes, and minimally impacted insulin secretion in vivo suggest a beneficial role of piceatannol in glucose handling.44,46,49 Piceatannol's role in insulin signaling may be tissue dependent as it blocked insulin signaling in adipocytes and improved it in endothelial cells under inflammatory stress.36,53 Further investigation of piceatannol's tissue-specific effects in insulin signaling and glucose uptake will provide greater understanding of its role in hyperglycemia. Clearly, as metabolic perturbations are the consequence of systemic interdependence, studies examining the whole-body effect of piceatannol are needed.
Oxidative stress and inflammation
Elevated FFAs due to aberrant activation of obesity-associated lipolysis in WAT contribute to insulin resistance by increasing oxidative stress and inflammation. Indeed, systemic inflammation and oxidative stress are thought to have a causative role in the etiology of insulin resistance.54–56 High levels of FFAs promote proinflammatory signaling pathways, such as nuclear factor-κB (NF-κB) and Toll-like receptor 4, and induce expression of the proinflammatory cytokines tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and IL-1β. High FFA levels also stimulate expression of monocyte chemotactic protein-1 (MCP-1), increase free radical concentration, and induce production of reactive oxygen species.54
Piceatannol has demonstrated significant radical scavenging activity, even up to 1250-fold higher than resveratrol.27 In human adipose tissue specifically, piceatannol lowered hydrogen peroxide.43 Although no difference in hydrogen peroxide generation was detected in adipose tissue of piceatannol-treated obese Zucker rats, lower amine-induced hydrogen peroxide generation was observed when piceatannol was added to these explants ex vivo.44 However, the same group found no change in protein oxidation (3-nitrotyrosine assay), lipid peroxidation (thiobarbituric acid-reactive substrate assay), or superoxide dismutase activity in kidney homogenates.57 Thus, while piceatannol shows antiradical activity in vitro, its effect in more physiologically relevant models remains to be proved.
Piceatannol may reduce inflammation by activating anti-inflammatory pathways. In human endothelial cells, piceatannol increased expression of heme oxygenase-1 (HO-1), an enzyme with antioxidant and anti-inflammatory properties, in a concentration-dependent manner through activation of nuclear factor erythroid 2-related factor 2 (Nrf2) transcriptional activity. Piceatannol's anti-inflammatory action was HO-1 dependent as inhibition of HO-1 abolished piceatannol's effect on TNF-α, IL-6, and NF-κB transcriptional activity and p65 phosphorylation.53 As further evidence, piceatannol increased HO-1 expression in macrophages, endothelial cells, mammary breast epithelial cells, rat liver, and neuronal cells.58–63 However, it must be noted that the role of HO-1 in metabolic disorders is not fully understood and has even been shown to be proinflammatory.64 Given this context, it is possible that piceatannol's anti-inflammatory effects may be mediated by its regulation of the HO-1 upstream activator Nrf2.61
Several studies provide evidence of piceatannol's inhibitory role in inflammatory pathways. As reviewed previously, FFAs promote inflammation; palmitic acid, specifically, is one such FFA with proinflammatory properties. Piceatannol prevented the inhibitory effects of palmitic acid on IRS-1 phosphorylation, glucose uptake, endothelial nitric oxide synthase (eNOS) phosphorylation, and nitric oxide (NO) production in human endothelial cells.53 In addition, piceatannol lowered LPS-induced protein expression of IL-6 and TNF-α and attenuated NK-κB and STAT3 signaling in macrophages; prevented IL-6 secretion and STAT3 signaling in prostate cancer cells; and inhibited the downstream IL-6 mediators STAT3 and STAT5 in human lymphocytes.58,65–67 Indeed, many others have employed piceatannol as a STAT3 inhibitor and observed decreased expression and signaling of IL-6 and other proinflammatory mediators.62,68–74 Together, these studies support an anti-inflammatory role of piceatannol.
Cardiovascular disease
As the role of piceatannol in CVD has been extensively reviewed recently by others, here we will focus on key themes and new additions to the literature.11 In addition, many of the mechanisms previously discussed in this review, such as insulin resistance, oxidative stress, and elevated FFAs, also play contributory roles in the development of CVD.54 A recent study in obese Zucker rats provides additional insight into piceatannol action in cardiac health. Piceatannol supplementation had no significant impact on heart weight:body ratio, cardiomyocyte transverse area, or degree of fibrosis, suggesting minimal effect on cardiac remodeling during obesity. However, an increase in the cardiac structural protein ephrin-B1 was observed, indicating that piceatannol may promote cardiac compensation and cardiac muscle structure during weight gain.44
Conclusion and Future Direction
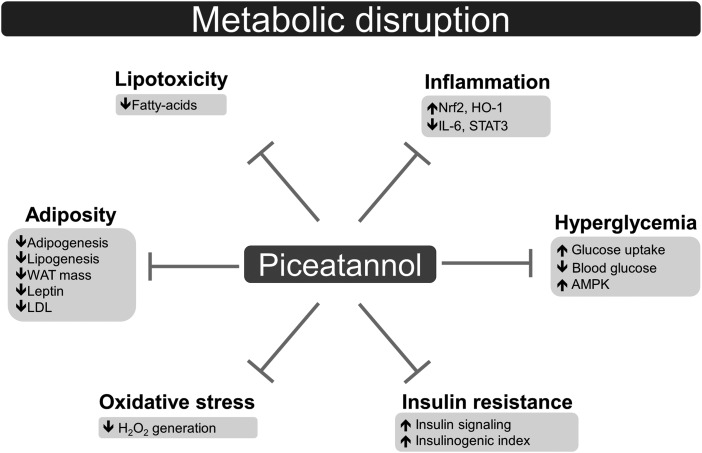
In this review, we highlight numerous putative mechanisms of piceatannol in metabolic disease, including inhibited adipogenesis, lowered lipid accumulation, lowered blood glucose, and attenuated oxidative stress and inflammation (Fig. 1). Although piceatannol has shown limited impact on body weight, treating symptoms of metabolic disease has therapeutic potential as adiposity itself is not always causative of metabolic disruption.75 It should be noted that although current evidence on piceatannol action in metabolic disorders is insufficient to recommend the use of this small molecule to treat and prevent human disease, the beneficial effects of piceatannol observed in several metabolic pathways provide a rationale for further study of this bioactive compound.
FIG. 1.
Mechanisms by which piceatannol may ameliorate metabolic disorders.
The large majority of available literature on piceatannol action is limited to cell and animal models, thus results must be interpreted with caution. In light of these models, limitations of phytochemical studies in general, such as bioavailability, food–matrix interactions, and effects of metabolites, must be taken into account when considering potential dietary relevance.76 Furthermore, individual variation in genetics and environment will also influence polyphenol metabolism. Indeed, more human studies are required to truly understand piceatannol's effect in metabolic disorders. Studies revealing piceatannol's mechanism and effects on signaling pathways in multiple tissues, crosstalk between metabolic organs, the gut microbiome, and insulin resistance will provide greater insights. Considering the complexity of metabolic disease and the current gaps in the literature, models that examine whole-body metabolism will provide the greatest value.
Acknowledgment
This work was supported, in part, by a grant from the National Institutes of Health, 5R03CA184544.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ: Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA 2015;313:1973–1974 [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation: IDF worldwide definition of the metabolic syndrome, 2015. www.idf.org/metabolic-syndrome (accessed September2016)
- 3.Kaur J: A comprehensive review on metabolic syndrome. Cardiol Res Pract 2014;2014:943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Baur JA, Pearson KJ, Price NL, et al. : Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006;444:337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lagouge M, Argmann C, Gerhart-Hines Z, et al. : Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1 alpha. Cell 2006;127:1109–1122 [DOI] [PubMed] [Google Scholar]
- 6.Liu K, Zhou R, Wang B, Mi MT: Effect of resveratrol on glucose control and insulin sensitivity: A meta-analysis of 11 randomized controlled trials. Am J Clin Nutr 2014;99:1510–1519 [DOI] [PubMed] [Google Scholar]
- 7.Carpene C, Gomez-Zorita S, Deleruyelle S, Carpene MA: Novel strategies for preventing diabetes and obesity complications with natural polyphenols. Curr Med Chem 2015;22:150–164 [DOI] [PubMed] [Google Scholar]
- 8.Walle T: Bioavailability of resveratrol. Ann NY Acad Sci 2011;1215:9–15 [DOI] [PubMed] [Google Scholar]
- 9.Setoguchi Y, Oritani Y, Ito R, et al. : Absorption and metabolism of piceatannol in rats. J Agric Food Chem 2014;62:2541–2548 [DOI] [PubMed] [Google Scholar]
- 10.Piotrowska H, Kucinska M, Murias M: Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat Res 2012;750:60–82 [DOI] [PubMed] [Google Scholar]
- 11.Tang YL, Chan SW: A review of the pharmacological effects of piceatannol on cardiovascular diseases. Phytother Res 2014;28:1581–1588 [DOI] [PubMed] [Google Scholar]
- 12.Seyed MA, Jantan I, Bukhari SN, Vijayaraghavan K: A comprehensive review on the chemotherapeutic potential of piceatannol for cancer treatment, with mechanistic insights. J Agric Food Chem 2016;64:725–737 [DOI] [PubMed] [Google Scholar]
- 13.Surh YJ, Na HK: Therapeutic potential and molecular targets of piceatannol in chronic diseases. Adv Exp Med Biol 2016;928:185–211 [DOI] [PubMed] [Google Scholar]
- 14.Vinas P, Martinez-Castillo N, Campillo N, Hernandez-Cordoba M: Directly suspended droplet microextraction with in injection-port derivatization coupled to gas chromatography-mass spectrometry for the analysis of polyphenols in herbal infusions, fruits and functional foods. J Chromatogr A 2011;1218:639–646 [DOI] [PubMed] [Google Scholar]
- 15.Matsui Y, Sugiyama K, Kamei M, et al. : Extract of passion fruit (Passiflora edulis) seed containing high amounts of piceatannol inhibits melanogenesis and promotes collagen synthesis. J Agric Food Chem 2010;58:11112–11118 [DOI] [PubMed] [Google Scholar]
- 16.Rimando AM, Kalt W, Magee JB, Dewey J, Ballington JR: Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J Agric Food Chem 2004;52:4713–4719 [DOI] [PubMed] [Google Scholar]
- 17.Ku KL, Chang PS, Cheng YC, Lien CY: Production of stilbenoids from the callus of Arachis hypogaea: A novel source of the anticancer compound piceatannol. J Agric Food Chem 2005;53:3877–3881 [DOI] [PubMed] [Google Scholar]
- 18.Cantos E, Espin JC, Fernandez MJ, Oliva J, Tomas-Barberan FA: Postharvest UV-C-irradiated grapes as a potential source for producing stilbene-enriched red wines. J Agric Food Chem 2003;51:1208–1214 [DOI] [PubMed] [Google Scholar]
- 19.Yang MH, Lin YJ, Kuo CH, Ku KL: Medicinal mushroom Ganoderma lucidum as a potent elicitor in production of t-resveratrol and t-piceatannol in peanut calluses. J Agric Food Chem 2010;58:9518–9522 [DOI] [PubMed] [Google Scholar]
- 20.Potter GA, Patterson LH, Wanogho E, et al. : The cancer preventative agent resveratrol is converted to the anticancer agent piceatannol by the cytochrome P450 enzyme CYP1B1. Br J Cancer 2002;86:774–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piver B, Fer M, Vitrac X, et al. : Involvement of cytochrome P450 1A2 in the biotransformation of trans-resveratrol in human liver microsomes. Biochem Pharmacol 2004;68:773–782 [DOI] [PubMed] [Google Scholar]
- 22.Niles RM, Cook CP, Meadows GG, Fu YM, McLaughlin JL, Rankin GO: Resveratrol is rapidly metabolized in athymic (Nu/Nu) mice and does not inhibit human melanoma xenograft tumor growth. J Nutr 2006;136:2542–2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li F, Sun Y, Song M, Wu X, Xiao H: Gastrointestinal biotransformation of resveratrol in mice. FASEB J 2016;30:145.7 [Google Scholar]
- 24.Roupe KA, Remsberg CM, Yanez JA, Davies NM: Pharmacometrics of stilbenes: Seguing towards the clinic. Curr Clin Pharmacol 2006;1:81–101 [DOI] [PubMed] [Google Scholar]
- 25.Roupe K, Teng XW, Fu X, Meadows GG, Davies NM: Determination of piceatannol in rat serum and liver microsomes: Pharmacokinetics and phase I and II biotransformation. Biomed Chromatogr 2004;18:486–491 [DOI] [PubMed] [Google Scholar]
- 26.Billack B, Radkar V, Adiabouah C: In vitro evaluation of the cytotoxic and anti-proliferative properties of resveratrol and several of its analogs. Cell Mol Biol Lett 2008;13:553–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murias M, Jager W, Handler N, et al. : Antioxidant, prooxidant and cytotoxic activity of hydroxylated resveratrol analogues: Structure-activity relationship. Biochem Pharmacol 2005;69:903–912 [DOI] [PubMed] [Google Scholar]
- 28.Kita Y, Miura Y, Yagasaki K: Antiproliferative and anti-invasive effect of piceatannol, a polyphenol present in grapes and wine, against hepatoma AH109A cells. J Biomed Biotechnol 2012;2012:672416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almeida L, Vaz-da-Silva M, Falcao A, et al. : Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res 2009;53:S7–S15 [DOI] [PubMed] [Google Scholar]
- 30.Brents LK, Medina-Bolivar F, Seely KA, et al. : Natural prenylated resveratrol analogs arachidin-1 and -3 demonstrate improved glucuronidation profiles and have affinity for cannabinoid receptors. Xenobiotica 2012;42:139–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin HS, Tringali C, Spatafora C, Wu C, Ho PC: A simple and sensitive HPLC-UV method for the quantification of piceatannol analog trans-3,5,3′,4′-tetramethoxystilbene in rat plasma and its application for a pre-clinical pharmacokinetic study. J Pharm Biomed Anal 2010;51:679–684 [DOI] [PubMed] [Google Scholar]
- 32.Messiad H, Amira-Guebailia H, Houache O: Reversed phase high performance liquid chromatography used for the physicochemical and thermodynamic characterization of piceatannol/β-cyclodextrin complex. J Chromatogr B Analyt Technol Biomed Life Sci 2013;926:21–27 [DOI] [PubMed] [Google Scholar]
- 33.Inagaki H, Ito R, Setoguchi Y, Oritani Y, Ito T: Administration of piceatannol complexed with α-cyclodextrin improves its absorption in rats. J Agric Food Chem 2016;64:3557–3563 [DOI] [PubMed] [Google Scholar]
- 34.Van Gaal LF, Mertens IL, De Block CE: Mechanisms linking obesity with cardiovascular disease. Nature 2006;444:875–880 [DOI] [PubMed] [Google Scholar]
- 35.Spalding KL, Arner E, Westermark PO, et al. : Dynamics of fat cell turnover in humans. Nature 2008;453:783–787 [DOI] [PubMed] [Google Scholar]
- 36.Kwon JY, Seo SG, Heo YS, et al. : Piceatannol, natural polyphenolic stilbene, inhibits adipogenesis via modulation of mitotic clonal expansion and insulin receptor-dependent insulin signaling in early phase of differentiation. J Biol Chem 2012;287:11566–11578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang X, Ordemann J, Muller JM, Dubiel W: The COP9 signalosome, cullin 3 and Keap1 supercomplex regulates CHOP stability and adipogenesis. Biol Open 2012;1:705–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geahlen RL, McLaughlin JL: Piceatannol (3,4,3′,5′-tetrahydroxy-trans-stilbene) is a naturally occurring protein-tyrosine kinase inhibitor. Biochem Biophys Res Commun 1989;165:241–245 [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Malbon CC: Gsα repression of adipogenesis via Syk. J Biol Chem 1999;274:32159–32166 [DOI] [PubMed] [Google Scholar]
- 40.Arakaki N, Kita T, Shibata H, Higuti T: Cell-surface H+-ATP synthase as a potential molecular target for anti-obesity drugs. FEBS Lett 2007;581:3405–3409 [DOI] [PubMed] [Google Scholar]
- 41.Kita T, Arakaki N: Contribution of extracellular ATP on the cell-surface F1F0-ATP synthase-mediated intracellular triacylglycerol accumulation. Biomed Res 2015;36:115–120 [DOI] [PubMed] [Google Scholar]
- 42.Arakaki N, Nagao T, Niki R, et al. : Possible role of cell surface H+-ATP synthase in the extracellular ATP synthesis and proliferation of human umbilical vein endothelial cells. Mol Cancer Res 2003;1:931–939 [PubMed] [Google Scholar]
- 43.Les Parellada F, Deleruyelle S, Cassagnes LE, et al. : Piceatannol and resveratrol share inhibitory effects on hydrogen peroxide release, monoamine oxidase and lipogenic activities in adipose tissue, but differ in their antilipolytic properties. Chem Biol Interact 2016;258:115–125 [DOI] [PubMed] [Google Scholar]
- 44.Hijona E, Aguirre L, Perez-Matute P, et al. : Limited beneficial effects of piceatannol supplementation on obesity complications in the obese Zucker rat: Gut microbiota, metabolic, endocrine, and cardiac aspects. J Physiol Biochem 2016;72:567–582 [DOI] [PubMed] [Google Scholar]
- 45.Tung YC, Lin YH, Chen HJ, et al. : Piceatannol exerts anti-obesity effects in C57BL/6 mice through modulating adipogenic proteins and gut microbiota. Molecules 2016;21:1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Minakawa M, Miura Y, Yagasaki K: Piceatannol, a resveratrol derivative, promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in L6 myocytes and suppresses blood glucose levels in type 2 diabetic model db/db mice. Biochem Biophys Res Commun 2012;422:469–475 [DOI] [PubMed] [Google Scholar]
- 47.Uchida-Maruki H, Inagaki H, Ito R, Kurita I, Sai M, Ito T: Piceatannol lowers the blood glucose level in diabetic mice. Biol Pharm Bull 2015;38:629–633 [DOI] [PubMed] [Google Scholar]
- 48.Mutabaruka MS, Aoulad Aissa M, Delalandre A, Lavigne M, Lajeunesse D: Local leptin production in osteoarthritis subchondral osteoblasts may be responsible for their abnormal phenotypic expression. Arthritis Res Ther 2010;12:R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oritani Y, Okitsu T, Nishimura E, Sai M, Ito T, Takeuchi S: Enhanced glucose tolerance by intravascularly administered piceatannol in freely moving healthy rats. Biochem Biophys Res Commun 2016;470:753–758 [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi K, Ishihara T, Khono E, Miyase T, Yoshizaki F: Constituents of stem bark of Callistemon rigidus showing inhibitory effects on mouse α-amylase activity. Biol Pharm Bull 2006;29:1275–1277 [DOI] [PubMed] [Google Scholar]
- 51.Towler MC, Hardie DG: AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res 2007;100:328–341 [DOI] [PubMed] [Google Scholar]
- 52.Saltiel AR, Kahn CR: Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001;414:799–806 [DOI] [PubMed] [Google Scholar]
- 53.Jeong SO, Son Y, Lee JH, et al. : Resveratrol analog piceatannol restores the palmitic acid-induced impairment of insulin signaling and production of endothelial nitric oxide via activation of anti-inflammatory and antioxidative heme oxygenase-1 in human endothelial cells. Mol Med Rep 2015;12:937–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boden G: Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes 2011;18:139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meigs JB, Larson MG, Fox CS, Keaney JF, Jr., Vasan RS, Benjamin EJ: Association of oxidative stress, insulin resistance, and diabetes risk phenotypes: The Framingham Offspring Study. Diabetes Care 2007;30:2529–2535 [DOI] [PubMed] [Google Scholar]
- 56.Samuel VT, Shulman GI: Mechanisms for insulin resistance: Common threads and missing links. Cell 2012;148:852–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Llarena M, Andrade F, Hasnaoui M, et al. : Potential renoprotective effects of piceatannol in ameliorating the early-stage nephropathy associated with obesity in obese Zucker rats. J Physiol Biochem 2015;72:555–566 [DOI] [PubMed] [Google Scholar]
- 58.Son Y, Chung HT, Pae HO: Differential effects of resveratrol and its natural analogs, piceatannol and 3,5,4′-trans-trimethoxystilbene, on anti-inflammatory heme oxigenase-1 expression in RAW264.7 macrophages. Biofactors 2014;40:138–145 [DOI] [PubMed] [Google Scholar]
- 59.Ke K, Sul OJ, Rajasekaran M, Choi HS: MicroRNA-183 increases osteoclastogenesis by repressing heme oxygenase-1. Bone 2015;81:237–246 [DOI] [PubMed] [Google Scholar]
- 60.Wung BS, Hsu MC, Wu CC, Hsieh CW: Piceatannol upregulates endothelial heme oxygenase-1 expression via novel protein kinase C and tyrosine kinase pathways. Pharmacol Res 2006;53:113–122 [DOI] [PubMed] [Google Scholar]
- 61.Lee HH, Park SA, Almazari I, Kim EH, Na HK, Surh YJ: Piceatannol induces heme oxygenase-1 expression in human mammary epithelial cells through activation of ARE-driven Nrf2 signaling. Arch Biochem Biophys 2010;501:142–150 [DOI] [PubMed] [Google Scholar]
- 62.Liu FC, Hwang TL, Lau YT, Yu HP: Mechanism of salutary effects of astringinin on rodent hepatic injury following trauma-hemorrhage: Akt-dependent hemeoxygenase-1 signaling pathways. PLoS One 2011;6:e25907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Son Y, Byun SJ, Pae HO: Involvement of heme oxygenase-1 expression in neuroprotection by piceatannol, a natural analog and a metabolite of resveratrol, against glutamate-mediated oxidative injury in HT22 neuronal cells. Amino Acids 2013;45:393–401 [DOI] [PubMed] [Google Scholar]
- 64.Jais A, Einwallner E, Sharif O, et al. : Heme oxygenase-1 drives metaflammation and insulin resistance in mouse and man. Cell 2014;158:25–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cho HJ, Shim JH, So HS, Park JHY: Mechanism underlying the anti-inflammatory action of piceatannol induced by lipopolysaccharide. J Korean Soc Food Sci Nutr 2012;41:1226–1234 [Google Scholar]
- 66.Kwon GT, Jung JI, Song HR, et al. : Piceatannol inhibits migration and invasion of prostate cancer cells: Possible mediation by decreased interleukin-6 signaling. J Nutr Biochem 2012;23:228–238 [DOI] [PubMed] [Google Scholar]
- 67.Su L, David M: Distinct mechanisms of STAT phosphorylation via the interferon-α/β receptor. Selective inhibition of STAT3 and STAT5 by piceatannol. J Biol Chem 2000;275:12661–12666 [DOI] [PubMed] [Google Scholar]
- 68.Schou M, Djurup R, Norris K, Flodgaard H: Identifying the functional part of heparin-binding protein (HBP) as a monocyte stimulator and the novel role of monocytes as HBP producers. Innate Immun 2011;17:60–69 [DOI] [PubMed] [Google Scholar]
- 69.Olsnes C, Stavang H, Olofsson J, Aarstad HJ: TNF-α is secreted by monocytes in transit to become macrophages, but not by peripheral blood monocytes, following OK-432 (lyophilized S. pyogenes) stimulation. Scand J Immunol 2007;66:684–693 [DOI] [PubMed] [Google Scholar]
- 70.Fritzenwanger M, Meusel K, Foerster M, Kuethe F, Krack A, Figulla HR: Cardiotrophin-1 induces interleukin-6 synthesis in human umbilical vein endothelial cells. Cytokine 2006;36:101–106 [DOI] [PubMed] [Google Scholar]
- 71.Jin CY, Moon DO, Lee KJ, et al. : Piceatannol attenuates lipopolysaccharide-induced NF-κB activation and NF-κB-related proinflammatory mediators in BV2 microglia. Pharmacol Res 2006;54:461–467 [DOI] [PubMed] [Google Scholar]
- 72.Richard N, Porath D, Radspieler A, Schwager J: Effects of resveratrol, piceatannol, tri-acetoxystilbene, and genistein on the inflammatory response of human peripheral blood leukocytes. Mol Nutr Food Res 2005;49:431–442 [DOI] [PubMed] [Google Scholar]
- 73.Ulanova M, Puttagunta L, Marcet-Palacios M, et al. : Syk tyrosine kinase participates in β1-integrin signaling and inflammatory responses in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2005;288:L497–L507 [DOI] [PubMed] [Google Scholar]
- 74.Dang O, Navarro L, David M: Inhibition of lipopolysaccharide-induced interferon regulatory factor 3 activation and protection from septic shock by hydroxystilbenes. Shock 2004;21:470–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stefan N, Häring HU, Hu FB, Schulze MB: Metabolically healthy obesity: Epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol 2013;1:152–162 [DOI] [PubMed] [Google Scholar]
- 76.Neilson AP, Ferruzzi MG: Bioavailability and metabolism of bioactive compounds from foods. In: Nutrition in the Prevention and Treatment of Disease (Coulston AM, Boushey CJ, Ferruzzi MG, eds.) Elsevier, Atlanta, GA, USA, 2013, pp. 407–420 [Google Scholar]
- 77.Miksits M, Sulyok M, Schuhmacher R, Szekeres T, Jager W: In-vitro sulfation of piceatannol by human liver cytosol and recombinant sulfotransferases. J Pharm Pharmacol 2009;61:185–191 [DOI] [PubMed] [Google Scholar]
- 78.Miksits M, Maier-Salamon A, Vo TP, et al. : Glucuronidation of piceatannol by human liver microsomes: Major role of UGT1A1, UGT1A8 and UGT1A10. J Pharm Pharmacol 2010;62:47–54 [DOI] [PubMed] [Google Scholar]
- 79.Zheng J, Ramirez VD: Piceatannol, a stilbene phytochemical, inhibits mitochondrial F0F1-ATPase activity by targeting the F1 complex. Biochem Biophys Res Commun 1999;261:499–503 [DOI] [PubMed] [Google Scholar]
- 80.Cordonier EL, Adjam R, Teixeira DC, et al. : Resveratrol compounds inhibit human holocarboxylase synthetase and cause a lean phenotype in Drosophila melanogaster. J Nutr Biochem 2015;26:1379–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wan X, Wang XB, Yang MH, Wang JS, Kong LY: Dimerization of piceatannol by Momordica charantia peroxidase and α-glucosidase inhibitory activity of the biotransformation products. Bioorg Med Chem 2011;19:5085–5092 [DOI] [PubMed] [Google Scholar]
- 82.Ashikawa K, Majumdar S, Banerjee S, Bharti AC, Shishodia S, Aggarwal BB: Piceatannol inhibits TNF-induced NF-κB activation and NF-κB-mediated gene expression through suppression of IκBα kinase and p65 phosphorylation. J Immunol 2002;169:6490–6497 [DOI] [PubMed] [Google Scholar]
- 83.Kawakami S, Kinoshita Y, Maruki-Uchida H, Yanae K, Sai M, Ito T: Piceatannol and its metabolite, isorhapontigenin, induce SIRT1 expression in THP-1 human monocytic cell line. Nutrients 2014;6:4794–4804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Howitz KT, Bitterman KJ, Cohen HY, et al. : Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003;425:191–196 [DOI] [PubMed] [Google Scholar]
- 85.Hong KS, Park JI, Kim MJ, et al. : Involvement of SIRT1 in hypoxic down-regulation of c-Myc and β-catenin and hypoxic preconditioning effect of polyphenols. Toxicol Appl Pharmacol 2012;259:210–218 [DOI] [PubMed] [Google Scholar]