Abstract
Background/Objectives: To investigate in adolescents the relationships between retinal vessel diameter, physical fitness, insulin sensitivity, and systemic inflammation.
Methods: We evaluated 157 adolescents, 112 with excessive weight and 45 lean, all without type 2 diabetes mellitus. All received detailed evaluations, including measurements of retinal vessel diameter, insulin sensitivity, levels of inflammation, and physical fitness.
Results: Overweight/obese adolescents had significantly narrower retinal arteriolar and wider venular diameters, significantly lower insulin sensitivity, and physical fitness. They also had decreased levels of anti-inflammatory and increased levels of proinflammatory markers as well as an overall higher inflammation balance score. Fitness was associated with larger retinal arteriolar and narrower venular diameters and these relationships were mediated by insulin sensitivity. We demonstrate that inflammation also mediates the relationship between fitness and retinal venular, but not arterial diameter; insulin sensitivity and inflammation balance score jointly mediate this relationship with little overlap in their effects.
Conclusions: Increasing fitness and insulin sensitivity and reducing inflammation among adolescents carrying excess weight may improve microvascular integrity. Interventions to improve physical fitness and insulin function and reduce inflammation in adolescents, a group likely to benefit from such interventions, may reduce not only cardiovascular disease in middle age, but also improve cerebrovascular function later in life.
Keywords: : cerebral microvasculature, fitness, inflammation, insulin sensitivity, obesity, retinal vessel health
Background
The prevalence of childhood obesity has increased significantly in the past 30 years with many adolescents at risk of becoming obese adults and developing metabolic syndrome (MetS) and type 2 diabetes mellitus (T2DM). We have also reported similar brain abnormalities in adolescents with obesity and MetS.(e.g.,1) Therefore, early intervention targeting microvascular health may help safeguard against future cardiovascular and neurodegenerative diseases.
Vascular manifestations of metabolic disease are useful early markers of cardiometabolic risk.2 Exercise is known to improve arteriolar dilation,2 increase insulin sensitivity,3 and reduce the systemic low-grade inflammation that accompanies cardiovascular disease.2,3 Hypertension, T2DM, and cardiovascular disease in older adults are associated with retinal arteriolar narrowing.4 Retinal vessel diameter, which can be measured noninvasively and quite economically, can thus be used as a possible proxy for cerebral microvascular integrity.5 Retinal microvasculature can be affected early in life; adolescents with low birth weights, a group at increased risk of T2DM in adulthood, were found to have narrower retinal arterioles.6 We have previously described associations between retinal arteriolar narrowing and preclinical white matter abnormalities among adolescents with MetS.1
Given that microvascular dysfunction is an early marker of cardiovascular disease7 and that endothelial function is highly dependent on level of fitness,8 physical fitness is important for retinal vessel health, and by extension cerebral microvascular health, in both adults2 and children.9 Abnormalities in microcirculation may be indicative of endothelial dysfunction, which has been linked to insulin resistance (IR).10 The link between IR and cardiovascular disease has been well established and physical fitness is believed to protect against premature cardiovascular disease mortality.11 Reducing circulating fasting insulin levels and weight has been found to improve arterial stiffness in adults12 and may have a similar effect in adolescents.13 We have previously shown that reductions in both fitness (maximal oxygen consumption-VO2 max-) and insulin sensitivity are associated with reductions in brain integrity and that the effect of fitness is mediated by IR. Thus, understanding the mechanisms behind retinal microcirculatory changes will be useful to design better strategies to prevent cardio- and cerebrovascular diseases.
Low cardiorespiratory fitness is a risk factor for obesity and has been linked to increased inflammatory cytokines and acute-phase reactants, even after controlling for body mass index (BMI), however, the exact mechanisms are unknown.14 Although obesity is associated with chronic low-grade inflammation in adults, and inflammation may be a contributor to the relationship between obesity and T2DM,4 findings on obesity-mediated inflammation in adolescents have been mixed.15 The anti-inflammatory effect of physical fitness is believed to improve endothelial function16; in obese adults regular exercise seems to be protective against early subclinical changes in retinal microvasculature.2 Although comprehensive data on retinal vessel health and inflammation in adolescents are lacking, IR has been suggested as a factor affecting retinal microcirculation,13 and specifically retinal vessel health has been shown to be more sensitive to the adverse effects of obesity and inflammation in children.17
While retinal vessel health has been well characterized in adolescents, few studies have examined, in the same set of individuals, the relationships between retinal vasculature (an excellent proxy of cerebral microvascular health), physical fitness, insulin sensitivity, and low-grade inflammation. In the present study, we sought to ascertain, for the first time, the relationships between obesity, cardiorespiratory fitness, and retinal vessel health and to determine if insulin sensitivity and/or low-grade inflammation mediate the known relationships between fitness and retinal vessel diameters.
Methods
Study Participants
The study was conducted at the Brain, Obesity, and Diabetes Laboratory of NYU School of Medicine and approved by the local Institutional Review Board. Of 165 nondiabetic adolescents evaluated (16–21 years of age), we included in the present analysis the 157 with physical fitness data, with 112 adolescents being overweight/obese (9 overweight and 103 obese) and 45 being lean. For participants <18 years of age, overweight/obesity was defined by standard BMI percentile scores. For those ≥18 years of age, adult BMI cutoffs were used. All participants had fasting blood data and C-reactive protein and fibrinogen determinations by our clinical laboratory, 80 of whom had retinal vessel evaluation and 102 had inflammatory cytokine data (see participant cohort diagram in Fig. 1).
Figure 1.
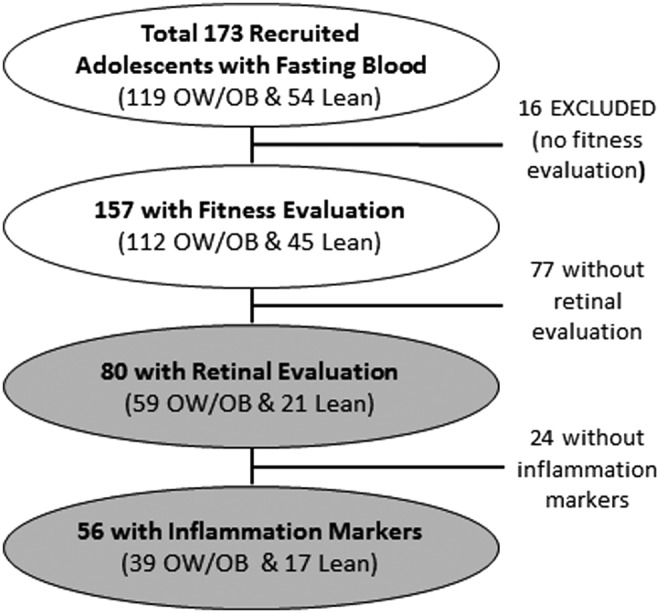
Diagram depicting participant recruitment flow/dropout.
We excluded from participation individuals with a diagnosis of T2DM or other significant medical (other than IR, polycystic ovary disease, dyslipidemia, and/or hypertension), psychiatric, or neurologic conditions.
Insulin sensitivity and cytokines
After a 10–12-hour overnight fast, blood samples were obtained from all study participants for the assessment of fasting glucose and insulin levels, liver enzymes, blood count, and levels of inflammatory markers. These fasting glucose and insulin blood levels for each participant were utilized to compute the quantitative insulin sensitivity check index (QUICKI).18 Inflammatory cytokines were measured from plasma by the Immune Monitoring Core of the NYU Langone Medical Center by Luminex assays using Multiplex plates (Millirose, Billerica, MA) and standard curves.
BMI and Anthropometric Measurements
Height and weight were measured using a Seca 700 Beam Scale of 500 lbs capacity, calibrated before each measurement, and used to calculate BMI (kg/m2) or BMI percentiles. Sitting blood pressure (BP) was measured on two separate occasions during one of the visits to the laboratory. These two readings were then averaged and the mean arterial blood pressure (MABP) was defined as DBP + (SBP–DBP)/3. Hypertension is defined as having BP ≥90th percentile for age, sex, and height for those <18 years of age19; for those of ≥18 years of age, we used adult criteria, BP ≥130 mm Hg, diastolic BP ≥85 mm Hg; or use of antihypertensive medication. Self-rating of sleep apnea was estimated from a sleep-related behavior questionnaire.20
Six-Minute Walk Test
Participants completed the 6-minute walk test (6MWT). The total distance covered during those 6 minutes was measured in meters and was recorded as the 6MWT score. Heart rate, BP, and blood oxygen saturation (using a finger pulse oximeter) were assessed at baseline, immediately following the test and at 1, 3, and 5 minutes after that.
Fitness or VO2 Max Estimation
VO2 max was estimated using the 6MWT algorithm, as described previously,21 which uses the total distance walked during the 6MWT, the participant's age, sex, weight in kg, and baseline heart rate in the computation. This VO2 max estimate has excellent correspondence with that obtained with direct measures of O2 consumption and CO2 production during an exercise paradigm.22
Retinal Vessel Caliber Measurements
Digital retinal photography was performed using a nonmydriatic 45 fundus camera (Canon CR4-45NM, Canon EOS Rebel 6.1MPix). Participants were seated in a dimly lit room for 5–10 minutes to allow pupillary dilation of 4 mm or greater to obtain an optimal image. The digital photographs were centered on the optic disc for both eyes using standardized settings and were processed as described previously.23 Briefly, we measured each of the six largest arterioles and venules for each eye.
The individual measurements of arterioles and venules were then combined into summary indices, based on the Revised Parr-Hubbard formula for summarizing retinal vessel diameters.24 We then derived a single number called a central retinal vessel equivalent for arterioles and venules (central retinal arteriolar equivalent [CRAE] and CRVE, respectively) that reflects the average arteriolar and venular diameters of that eye.24,25
Retinal vessel measurements were performed on both eyes of all participants when possible and then averaged. Mean CRAE and CRVE for both eyes were then used in our analyses. Given the general absence of cataracts in this age group, all retinal photographs were gradable. Our method has high inter-rater reliability with intraclass correlation coefficients of 0.83 for CRAE and 0.88 for CRVE.26 The average CRAE and CRVE, after adjusting for overall vessel size (by residualizing the CRAE to the CRVE and vice versa, and thus adjust for individual variability in vessel size and because we did not refract each individual's eyes), were used for hypothesis testing.
Estimation of an Inflammation Balance Scores
The inflammatory process is putatively the result of the balance between pro- and anti-inflammatory influences. To construct an inflammation balance score that takes into account the relative contributions of individual markers (Table 1 for the list of pro- and anti-inflammatory markers), we derived the beta weights using a logistic regression for the same markers studied here from a larger reference set of 309 adolescents (89 from the current study and 220 from another study,14 of predominantly Hispanic and African American origin). To compute the balance score for this study, we removed outliers within groups, computed z-scores utilizing the lean group as reference, and finally applied the beta weights derived from the larger set (see Table 1 in Adabimohazab et al. [2016])14 and created the inflammation balance score as the algebraic mean of the weighted z-scores of the eight markers.
Table 1.
Demographics, Endocrine Data, and Key Study Variables
| Variables | Overweight/obese, (n = 112), M ± SD | Lean, (n = 45), M ± SD | p | Effect size |
|---|---|---|---|---|
| Age (years)a | 19.67 ± 1.58 | 19.29 ± 1.61 | 0.12 | −0.12 |
| (16.04–22.05) | (16.70–21.96) | |||
| Gender | 68 F/44 M | 26 F/19 M | 0.74 | −0.06 |
| SESa | 3.15 ± 1.22 | 3.40 ± 1.09 | 0.30 | −0.08 |
| Ethnicity (Black/Hispanic/White/Asian & Others) | 38/47/17/10 | 15/15/5/10 | 0.21 | |
| Education (years completed)a | 12.88 ± 1.50 | 12.62 ± 1.68 | 0.27 | −0.09 |
| Body mass index (kg/m2) | 34.80 ± 4.91 | 21.28 ± 1.77 | <0.001 | 3.17 |
| QUICKI | 0.325 ± 0.024 | 0.371 ± 0.027 | <0.001 | −1.82 |
| Fasting glucose (mmol/L) | 4.79 ± 0.40 | 4.47 ± 0.30 | <0.001 | 0.87 |
| Fasting insulin (μIU/mL)a | 113.76 ± 67.02 | 48.48 ± 20.21 | <0.001 | −0.62 |
| MABP | 82.935 ± 7.576 | 75.585 ± 6.717 | <0.001 | 1.00 |
| Systolic BP (mm Hg) | 113.98 ± 9.97 | 103.84 ± 8.26 | <0.001 | 1.07 |
| Diastolic BP (mm Hg) | 67.41 ± 7.82 | 61.46 ± 7.23 | <0.001 | 0.78 |
| Self-reported sleep apnea scorea | 0.25 ± 0.15 | 0.16 ± 0.12 | 0.001 | −0.27 |
| VO2 maximum (mL/kg/min)a | 31.23 ± 6.22 | 45.32 ± 3.81 | <0.001 | −2.50 |
| 6MWT total distance walked (m) | 553.25 ± 56.86 | 626.54 ± 49.68 | <0.001 | −1.33 |
| 6MWT baseline heart rate (beats/min) | 83.96 ± 11.81 | 78.44 ± 11.99 | 0.01 | 0.47 |
| CRAE (μm)b | 181.31 ± 15.61 | 196.75 ± 21.83 | <0.001 | −1.68 |
| CRVE (μm)b | 290.29 ± 25.13 | 270.10 ± 27.88 | <0.001 | 1.60 |
| Fibrinogen (μmol/L) | 12.60 ± 2.79 | 9.75 ± 2.14 | <0.001 | 1.09 |
| Inflammation composite scorea | 0.652 ± 0.597 | 0.002 ± 0.135 | <0.001 | −0.66 |
| CRP (nmol/L)a | 51.16 ± 47.74 | 5.53 ± 8.93 | <0.001 | −0.63 |
| IFN-γ (pg/mL)a | 3.686 ± 4.718 | 5.188 ± 8.800 | 0.59 | −0.05 |
| IL-6 (pg/mL)a | 3.981 ± 3.715 | 1.654 ± 1.544 | <0.001 | −0.36 |
| Resistin (ng/mL)a | 64274.216 ± 25977.832 | 47924.900 ± 21101.325 | <0.01 | −0.29 |
| TNF-α (pg/mL) | 4.706 ± 1.787 | 3.777 ± 1.924 | 0.02 | 0.51 |
| Adiponectin (ng/mL)a | 22453571.875 ± 14134044.127 | 33529334.218 ± 18426311.510 | 0.001 | −0.33 |
| IL-4 (pg/mL)a | 5.615 ± 8.462 | 7.807 ± 12.396 | 0.20 | −0.13 |
| IL-10 (pg/mL)a | 12.778 ± 18.382 | 12.129 ± 11.580 | 0.86 | −0.02 |
t-tests were used for normally distributed variables (effect sizes were expressed as Cohen's d); otherwise.
The Mann–Whitney U test was used (effect sizes were expressed as r).
Raw vessel diameter values are presented. Group comparison was evaluated using the CRAE value residualized to CRVE when evaluating CRAE and vice versa.
6MWT, 6-minute walk test; BP, blood pressure; CRAE, central retinal arteriolar equivalent; CRVE, central retinal venular equivalent; IFN-γ, interferon gamma; IL-6, interleukin-6; TNF-α, tumor necrosis factor alpha; MABP, mean arterial blood pressure; SD, standard deviation.
Statistical Analyses
For all the important variables in our analyses, outliers with values ≥3 standard deviation from the mean of their group were removed variable wise. For descriptive purposes and to ensure group balance, we conducted two-tailed comparisons of the key variables between groups using independent sample t-tests (effect size expressed by Cohen d) for normally distributed continuous variables; otherwise, the Mann–Whitney U test (effect size expressed by r) was used (Table 1). The CRAE and CRVE values presented in Table 1 are the estimated absolute measurements; for the analyses, to adjust for overall vessel diameter, we residualized CRAE to CRVE, and vice versa, by means of linear regression analyses and utilized the unstandardized residuals.
For retinal vessel parameters and key explanatory variables that were non-normally distributed, natural log-transformed data were used in the correlation and mediation analyses. Spearman's rho (rs) partial correlational analyses (controlling for age, sex, and MABP) were used to establish associations of residualized retinal measures with VO2 max, QUICKI, and inflammation balance score. In addition to age, we controlled for sex in our analyses because males are known to have higher VO2 max values due to their lower body fat percentage27 and higher hemoglobin content,28 and also adjusted for MABP because prior reports have demonstrated relationships between MABP and retinal vessel diameter.29
Based on the existing literature, we predicted that lower VO2 max and QUICKI values and higher inflammation balance levels would be related to narrower retinal arteriolar and larger venular diameters. We tested whether QUICKI or inflammation balance score mediates the relationship between physical fitness and retinal health using the Sobel test30 and the bias corrected nonparametric bootstrapping procedure with 5000 bootstrapped samples, at 95% confidence interval.31 The indirect (mediation) effect is significant if the confidence interval does not contain zero.
Results
Demographics and Key Study Variables
Table 1 presents demographics, endocrine data, and key variables for study participants separated by the BMI group (112 overweight/obese vs. 45 lean). Overweight/obese adolescents had significantly shorter distance walked in the 6MWT and higher baseline heart rate, resulting in lower estimated VO2 max. As expected, overweight/obese adolescents had significantly narrower retinal arteriolar and larger venular diameters, lower insulin sensitivity as estimated by lower values on the QUICKI, and higher MABP. Thirteen overweight/obese adolescents had a history of hypertension, but only five reached threshold for hypertension when evaluated at our laboratory. None of the adolescents was on hypertensive medication while being evaluated at our laboratory. There was one adolescent who had previously been on one, but it had been discontinued before study participation, and his BP was within the normal range when being evaluated at our laboratory. None of the lean adolescents was hypertensive or had a history of hypertension. Overweight/obese adolescents also had higher obstructive sleep apnea determined by a self-rated questionnaire.20
Inflammation Balance Score
As expected, overweight/obese adolescents had significantly elevated fibrinogen, C-reactive protein, interleukin-6, resistin, and tumor necrosis factor alpha (TNF-α), and had significantly lower levels of the anti-inflammatory marker adiponectin. The groups did not differ on interferon gamma, IL-10, or IL-4. Overweight/obese adolescents had evidence of a low-grade inflammatory state as indicated by an overall higher inflammatory balance score.14
Associations with Retinal Vessel Health
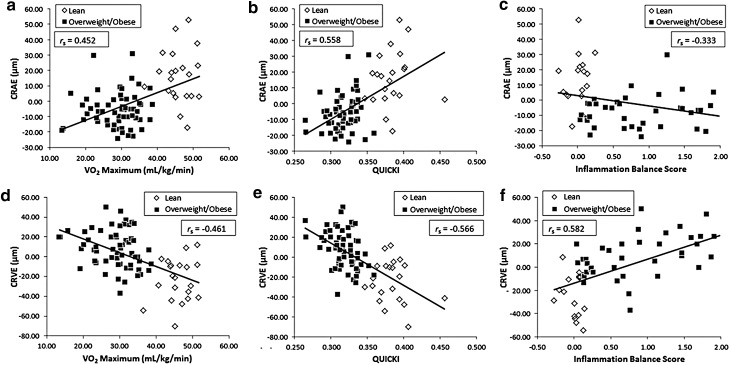
After accounting for age, sex, and MABP, retinal arteriolar diameters were positively associated with cardiovascular fitness (VO2 max, rs[75] = 0.452, p < 0.0001) and QUICKI (rs[75] = 0.558, p < 0.0001) and negatively associated with inflammation balance score (rs[51] = −0.333, p = 0.02) (Fig. 2a–c). In contrast, retinal venular diameters were inversely associated with VO2 max (rs[75] = −0.461, p < 0.0001) and QUICKI (rs[75] = −0.566, p < 0.0001), and as expected, correlated strongly and positively with inflammation balance scores (rs[51] = 0.582, p < 0.001) (Fig. 2d–f).
Figure 2.
VO2 max, QUICKI, and inflammation balance score are strongly associated with residualized retinal arterial (a–c) and venular (d–f) diameters. Spearman's rho coefficients presented in the plots are after adjusting for age, sex, and MAP.
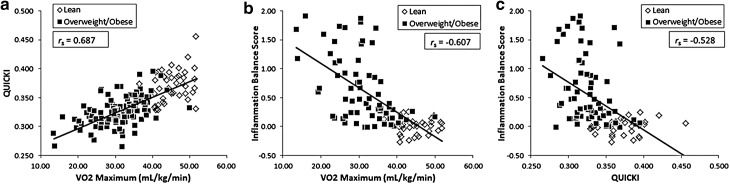
Consistent with our previous report in adolescents, VO2 max correlated positively with QUICKI (rs[152] = 0.687, p < 0.001; see Fig. 3) and as predicted, correlated negatively with inflammation balance score (rs[97] = −0.607, p < 0.001). Higher inflammation balance score was related to lower insulin sensitivity (QUICKI, rs[97] = −0.528, p < 0.001). We also explored whether there was an association between BMI and inflammation balance score, and after accounting for age and sex, found a strong positive association (rs[98] = 0.699, p < 0.0001). This relationship remained significant when only including overweight/obese adolescents (rs[65] = 0.453, p < 0.0001).
Figure 3.
Associations between VO2 max, QUICKI, and inflammation balance score independent of age, sex, and MAP.
Do Insulin Sensitivity and/or Inflammation Balance Score Mediate the Relationship between VO2 max and Retinal Vessel Diameter?
QUICKI as the mediator
Central retinal arteriolar equivalent
When using QUICKI as the mediator, the results showed that in the subset of 80 adolescents, the total effect of VO2 max on CRAE was significant independent of age, sex, and MABP (effect = 0.98, p < 0.001, 95% CI[0.59–1.37]). Whereas the direct effect of VO2 max was nonsignificant (effect = 0.21, p = 0.48, 95% CI[−0.38 to 0.80]), the indirect effect was significant, indicating that QUICKI mediated the effect of VO2 max on CRAE (effect = 0.77, p = 0.002, 95% CI[0.34–1.33]).
Central retinal venular equivalent
Similarly, we found that in the same 80 adolescents, the total effect of VO2 max on CRVE was significant and independent of age, sex, and MABP (effect = −1.34, p < 0.001, 95% CI[−1.92 to −0.76]). Whereas the direct effect of VO2 max was nonsignificant (effect = −0.16, p = 0.70, 95% CI[−1.02 to 0.69]), the indirect effect was significant with QUICKI also mediating the relationship between VO2 max and CRVE (effect = −1.18, p < 0.001, 95% CI[−1.93 to −0.58]).
Inflammation as the mediator
Because a smaller subset of 56 participants also had the inflammatory markers, we first confirmed that for this subset of study participants the partial correlations between the key variables remained largely unchanged, which they did.
Central retinal arteriolar equivalent
The mediation analysis showed an overall significant model (total effect = 0.86, p < 0.001, 95% CI[0.41–1.32]). The direct effect of VO2 max on CRAE was significant (effect = 0.89, p = 0.003, 95% CI[0.33–1.45]), but there was no mediation effect by the inflammation balance score (effect = −0.03, p = 0.88, 95% CI[−0.29 to 0.19]).
Central retinal venular equivalent
In contrast with CRAE and consistent with previous literature, inflammation balance score partially mediated the effect of VO2 max on CRVE in the same subset of 56 (total effect = −1.33, p = 0.0001, 95% CI[−1.95 to −0.70]; direct effect = −0.86, p = 0.02, 95% CI[−1.59 to −0.13]; indirect effect = −0.46, p = 0.05, 95%CI[−1.11 to −0.06]).
Both QUICKI and inflammation as mediators of retinal vein width (CRVE)
Since QUICKI and inflammation individually mediated the effect of VO2 max on CRVE and they only shared 25% of variance after accounting for age, sex, and MABP (rs[51] = −0.50, p < 0.001), we explored their joint effect on CRVE by carrying out a single mediation analysis. First, we confirmed that in this subset of 56 adolescents, QUICKI remained a significant mediator in the relationship between VO2 max and CRVE (direct effect = −0.41, p = 0.44; indirect effect = −0.91, p = 0.04, 95% CI[−1.87 to −0.14]) and that these results were also consistent with those for the larger set of 80.
We then repeated the analysis, this time with inflammation and QUICKI jointly as the mediators, and found that with the addition of QUICKI as a comediator, the direct effect of VO2 max on CRVE remained nonsignificant (effect = −0.05, p = 0.92, 95% CI[−1.14,1.04]), whereas the indirect effects were significant for both QUICKI (effect = −0.84, p = 0.05, 95% CI[−1.79 to −0.07]) and the inflammation balance score (effect = −0.43, p = 0.05, 95% CI[−1.06 to −0.05]), respectively. The mediation results are summarized in Figure 4.
Figure 4.
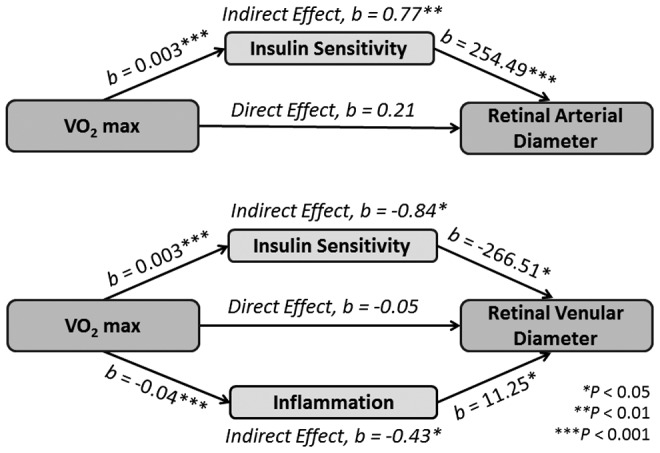
Summary of mediation results.
Conclusions
As hypothesized, we observed among overweight/obese adolescents reduced retinal arteriolar and increased venular diameters, lower VO2 max, lower insulin sensitivity, as well as elevated proinflammatory and reduced anti-inflammatory markers. In line with previous reports showing that regular aerobic exercise improves retinal microcirculation,(e.g.,2) our data suggest that these relationships are mediated by insulin sensitivity for both retinal arteries and veins. We also demonstrate for the first time that in adolescents low-grade inflammation, as expressed by the weighted balance of pro- and anti-inflammatory markers toward a proinflammatory state, partially mediates the relationship between cardiovascular fitness and retinal venular, but not arteriolar, health. Importantly, these jointly mediated effect of insulin sensitivity and inflammation on the retinal vein size were not overlapping. These data emphasize the importance of physical fitness in reducing microvascular alterations, and thus lowering the risk of cardio- and cerebrovascular diseases. Previous work has suggested that both QUICKI and VO2 max serve as proxies for brain structural integrity.32 The current data extend this work by suggesting that chronic low-grade inflammation, in addition to insulin sensitivity, is also a potential marker of microvascular health in adolescents.
Smaller retinal arterial and larger venular diameters indicate greater risk of microvascular impairment.4 In this study, we present robust findings describing strong associations between poor physical fitness and reduced retinal arterial and increased venular diameters, which are consistent with prior reports suggesting that frequent physical activity reduces markers associated with cardiovascular disease.33 Consistent with our prior reports of associations among QUICKI score, VO2 max and retinal arterial health,16,34 the current data suggest that increasing physical activity, in addition to improving insulin sensitivity in adolescents, can protect against microvascular impairment and future cardiovascular disease and perhaps late-life neurodegeneration. Of note, our novel findings that low-grade systemic inflammation is strongly associated with poor physical fitness and that it is a partial mediator of the relationship between physical fitness and retinal venular health, are consistent with adult data showing that inflammation mediates cardiovascular disease.35 Importantly, by testing both insulin sensitivity and inflammation in one single mediation analysis, we demonstrate for the first time that both contribute independently to retinal venular health.
It is commonly believed that inflammation plays a vital role in the association between obesity and metabolic disease.36 Other studies have found that inflammatory processes alter metabolic pathways and increase the risk of diabetes37 and that retinal vessel caliber and diabetes are linked.38 Therefore, we selected, a priori, inflammatory markers that have been previously associated to retinal vessel caliber and physical fitness. As expected, our findings that inflammation correlated more strongly with retinal venular than arterial health are also consistent with the existing literature that retinal venular width is more sensitive to inflammation than arterial width.41 These results contrast with prior work on adolescents demonstrating no such associations, but in that work, physical fitness was estimated from measures of motor abilities.13 Furthermore, our current findings also differ from a prior report from our laboratory, where we failed to find an association between individual inflammatory markers (did not ascertain the inflammation balance) and IR in a different and smaller group of adolescents.15
One potential limitation of the current study is how representative our sample is relative to the general population. Although our sample was not derived from a clinical setting, but recruited directly from the community, our goal was to study obesity and the sample was enriched accordingly resulting in an imbalance in the number of overweight/obese participants relative to lean participants (112 vs. 45), which may limit the generalizability of our findings. Obstructive sleep apnea is commonly related with obesity and has been associated with retinal vessel problems in adults.40 However, adolescents are unlikely to have clinically significant obstructive sleep apnea, and as expected in this age group, self-reported sleep apnea did not correlate with any of the key study variables (data not shown). Therefore, we did not control for it in our mediation analyses. Future work should address possible confounding effects of subclinical obstructive sleep apnea assessed more objectively in the sleep laboratory.
The 6MWT provides an estimate of VO2 max when a direct measurement with a graded treadmill test measuring total oxygen consumption, the gold standard, is not feasible. The 6MWT was originally designed to evaluate functional capacity in patients with chronic respiratory disease and heart failure, but has since been used among healthy individuals.21 Although some studies have validated the 6MWT in children and adolescents against the VO2 max from the standard treadmill test,41 the validation has not been extensive. However, over 80% (128/157) of our adolescents were between 18 and 21, close to being adults, therefore, the lack of multiple replications of the validation in children for the 6MWT is not a significant concern.
Our method, deriving an overall balance of chronic low-grade inflammation from a sample of over 300 adolescents, is novel and robust,14 but will require validation. Some investigators consider leptin a proinflammatory cytokine, however, given that it is an adipokine and its role in inflammation remains controversial, we did not include it in our estimation of the inflammation balance. Prospective studies are necessary to monitor changes in inflammatory balance with age and increasing duration of obesity so as to better determine when inflammation may begin to affect cerebrovascular health.
Of interest, we report, for the first time, that in adolescents increased fitness is related to lower levels of inflammation and higher levels of insulin sensitivity, both of which are predictive of retinal vessel health. Impaired glucose control and low-grade inflammation are known to contribute to cerebrovascular abnormalities in T2DM, and these comorbidities provide possible mechanistic links between T2DM and dementia.
In sum, these data suggest that increasing physical fitness and insulin sensitivity and reducing inflammation among adolescents carrying excess weight may improve microvascular integrity and help safeguard future cerebrovascular function and potentially protect against neurodegenerative disease later in life.
Acknowledgments
This work was funded by the National Institutes of Health Grant No. DK 083537 and also funded in part by Grant No. 1UL1RR029893 from the National Center for Research Resources.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Yau PL, Kim M, Tirsi A, Convit A. Retinal vessel alterations and cerebral white matter microstructural damage in obese adolescents with metabolic syndrome. JAMA Pediatr 2014;168:e142815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanssen H, Nickel T, Drexel V, et al. . Exercise-induced alterations of retinal vessel diameters and cardiovascular risk reduction in obesity. Atherosclerosis 2011;216:433–439 [DOI] [PubMed] [Google Scholar]
- 3.Keshel TE, Coker RH. Exercise training and insulin resistance: A current review. J Obes Weight Loss Ther 2015;5:S5–003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong TY, Islam FM, Klein R, et al. . Retinal vascular caliber, cardiovascular risk factors, and inflammation: The multi-ethnic study of atherosclerosis (MESA). Invest Ophthalmol Vis Sci 2006;47:2341–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwa VI, van der Sande JJ, Stam J, et al. ; Amsterdam Vascular Medicine Group. Retinal arterial changes correlate with cerebral small-vessel disease. Neurology 2002;59:1536–1540 [DOI] [PubMed] [Google Scholar]
- 6.Gopinath B, Baur LA, Wang JJ, et al. . Smaller birth size is associated with narrower retinal arterioles in early adolescence. Microcirculation 2010;17:660–668 [DOI] [PubMed] [Google Scholar]
- 7.Hadi HA, Carr CS, Al Suwaidi J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag 2005;1:183–198 [PMC free article] [PubMed] [Google Scholar]
- 8.Bruyndonckx L, Hoymans V, De Guchtenaere A, et al. . Diet, exercise, and endothelial function in obese adolescents. Pediatrics 2015;135:9. [DOI] [PubMed] [Google Scholar]
- 9.Imhof K, Zahner L, Schmidt-Trucksass A, Faude O, Hanssen H. Influence of physical fitness and activity behavior on retinal vessel diameters in primary schoolchildren. Scand J Med Sci Sports 2016;26:731–738 [DOI] [PubMed] [Google Scholar]
- 10.Steinberg HO, Chaker H, Leaming R, et al. . Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest 1996;97:2601–2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiroma EJ, Lee IM. Physical activity and cardiovascular health: Lessons learned from epidemiological studies across age, gender, and race/ethnicity. Circulation 2010;122:743–752 [DOI] [PubMed] [Google Scholar]
- 12.Hughes TM, Althouse AD, Niemczyk NA, et al. . Effects of weight loss and insulin reduction on arterial stiffness in the SAVE trial. Cardiovasc Diabetol 2012;11:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegrist M, Hanssen H, Neidig M, et al. . Association of leptin and insulin with childhood obesity and retinal vessel diameters. Int J Obes (Lond) 2014;38:1241–1247 [DOI] [PubMed] [Google Scholar]
- 14.Adabimohazab R, Garfinkel A, Milam EC, et al. . Does inflammation mediate the association between obesity and insulin resistance? Inflammation 2016;39:994–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen JI, Maayan L, Convit A. Preliminary evidence for obesity-associated insulin resistance in adolescents without elevations of inflammatory cytokines. Diabetol Metab Syndr 2012;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross N, Yau PL, Convit A. Obesity, fitness, and brain integrity in adolescence. Appetite 2015;93:44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanssen H, Siegrist M, Neidig M, et al. . Retinal vessel diameter, obesity and metabolic risk factors in school children (JuvenTUM 3). Atherosclerosis 2012;221:242–248 [DOI] [PubMed] [Google Scholar]
- 18.Katz A, Nambi SS, Mather K, et al. . Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000;85:2402–2410 [DOI] [PubMed] [Google Scholar]
- 19.Adolescents NHBPEPWGoHBPiCa. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004;114:555–576 [PubMed] [Google Scholar]
- 20.Mindell JA, Owens JA. A clinical Guide to Pediatric Sleep: Diagnosis and Management of Sleep Problems. Philadelphia: Lippincott Williams & Wilkins, 2003 [Google Scholar]
- 21.Burr JF, Bredin SS, Faktor MD, Warburton DE. The 6-minute walk test as a predictor of objectively measured aerobic fitness in healthy working-aged adults. Phys Sportsmed 2011;39:133–139 [DOI] [PubMed] [Google Scholar]
- 22.Cataneo DC, Kobayasi S, Carvalho LR, et al. . Accuracy of six minute walk test, stair test and spirometry using maximal oxygen uptake as gold standard. Acta Cir Bras 2010;25:194–200 [DOI] [PubMed] [Google Scholar]
- 23.Tirsi A, Bruehl H, Tsui W, et al. . Retinal vessel abnormalities are associated with elevated fasting insulin levels and cerebral atrophy in non-diabetic individuals. Ophthalmology 2009;116:1175–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knudtson MD, Lee KE, Hubbard LD, et al. . Revised formulas for summarizing retinal vessel diameters. Curr Eye Res 2003;27:143–149 [DOI] [PubMed] [Google Scholar]
- 25.Wong TY, Knudtson MD, Klein R, et al. . Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: Methodology, correlation between eyes, and effect of refractive errors. Ophthalmology 2004;111:1183–1190 [DOI] [PubMed] [Google Scholar]
- 26.Tirsi A, Bruehl H, Sweat V, et al. . Retinal vessel abnormalities are associated with elevated fasting insulin levels and cerebral atrophy in nondiabetic individuals. Ophthalmology 2009;116:1175–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saygin O, Zorba E, Karacabey K, Mengutay S. Gender and maturation differences in health-related physical fitness and physical activity in Turkish children. Pak J Biol Sci 2007;10:1963–1969 [DOI] [PubMed] [Google Scholar]
- 28.Murphy W. The sex difference in haemoglobin levels in adults—mechanisms, causes, and consequences. Blood Rev 2014;28:7. [DOI] [PubMed] [Google Scholar]
- 29.Sharrett AR, Hubbard LD, Cooper LS, et al. . Retinal arteriolar diameters and elevated blood pressure: The Atherosclerosis Risk in Communities Study. Am J Epidemiol 1999;150:263–270 [DOI] [PubMed] [Google Scholar]
- 30.Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol Methodol 1982;13:290–312 [Google Scholar]
- 31.Hayes AF, Preacher KJ. Quantifying and testing indirect effects in simple mediation models when the constituent paths are nonlinear. Multivariate Behav Res 2010;45:627–660 [DOI] [PubMed] [Google Scholar]
- 32.Castro MG, Venutolo C, Yau PL, Convit A. Fitness, insulin sensitivity, and frontal lobe integrity in adults with overweight and obesity. Obesity (Silver Spring) 2016;24:1283–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silva LR, Cavaglieri C, Lopes WA, et al. . Endothelial wall thickness, cardiorespiratory fitness and inflammatory markers in obese and non-obese adolescents. Braz J Phys Ther 2014;18:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tirsi A, Duong M, Tsui W, et al. . Retinal vessel abnormalities as a possible biomarker of brain volume loss in obese adolescents. Obesity (Silver Spring) 2013;21:E577–E585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res 2005;96:939–949 [DOI] [PubMed] [Google Scholar]
- 36.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 2011;121:2111–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luft VC, Schmidt MI, Pankow JS, et al. . Chronic inflammation role in the obesity-diabetes association: A case-cohort study. Diabetol Metab Syndr 2013;5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen TT, Wang JJ, Sharrett AR, et al. . Relationship of retinal vascular caliber with diabetes and retinopathy: The Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2008;31:544–549 [DOI] [PubMed] [Google Scholar]
- 39.Ikram MK, De Jong FJ, Vingerling JR, et al. . Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci 2004;45:2129–2134 [DOI] [PubMed] [Google Scholar]
- 40.Shankar A, Peppard PE, Young T, et al. . Sleep-disordered breathing and retinal microvascular diameter. Atherosclerosis 2013;226:124–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li AM, Yin J, Yu CC, et al. . The six-minute walk test in healthy children: Reliability and validity. Eur Respir J 2005;25:1057–1060 [DOI] [PubMed] [Google Scholar]