Abstract
Background:
Hyaluronic acid gels are used to restore volume to the midface, but there are few data published on this use in Asian subjects.
Methods:
This study evaluated the safety and effectiveness in Chinese subjects of Juvéderm Voluma, a 20-mg/ml hyaluronic acid gel formulated for midface volumizing. This prospective, multicenter study randomized 119 subjects aged 18 years or older to a treatment group and 27 subjects to a no-treatment control group. The primary effectiveness endpoint was the objectively measured magnitude of change from baseline in volume of the midface area (right and left combined) calculated by digital analysis at month 6 using three-dimensional images for all subjects in both groups. Effectiveness was protocol-defined as a mean change for the treatment group that was significantly greater than that for the control group at month 6 using a one-side two-group t test performed at the 5 percent level.
Results:
With a median volume of 2 ml of Voluma injected, the primary effectiveness endpoint was met, with the mean change from baseline to 6 months in malar volume for the treatment group (1.83 ml) being significantly greater than that for the control group (0.11 ml; p < 0.001). The secondary effectiveness endpoints of responder rate (malar volumization rated improved or much improved) using the Global Aesthetic Improvement Scale as assessed at month 6 by the investigator and by the subject were 98.2 and 93.8 percent, respectively. The most common treatment-related adverse events were mild injection-site swelling and bruising.
Conclusion:
Juvéderm Voluma is effective and well tolerated for midface augmentation in Chinese subjects.
CLINICAL QUESTION/LEVEL OF EVIDENCE:
Therapeutic, II.

In Asia, facial contouring has become a popular dermatology procedure.1 The Asian face is typically more broad and flat compared with that of Caucasians, and Asians are increasingly seeking procedures to obtain more projected, three-dimensional facial features, including more prominent cheeks.2,3 Most Asian patients focus on cosmetic rejuvenation and reshaping to achieve specific facial characteristics within their own ethnic facial aesthetic spectrum, such as a slender, oval face and increased projection of the anterior aspects of the cheeks with adequate midface support and malar highlights.4
The cheeks and other aspects of the midface region undergo changes that reflect aging. Facial volume loss caused by decreases in and displacement of underlying fat is a key contributor to signs of aging in the face.5,6 With age, volume changes in fat pads may alter midface structural features. Loss of volume in deep cheek fat can contribute to hollowing of the cheeks and malar fat pad ptosis.7,8
Hyaluronic acid dermal fillers are established agents for restoring facial volume in the midface region.9 These agents, which reduce the appearance of sagging skin and skin folds by replacing hyaluronic acid, are used in facial rejuvenation and augmentation procedures.9 They have the capacity to fill and lift facial tissue and to provide a durable, natural-appearing contour to the face.10 There is a growing acceptance and use of such procedures by Asians, yet there is a lack of publications in the literature on nonsurgical rejuvenation of the midface in Asian subjects.11,12
The use of dermal fillers has become popular in Asia; according to a 2012 survey of members of the Korean Academy of Anti-Aging Dermatology, midface augmentation has quickly become a highly popular indication.12 However, there are few consensus guidelines on nonsurgical antiaging procedures in Asians overall.12,13 Because there are differences in facial anatomy based on ethnicity and race, and differences in aesthetic concepts and goals, data from Asian subjects are needed to help guide treatment.13,14
The hyaluronic acid dermal filler Juvéderm Voluma (Allergan plc, Dublin, Ireland) is a sterile, biodegradable, viscoelastic, homogenized dermal filler consisting of cross-linked hyaluronic acid in a 20-mg/ml concentration in physiologic buffer without lidocaine; it is produced by Streptococcus equi and is cross-linked using 1,4-butanediol diglycidyl ether. Midface volumizing with Voluma with and without 0.03% lidocaine has been studied extensively in Europe, the United States, Canada, and Australia.15–17 These studies showed high rates of treatment response and satisfaction based on investigator and subject assessment, with clinically meaningful volume correction noticeable up to 2 years after treatment.15–18
This study constitutes the first multicenter trial with a hyaluronic acid filler product focusing on midface augmentation conducted in China. The objective of this study was to evaluate the safety and effectiveness of Voluma injectable gel for use in malar augmentation in a Chinese population.
SUBJECTS AND METHODS
Study Design
This was a prospective, multicenter, randomized, no-treatment–controlled study. The study used a no-treatment control group because there were no hyaluronic acid fillers approved by the China Food and Drug Administration for malar augmentation in the People’s Republic of China at the time the study was conducted. The study was conducted in accordance with the ethics committee at each participating site, with approval from an ethics committee before initiation, and in compliance with the China Food and Drug Administration and the International Conference on Harmonisation principles of good clinical practice. Each subject provided written informed consent before enrollment; subject privacy-related documentation was obtained in accordance with People’s Republic of China and local privacy requirements where applicable.
Subjects
Study subjects had to be of Chinese ancestry and aged 18 years or older. By the investigators’ assessment, each subject had to require greater than or equal to 1 ml total volume of Voluma at initial treatment and less than or equal to 9 ml in total to achieve a meaningful improvement and/or change in their aesthetic appearance. Exclusion criteria included facial characteristics that would affect study measurements (i.e., moustache, beard, scarring), facial plastic surgery within the previous 12 months, previous tissue grafting/augmentation with permanent or semipermanent fillers in any part of the face or fat injections in the midface, various other facial aesthetic treatments and procedures during the previous 6 months, or plans to undergo any aesthetic facial procedure or dental operation during the study. Other exclusion criteria were a history of anaphylaxis; multiple severe allergies; allergy to hyaluronic acid products or streptococcal protein, or plans to undergo desensitization during the study; and any other condition or situation that in the investigators’ opinion could put the subject at risk, interfere with study participation, or confound study results.
Study Treatment
Three training physicians who had experience in midface injection with Voluma provided product-specific injection training and oversight of each investigator’s first one to two subjects, who were designated as “run-in” subjects. The remaining subjects were randomized in a 4:1 ratio to receive Voluma or be in the delayed-treatment control group (who could receive Voluma at month 6).
The injection location and intended injection volume were determined by the investigator. The study treatment, Voluma, was supplied in prefilled syringes containing 1.0 ml of product and fitted with 27-gauge, half-inch needles. Pretreatment anesthetic was applied if needed (i.e., ice, topical anesthetic, or local anesthetic). Voluma was injected deeply into the subcutaneous and/or supraperiosteal plane within the medial malar region (Fig. 1); areas adjacent to the target treatment area could also be injected except for the tear troughs, nasolabial folds, and upper lip. Injections were performed in multiple locations using a volume of less than or equal to 0.25 ml at each point for even distribution and to reduce risk of product pooling. The minimum total initial treatment volume to be injected into the two sides of the face was 1.0 ml. After injection, the injected areas were massaged/molded to optimize tissue integration of the product.
Fig. 1.
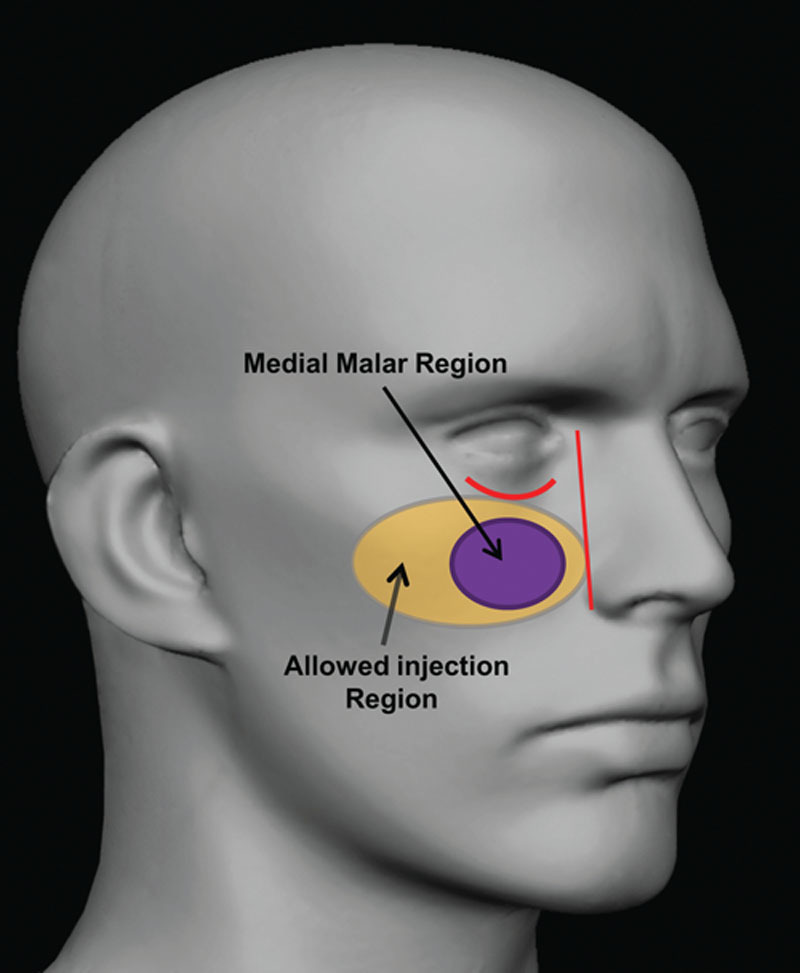
Treatment areas.
Touch-up treatment could be administered 30 days after the initial treatment according to the agreement of the subject and investigator to achieve optimal correction. The maximum total volume permissible (right and left malar regions and the initial and touch-up treatments combined) was 9.0 ml.
Effectiveness Endpoints
The primary effectiveness endpoint was the change from baseline in volume of the midface area (right and left combined) at month 6, calculated by digital analysis of each subject’s three-dimensional images at baseline and after treatment for subjects randomized to Voluma and control. The three-dimensional images were obtained using the Vectra M3 stereophotogrammetry system from Canfield Scientific, Inc. (Fairfield, N.J.) using a specific protocol. The three-dimensional images were collected at baseline (before injections) and at months 1, 3, and 6. The three-dimensional data were analyzed by blinded Canfield Scientific personnel. Briefly, multiple areas of interest on specific facial features were marked as landmarks on baseline three-dimensional images. A validated software algorithm was used to determine changes in volume by overlaying the baseline landmarks onto images from months 1, 3, and 6 to assess volume change in each area of interest.
Secondary endpoints included investigator and subject assessments of overall aesthetic improvement using the Global Aesthetic Improvement Scale (2 = much improved; 1 = improved; 0 = no change; −1 = worse; and −2 = much worse) by comparison of baseline photographs to subjects’ facial features at each posttreatment assessment. The study included several additional assessments. The mean change in volume from baseline in the medial malar area at months 1 and 3 for the treatment group was determined. A subject self-assessment of treatment was used in which subjects responded to five questions/statements pertaining to treatment outcomes. The first was “Are you satisfied with your treatment outcome?” Possible answers and corresponding scores were “very satisfied,” “satisfied,” “neutral,” “dissatisfied,” or “very dissatisfied.” The second question was “Would you recommend the treatment to a friend?” Possible answers were “yes” or “no.” The other three statements were as follows: “The treatment made my cheeks look fuller,” “The treatment improved my facial contouring,” and “The treatment made me feel more attractive.” The response scale for these three statements consisted of “strongly agree,” “agree,” “neither agree nor disagree,” “disagree,” and “strongly disagree.”
Safety Assessments
Adverse events observed by the investigator or reported by or solicited from the subject for the pretreatment and follow-up periods were categorized using the Medical Dictionary for Regulatory Activities Version 17. Typical or expected side effects (e.g., tenderness, swelling, firmness, lumps/bumps, bruising, redness, pain, discoloration, and/or itching) were reported but not recorded as adverse events unless they persisted to the next scheduled follow-up visit, interfered with the subject’s normal activities, or required medical or procedural treatment. Other safety assessments included device/needle problems/malfunction.
Statistical Analyses
All effectiveness analyses were performed for the modified intent-to-treat population of the treatment group, which included all subjects randomized to the treatment group who received one or more study treatments and all control subjects who completed at least one follow-up visit. The primary effectiveness endpoint was also analyzed for the treatment and control groups in the per-protocol population, which consisted of all subjects in the modified intent-to-treat population who had no significant protocol deviations affecting the primary endpoint. For the primary effectiveness endpoint, a one-sided two-group t test was performed at the 5 percent level using an analysis of variance model to test the hypothesis that the change in volume from baseline for the treatment group would be greater than that for the control group at month 6. Responder rates for improvement on the Global Aesthetic Improvement Scale (secondary endpoint) were defined as the proportion of subjects with ratings of improved or much improved. Summary statistics for categorical and continuous variables were used. For continuous data, a two-sample t test was used for between-group comparisons. The incidence of treatment-related adverse events was tabulated. All statistical analyses were performed using SAS version 9.2 or higher (SAS Institute, Inc., Cary, N.C.).
RESULTS
Demographics and Subject Disposition
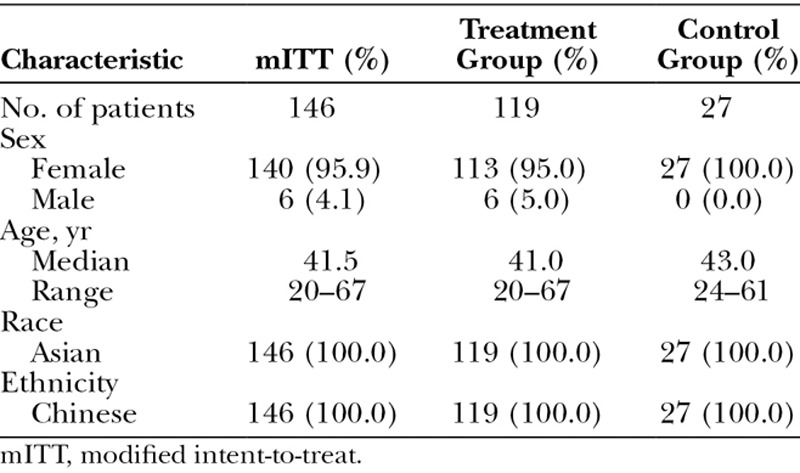
Nearly all subjects were female, and all were ethnic Chinese Asians. The median age was 41.5 years; approximately three-quarters of the subjects (76 percent) were aged between 30 and 50 years. Treatment and control groups were well matched in demographics (Table 1). A total of 190 subjects were enrolled; nine of these were screen failures, and 21 of the enrollees were treated as run-in subjects. A total of 160 subjects were randomized, with 11 in the treatment group discontinuing before treatment and three in the control group discontinuing before the month-1 visit, leaving 146 subjects in the modified intent-to-treat population (treatment, n = 119; control, n = 27). The safety population included 119 subjects in the treatment group and 22 in the control group who received treatment at the end of the control period. A total of 113 subjects in the treatment group (95.0 percent) completed the final month-12 visit, and 21 subjects in the control group (95.5 percent) completed the final month-6 visit after receiving treatment.
Table 1.
Demographics of the Modified Intent-to-Treat Population
Treatment Characteristics
Treatment characteristics (safety population) are summarized in Table 2. Forty-one subjects (34.5 percent) in the treatment group received touch-up treatment. Anesthetic was administered to 81.6 percent of subjects for the initial treatment and 60.4 percent for the touch-up treatment. Ice was the most frequently used type of anesthetic, followed by topical anesthetic. Ice was administered for a median duration of 20.0 minutes for the initial treatment and 15.0 minutes for the touch-up treatment. Topical anesthetic was administered for a median of 40.0 minutes for the initial treatment and 35.0 minutes for the touch-up treatment.
Table 2.
Treatment Characteristics of the Safety Population
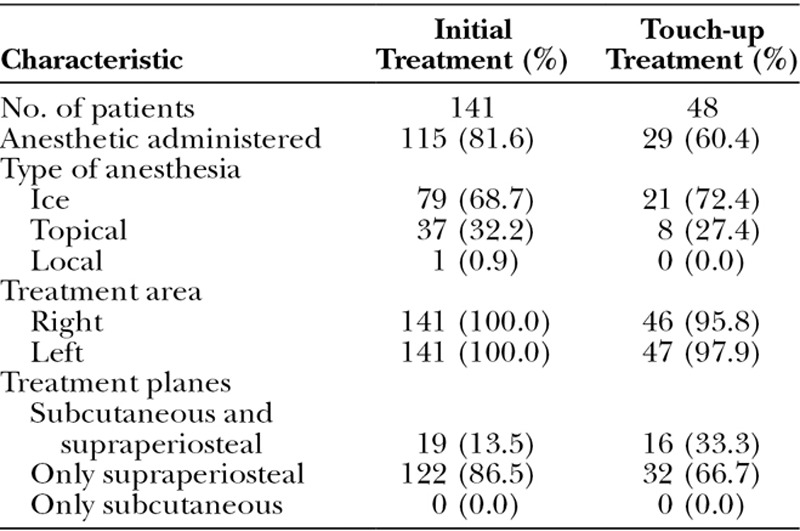
Median volume injected (both sides of the face) for the treatment group was 2.0 ml (range, 1.0 to 5.7 ml) overall, with 1.7 ml (range, 1.0 to 4.0 ml) for the initial treatment and 1.1 ml (range, 0.3 to 2.4 ml) for the touch-up treatment. Most initial (86.5 percent) and touch-up (66.7 percent) treatments were administered in the supraperiosteal plane only (Table 2). After all treatments, the treatment area was massaged or molded.
Effectiveness
The primary effectiveness endpoint was met, with the mean change from baseline to 6 months for the modified intent-to-treat population in malar volume for the treatment group of 1.83 ml being significantly greater than that for the control group (0.11 ml; p < 0.001) (Fig. 2). Analysis of data from the per-protocol population also showed a significantly greater change at month 6 from baseline in malar volume for the treatment group compared with the control group (1.84 ml versus 0.07 ml; p < 0.001).
Fig. 2.
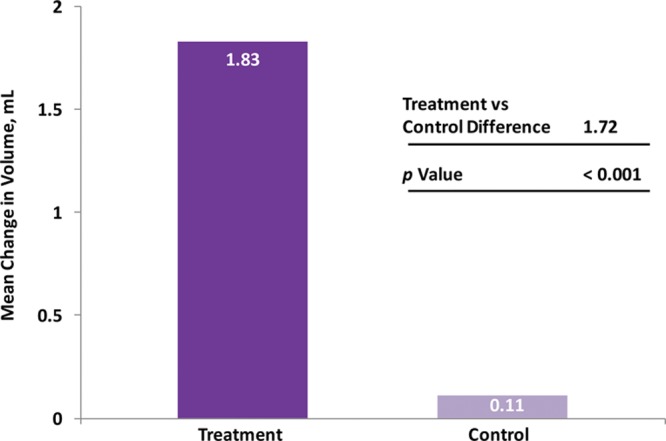
Mean change in malar area volume from baseline at the month-6 visit (modified intent-to-treat population).
Responder rates (subjects rated improved or much improved) using the Global Aesthetic Improvement Scale at month 6 as assessed by the investigator and the subject were 98.2 percent and 93.8 percent, respectively (Fig. 3). Assessments by the investigator showed responder rates of 98.1 percent at month 1 and 99.1 percent at month 3 (Fig. 3). Assessments by the subjects showed responder rates of 97.2 and 97.3 percent for months 1 and 3, respectively (Fig. 3). Additional effectiveness endpoints showed mean changes in malar volume at months 1 and 3 for the modified intent-to-treat population of 2.09 and 1.84 ml, respectively (Fig. 4). At month 6, 90.4 percent of the treatment group subjects were satisfied or very satisfied with the treatment outcomes (Fig. 5), and 96.5 percent stated that they would recommend the treatment to a friend (Fig. 6). From month 1 to month 6, consistently high proportions of subjects agreed or strongly agreed that their cheeks appeared fuller, their facial contour was improved, and they felt more attractive (Fig. 7). Photographic examples of aesthetic features before and 6 months after treatment are provided in Figure 8. Canfield three-dimensional digital images used in the scanning process that illustrate the change in volume with Voluma treatment are provided in Figure 9.
Fig. 3.
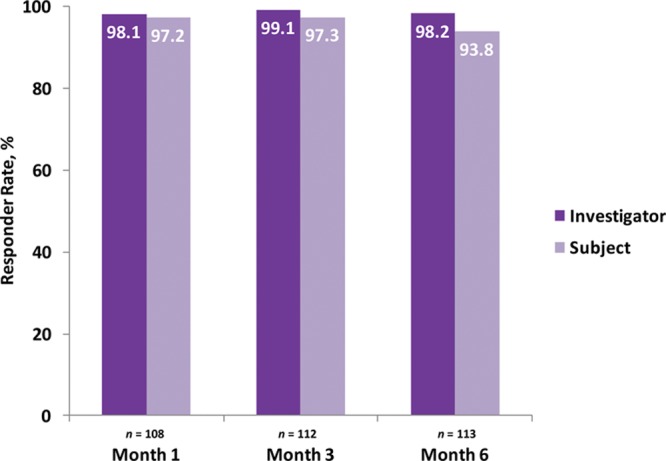
Investigator- and subject-assessed responder rates based on the Global Aesthetic Improvement Scale (treatment group; modified intent-to-treat population).
Fig. 4.
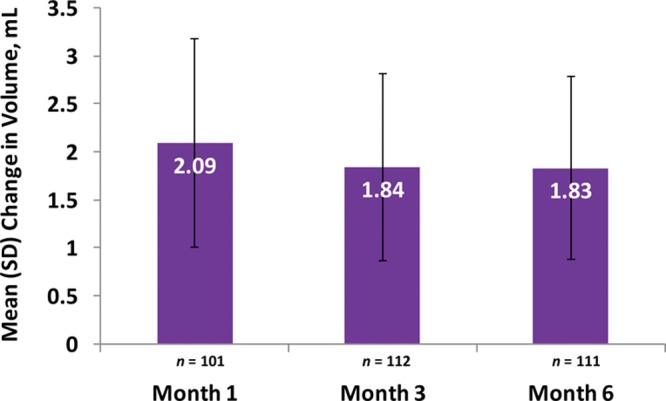
Mean (SD) change from baseline in malar volume by visit (treatment group; modified intent-to-treat population).
Fig. 5.
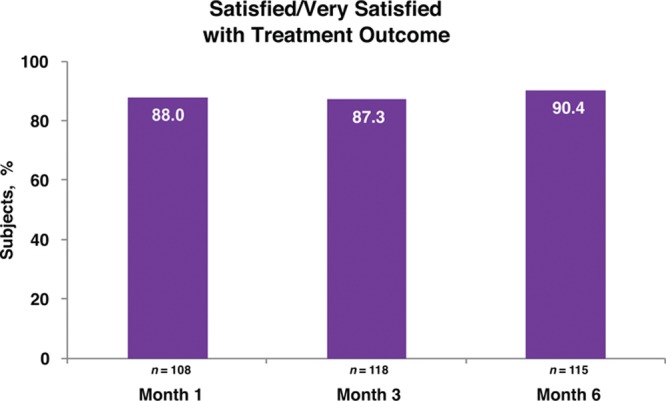
Subject self-assessment of treatment satisfaction (treatment group; modified intent-to-treat population).
Fig. 6.
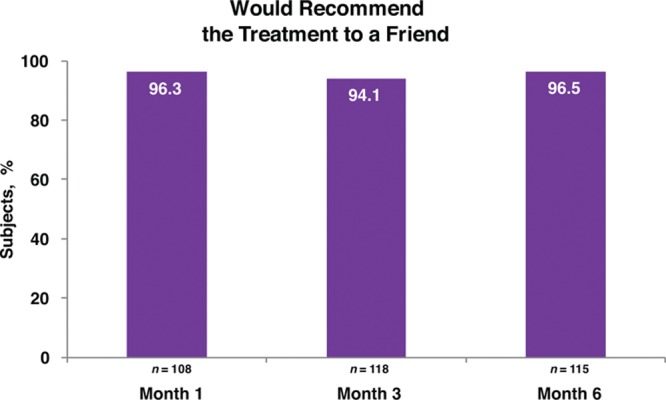
Subject self-assessment of willingness to recommend treatment (treatment group; modified intent-to-treat population).
Fig. 7.
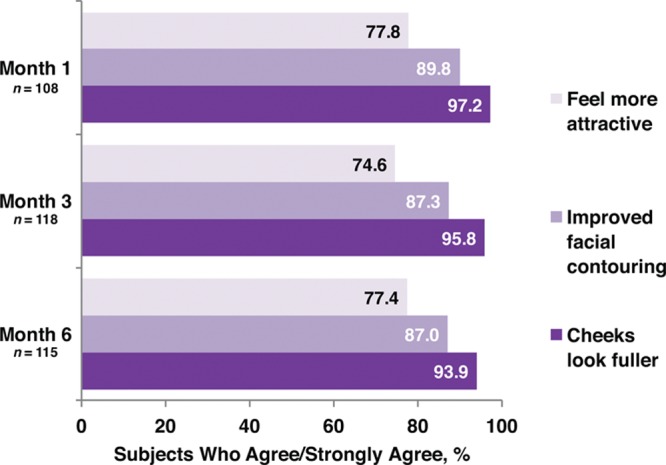
Subject self-assessment of treatment outcomes (treatment group; modified intent-to-treat population).
Fig. 8.
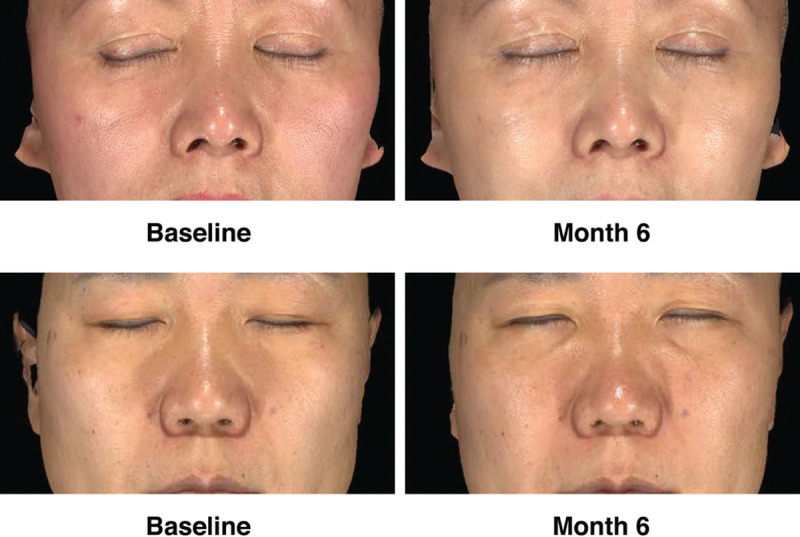
Aesthetic outcomes shown in conventional photographs at baseline and month 6. (Above) A 58-year-old female subject. The subject received 1.5 ml of Voluma at initial treatment and 1.5 ml of Voluma at touch-up treatment. (Below) A 50-year-old female subject. The subject received 5.5 ml of Voluma at initial treatment and 1.3 ml at touch-up treatment.
Fig. 9.
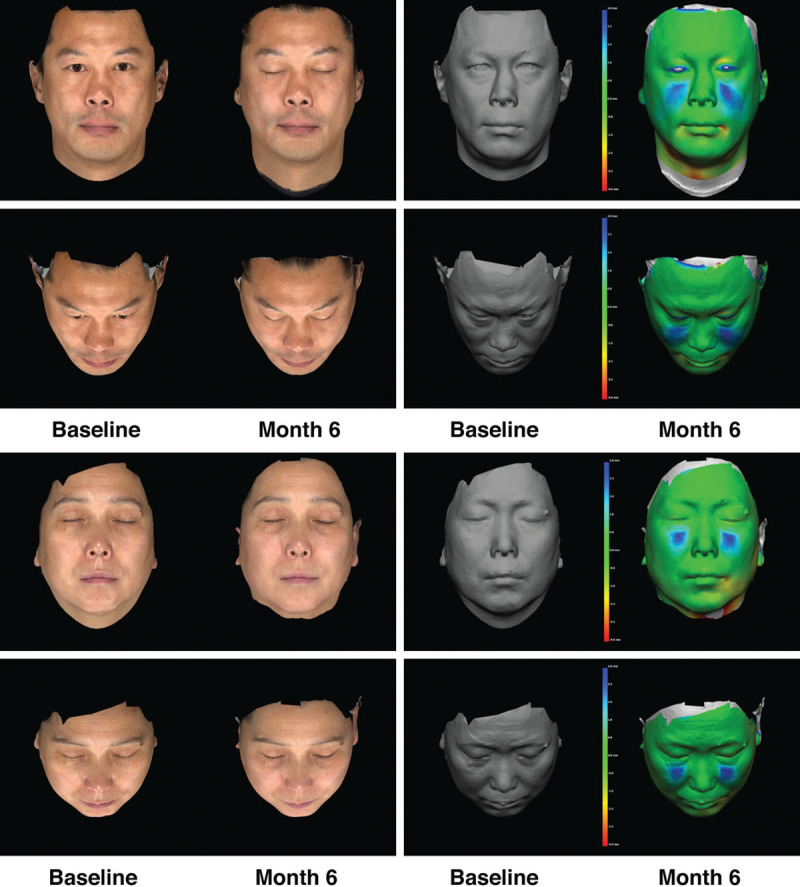
Conventional photographs at baseline and month 6 (left) and Canfield three-dimensional scanning images at baseline and month 6 (right) for both full-face and oblique angles. (Above) A 45-year-old male subject at baseline and at month 6. The subject received a total of 2.5 ml of Voluma. Midface volume change at month 6 by three-dimensional imaging was 4.97 ml. (Below) A 50-year-old female subject at baseline and at month 6. The subject received a total of 3.2 ml of Voluma. Midface volume change at month 6 by three-dimensional imaging was 4.10 ml. The color-mapping digital image (far right) shows the distance between the two surfaces (before and after treatment) based on a color scale where green = 0 distance, red = negative distance, and blue = positive distance.
Safety
Treatment-related adverse events occurred in 23 subjects, for a total of 38 events; these occurred at the injection site and consisted of swelling (17 events), bruising (12 events), injection-site pain (eight events), and induration (one event). Most of the treatment-related adverse events were mild in severity; 60.5 percent lasted 14 days or less, and 78.9 percent lasted 30 days or less. None of the treatment-related adverse events required medications or procedures, and all resolved without sequelae within 60 days. One serious adverse event (endometrial hypertrophy) was not considered related to treatment. No subjects discontinued because of an adverse event, and no deaths occurred.
DISCUSSION
In this study, injection of Voluma produced a significant increase in malar volume at month 6 as evaluated using an objective three-dimensional volume analysis. Subjective analyses confirmed the effectiveness, with consensus between investigators and subjects for high rates of improvement on the Global Aesthetic Improvement Scale. In addition, more than 80 percent of subjects were satisfied or very satisfied with their treatment, and more than 90 percent stated that they would recommend the treatment to a friend. Treatment was well tolerated.
These findings are similar to those reported in two retrospective studies that included midface volumizing in Asian subjects. One study reported data from young adult women (mean age, 29.4 years) in the Republic of Korea using Voluma for injection in the glabellar, malar, and chin areas.3 More than 95 percent of physicians and subjects rated treatment on the Global Aesthetic Improvement Scale as “improved,” “much improved,” or “very much improved.” Another study exclusively assessed midface volumizing in 11 male and female subjects in the Republic of Korea with a mean age of 41.5 years using a poly-l-lactic acid filler.19 More than 90 percent of subjects and investigators rated improvement of subjects’ cheeks to be “excellent” or “good.”
The current findings of effectiveness in this Asian subject population are consistent with findings for U.S., European, and Australian subjects in studies of midface volumizing with Voluma. A total of 92.8 percent of subjects in a multicenter study at U.S. and Canadian sites rated their midface volume as improved or much improved on the Global Aesthetic Improvement Scale at month 6.16 Likewise, investigator and subject ratings of satisfied/very satisfied with treatment in a study in Australian subjects were 100 percent and 98 percent, respectively, at week 8; and 88.2 and 79.4 percent, respectively, at week 52.17 Subjects in the current study received a smaller injection volume compared with those in the European study of Voluma [mean of 1.9 ml for initial treatment and 2.3 ml including touch-up treatment compared with 3.8 ml (1.9 ml/side) in the European study].15 The smaller injection volume is explained by the fact that the faces of individuals of Chinese ancestry are typically wider compared with the faces of individuals of European ancestry; as a result, investigators avoided deep lateral injections into Chinese subjects with a prominent zygoma and soft tissue. This resulted in a smaller, more narrow target injection area to achieve optimized outcomes.
Most treatment-related adverse events were mild and resolved spontaneously within 2 weeks. The most common treatment-related adverse events were mild swelling and bruising at the injection site. Approximately two-thirds of subjects received anesthetic treatment with ice. The use of ice required approximately half as much time as for administration of topical anesthetic. In addition, ice may reduce swelling and bruising. A recent study evaluated the use of a contact topical cooling device on one side of the face before injection of a hyaluronic acid gel for correction of nasolabial folds compared with the application of a noncooled applicator on the other side of the face. The use of the contact topical cooling device was associated with a significantly lower incidence of ecchymosis immediately after the procedures and at 1 and 3 hours after the procedure and showed a 66 percent mean reduction in ecchymosis on the following day, which failed to reach statistical significance (p = 0.09).20
In this trial, the majority of subjects were between 30 and 50 years of age, so meaningful conclusions for subjects younger than 30 or older than 50 years should be drawn with caution. Effectiveness data were collected through 6 months; additional data over longer follow-up periods may provide perspective on the overall duration of effect of Voluma in this subject population. In addition, the measurement area for three-dimensional volume change in the midface was smaller than the disposition of Voluma after injection; therefore, expansion of the measurement area in future trials of Voluma may more precisely capture volume changes.
CONCLUSIONS
The results of this study demonstrate that Voluma injected in the subcutaneous and/or supraperiosteal plane was safe and effective for malar augmentation in Chinese subjects. The safety and effectiveness demonstrated in this study were comparable to those reported in studies of individuals of other ethnic backgrounds.
PATIENT CONSENT
Li Dong, M.D., represents that he has obtained or made every effort to obtain all necessary and appropriate patient releases.
ACKNOWLEDGMENTS
This study was sponsored by Allergan, Inc. (Irvine, Calif.). Writing and editorial assistance was provided to the authors by Annette F. Skorupa, Ph.D., of Peloton Advantage (Parsippany, N.J.) and was funded by Allergan, Inc. The authors wish to thank Dr. Haihuan Ma, who served as a principal investigator, Dr. Xue Zhiqiang, who served as a subinvestigator responsible for Juvéderm Voluma injection and subject recruitment, and Drs. Wilson W. S. Ho, Steven Liu, and Liyang Chen, who acted as injection trainers for all treating investigators in this study. All authors met the International Committee of Medical Journal Editors authorship criteria. Neither honoraria nor payments were made for authorship.
Footnotes
Presented at the International Master Course on Aging Skin, in Bali, Indonesia, July 31 through August 2, 2015.
Disclosure: Drs. Dong Li, Qin Li, Jiaming Sun, Yan Wu, Xiaojun Wang, Shuzhong Guo, and Haihuan Ma have served as investigators for Allergan, Inc. Dr. Yi Jia is an employee of Allergan China, Beijing, China. Diane K. Murphy is an employee and stockholder of Allergan, Inc.
REFERENCES
- 1.Lee JH, Choi YS, Kim SM, Kim YJ, Rhie JW, Jun YJ. Efficacy and safety of porcine collagen filler for nasolabial fold correction in Asians: A prospective multicenter, 12 months follow-up study. J Korean Med Sci. 2014;29(Suppl 3):S217–S221.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park H, Chun KW, Kye MS, Dhong ES, Yoon ES. Midface augmentation using bony segments obtained from sagittal splitting angle ostectomy in Asians. Plast Reconstr Surg. 2008;121:578–586.. [DOI] [PubMed] [Google Scholar]
- 3.Bae JM, Lee DW. Three-dimensional remodeling of young Asian women’s faces using 20-mg/ml smooth, highly cohesive, viscous hyaluronic acid fillers: A retrospective study of 320 patients. Dermatol Surg. 2013;39:1370–1375.. [DOI] [PubMed] [Google Scholar]
- 4.Wu WTL. Lee P, Chen YR, Li QF, et al. Botulinum toxin A injections for facial rejuvenation and reshaping. In: Aesthetic Plastic Surgery in Asians: Principles and Techniques Volume I and II. 2015:1st Ed Boca Raton, Fla: CRC Press; 149–169.. [Google Scholar]
- 5.Lam SM. Volumetric rejuvenation: General concepts. Facial Plast Surg. 2015;31:15–21.. [DOI] [PubMed] [Google Scholar]
- 6.Gierloff M, Stöhring C, Buder T, Gassling V, Açil Y, Wiltfang J. Aging changes of the midfacial fat compartments: A computed tomographic study. Plast Reconstr Surg. 2012;129:263–273.. [DOI] [PubMed] [Google Scholar]
- 7.Coleman SR, Grover R. The anatomy of the aging face: Volume loss and changes in 3-dimensional topography. Aesthet Surg J. 2006;26(Suppl):S4–S9.. [DOI] [PubMed] [Google Scholar]
- 8.Rohrich RJ, Pessa JE, Ristow B. The youthful cheek and the deep medial fat compartment. Plast Reconstr Surg. 2008;121:2107–2112.. [DOI] [PubMed] [Google Scholar]
- 9.Jones D. Volumizing the face with soft tissue fillers. Clin Plast Surg. 2011;38:379–390, v.. [DOI] [PubMed] [Google Scholar]
- 10.Borrell M, Leslie DB, Tezel A. Lift capabilities of hyaluronic acid fillers. J Cosmet Laser Ther. 2011;13:21–27.. [DOI] [PubMed] [Google Scholar]
- 11.Ho SG, Chan HH. The Asian dermatologic patient: Review of common pigmentary disorders and cutaneous diseases. Am J Clin Dermatol. 2009;10:153–168.. [DOI] [PubMed] [Google Scholar]
- 12.Lee SK, Kim HS. Recent trend in the choice of fillers and injection techniques in Asia: A questionnaire study based on expert opinion. J Drugs Dermatol. 2014;13:24–31.. [PubMed] [Google Scholar]
- 13.Ahn BK, Kim YS, Kim HJ, Rho NK, Kim HS. Consensus recommendations on the aesthetic usage of botulinum toxin type A in Asians. Dermatol Surg. 2013;39:1843–1860.. [DOI] [PubMed] [Google Scholar]
- 14.Kim JT. Preface: Recent wave in the field of Korean plastic surgery. J Korean Med Sci. 2014;29(Suppl 3):S166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann K. Juvéderm Voluma Study Investigators Group. Volumizing effects of a smooth, highly cohesive, viscous 20-mg/mL hyaluronic acid volumizing filler: Prospective European study. BMC Dermatol. 2009;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones D, Murphy DK. Volumizing hyaluronic acid filler for midface volume deficit: 2-year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013;39:1602–1612.. [DOI] [PubMed] [Google Scholar]
- 17.Callan P, Goodman GJ, Carlisle I, et al. Efficacy and safety of a hyaluronic acid filler in subjects treated for correction of midface volume deficiency: A 24 month study. Clin Cosmet Investig Dermatol. 2013;6:81–89.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Few J, Cox SE, Paradkar-Mitragotri D, Murphy DK. A multicenter, single-blind randomized, controlled study of a volumizing hyaluronic acid filler for midface volume deficit: Patient-reported outcomes at 2 years. Aesthet Surg J. 2015;35:589–599.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suh DH, Lee SJ, Kim SM, Lee JD, Kim HS. The safety and efficacy of poly-L-lactic acid on sunken cheeks in Asians. J Cosmet Laser Ther. 2014;16:180–184.. [DOI] [PubMed] [Google Scholar]
- 20.Nestor MS, Ablon GR, Stillman MA. The use of a contact cooling device to reduce pain and ecchymosis associated with dermal filler injections. J Clin Aesthet Dermatol. 2010;3:29–34.. [PMC free article] [PubMed] [Google Scholar]


