Highlight
Phloem-specific expression and peptidyl-prolyl isomerase (PPI) activity are required for Cyclophilin1 action as a long-distance signal molecule, and auxin response modulation depends on activity of the trafficking protein in the target tissue.
Keywords: Auxin, cyclophilin, diageotropica, phloem, root development, Solanum lycopersicum.
Abstract
Tomato (Solanum lycopersicum) diageotropica (dgt) mutants, containing a single mutation in the Cyclophilin1 (SlCyp1) gene, are auxin-insensitive, exhibiting a pleiotropic phenotype including lack of geotropism, abnormal xylem structure, lack of lateral roots (LRs), and elevated shoot-to-root ratio. SlCyp1 is a putative peptidyl-prolyl isomerase that can traffic from shoot to root, where it induces changes in auxin response, LR formation, and xylem development, suggesting it has a role as a long-distance signaling molecule. Here, we explored the mechanism underlying SlCyp1 function in the phloem. Expression of SlCyp1 under a phloem-specific (AtSuc2) promoter in dgt plants partially restored the wild-type phenotype, including lateral root development, root branching, and xylem morphology. The observed developmental changes were associated with physiological alternations at the whole-plant level, including a reduction in shoot-to-root ratio, enhanced transpiration, and elevated photosynthetic rates. Conversely, phloem-specific expression of SlCyp1 active-site mutants did not restore the wild-type phenotype. Local inhibition of cyclophilin functioning in the target tissue reduced auxin sensitivity, suggesting that its enzymatic activity in the distant organ is required for its action as a long-distance signalling agent. The data presented suggest that SlCyp1 is a signal molecule trafficking from shoot to root where its activity is required for auxin-mediated lateral root development.
Introduction
Virtually all aspects of plant growth and development are dominated by auxins, a class of growth regulators (Woodward and Bartel, 2005). In roots, auxins control numerous developmental processes including cell division and elongation, cellular differentiation, root hair formation, and lateral root (LR) development (Teale et al., 2006; Overvoorde et al., 2010). The tomato (Solanum lycopersicum) diageotropica (dgt) mutant is auxin-insensitive, characterized by a pleiotropic phenotype including lack of geotropism, hyponastic leaves, impaired secondary growth, malformed secondary xylem vessels, short roots, and lack of LRs (Zobel, 1973, 1974). Several physiological studies have established that while the dgt mutant is not impaired in IAA synthesis (Fujino et al., 1988) or uptake (Daniel et al., 1989; Muday et al., 1995), it is unresponsive to auxin (Kelly and Bradford, 1986) and exhibits a partial reduction in polar auxin transport (Ivanchenko et al., 2015). Corresponding to the auxin-insensitive phenotype, the dgt mutation is associated with a reduction in the levels of auxin-responsive transcripts, including small-auxin-up-regulated-RNA (SAUR) (Mito and Bennett, 1995), Aux/IAA, and ACC synthase (Nebenführ et al., 2000; Balbi and Lomax, 2003). Recent morphological studies have established that the absence of LRs in dgt mutants is due to an inability to form LR primordia from dividing pericycle cells (Ivanchenko et al., 2006). A more recent study indicated that dgt mutant root caps contain elevated levels of hydrogen peroxide (H2O2), suggesting that this mutation inhibits the auxin response by modulating reactive oxygen species (ROS) levels at the root tip (Ivanchenko et al., 2013).
Mapping of the dgt mutation established its location in the tomato Cyclophilin1 (SlCyp1) gene (Oh et al., 2006). Cyclophilins are chaperones that catalyze protein folding by peptidyl-prolyl cis-trans isomerase (PPI) activity and are involved in various cellular signaling pathways in different organisms (Wang and Heitman, 2005). Research conducted over the last few decades has demonstrated the involvement of cyclophilins in numerous biological processes. These include metabolic responses to oxidative stress and light intensity (Dominguez-Solis et al., 2008), abiotic stress tolerance (Ruan et al., 2011), plant–pathogen interactions (Deng et al., 1998), and control over plant development and phase change (Berardini et al., 2001). SlCyp1 orthologue mutants have been discovered in the moss Physcomitrella patens (Lavy et al., 2012) and rice (Kang et al., 2013; Zheng et al., 2013), exhibiting phenotypes similar to those of the tomato dgt, including auxin insensitivity (Lavy et al., 2012) and lack of LR formation (Kang et al., 2013; Zheng et al., 2013), suggesting that Cyp1 has a conserved role in the auxin response pathway across the plant kingdom. Recent insights into the biochemical mechanism underlying the role of cyclophilins in auxin response were provided by studying the Cyp1 orthologue from rice, LATERAL ROOTLESS 2 (LRT2) (Jing et al., 2015). The authors concluded that LRT2 directly interacts with the rice Aux/IAA protein, OsIAA11. This interaction further leads to the isomerization of the OsIAA11 proline peptide bond C105T106, which destabilizes OsIAA11 and thereby promotes the auxin signal.
Cyclophilin activity has been extensively studied in humans. This is mostly due to the fact that human Cyclophilin A (HsCypA) was identified as the target of the immunosuppressive drug Cyclosporin A (CsA) (Handschumacher et al., 1984), which inhibits its PPI activity (Takahashi et al., 1989). Site-directed mutagenesis studies on HsCypA defined the residues required for either PPI activity or the CsA binding affinity of the protein (Zydowsky et al., 1992). Mutations in two active site residues, R55A and H126A, resulted in only 1% of the wild-type PPI activity, leaving the CsA binding affinity undisturbed. However, the W121A mutation retained 8.7% of the catalytic function, but CsA affinity was reduced up to 400-fold. The W121 residue was also found to be the major determinant of CsA binding affinity in several other studies (Liu et al., 1991; Dorfman et al., 1997).
It is now evident that the phloem translocation stream contains a distinct population of macromolecules, including mRNAs and proteins, that possess putative functions as long-distance signaling agents (Turgeon and Wolf, 2009; Ham and Lucas, 2013; Spiegelman et al., 2013). Interestingly, cyclophilins have been found to be prominent in the phloem translocation stream. Members of this protein family have been detected in the phloem sap of several monocots and dicots species, including Triticum aestivum, Oryza sativa, Ricinus communis, Cucurbita maxima, Lupinus albus, and Brassica oleracea (Schobert et al., 1998; Barnes et al., 2004; Aki et al., 2008; Lin et al., 2009; Rodriguez-Medina et al., 2011; Anstead et al., 2013). RcCyp1, isolated from the phloem sap of Ricinus communis, was found to possess biochemical PPI activity, and mediate its own cell-to-cell movement (Gottschalk et al., 2008).
We have recently established that the SlCyp1 protein can traffic long-distance through the phloem translocation stream from the shoot to the root, where it induces changes in xylem morphology, LR development, auxin response, and shoot-to-root ratio (Spiegelman et al., 2015). While these findings suggest that SlCyp1 acts as a long-distance signal, additional evidence is required to establish the specific role this protein plays in the phloem, and whether its activity in the target site is required for its evident role in inter-organ communication.
In the current study, we further examined the specific role that SlCyp1 plays in the phloem of tomato plants. Expression of the wild-type SlCyp1 gene in the phloem of the dgt mutant background resulted in partial restoration of the wild-type phenotype, including rescue of secondary xylem and LR development, an increase in root branching, and a decrease in shoot-to-root ratio. The observed developmental changes probably occurred through an increase in auxin sensitivity. Site-directed mutations indicated that PPI activity is essential for SlCyp1 activity in the phloem, and grafting experiments showed that Cyp1 expression in the shoot phloem is sufficient to rescue the root phenotype. Importantly, application of CsA to the rootstock only reduced the recovered auxin sensitivity following scion-to-rootstock trafficking of SlCyp1. These results indicate that, in addition to its long-distance trafficking, activity of the trafficking protein in the target tissue is required to exert developmental changes.
Materials and methods
Plant material and grafting protocol
Tomato (Solanum lycopersicum) diageotropica (dgt) mutants and their respective control VFN8 plants were obtained from the Tomato Genetics Resource Center (TGRC) at UC Davis. Plants were grown in a soil mixture in 10-cm diameter plastic pots. Growth chamber conditions were a temperature of 25 ± 2/18 ± 2 °C day/night, and light intensity of 180 µmol m–2 s–1. Photoperiod was set at 12 h. For western blot experiments, different plant organs were collected from Cucurbita maxima (cv. Tripoli), Cucumis sativus (cv. Beit Alfa). and Solanum lycopersicum (cv. M82) at 4 weeks after sowing. For the different physiological measurements performed on dgt, VFN8, and SlCyp1-PX transgenic dgt lines, samples were collected 1 month after sowing. Reciprocal grafting experiments were performed with 4-week-old chamber-grown dgt and SlCyp1-PX plants using 0.6-mm wide plastic grafting tubes. Graftings were performed ~0.5cm under the cotyledons, resulting in source–sink relations between the scion and the rootstock, respectively. Measurements were taken 4 weeks after the time of grafting.
Gravitropic response assay
Seeds were placed on moist Whatman paper in the dark at room temperature. When root length reached 2–5 mm, the intact seedlings were transferred to 10 × 10 cm 1% agar plates containing Nitch medium supplemented with vitamins (Duchefa Biochemie). Seedlings were placed horizontal to the gravity vector and grown in the same orientation for 48 h, after which the angles of curvature were measured.
Cloning, site-directed mutagenesis, and plant transformation
To generate transgenic tomato plants expressing SlCyp1 specifically in the phloem (SlCyp1-PX, where PX stands for phloem-expression), the SlCyp1 open reading frame (ORF) was amplified from total cDNA of a cv. M82 leaf using extension primers adding a 6xHis tag sequence at the 5′ of the Cyp1 ORF, and PstI and HindIII sites at the 5′ and 3′, respectively (for primers, see Table 1). The PCR product was cloned downstream to the Arabidopsis thaliana Suc2 promoter sequence (Truernit and Sauer, 1995) and upstream to the OCS terminator in the pBJ36-pSuc2 vector (kindly supplied by Yuval Eshed, Weizmann Institute of Science, Israel), forming pBJ36-pSuc2:6xHis-SlCyp1. To generate the active-site SlCyp1 mutants, site-directed mutagenesis was performed on the pBJ36-pSuc2:6xHis-SlCyp1 plasmid using the QuikChange Site-Directed Mutagenesis Kit (Agilent) according to the manufacturer’s protocol. Sense and antisense primers (Table 1) were designed to introduce the mutations in each of the targeted amino acids: R62A, H133Q, and W128A. The pSuc2:6xHis-SlCyp1:OCSter segment, as well as the mutant segments, were then cloned into the binary vector pART27 (Gleave, 1992) using two NotI restriction sites. The binary plasmids were then transformed into Agrobacterium tumerfaciens, GV3101 strain. Cotyledon transformation was performed to tomato dgt mutants according to McCormick (1991).
Table 1.
Primers used for cloning of pSuc2:6xHis-Cyp1, real-time PCR experiments and site-directed mutagenesis. Bold letters indicate the corresponding amino acid codon changed.
| Gene | Forward | Reverse |
|---|---|---|
| pSuc2:6xHis-Cyp1 | CAAATACGTGAAGGTAGCAGTTGAC | ACACCATTTGTAAGGTCCATAAGCT |
| IAA10 | GACTTCTCAAAAGCTTGATCGAGAG | TGAAATCTTTCATTCCTTGGACAA |
| IAA11 | AAAGAACAGTTTTAACGGACGTGAA | GACTTATCTGCATCCTCCAATGCT |
| Tubulin | GAAAGCCTACCATGAGCAGC | CTTTGGCACAACATCACCAC |
| R62A | GGCTCAACCTTCCACGCTGTGATCCCAGGGTT | AACCCTGGGATCACAGCGTGGAAGGTTGAGCC |
| H133Q | GGCTCAACGGAAAGCAAGTCGTGTTTGGACAAG | CTTGTCCAAACACGACTTGCTTTCCGTTGAGCC |
| W128A | GTACCGCTAAGACTGAGGCGCTCAACGGAAAGCACG | CGTGCTTTCCGTTGAGCGCCTCAGTCTTAGCGGTAC |
Protein extraction and western blot analysis
Tissue samples of ~200 mg were taken from different plant organs. Samples were ground in liquid nitrogen using a mortar and pestle and then suspended in 500 µl of X4 protein sample buffer (40% glycerol: 8% SDS: 4% β-mercaptoethanol, pH6.8). Samples were centrifuged (4 °C, 10 000g) and supernatants were collected for protein analysis. Protein extracts were further separated by SDS-PAGE with 15% acrylamide in a Bio-Rad mini-gel system and electroblotted. Blots were blocked and incubated with 1:1000 diluted rabbit polyclonal antibodies directed against A. thaliana cyclophilin AtCYP18-3 (kindly provided by Charles Gasser, UC Davis) in 5% milk–TBS solution. Horseradish peroxidase conjugated goat anti-rabbit antibody (Sigma-Aldrich, http://www.sigmaaldrich.com) was used as the secondary antibody (dilution 1:50 000). Detection was performed by film exposure following ECL (Enhanced Chemilluminescence System, Thermo Scientific, http://www.thermoscientific.com) incubation.
Light microscopy
Stem samples were collected ~2.5 cm below the cotyledons and root samples were collected ~7 cm above the root cap. Samples were harvested and trimmed to 5 mm length, and were then fixed in FAA solution (10% formaldehyde: 5% acetic acid: 85% ethanol), subjected to vacuum for 30 min, and dehydrated at room temperature in a graded ethanol series (30 min each at 50, 70, 90, 95, and 100%). Samples were infiltrated and embedded in paraffin according to Ruzin (1999). Paraffin-embedded tissue was then cut by microtome (Leica RM2245) into 15–18-µm sections and transferred to microscope slides. Slides were deparaffinized twice for 10 min each in 100% Histocler (Gadot, http://www.gadot.com), followed by one wash in 100% ethanol (2 min), and then were air-dried under the chemical fume hood for 5 min. Sections were stained with safranin and fast green dye (Ruzin, 1999). The sections were observed with a light microscope (Olympus BX50, ×50–100 magnifications).
Immunolocalization
SlCyp1 immunolocaliztion was performed as previously described by Paciorek et al. (2006), with the following modifications. Stem samples were collected from VFN8 plants below the cotyledons, and trimmed to 5 mm length. Samples were then fixed in a paraformaldehyde solution (4% formaldehyde, 5% acetic acid in 1×PBS), infiltrated and embedded in Wax (90% PEG, 10% 1-hexadecanol) and then sectioned (15 μm thick) in a cooled microtome (Leica RM2245). Sections were transferred to microscope slides for incubation with 1:100-diluted rabbit polyclonal antibodies directed against A. thaliana cyclophilin AtCYP18-3 (1×PBS containing 2% BSA). Fluorescein isothiocyanate (FITC) -conjugated goat anti-rabbit (Sigma-Aldrich) was used as the secondary antibody (dilution 1:50).
Confocal microscopy
Observations and acquisition of images were performed with an Olympus IX-81 confocal laser scanning microscope (CLSM; FV 500, Olympus Optical Co., Tokyo, Japan) equipped with a 405-nm diode laser, 488-nm argon-ion laser, and a UPlanApo 20× NA 0.7 objective. Cell walls were excited by 405 nm light and the emission was collected through a BA 430–460 filter. FITC was excited by 488 light and emissions collected through BA 515–525 barrier filter.
IAA and CsA response assays
Stem segments were excised from the different rootstocks at 3 weeks after grafting. Segments were taken from 1.5–5 cm below the graft union. These sections were cut to short pieces of about 0.5 cm and incubated in a MES-sucrose solution [10 mM MES buffer, 1% (w/v) sucrose, pH 6.0] for 2 h to deplete endogenous auxin. Segments were then transferred to a 100-mM sodium potassium citrate buffer (pH 4.6) (control) or buffer containing either 0.1 mM of indole-3-acetic acid (IAA) or 0.1 mM IAA + 10 µM cyclosporin A (CsA). Samples were gently shaken for 2 h at room temperature. After incubation, they were soaked and immediately frozen in liquid nitrogen and stored at –80°C for later analysis.
RNA isolation and quantitative RT-PCR
Samples of stem segments 5-cm long were taken 0.5 cm above the root–shoot junction from plants of dgt, SlCyp1-PX lines 2 and 9, and VFN8. Samples were frozen in liquid nitrogen and stored at –80 °C until use. Total RNA was extracted from 500 mg of the samples, using Tri-reagent (Sigma-Aldrich, http://sigmaaldrich.com) according to the manufacturer’s protocol. RNA was quantified by NanoDrop 2000C analyser (ThermoFisher Scientific, http://thermofisher.com). cDNA was prepared from the same amounts of RNA (1 µg) per sample pretreated with 1 unit µg–1 of RQ1 DNAse (Promega, http://promega.com), using the Verso cDNA synthesis kit (ThermoFisher Scientific). Real-time RT-PCR reactions were carried out using 0.5 µl of 2.5 pmol of each primer (see Table 1), 4 µl cDNA, and 5 µl ABsoluteTM Blue QPCR SYBER® Green ROX Mix. PCR conditions were 95 °C for 15 min (enzyme activation), and then the following cycle, repeated 45 times: 95 °C for 10 s, 59 °C for 15 s, and 72 °C for 25 s. The obtained cycle temperature (CT) values were analysed with Rotor-Gene 6000 Series software by averaging the two independently calculated normalized expression values of the duplicates. The calculated IAA10 and IAA11 numerical values were divided by the values obtained for the housekeeping gene tubulin in each respective sample (for primers, see Table 1).
Gas exchange measurements
Gas exchange measurements were taken using the LI-6400 portable gas-exchange system (Li-Cor). Photosynthesis, CO2 concentrations in the substomatal cavities (Ci), stomatal conductance, and transpiration rates were measured in young source leaves (4th leaf from the apex) of 6-week-old tomato plants. Plants were grown in an environmentally controlled greenhouse. Measurements were performed at around noon in a constant CO2 concentration of 390 ppm and constant photosynthetic photon flux density (PPFD) of 1200 µmol m–2 s–1. For PPFD response curve, plants were subjected to the following radiation levels: 200, 400, and 800 µmol m–2 s–1.
Results
Expression patterns of SlCyp1 in plants
Previous research has established that Cyp1 proteins are present within the phloem sap of different species (Schobert et al., 1998; Gottschalk et al., 2008) and that the SlCyp1 gene is expressed in the tomato vasculature and lateral root primordia (Ivanchenko et al., 2015). However, it is not clear if Cyp1 is selectively accumulated in the phloem sap or constitutively expressed in other plant tissues and secreted passively into the phloem translocation stream. To determine the organs in which Cyp1 is accumulated, expression pattern analyses were performed.
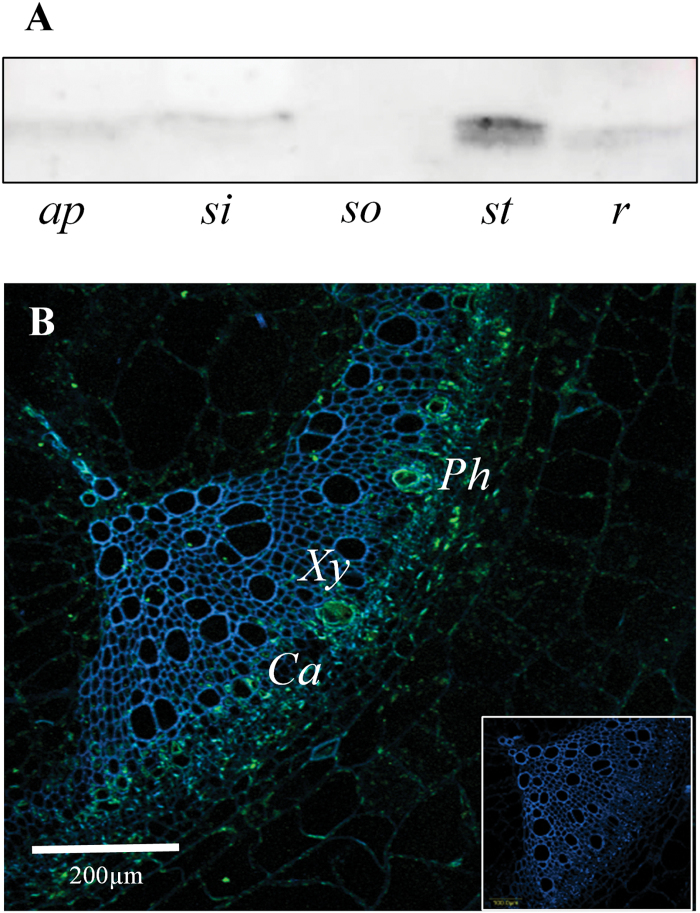
In tomato, the highest accumulation of Cyp1 was evident in stems (Fig. 1A). A strong Cyp1 signal was obtained in the phloem sap and apices of cucurbit plants (see Supplementary Fig. S1). Cyp1 was also detected, to a lesser extent, in sink leaves, and lower levels of Cyp1 were observed in source leaves and in roots (Fig. 1A; Supplementary Fig. S1). While phloem sap can be easily collected from cucurbits, it cannot be obtained from tomatoes. Therefore, immunolocalization analysis was performed on tomato stem cross-sections (Fig. 1B). In agreement with previous results (Spiegelman et al., 2015), this analysis indicated that SlCyp1 localizes mainly in areas peripheral to the xylem and phloem regions.
Fig. 1.
Spatial distribution of Cyp1 proteins in different organs. (A) Anti-cyclophilin western-blot analyses on protein extracts from different tomato organs using an anti-AtCyp18-3/ROC1 antiserum. Proteins were extracted from the following organs: shoot apex (ap), sink leaf (si), source leaf (so), stem (st), and root (r). (B) SlCyp1 immunolocalization in a transverse section of tomato stem. The green signal indicates SlCyp1 and the blue signal indicates xylem autofluorescence. Note that SlCyp1 localizes mainly to the phloem (Ph), cambium (Ca), and developing xylem (Xy) vessels. Inset: negative control. Images were obtained using an Olympus IX-81 confocal microscope.
Partial restoration of wild-type phenotype by companion-cell-specific expression of SlCyp1 in dgt mutants
To further explore the functional role of SlCyp1 in the phloem, dgt mutants were transformed with a 6xHis-tagged SlCyp1 gene under the companion-cell-specific AtSuc2 promoter from Arabidopsis (SlCyp1-PX plants). This promoter was also found to be phloem-specific in tomato plants (Golan et al., 2013). Out of five independent transgenic SlCyp1-PX dgt lines, two were characterized as representatives: SlCyp1-PX-2 and SlCyp1-PX-9, exhibiting low and high levels of the 6xHis-SlCyp1 transcripts, respectively (see Supplementary Fig. S2). Interestingly, while the dgt mutants exhibited a typical droopy stature, SlCyp1-PX plants were characterized by partial restoration of the wild-type phenotype, similar to that of VFN8 control plants (Fig. 2A). Moreover, the SlCyp1-PX plants had more shoot biomass (Fig. 2C) and were taller than the dgt mutants (Supplementary Fig. S2). In addition, SlCyp1-PX plants had wider stems than the dgt plants, as indicated by their diameter (Supplementary Fig. S2).
Fig. 2.
Effect of phloem-specific expression of SlCyp1 on plant growth and development. Expression of SlCyp1 under the AtSuc2 promoter in dgt mutant lines (SlCyp1-PX lines) results in phenotypic recovery. The images show shoots (A) and roots (B) of 4-week-old plants. Data for shoot fresh weight (C), root fresh weight (D), and shoot-to-root ratio (E) of dgt mutants (black bars), SlCyp1-PX-2 (dark grey bars), SlCyp1-PX-9 (light grey bars), and VFN8 control plants (white bars). Data represent means of 10 replications (±SE). Identical letters indicate no significant differences between genotypes at P<0.05 by Tukey’s HSD-test. (This figure is available in colour at JXB online.)
One of the most prominent characteristics of the dgt mutant is impaired root growth and lack of LRs (Fig. 2B). Interestingly, the transgenic SlCyp1-PX plants had significantly higher root volume and length (Fig. 2B; Supplementary Fig. S2) than the dgt mutants, but not to the level of VFN8 plants. This increase in root volume was due to induction of LR development (Fig. 2B). The enhanced root growth and branching led to an increase in root biomass (Fig. 2D), as compared to that of the dgt mutants. Interestingly, the enhanced root growth and branching in SlCyp1-PX plants led to a lower shoot-to-root ratio than that of dgt mutants (Fig. 2E). These results suggest that phloem expression of SlCyp1 may be required to balance root growth with that of the shoot, leading to a controlled shoot-to-root ratio.
Notably, restoration of the wild-type phenotype in the plant line SlCyp1-PX-9 was stronger than in the line SlCyp1-PX-2. This suggests that the rescue of the dgt mutant phenotype is a result of the level of SlCyp1 expression in the phloem (see Supplementary Fig. S2).
Phloem-specific expression of SlCyp1 promotes xylem development, water transport, and photosynthetic activity
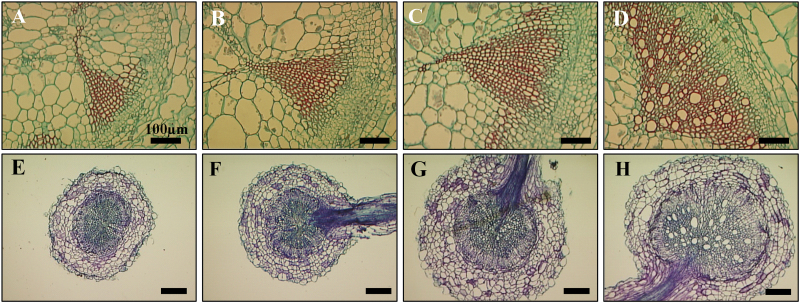
An additional set of experiments was aimed at studying the effect of phloem-specific SlCyp1 expression on xylem development. Transverse sections showed that the dgt mutants are characterized by a severely impaired xylem system, with the number of vessels being reduced, and those that are present being more narrow and fibrous (Fig. 3A and E) compared with the wide xylem vessels of the VFN8 control plants (Fig. 3D and H) (see also Spiegelman et al., 2015). Phloem-specific expression of SlCyp1 resulted in partial rescue of xylem development in both the SlCyp1-PX lines (Fig. 3B, C and F, G), which exhibited an increased number of xylem vessels that were slightly wider than those of the dgt mutants (Fig. 3A, E). In addition to restoration of xylem development, the SlCyp1-PX lines were characterized by LR formation (Fig. 3F, G), similar to that of the VFN8 control plants (Fig. 3H).
Fig. 3.
Ultrastructural changes in stems and roots in dgt, control, and transgenic plants expressing SlCyp1 in the phloem. Transverse sections of stems (A–D) and roots (E–H) in dgt mutants (A, E), SlCyp1-PX-2 (B, F), SlCyp1-PX-9 (C, G), and VFN8 control plants (D, H). Stems of dgt mutants exhibit small numbers of narrow and fibrous xylem vessels (A, E) compared to VFN8 control plants (D, H). Note the lateral-root sites of origin (F–H). Stem sections were excised ~3 cm below the cotyledons. Root sections were excised ~2 cm below the shoot–root junction. Scale bars = 100 µm.
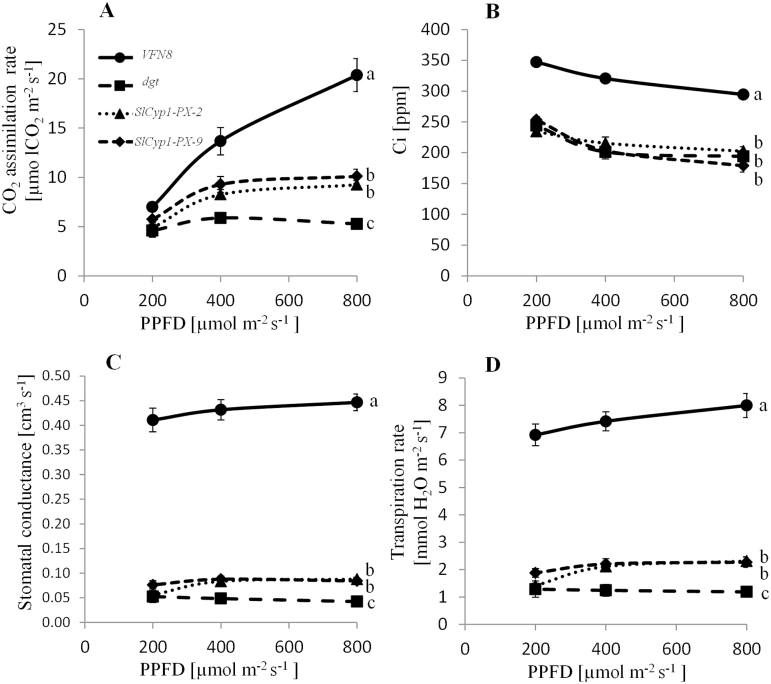
It is logical to assume that the lack of lateral root and aberrant xylem vessels in the dgt mutant leads to water deficit that further affects transpiration rate and photosynthetic activity. As anticipated, the photosynthetic rate of the dgt mutants was lower than that of VFN8 control plants, with limited response to increasing photosynthetic photon flux density (PPFD) (Fig. 4A). In concordance with the developmental recovery, photosynthetic rates of SlCyp1-PX plants were higher than those of dgt mutants (Fig. 4A). Interestingly, while CO2 concentrations in the substomatal cavities (Ci) were similar in the SlCyp1-PX plants and dgt mutants (Fig. 4B), stomatal conductance and transpiration rates were significantly higher in SlCyp1-PX plants (Fig. 4C, D). These results suggest that the reduced photosynthetic activity in the dgt mutants is not due to impairment in the carboxylation activity, as CO2 homeostasis at the substomatal cavities is not disturbed. The deficient photosynthesis can rather be explained by lower CO2 diffusion as a result of stomatal closure, which is associated with inhibited transpiration and root activity.
Fig. 4.
Effect of phloem-specific expression of SlCyp1 on photosynthesis and leaf gas exchange parameters. Data for photosynthesis (A), CO2 concentrations in the substomatal cavities (Ci) (B), stomatal conductance (C), and transpiration rate (D) of dgt mutants, VFN8 control plants, SlCyp1-PX-2, and SlCyp1-PX-9 are presented. Data represent means of four biological replications (±SE). Identical letters indicate no significant differences between genotypes at P<0.05 by Tukey’s HSD-test.
Restoration of auxin response by phloem-specific expression of SlCyp1 depends on its PPI active site
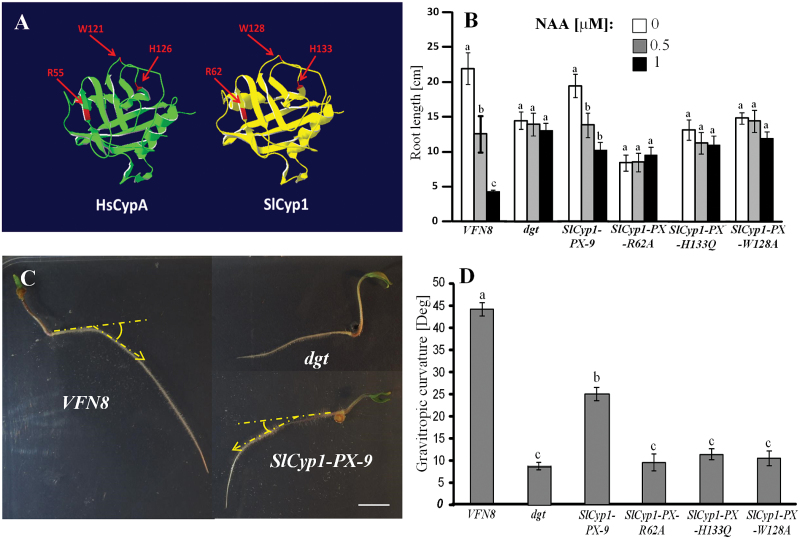
The pleiotropic phenotype of the dgt mutants is caused by the lack of auxin sensitivity (Zobel, 1973; Kelly and Bradford, 1986). It is therefore hypothesized that the PPI activity of the phloem-expressed SlCyp1 plays a role in modulating auxin response. To test this hypothesis, we first identified the amino acid residues that are important for SlCyp1 biochemical function. Previous mutational analysis of the human HsCypA established that substituting histidine 126 with glutamine (H126) or arginine 55 with alanine (R55A) result in a >99% loss of PPI activity (Zydowsky et al., 1992; Chatterji et al., 2009). The tryptophan 121 to alanine mutation (W121A) resulted in an 80- to 400-fold reduction in cyclosporine binding affinity and a 92% reduction in PPI activity (Bossard et al., 1991; Zydowsky et al., 1992). To determine the analogous residues in SlCyp1, we used a combination of protein sequence alignment (see Supplementary Fig. S3) and 3D homology modeling (Fig. 5A) with HsCypA. Notably, the identified active-site residues in HsCypA are conserved in SlCyp1: residues R62, W128, and H133 in SlCyp1 are analogous to HsCypA R55, W121, and H126, respectively (Fig. 5A; Supplementary Fig. S3). We further introduced these mutations to SlCyp1 using site-directed mutagenesis, and generated transgenic dgt mutants expressing each of the mutated genes under the AtSuc2 promoter. The lines were named according to the specific mutation: SlCyp1-PX-R62A, SlCyp1-PX-W128A, and SlCyp1-PX-H133Q.
Fig. 5.
The PPI active site is required for SlCyp1 function in the phloem to control auxin response. (A) 3D structure of HsCypA (left) and a homology-based model of SlCyp1 (right). The shaded areas at the end of the arrows indicate the active-site residues that were targeted for mutagenesis in HsCypA (Zydowsky et al., 1992) and the corresponding amino-acids in SlCyp1. 3D modeling was performed according to Arnold et al. (2006). (B) Primary root length of VFN8, dgt, SlCyp1-PX-9, SlCyp1-PX-R62A, SlCyp1-PX-H133Q, and SlCyp1-PX-W128A tomato seedlings grown on various concentrations of NAA: 0 µM (white bars), 0.5 µM (grey bars), or 1 µM (black bars). Tomato seeds were sown on standard germination paper soaked with distilled water containing each of the indicated NAA concentrations. Root length measurements were taken 14 d after sowing. (C) Images showing the gravitropic response of VFN8, dgt, and SlCyp1-PX-9 seedlings 48 h after a gravity stimulus. Soon after the emergence of the radicle (2–5 mm) seedlings were oriented horizontal to the gravity axis (represented by the dashed lines) and allowed to grow for 48 h, after which the angle of curvature (represented by the dashed arrows) was measured. Scale bar = 1 cm. (D) Gravitropic curvature angles of VFN8, dgt, SlCyp1-PX-9, SlCyp1-PX-R62A, SlCyp1-PX-H133Q, and SlCyp1-PX-W128A 48 h after the gravity stimulus. The data represent the means of six biological replications (±SE). Identical letters indicate no significant differences between each replicate at P<0.05 by Tukey’s HSD-test. (This figure is available in colour at JXB online.)
To determine auxin response capacity, the dgt mutants, the various transgenic lines, and the VFN8 control plants were germinated on different concentrations of naphthalene acetic acid (NAA) (Fig 5B). While the response of the VFN8 control plants to NAA was dramatic and resulted in clear restriction of root elongation, the dgt mutants did not respond to NAA application and root length remained similar at all NAA concentrations. SlCyp1-PX-9 plants exhibited partial restoration of the response to auxin, resulting in a significant inhibition of root elongation under higher NAA concentrations (Fig 5B); however, none of the three SlCyp1 active-site mutants responded to NAA. The restored auxin response of plants expressing the native SlCyp1 (SlCyp1-PX-9) is in agreement with the transcription levels of IAA10, an auxin- responsive Aux/IAA gene, which were significantly higher in SlCyp1-PX-9 and SlCyp1-PX-2 plants than in dgt mutants (see Supplementary Fig. S4).
Another process indicative of auxin activity is gravitropic response. We tested gravitropic response in SlCyp1-PX-9 plants and in dgt plants expressing the mutated SlCyp1. Here, minimal gravitropic response was observed in SlCyp1-PX-R62A, SlCyp1-PX-W128A, and SlCyp1-PX-H133Q plants, similar to that of the dgt mutants (Fig. 5D). Partial gravitropism recovery was observed in SlCyp1-PX-9 seedlings as compared to the VFN8 control plant (Fig 5C, D). Collectively, these results suggest that SlCyp1 biochemical activity in the phloem plays an important role in controlling auxin activity.
Phloem-expression of SlCyp1 in the shoot is sufficient to restore root growth and shoot-to-root ratio in dgt mutants
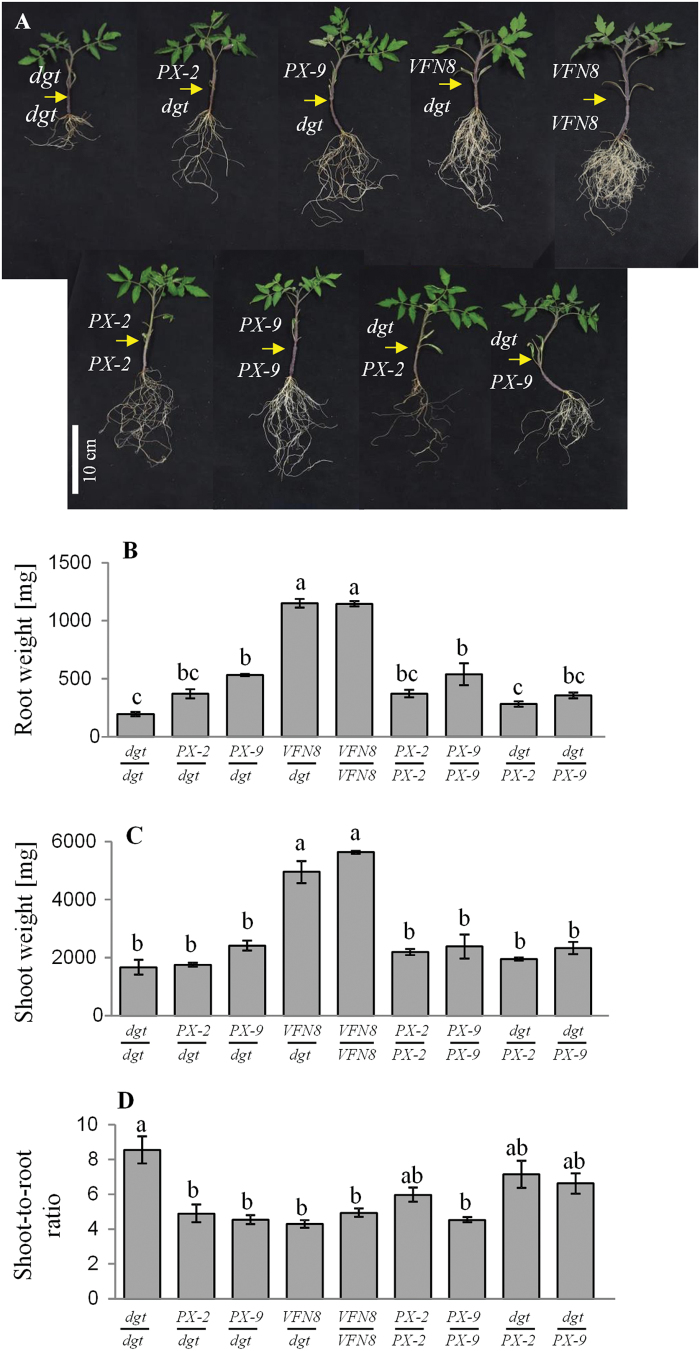
Grafting experiments have established that long-distance trafficking of the SlCyp1 protein from the VFN8 scion to the dgt rootstock restored auxin response and development of lateral roots (Spiegelman et al., 2015). To determine if exclusive expression of SlCyp1 in the shoot phloem can rescue the dgt root phenotype, grafting experiments were performed using dgt, SlCyp1-PX-9, SlCyp1-PX-2, and VFN8 control plants. dgt rootstocks grafted onto dgt scions had under-developed root systems and lacked LRs, as compared to homografted SlCyp1-PX-9 and VFN8 plants (Fig. 6A, B). In marked contrast, the shoot biomass of SlCyp1-PX homografted plants was not significantly different from that of dgt homografts (Fig. 6C). Consequently, the shoot-to-root ratio of the homografted dgt plants was significantly higher than that of the homogratferd SlCyp1-PX plants (Fig. 6D). Consistent with the previous results, when VFN8 scions were grafted onto dgt rootstocks, a recovery in dgt root growth was evident (Fig. 6A), resulting in elevated root biomass (Fig. 6B). Of note here is that a recovery in dgt root system was also observed when dgt rootstocks were grafted onto SlCyp1-PX scions (Fig. 6A), leading to a significant increase in root biomass (Fig. 6B). In contrast with the differences in root biomass accumulation, no differences in shoot biomass were evident between the SlCyp1-PX scions grafted onto dgt rootstocks and homografted dgt scions (Fig. 6C). This resulted in reduced shoot-to-root ratios in dgt rootstocks grafted onto SlCyp1-PX scions compared to homografted dgt rootstocks (Fig. 6D). When dgt scions were grafted on SlCyp1-PX rootstocks, root weight was lower compared to homografted SlCyp1-PX plants (Fig. 6A, B), and the shoot-to-root ratio was not different from that of the homografted dgt mutants (Fig. 6D). These results indicated that phloem-specific expression of SlCyp1 in the shoot is sufficient to activate a SlCyp1-derived long-distance signal to promote root growth and reduce shoot-to-root ratios. Conversely, when SlCyp1 is absent from the shoot, a reduction in root growth is observed, causing elevated shoot-to-root ratio.
Fig. 6.
Reciprocal graftings of SlCyp1 phloem-expressing plants with dgt mutants affect root development and shoot-to-root ratio. Reciprocal grafting experiment using dgt mutants grafted with SlCyp1-PX-2 (PX-2), SlCyp1-PX-9 (PX-9), and VFN8 plants. (A) Images showing each of the different reciprocal grafts, as indicated. The arrows mark the grafting point in each plant. Root weight (B), shoot weight (C), and shoot-to-root ratio (D) were measured 4 weeks after grafting. The data represent the means of five biological replications (±SE). Identical letters indicate no significant differences between each graft at P<0.05 by Tukey’s HSD-test. (This figure is available in colour at JXB online.)
Auxin response in hetereografted dgt rootstock is suppressed by CsA, a cyclophilin inhibitor
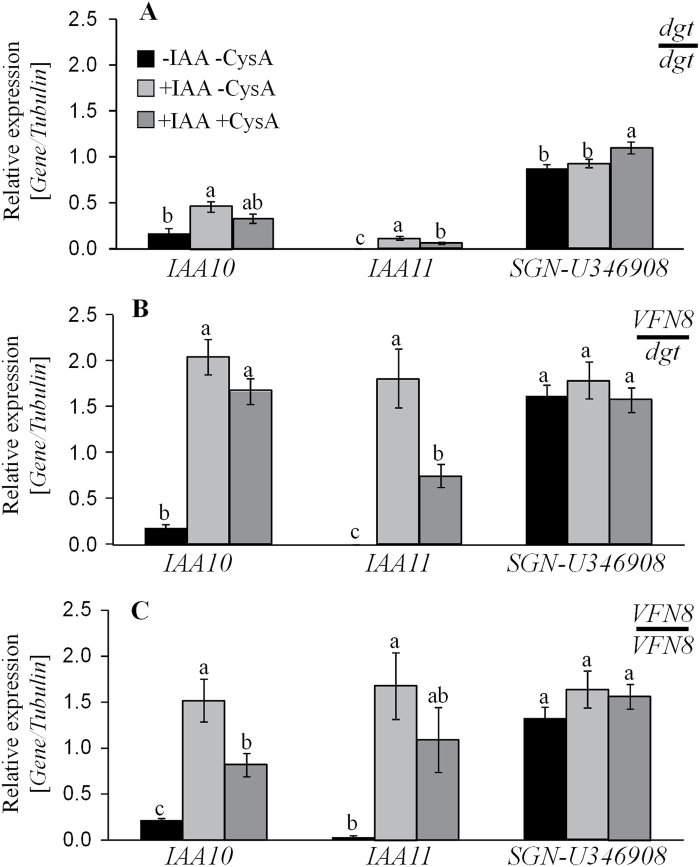
Given that SlCyp1 is a long-distance trafficking protein (Spiegelman et al., 2015), it is logical to assume that restoration of auxin sensitivity in dgt rootstocks grafted to VFN8 or SlCyp1-PX tomato scions is a result of SlCyp1 trafficking to the heterografted dgt rootstock. However, as PPI activity is required for the functioning of the protein, restoration of auxin response may also be explained by trafficking of an additional molecule, which is subjected to the chaperonin activity of SlCyp1. To explore whether SlCyp1 activity is required in the target tissue (root) or only in the shoot, auxin response was tested in rootstock stems (1.5–5 cm below the graft union) from VFN8–dgt reciprocal graftings in the presence of the cyclophilin inhibitor CsA. Sections of rootstock stem were incubated in an IAA solution with or without the addition of CsA. In agreement with earlier findings (Nebenführ et al., 2000; Spiegelman et al., 2015), auxin treatment caused a significant increase in the expression of both IAA10 and IAA11 in homografted VFN8 rootstock stems with minimal effect on expression of these genes in homografted dgt rootstocks (Fig. 7A, C). A significant increase in expression of the Aux/IAA transcripts was evident in dgt rootstocks grafted to VFN8 scions (Fig. 7B). Application of CsA resulted in reduction of the IAA-induced up-regulation of both IAA10 and IAA11. This reduction was statistically significant for the levels of IAA10 in homografted VFN8 rootstocks (Fig 7C) and for the levels of IAA11 in dgt rootstocks grafted to VFN8 scions (Fig. 7B). Levels of the control housekeeping gene, SGN-U346908, were similar under all IAA and CsA treatments, suggesting that CysA inhibited specifically the expression of auxin response genes (Fig. 7A–C). These results suggest that the restoration of auxin response in dgt rootstocks grafted to VFN8 scions is attributable to the long-distance trafficking and activity of SlCyp1 at the target site.
Fig. 7.
Cyclosporin A inhibits auxin-induced expression of Aux/IAA genes. Relative expression of IAA10, IAA11, and the SGN-U346908 control gene determined in stem sections taken from rootstocks of the following scion/rootstock combinations: dgt/dgt (A), VFN8/dgt (B), and VFN8/VFN8 (C). Transcription levels were measured following one of the indicated treatments: incubation in control solution (black bars), in a solution containing 0.1 mM IAA (light grey bars), or in a solution containing 0.1 mM IAA + 10 µM CysA (dark grey bars). Transcription levels were normalized using tubulin as an internal control. To verify that IAA or CysA does not have a general impact on gene expression SGN-U346908, which was characterized as stable housekeeping gene (Expósito-Rodríguez et al., 2008), was selected as an internal control. Data represent means (±SE) (n=5 independent experiments). A Student’s t-test indicated a significant (P<0.05) effect of the chemical treatment on the expression levels within each grafting combination. Identical letters indicate no significant differences between each graft at P<0.05 by Tukey’s HSD-test.
Discussion
Proteins from the cyclophilin family have been identified in the phloem sap of various species (Schobert et al., 1998; Barnes et al., 2004; Aki et al., 2008; Lin et al., 2009; Rodriguez-Medina et al., 2011; Anstead et al., 2013). However, their function in the sieve tube and in the phloem translocation stream has been unclear. More recent evidence has established that SlCyp1 long-distance trafficking from shoot to root can alter auxin response, xylem morphology, and root development (Spiegelman et al., 2015). The current study focused on the specific activity of SlCyp1 in the phloem, and its function in long-distance signaling. Phloem-specific expression of SlCyp1 was sufficient to partially restore the normal phenotype in the auxin-insensitive dgt mutant. This includes rescue of shoot and root growth, LR development (Fig. 2), xylem-vessel formation (Fig. 3), and transpiration and photosynthetic rates (Fig. 4). Rescue of the dgt root phenotype could also be partially achieved by the grafting of SlCyp1-PX plants onto dgt mutant rootstocks. Moreover, inhibition of cyclophilin activity in the target tissue resulted in reduced auxin response. These observations are consistent with the idea that trafficking of SlCyp1 in the phloem, and not of a downstream molecule, acts as a shoot-to-root mobile signal (Spiegelman et al., 2015).
Phloem-specific expression of SlCyp1 in dgt plants did not fully restore the wild-type phenotype. One possible explanation can be attributed to the nature of the promoter. The AtSuc2 promoter, which is a constitutive and CC-specific (Truernit and Sauer, 1995), may be weaker than the endogenous SlCyp1 promoter. It should be taken into account that the endogenous SlCyp1 promoter is not CC-specific and is also active in the surrounding cell layers (Ivanchenko et al., 2015) from which SlCyp1 proteins could be loaded to the phloem. It is possible that the level of SlCyp1 accumulated in the sieve tube, when expressed under the AtSuc2 promoter, is lower than that of wild-type tomato plants. Another explanation for the lack of full complementation is that SlCyp1 is subjected to post-transcriptional regulation limiting its expression or activity. Nevertheless, the results presented here indicate that expression of SlCyp1 exclusively in the phloem is sufficient for its influence over developmental and physiological process in distant tissues.
Several studies have established that phloem-specific expression of auxin-responsive genes can modify root architecture. In Arabidopsis, phloem expression of the IAA18 transcription factor negatively regulates LR formation (Notaguchi et al., 2012). In tomato, phloem-specific expression of an Aux/IAA transcript, F-308 (Omid et al., 2007), resulted in a dramatic increase in the number of LRs and root biomass (Golan et al., 2013). Auxin response in the phloem was found to be critical for LR development in maize (Jansen et al., 2012), with an auxin-response maximum in the protophloem acting as a trigger preceding LR organogenesis, while abolishment of this phloem-specific auxin response resulted in random cellular divisions (Jansen et al., 2012). Interestingly, uncontrolled cell division rather than the differentiation of LR primordia was also observed when dgt mutants were treated with auxin (Ivanchenko et al., 2006). Taken together, the data suggest that the phloem plays an important role in linking auxin-induced differentiation with cell division. Our results, demonstrating that auxin response is affected by the accumulation of SlCyp1 in the phloem prior to LR differentiation, support this concept. It is important to note that SlCyp1 is expressed in the phloem of our transgenic dgt mutants, while LRs emerge from pericycle cells adjacent to xylem poles. This implies that either SlCyp1 or an unknown downstream signal move locally from the phloem to the sites of LR initiation. It is logical to assume that this signal provides an important connecting link between phloem activity and local auxin responses in LR formation.
PPI activity of various plant cyclophilins, including SlCyp1, has been demonstrated in vitro (Grebe et al., 2000; Gottschalk et al., 2008; Iki et al., 2012; Kaur et al., 2015); however, little is known about the biological significance of in vivo PPI activity in plants. Our results suggest that the conserved amino acid residues required for PPI activity of HsCypA (Zydowsky et al., 1992) are also essential for SlCyp1 function in the tomato phloem. Interestingly, a recent study established that the rice orthologue of SlCyp1, LRT2, catalyzes the cis/trans isomerization of a proline peptide bond in OsIAA11 to promote its degradation and stimulate auxin response (Jing et al., 2015). This result may point to a biochemical link between cyclophilin PPI activity and regulation of auxin signaling.
The observed recovery in transpiration and photosynthetic rates in dgt plants expressing SlCyp1 in the phloem can be largely attributed to the partial rescue of secondary xylem vessels and LR development. The formation of a branched root system and wider xylem vessels allowed improved water transport that in turn affected stomatal conductance and transpiration rates. Insights into the biological mechanism connecting auxin response, xylem-vessel diameter, and photosynthesis were provided by a study conducted on poplar trees subjected to salt stress. These trees exhibited a significant reduction in xylem-vessel diameter and lower transpiration rate that was associated with reduced concentration of free auxin in the xylem. Overexpression of the poplar auxin-amidohydrolase, ILL3, which releases active IAA from storage auxin conjugates, in Arabidopsis plants alleviated the sensitivity to salt stress (Junghans et al., 2006). It can be hypothesized that SlCyp1 functions in the phloem to modulate auxin-mediated development of xylem vessels for the coordination of root water uptake with xylem water transport and transpiration.
Collectively, the research presented here established that SlCyp1 plays a role in the tomato phloem to regulate several auxin-mediated developmental processes, including xylem development, LR formation, and root branching, that affect transpiration rate and photosynthetic activity. The functioning of SlCyp1 as a long-distance signal molecule depends on its PPI activity. Moreover, the trafficking protein must be active at the target sites in order to exert its influence on auxin response. Nevertheless, at this point we cannot rule out the possibility that SlCyp1 functions in the phloem as a complex with additional protein(s) that are potentially involved in the auxin response pathway.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Spatial distribution of Cyp1 proteins in different organs.
Fig. S2. Effect of phloem-specific expression of SlCyp1 on shoot and root growth.
Fig. S3. Homology of the human CypA and SlCyp1.
Fig. S4. Phloem-expression of SlCyp1 restores auxin response in dgt roots.
Supplementary Material
Acknowledgments
This paper is a contribution from the Uri Kinamon Laboratory, and ZS was supported by a scholarship from the Kinamon Foundation. SO was supported by a scholarship from the PBC.
References
- Aki T, Shigyo M, Nakano R, Yoneyama T, Yanagisawa S. 2008. Nano scale proteomics revealed the presence of regulatory proteins including three FT-like proteins in phloem and xylem saps from rice. Plant & Cell Physiology 49, 767–790. [DOI] [PubMed] [Google Scholar]
- Anstead JA, Hartson SD, Thompson GA. 2013. The broccoli (Brassica oleracea) phloem tissue proteome. BMC Genomics 14, 764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold K, Bordoli L, Kopp J, Schwede T. 2006. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22, 195–201. [DOI] [PubMed] [Google Scholar]
- Balbi V, Lomax TL. 2003. Regulation of early tomato fruit development by the diageotropica gene. Plant Physiology 131, 186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes A, Bale J, Constantinidou C, Ashton P, Jones A, Pritchard J. 2004. Determining protein identity from sieve element sap in Ricinus communis L. by quadrupole time of flight (Q-TOF) mass spectrometry. Journal of Experimental Botany 55, 1473–1481. [DOI] [PubMed] [Google Scholar]
- Berardini TZ, Bollman K, Sun H, Poethig RS. 2001. Regulation of vegetative phase change in Arabidopsis thaliana by cyclophilin 40. Science 291, 2405–2407. [DOI] [PubMed] [Google Scholar]
- Bossard MJ, Koser PL, Brandt M, Bergsma DJ, Levy MA. 1991. A single Trp121 to Ala121 mutation in human cyclophilin alters cyclosporin A affinity and peptidyl-prolyl isomerase activity. Biochemical and Biophysical Research Communications 176, 1142–1148. [DOI] [PubMed] [Google Scholar]
- Chatterji U, Bobardt M, Selvarajah S, Yang F, Tang H, Sakamoto N, Vuagniaux G, Parkinson T, Gallay P. 2009. The isomerase active site of cyclophilin A is critical for hepatitis C virus replication. The Journal of Biological Chemistry 284, 16998–17005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel SG, Rayle DL, Cleland RE. 1989. Auxin physiology of the tomato mutant diageotropica. Plant Physiology 91, 804–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Chen L, Wood DW, Metcalfe T, Liang X, Gordon MP, Comai L, Nester EW. 1998. Agrobacterium VirD2 protein interacts with plant host cyclophilins. Proceedings of the National Academy of Sciences, USA 95, 7040–7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Solis JR, He Z, Lima A, Ting J, Buchanan BB, Luan S. 2008. A cyclophilin links redox and light signals to cysteine biosynthesis and stress responses in chloroplasts. Proceedings of the National Academy of Sciences, USA 105, 16386–16391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman T, Weimann A, Borsetti A, Walsh CT, Göttlinger HG. 1997. Active-site residues of cyclophilin A are crucial for its incorporation into human immunodeficiency virus type 1 virions. Journal of Virology 71, 7110–7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Expósito-Rodríguez M, Borges AA, Borges-Pérez A, Pérez JA. 2008. Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biology 8, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino DW, Nissen SJ, Jones AD, Burger DW, Bradford KJ. 1988. Quantification of indole-3-acetic acid in dark-grown seedlings of the diageotropica and epinastic mutants of tomato (Lycopersicon esculentum Mill.). Plant Physiology 88, 780–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleave AP. 1992. A versatile binary vector system with a T-DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Molecular Biology 20, 1203–1207. [DOI] [PubMed] [Google Scholar]
- Golan G, Betzer R, Wolf S. 2013. Phloem-specific expression of a melon Aux/IAA in tomato plants alters auxin sensitivity and plant development. Frontiers in Plant Science 4, 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk M, Dolgener E, Xoconostle-Cázares B, Lucas WJ, Komor E, Schobert C. 2008. Ricinus communis cyclophilin: functional characterisation of a sieve tube protein involved in protein folding. Planta 228, 687–700. [DOI] [PubMed] [Google Scholar]
- Grebe M, Gadea J, Steinmann T, Kientz M, Rahfeld JU, Salchert K, Koncz C, Jürgens G. 2000. A conserved domain of the arabidopsis GNOM protein mediates subunit interaction and cyclophilin 5 binding. The Plant Cell 12, 343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham BK, Lucas WJ. 2013. The angiosperm phloem sieve tube system: a role in mediating traits important to modern agriculture. Journal of Experimental Botany 65, 1799–1816. [DOI] [PubMed] [Google Scholar]
- Handschumacher RE, Harding MW, Rice J, Drugge RJ, Speicher DW. 1984. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science 226, 544–547. [DOI] [PubMed] [Google Scholar]
- Iki T, Yoshikawa M, Meshi T, Ishikawa M. 2012. Cyclophilin 40 facilitates HSP90-mediated RISC assembly in plants. The EMBO Journal 31, 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanchenko MG, Coffeen WC, Lomax TL, Dubrovsky JG. 2006. Mutations in the Diageotropica (Dgt) gene uncouple patterned cell division during lateral root initiation from proliferative cell division in the pericycle. The Plant Journal 46, 436–447. [DOI] [PubMed] [Google Scholar]
- Ivanchenko MG, den Os D, Monshausen GB, Dubrovsky JG, Bednárová A, Krishnan N. 2013. Auxin increases the hydrogen peroxide (H2O2) concentration in tomato (Solanum lycopersicum) root tips while inhibiting root growth. Annals of Botany 112, 1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanchenko MG, Zhu J, Wang B, et al. 2015. The cyclophilin A DIAGEOTROPICA gene affects auxin transport in both root and shoot to control lateral root formation. Development 142, 712–721. [DOI] [PubMed] [Google Scholar]
- Jansen L, Roberts I, De Rycke R, Beeckman T. 2012. Phloem-associated auxin response maxima determine radial positioning of lateral roots in maize. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 367, 1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing H, Yang X, Zhang J, et al. 2015. Peptidyl-prolyl isomerization targets rice Aux/IAAs for proteasomal degradation during auxin signalling. Nature Communications 22, 6. [DOI] [PubMed] [Google Scholar]
- Junghans U, Polle A, Düchting P, Weiler E, Kuhlman B, Gruber F, Teichmann T. 2006. Adaptation to high salinity in poplar involves changes in xylem anatomy and auxin physiology. Plant, Cell & Environment 29, 1519–1531. [DOI] [PubMed] [Google Scholar]
- Kang B, Zhang Z, Wang L, Zheng L, Mao W, Li M, Wu Y, Wu P, Mo X. 2013. OsCYP2, a chaperone involved in degradation of auxin-responsive proteins, plays crucial roles in rice lateral root initiation. The Plant Journal 74, 86–97. [DOI] [PubMed] [Google Scholar]
- Kaur G, Singh S, Singh H, et al. 2015. Characterization of peptidyl-prolyl cis-trans isomerase- and calmodulin-binding activity of a cytosolic Arabidopsis thaliana cyclophilin AtCyp19-3. PloS ONE 10, e0136692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MO, Bradford KJ. 1986. Insensitivity of the diageotropica tomato mutant to auxin. Plant Physiology 82, 713–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavy M, Prigge MJ, Tigyi K, Estelle M. 2012. The cyclophilin DIAGEOTROPICA has a conserved role in auxin signaling. Development 139, 1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MK, Lee YJ, Lough TJ, Phinney BS, Lucas WJ. 2009. Analysis of the pumpkin phloem proteome provides insights into angiosperm sieve tube function. Molecular & Cellular Proteomics 8, 343–356. [DOI] [PubMed] [Google Scholar]
- Liu J, Chen CM, Walsh CT. 1991. Human and Escherichia coli cyclophilins: sensitivity to inhibition by the immunosuppressant cyclosporin A correlates with a specific tryptophan residue. Biochemistry 30, 2306–2310. [DOI] [PubMed] [Google Scholar]
- McCormick S. 1991. Transformation of tomato with Agrobacterium tumefaciens. In: Lindsey K. ed. Plant tissue culture manual, Supplement 7, Section B. Springer, 311–319. [Google Scholar]
- Mito N, Bennett AB. 1995. The diageotropica mutation and synthetic auxins differentially affect the expression of auxin-regulated genes in tomato. Plant Physiology 109, 293–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muday GK, Lomax TL, Rayle DL. 1995. Characterization of the growth and auxin physiology of roots of the tomato mutant, diageotropica. Planta 195, 548–553. [DOI] [PubMed] [Google Scholar]
- Nebenführ A, White TJ, Lomax TL. 2000. The diageotropica mutation alters auxin induction of a subset of the Aux/IAA gene family in tomato. Plant Molecular Biology 44, 73–84. [DOI] [PubMed] [Google Scholar]
- Notaguchi M, Wolf S, Lucas WJ. 2012. Phloem-mobile Aux/IAA transcripts target to the root tip and modify root architecture. Journal of Integrative Plant Biology 54, 760–772. [DOI] [PubMed] [Google Scholar]
- Oh K, Ivanchenko MG, White TJ, Lomax TL. 2006. The diageotropica gene of tomato encodes a cyclophilin: a novel player in auxin signaling. Planta 224, 133–144. [DOI] [PubMed] [Google Scholar]
- Omid A, Keilin T, Glass A, Leshkowitz D, Wolf S. 2007. Characterization of phloem-sap transcription profile in melon plants. Journal of Experimental Botany 58, 3645–3656. [DOI] [PubMed] [Google Scholar]
- Overvoorde P, Fukaki H, Beeckman T. 2010. Auxin control of root development. Cold Spring Harbor Perspectives in Biology 2, a001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorek T, Sauer M, Balla J, Wiśniewska J, Friml J. 2006. Immunocytochemical technique for protein localization in sections of plant tissues. Nature Protocols 1, 104–107. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Medina C, Atkins CA, Mann AJ, Jordan ME, Smith PM. 2011. Macromolecular composition of phloem exudate from white lupin (Lupinus albus L.). BMC Plant Biology 11, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan SL, Ma HS, Wang SH, et al. 2011. Proteomic identification of OsCYP2, a rice cyclophilin that confers salt tolerance in rice (Oryza sativa L.) seedlings when overexpressed. BMC Plant Biology 11, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzin SE. 1999. Plant microtechnique and microscopy. New York:Oxford University Press. [Google Scholar]
- Schobert C, Baker L, Szederkényi J, Großmann P, Komor E, Hayashi H, Chino M, Lucas WJ. 1998. Identification of immunologically related proteins in sieve-tube exudate collected from monocotyledonous and dicotyledonous plants. Planta 206, 245–252. [Google Scholar]
- Spiegelman Z, Golan G, Wolf S. 2013. Don’t kill the messenger: Long-distance trafficking of mRNA molecules. Plant Science 213, 1–8. [DOI] [PubMed] [Google Scholar]
- Spiegelman Z, Ham BK, Zhang Z, Toal TW, Brady SM, Zheng Y, Fei Z, Lucas WJ, Wolf S. 2015. A tomato phloem-mobile protein regulates the shoot-to-root ratio by mediating the auxin response in distant organs. The Plant Journal 83, 853–863. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Hayano T, Suzuki M. 1989. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature 337, 473–475. [DOI] [PubMed] [Google Scholar]
- Teale WD, Paponov IA, Palme K. 2006. Auxin in action: signalling, transport and the control of plant growth and development. Nature Reviews 7, 847–859. [DOI] [PubMed] [Google Scholar]
- Truernit E, Sauer N. 1995. The promoter of the Arabidopsis thaliana SUC2 sucrose-H+ symporter gene directs expression of beta-glucuronidase to the phloem: evidence for phloem loading and unloading by SUC2. Planta 196, 564–570. [DOI] [PubMed] [Google Scholar]
- Turgeon R, Wolf S. 2009. Phloem transport: cellular pathways and molecular trafficking. Annual Review of Plant Biology 60, 207–221. [DOI] [PubMed] [Google Scholar]
- Wang P, Heitman J. 2005. The cyclophilins. Genome Biology 6, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AW, Bartel B. 2005. Auxin: regulation, action, and interaction. Annals of Botany 95, 707–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Li S, Ren B, Zhang J, Ichii M, Taketa S, Tao Y, Zuo J, Wang H. 2013. LATERAL ROOTLESS2, a cyclophilin protein, regulates lateral root initiation and auxin signaling pathway in rice. Molecular Plant 6, 1719–1721. [DOI] [PubMed] [Google Scholar]
- Zobel RW. 1973. Some physiological characteristics of the ethylene-requiring tomato mutant diageotropica. Plant Physiology 52, 385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel RW. 1974. Control of morphogenesis in the ethylene-requiring tomato mutant, diageotropica. Canadian Journal of Botany 52, 735–741. [Google Scholar]
- Zydowsky LD, Etzkorn FA, Chang HY, Ferguson SB, Stolz LA, Ho SI, Walsh CT. 1992. Active site mutants of human cyclophilin A separate peptidyl-prolyl isomerase activity from cyclosporin A binding and calcineurin inhibition. Protein Science 1, 1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.