Highlight
This article comments on:
Veromann-Jürgenson L-L, Tosens T, Laanisto L, Niinemets Ü. 2017. Extremely thick cell walls and low mesophyll conductance: welcome to the world of ancient living! Journal of Experimental Botany 68, 1639–1653.
Keywords: Cell wall elasticity, cell wall thickness, evolutionary constraints, gymnosperms, leaf anatomy, mesophyll conductance, photosynthesis, Rubisco, stomatal conductance
Mesophyll conductance to CO 2 – a key factor in plant photosynthesis – is strongly influenced by leaf anatomy. In this issue, Veromann-Jürgenson et al. (pages 1639–1653) provide evidence of the conservation of ancient structural traits (extremely thick cell walls) in evolutionarily old taxa that suggest apparent evolutionary constraints on CO 2 fixation. This opens the way for integrated approaches combining evolutionary constraints of diffusive, structural and biochemical factors on plant photosynthesis.
For many decades, the rate of CO2 diffusion through stomata (stomatal conductance, gs) and the capacity of photosynthetic machinery to convert light to biochemical energy and fix CO2 into sugars (biochemical capacity) were considered the only two factors constraining plant photosynthesis. However, pioneer studies already suggested that CO2 diffusion from sub-stomatal cavities to carboxylation sites inside chloroplasts (mesophyll conductance, gm) could also limit photosynthesis (Nobel, 1970). There is now an increasing interest among plant physiologists in studying the role of gm as the third major player involved in controlling the rate of photosynthesis, and this is reflected in the number of studies recently published addressing the ecophysiological significance of gm and its regulatory mechanisms (see Flexas et al., 2012, and references therein).
Large variations in gm among species or plant groups can be explained through the existence of several barriers to CO2 diffusion across the mesophyll (including air, cell walls, lipid membranes, cytoplasm and chloroplast stroma) differing in nature and size (Evans et al., 2009; Terashima et al., 2011). Recently, a small number of studies have quantified the importance of different leaf anatomical traits in determining the variability in gm and photosynthesis among species (Tomás et al., 2013; Peguero-Pina et al., 2016a ; Peguero-Pina et al., 2017) or even within the same species growing under contrasting environmental conditions (Terashima et al., 2011; Tosens et al., 2012a ; Peguero-Pina et al., 2016b , c). These analyses showed that gm was most strongly correlated with the chloroplast surface area facing intercellular air spaces (Sc/S), thickness of the mesophyll cell walls (Tcw), and chloroplast size; however, depending on foliage structure, the overall importance of gm in constraining photosynthesis and the importance of different anatomical traits in the restriction of CO2 diffusion varied (Evans et al., 2009; Terashima et al., 2011; Tosens et al., 2012b ).
Ancient structural traits constrain photosynthesis in old taxa
Mesophyll conductance has been estimated for more than 100 species from all major plant groups, but mainly spermatophytes (angiosperms and gymnosperms), with little data for ferns, liverworts and hornworts (Flexas et al., 2012; Carriquí et al., 2015; Tosens et al., 2016). Considerable variations in gm and its underlying traits among different plant groups have supported the hypothesis that an evolutionary trend exists towards higher gm together with the diversification of embryophytes (Flexas et al., 2012; Carriquí et al., 2015). However, there is still a significant knowledge gap concerning phylogenetic/evolutionary trends in gm.
The number of studies concerning gm in gymnosperms is surprisingly limited, in spite of the great importance of coniferous forests throughout the world (Breckle, 2002). Specifically, gm had only been estimated in 13 conifer species before the study by Veromann-Jürgenson et al. (2017; see also references therein). Although gymnosperms show the lowest gm values across spermatophytes (Flexas et al., 2012), available data show a high degree of interspecific variation and suggest the primary role of gm as a limiting factor for net CO2 assimilation in conifers. However, as pointed out by Veromann-Jürgenson et al. (2017), information about gm with its underlying structural traits is especially limited in conifers, and only Peguero-Pina et al. (2012, 2016b ) had previously correlated gm with ultrastructural needle anatomy in species belonging to this plant group.
In this context, Veromann-Jürgenson et al. (2017) have characterized the structural traits (i.e. Sc/S, chloroplast size and Tcw) that are mainly responsible for low gm and photosynthetic performance in several evolutionarily old gymnosperms and herbaceous species with contrasting phylogenetic age. These authors have found, for the first time, striking evidence about the effect of divergence time on structure and physiology, and specifically a negative correlation between estimated evolutionary age of the plant genus and area-based photosynthesis (AN). However, as they recognize, this statement should be treated with caution because species’ evolutionary adaptation to prevailing environmental conditions can actually drive photosynthetic capacity more strongly than their evolutionary age (Tosens et al., 2016). Regarding CO2 diffusion across the mesophyll, although gm itself was not related to plant evolutionary age, the lowest gm values (which scaled positively with AN regardless of evolutionary age) were observed for the oldest genera.
The most significant conclusion emerging from the study of Veromann-Jürgenson et al. (2017) is that the preservation of old traits suggests constraints on evolution due to the co-occurrence of low gm and AN and the corresponding high Tcw for species with widely contrasting ecological strategies. Thereby, these authors hypothesize that (i) the high-CO2 atmosphere when several of these thick-cell-walled species evolved (about 65–200 million years ago) suggests a lower control of diffusional limitations on the rate of photosynthesis, and (ii) the preservation of this ancient trait in spite of the gradual CO2 decrease through evolution has led to stronger control of foliage assimilation rates by gm (Box 1).
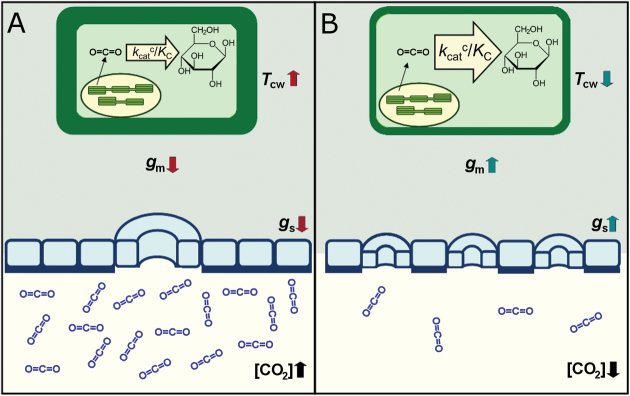
Box 1. Mesophylls of evolutionarily old or modern species which have evolved under different CO2 concentrations
The schematic representation shows the mesophyll of (A) an evolutionarily old species which evolved under high CO2 concentration and (B) an evolutionarily modern species which evolved under low CO2 concentration. Photosynthesis in evolutionarily old species at current CO2 concentrations could be constrained by low values of stomatal conductance (gs) (due to larger stomatal size but lower stomatal density: Franks and Beerling, 2009), low values of mesophyll conductance (gm) (due to extremely thick cell walls, Tcw: Veromann-Jürgenson et al., 2017), and lower carboxylase catalytic efficiency (kcatc/Kc) (Galmés et al., 2014).
Integrated approaches: the way forward
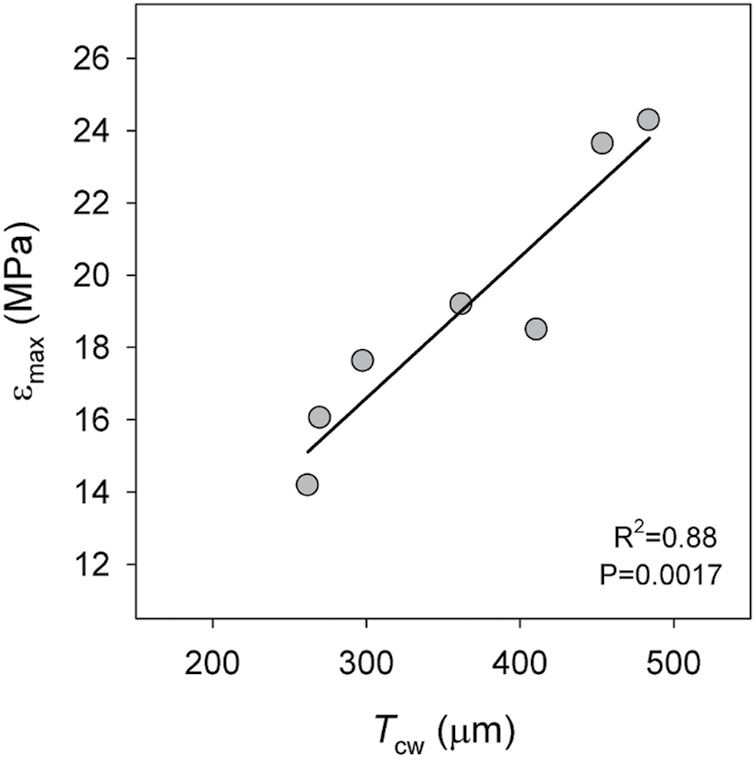
The phylogenetic trend consisting of a reduction of the cell wall thickness through evolution from bryophytes to angiosperms was recently considered by Carriquí et al. (2015), who suggested that this reduction was probably crucial to allow plants to achieve larger photosynthetic rates albeit at the expense of a reduction in desiccation tolerance. Increased values of cell wall thickness have been related to a greater ability to preserve the structure of the cells under water stress (Proctor and Tuba, 2002; Carriquí et al., 2015). Related to this, Corcuera et al. (2002) suggested that cell wall thickness may be associated with the maximum bulk modulus of elasticity ( εmax), one of the main physiological traits related to the functional role of the cell wall. Higher εmax values are seen as an efficient mechanism for plant performance under dry climates, as low cell-wall elasticity (i.e. high εmax) would allow a rapid recovery after a decrease in soil water content (Corcuera et al., 2002). To the best of our knowledge, there are no published studies empirically relating higher εmax values with increasing cell wall thickness. However, there does seem to be a positive trend between both parameters when values of cell wall thickness are plotted against εmax for several oak species (Box 2). Additional studies including simultaneous measurements of both parameters in a larger number of species from different genera are required for understanding the ultimate causal factors involved in this trade-off.
Box 2. Cell wall thickness and maximum bulk modulus of elasticity
The graph shows the relationship between cell wall thickness (Tcw) and the maximum bulk modulus of elasticity ( εmax) for several Quercus species. Mean values of εmax are from Corcuera et al. (2002); mean values of cell wall thickness are from Peguero-Pina et al. (2016a , 2017).
Besides gm, Veromann-Jürgenson et al. (2017) found that AN also depended strongly on gs, which correlated negatively with the age of the genus. This empirical result is supported by Franks and Beerling (2009), who stated that periods of falling atmospheric CO2 challenged plants with diminished CO2 availability, inducing a selection for higher maximum gs through a trend towards smaller stomatal size and higher density, thereby alleviating the negative impact of diminishing CO2 on photosynthesis (Box 1). This co-regulation between gm and gs is to some extent expected (Flexas et al., 2012) because CO2 and water vapour share, in part, diffusion pathways in the mesophyll (Evans et al., 2009; Terashima et al., 2011).
Beyond diffusive components (i.e. gs and gm), other factors also determine the rate of plant photosynthesis, such as the enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco). Galmés et al. (2014) found evolutionary trends in relation to atmospheric CO2 when analyzing the variability in Rubisco kinetics in different plant species. These authors confirmed that evolution of Rubisco towards increased affinity for CO2 (Kc falling) and increased carboxylase catalytic efficiency (kcatc/Kc) in land plants is likely to have been complementary to falling CO2/O2 ratios, as well as to adaptations in leaf architecture, morphology and conductance (Beerling et al., 2001; Franks and Beerling, 2009; Haworth et al., 2011) (Box 1).
Veromann-Jürgenson et al. (2017) provide an interesting starting point for further studies on the role of phylogenetic aspects in plant physiological performance (i.e. the influence of the age on photosynthesis associated with the preservation of ancient traits in evolution, such as extremely thick cell walls). Currently, the way forward is through the implementation of integrated approaches that combine evolutionary constraints of diffusive, structural and biochemical factors on plant photosynthetic performance, together with other functional traits (e.g. plant hydraulics).
References
- Beerling DJ, Osborne CP, Chaloner WG. 2001. . Evolution of leaf-form in land plants linked to atmospheric CO2 decline in the late Palaeozoic era. Nature 410 , 352 – 354 . [DOI] [PubMed] [Google Scholar]
- Breckle SW. 2002. . Walter’s vegetation of the earth . Berlin, Heidelberg: : Springer; . [Google Scholar]
- Carriquí M, Cabrera HM, Conesa MÀ, et al. . 2015. . Diffusional limitations explain the lower photosynthetic capacity of ferns as compared with angiosperms in a common garden study. Plant, Cell & Environment 38 , 448 – 460 . [DOI] [PubMed] [Google Scholar]
- Corcuera L, Camarero JJ, Gil-Pelegrín E. 2002. . Functional groups in Quercus species derived from the analysis of pressure-volume curves. Trees – Structure and Function 16 , 465 – 472 . [Google Scholar]
- Evans JR, Kaldenhoff R, Genty B, Terashima I. 2009. . Resistances along the CO2 diffusion pathway inside leaves. Journal of Experimental Botany 60 , 2235 – 2248 . [DOI] [PubMed] [Google Scholar]
- Flexas J, Barbour MM, Brendel O, et al. . 2012. . Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Science 193–194 , 70 – 84 . [DOI] [PubMed] [Google Scholar]
- Franks PJ, Beerling DJ. 2009. . Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proceedings of the National Academy of Sciences, USA 106 , 10343 – 10347 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmés J, Kapralov MV, Andralojc PJ, Conesa MÀ, Keys AJ, Parry MA, Flexas J. 2014. . Expanding knowledge of the Rubisco kinetics variability in plant species: environmental and evolutionary trends. Plant, Cell & Environment 37 , 1989 – 2001 . [DOI] [PubMed] [Google Scholar]
- Haworth M, Elliott-Kingston C, McElwain JC. 2011. . Stomatal control as a driver of plant evolution. Journal of Experimental Botany 62 , 2419 – 2423 . [DOI] [PubMed] [Google Scholar]
- Nobel PS. 1970. . Internal leaf area and cellular CO2 resistance: photosynthetic implications of variations with growth conditions and plant species. Physiologia Plantarum 40 , 137 – 144 . [Google Scholar]
- Peguero-Pina JJ, Flexas J, Galmés J, Niinemets U, Sancho-Knapik D, Barredo G, Villarroya D, Gil-Pelegrín E. 2012. . Leaf anatomical properties in relation to differences in mesophyll conductance to CO(2) and photosynthesis in two related Mediterranean Abies species. Plant, Cell & Environment 35 , 2121 – 2129 . [DOI] [PubMed] [Google Scholar]
- Peguero-Pina JJ, Sancho-Knapik D, Flexas J, Galmés J, Niinemets Ü, Gil-Pelegrín E. 2016. b Light acclimation of photosynthesis in two closely related firs (Abies pinsapo Boiss. and Abies alba Mill.): the role of leaf anatomy and mesophyll conductance to CO2. Tree Physiology 36 , 300 – 310 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peguero-Pina JJ, Sisó S, Fernández-Marín B, Flexas J, Galmés J, García-Plazaola JI, Niinemets Ü, Sancho-Knapik D, Gil-Pelegrín E. 2016. c Leaf functional plasticity decreases the water consumption without further consequences for carbon uptake in Quercus coccifera L. under Mediterranean conditions. Tree Physiology 36 , 356 – 367 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peguero-Pina JJ, Sisó S, Flexas J, Galmés J, García-Nogales A, Niinemets Ü, Sancho-Knapik D, Saz MÁ, Gil-Pelegrín E. 2017. . Cell-level anatomical characteristics explain high mesophyll conductance and photosynthetic capacity in sclerophyllous Mediterranean oaks. New Phytologist . doi: 10.1111/nph.14406 . [DOI] [PubMed] [Google Scholar]
- Peguero-Pina JJ, Sisó S, Sancho-Knapik D, Díaz-Espejo A, Flexas J, Galmés J, Gil-Pelegrín E. 2016. a Leaf morphological and physiological adaptations of a deciduous oak (Quercus faginea Lam.) to the Mediterranean climate: a comparison with a closely related temperate species (Quercus robur L.). Tree Physiology 36 , 287 – 299 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor MCF, Tuba Z. 2002. . Poikilohydry and homoihydry: antithesis or spectrum of possibilities? New Phytologist 156 , 327 – 349 . [DOI] [PubMed] [Google Scholar]
- Terashima I, Hanba YT, Tholen D, Niinemets Ü. 2011. . Leaf functional anatomy in relation to photosynthesis. Plant Physiology 155 , 108 – 116 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás M, Flexas J, Copolovici L, Galmés J, Hallik L, Medrano H, Ribas-Carbó M, Tosens T, Vislap V, Niinemets Ü. 2013. . Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: quantitative limitations and scaling up by models. Journal of Experimental Botany 64 , 2269 – 2281 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosens T, Niinemets U, Vislap V, Eichelmann H, Castro Díez P. 2012. a Developmental changes in mesophyll diffusion conductance and photosynthetic capacity under different light and water availabilities in Populus tremula: how structure constrains function. Plant, Cell & Environment 35 , 839 – 856 . [DOI] [PubMed] [Google Scholar]
- Tosens T, Niinemets Ü, Westoby M, Wright IJ. 2012. b Anatomical basis of variation in mesophyll resistance in eastern Australian sclerophylls: news of a long and winding path. Journal of Experimental Botany 63 , 5105 – 5119 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosens T, Nishida K, Gago J, et al. . 2016. . The photosynthetic capacity in 35 ferns and fern allies: mesophyll CO2 diffusion as a key trait. New Phytologist 209 , 1576 – 1590 . [DOI] [PubMed] [Google Scholar]
- Veromann-Jürgenson L-L, Tosens T, Laanisto L, Niinemets Ü. 2017. . Extremely thick cell walls and low mesophyll conductance: welcome to the world of ancient living! Journal of Experimental Botany 68 , 1639–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]