Highlight
Stomatal conductance of two species (a broadleaf and a conifer) increased with increasing temperature. This response was independent of carbon metabolism, plant water status, or vapour pressure difference.
Keywords: Ball–Berry model, elevated temperature, evaporative cooling, global change, heat waves, stomatal conductance.
Abstract
The effect of temperature on stomatal conductance (gs) and corresponding gas exchange parameters was studied in two tree species with contrasting leaf anatomy and ecophysiology—a broadleaf angiosperm, Populus deltoides x nigra (poplar), and a needle-leaf gymnosperm, Pinus taeda (loblolly pine). Experiments were conducted in growth chambers across a leaf temperature range of 19–48°C. Manipulations of temperature were done in well-watered and drought soil conditions and under ambient (400 ppm) and elevated (800 ppm) air CO2 concentrations. Increases in leaf temperature caused stomatal opening at both ambient and elevated [CO2]. The gs increased by 42% in poplar and by 40% in loblolly pine when leaf temperature increased from 30°C to 40°C at a vapour pressure difference of 1 kPa. Stomatal limitation to photosynthesis decreased in elevated temperature in loblolly pine but not in poplar. The ratio of net photosynthesis to gs depended on leaf temperature, especially at high temperatures. Evaporative cooling of transpiring leaves resulted in reductions in leaf temperature up to 9°C in well-watered poplar but only 1°C in drought-stressed poplar and in loblolly pine. As global mean temperatures rise and temperature extremes become more frequent and severe, understanding the effect of temperature on gs, and modelling that relationship, will become increasingly important.
Introduction
Plant stomata play a key role in water and carbon cycles. On average, plant transpiration accounts for 61% of global evapotranspiration (Schlesinger and Jasechko, 2014). In other words, most water moving from terrestrial ecosystems into the atmosphere passes through plants and the precise amount is regulated by stomata. At the same time, stomatal conductance (gs) is a key factor determining the rate of net photosynthesis (A) and, therefore, the global carbon cycle and plant carbon metabolism. As a result, stomatal regulation is one of the main factors which determine local growth and survival of plants and global cycles of mass and energy. Stomatal conductance is so important that it has become central to many models from the leaf level (Ball et al., 1987; Leuning et al., 1995; Jarvis and Davies, 1998; Tuzet et al., 2003), to the tree- and forest-stand level (Mirfenderesgi et al., 2016; Xu et al., 2016), and even up to the global level (Niyogi et al., 2009; Berry, 2012; Verhoef and Egea, 2014). However, the conditions in which plants grow are changing and we still do not know enough about plant stomatal regulation to predict future stomatal responses of plant species and their effects at ecosystem and global scales (Lin et al., 2015).
Temperature is one of the most variable factors in the environment and it affects many plant physiological processes, yet little is known about its effect on gs, especially at high temperatures (Teskey et al., 2015). Historically, temperatures over 40°C have been recorded in many places in North America. It has been predicted that mean maximum summer temperatures will increase 5°C in the eastern US within this century (Lynn et al., 2007). Here, we studied effects of temperature on the leaf gas exchange of two North American tree species, Pinus taeda (loblolly pine) and Populus deltoides x nigra (hybrid poplar). Loblolly pine is native to the south-eastern US where the highest temperatures recorded among the 12 states in the region range from 43 to 49°C, with a mean maximum temperature for all 12 states of 45°C (National Climatic Data Center, 2016). Hybrid poplar is widely planted in the Northern Great Plains, which includes the states of Nebraska, Wyoming, Montana, North Dakota and South Dakota. The highest recorded temperatures in those states range from 46 to 49°C with a mean of 48°C. In addition to increases in mean air temperature (Ta), the frequency of extreme temperatures and the severity of heat waves have also increased, and are likely to increase further (Meehl and Tebaldi, 2004; Perkins et al., 2012). Summertime extreme temperatures associated with prolonged heat waves now impact approximately 10% of land surfaces, up from 1% in the 1960s (Hansen et al., 2012). Over recent decades record-breaking monthly temperature extremes have occurred five times more often than during the late 19th through the mid-20th century (Coumou and Robinson, 2013). Heat waves are usually associated with low precipitation and soil drought (Ciais et al., 2005; Stéfanon et al., 2014). However, the frequency of heat waves during wet periods is also increasing. When temperature and precipitation were compared between the periods of 1951–1977 and 1978–2004, it was apparent that both wet/hot and dry/hot conditions were increasing substantially worldwide (Hao et al., 2013). Effects of the increasing frequency and severity of extreme temperature events on gs are largely unknown.
Results of experiments that examined the direct dependence of gs on temperature have not been consistent. Previous studies have reported a complete range of responses to increased temperature, including stomatal opening (Schulze et al., 1974; Freeden and Sage, 1999; Lu et al., 2000; Mott and Peak, 2010), no significant response (Teskey et al., 1986; Sage and Sharkey, 1987; Cerasoli et al., 2014; von Caemmerer and Evans, 2015), and stomatal closure (Raven et al., 2005; Weston and Bauerle, 2007; Lahr et al., 2015). A peaked response with maximum gs at 20°C (Way et al., 2011) or more complex responses with one peak between 20 and 30°C and an additional increase at extremely high temperatures (Slot et al., 2016) have also been described. One possible explanation for these inconsistent results is that to isolate the direct effect of temperature on gs requires a well-controlled environment, which is often hard to achieve, particularly with respect to vapour pressure difference (VPD). In addition, differences in sensitivity to heat are likely related to species, whether plants were grown in the laboratory or in the field, and the range of measurement temperature (Slot et al., 2016).
It has been well established that plants regulate rates of transpiration and photosynthesis in parallel, maintaining a balance between gs and A (Lawson et al., 2011). Therefore, the effect of temperature on stomata is often considered to be indirect, through VPD, transpiration, leaf water potential, or the effect of temperature on photosynthesis or intercellular [CO2] (Ci). This parallel regulation results in the conservation Ci at a given atmospheric [CO2] (Ca) and a close correspondence between gs and A (Wong et al., 1979; Hetherington and Woodward, 2003). The latter relationship has been central to several models of stomatal control of photosynthesis (Farquhar and Wong, 1984; Ball et al., 1987; Leuning, 1995; Buckley et al., 2003), which assume that the ratio of gs correlates with A over a wide range of environmental conditions. However, some studies indicated that this relationship was decoupled under extreme temperature during heat waves, such that A decreased, but gs did not. For example, during an imposed heat wave in which daily maximum Ta ranged from 47 to 53°C and VPD ranged from 6 to 8 kPa, Pinus taeda and Quercus rubra seedlings exhibited progressively lower A on each day of the heat wave but almost no change in gs (Ameye et al., 2012). Similarly, gs of Acer rubrum changed very little across a temperature range of 35 to 48°C (Weston and Bauerle, 2007). In a study of five species, gs either increased or did not decline as Ta increased from 20 to 40°C, even though A initially increased from 20 to 30°C and then decreased (von Caemmerer and Evans, 2015). Collectively these studies suggest that the mechanism modulating stomatal aperture may be independent of A at higher temperatures. However, because VPD varied with temperature in all of these studies, it could not be determined to what degree the observed changes in gs were due to a change in VPD or in the A, or were a direct response to temperature.
In this study, we addressed the following questions: (i) What is the direct effect of moderate to high temperature on gs? (ii) Is the effect of moderate to high temperature on gs altered by water stress or Ca? (iii) How does the response of gs to temperature link to other related factors such as A, Ci, and water status (transpiration, water potential), and how does the correlation between gs and A, which is crucial to many models, change with temperature? (iv) What is the magnitude of evaporative cooling under extreme temperatures? To answer these questions we performed leaf gas exchange measurements on two contrasting tree species: poplar (Populus deltoides x nigra) and loblolly pine (Pinus taeda) across a range of temperature and humidity and under well-watered and drought-stress conditions.
Material and methods
Growth chambers and tree material
Trees were grown, and measurements conducted, in two walk-in growth chambers (EGC 36, Environmental Growth Chambers, Chagrin Falls, OH, USA) at the University of Georgia campus in Athens, GA, USA. Prior to the start of experimental treatments, the trees were grown in the chambers for 30 days at 26°C/ 23°C (day/night) Ta, 1700/560 Pa (day/night) air VPD, and a daily light period of 13 hours. Photosynthetically active radiation (PAR) in the chambers was 520 μmol m−2 s−1. Air speed in each chamber was maintained at 1 m s−1. During the growth period the Ca was maintained above 400 µmol mol−1 as follows: a CO2 sensor (GMM 220, Vaisala, Helsinki, Finland) monitored [CO2] in each chamber and controlled a solenoid valve that released CO2 from a compressed gas cylinder into the chamber whenever the [CO2] fell below the 400 µmol mol−1 set point. Although this procedure prevented the [CO2] from decreasing below 400 µmol mol−1 during periods of active photosynthesis, it did not prevent increases above 400 µmol mol−1. To mitigate the build-up of CO2 in the chambers, the exterior room windows were fully opened and a large exhaust fan was placed in one window. We estimate that daytime ambient [CO2] in the chambers was typically between 400 and 475 µmol mol−1.
Measurements were made on clones of two tree species: a poplar (Populus deltoides x nigra) clone obtained as cuttings (OP-367, hybridpoplars.com, Glenmoore, PA, USA) and a loblolly pine (Pinus taeda) clone from the South Carolina Coastal Plain (Arborgen, Ridgeville, SC, USA). Two-year-old loblolly pine saplings, originally grown in 4-L pots in a greenhouse under natural temperature fluctuations with temperatures commonly reaching ~ 40°C, and poplar cuttings were planted in March 2014 into 15-L pots in a potting medium (Cofer’s Nursery Mix, Cofer’s, Athens, GA, USA). Each pot was fertilized with 40 g of 15-9-12 extended release fertilizer (Osmocote Plus #903286, Scotts-Sierra Horticultural Products, Marysville, OH, USA) and 0.2 g of chelated iron (Sprint 138, Becker Underwood, Ames, IA, USA). Trees were watered daily to full soil water capacity. At the beginning of the experiment, in April 2014, the mean stem height of the poplars was 1.05 m, and the diameter 10 cm above soil was 9.2 mm. The mean height and diameter of the loblolly pines were 1.1 m and 13.9 mm, respectively.
Gas exchange measurements
Measurements of light-saturated net photosynthesis (Asat), gs, rate of transpiration (E), and Ci were made with a portable photosynthesis system equipped with a CO2 mixer (LI-6400–20, LiCor Biosciences, Lincoln, NE, USA). Leaf cuvette conditions were set as follows: block temperature was set at ambient (growth chamber) temperature; [CO2] was set at either 400 µmol mol−1 or 800 µmol mol−1, equal to the concentration in the growth chamber; relative humidity was maintained equal to that in the growth chamber; and PAR was set at 1200 µmol m−2 s−1, resulting in light-saturated photosynthesis and no decline as a result of photorespiration (see figure 2 in Ingwers et al. (2016) for the photosynthetic light response curve of loblolly pine trees of the same clone measured in the same growth chambers). Measurements of loblolly pine foliage were made on two fully developed fascicles (six needles total) of the second flush attached to the main stem. The needles were arranged in the cuvette on a flat plane with equal spacing between needles to maximize light interception. After the gas exchange measurement, the widths of each of three sides of the needles were measured with a scale loupe and used to calculate the foliage area in the cuvette. For poplar, measurements were made on approximately the 30th leaf from the top of the plant. Gas exchange measurements were performed on six trees of each species (n=6). Gas exchange results were calculated on a total surface area basis for loblolly pine and a one-sided surface area basis for poplar.
Experimental setup
Responses to changes in temperature and VPD under various [CO2] and soil moisture
To determine stomatal responses to temperature and VPD, Ta in the growth chamber was controlled at 20, 30, 40, or 49°C and relative humidity was changed from approximately 30 to 80% at each temperature. The sequence of the temperature changes was chosen randomly and individual trees were excluded from further measurements after they had been subjected to 49°C. Six trees were allowed to acclimate for at least 45 minutes after each change in environmental conditions. At every measurement, gs, A, E, and Ci were recorded. To ensure high water availability, during the measurement period the base of each pot was placed in a 5-cm-tall container that was kept full of water. Pre-light water potential (ΨP) and water potential at varying Ta and VPD in the light were measured on foliage using a pressure chamber (model 700, PMS Instrument, Albany, OR, USA). Mean ΨP was −0.28 ± 0.02 and −0.13 ± 0.02 MPa (mean ± standard error) for loblolly pine and poplar, respectively. Measurements were conducted under ambient [CO2] (400 µmol mol−1) and elevated [CO2] (800 µmol mol−1). For measurements under elevated [CO2], the [CO2] was increased in the growth chamber to 800 µmol mol−1 as described above by reprogramming the set point of the CO2 sensor. The plants were allowed to equilibrate to elevated [CO2] for 24 hours prior to measurements.
In a subsequent experiment the effect of soil water deficit on the stomatal response to temperature was investigated. After withholding water for 5 days, the mean ΨP of the poplar plants was −0.81 ± 0.10 MPa. After withholding water for 12 days, the mean ΨP of the loblolly pine plants was −0.97 ± 0.06 MPa. On those days, measurements were made using the same combinations of temperature and humidity as in the first experiment. The effect of water deficit was studied only at ambient [CO2]. The first experiment and this experiment were conducted on different trees (n=6 for each experiment).
Effect of Ci on Asat at various temperatures
Under well-watered conditions, A/Ci curves were measured in the growth chamber on six trees of each species. The VPD was held constant at 1.2 kPa at a leaf temperature (Tl) of 20°C and 3.5 kPa at a Tl of 30°C and 40°C both in the growth chamber and the cuvette. PAR in the cuvette was set at 1200 µmol m−2 s−1. The [CO2] in the cuvette was manipulated from 50 to 100 µmol mol−1 and then in 100 µmol mol−1 steps to 1800 µmol mol−1. The A/Ci Curve Fitting Utility, version 1.1 (Long and Bernacchi, 2003) was used to determine the maximum rate of Rubisco carboxylation (Vcmax, μmol m−2 s−1), maximum rate of photosynthetic electron transport (Jmax, μmol m−2 s−1), maximum rate of triose-phosphate utilization (VTPU, μmol m−2 s−1), and day respiration in the absence of mitochondrial respiration (Rd*, μmol m−2 s−1).
Stomatal limitation to photosynthesis (Ls) was estimated at [CO2] 400 µmol mol−1 from fitted curves using the equation:
| (1) |
where A0 is the light-saturated net photosynthesis rate that would occur at infinite gs (Farquhar and Sharkey, 1982).
Cooling effect
Under lighted conditions, the cooling effect of transpiration was estimated as the difference between the temperature of normal transpiring foliage and foliage greased with petroleum jelly to prevent transpiration (Jones et al., 2002) at the same position on the plant. Leaves and needles were chosen for this comparison at a position on the plant close to the point where gas exchange was measured. Tl was measured with an infrared thermometer (Model 561, Fluke, Everett, WA, USA) with emissivity set to 0.97.
Statistical analysis
Prior to the analyses, the normality of data was determined using the Shapiro–Wilk test. We used linear and non-linear multiregression analysis to describe the dependence of gs on external factors (i.e. Tl, VPD). A least-squares regression was used to fit the 3D models to the data. Models used to fit data are listed in Supplementary Table S1 (available at JXB online). An F-test was used to test significance of model parameters. Analysis of the generalized linear model was used to test for differences among independent variables and a dependent variable when VPD was a continuous predictor. Tests were performed at α=0.05. Most statistical analyses were performed using SigmaPlot 12.5 software (Systat, San Jose, CA, USA) with the exception of the generalized linear model analysis, which was done in Statistica 12 (StatSoft, Tulsa, OK, USA).
Results
Responses of gs, E, and Asat to Tl and VPD
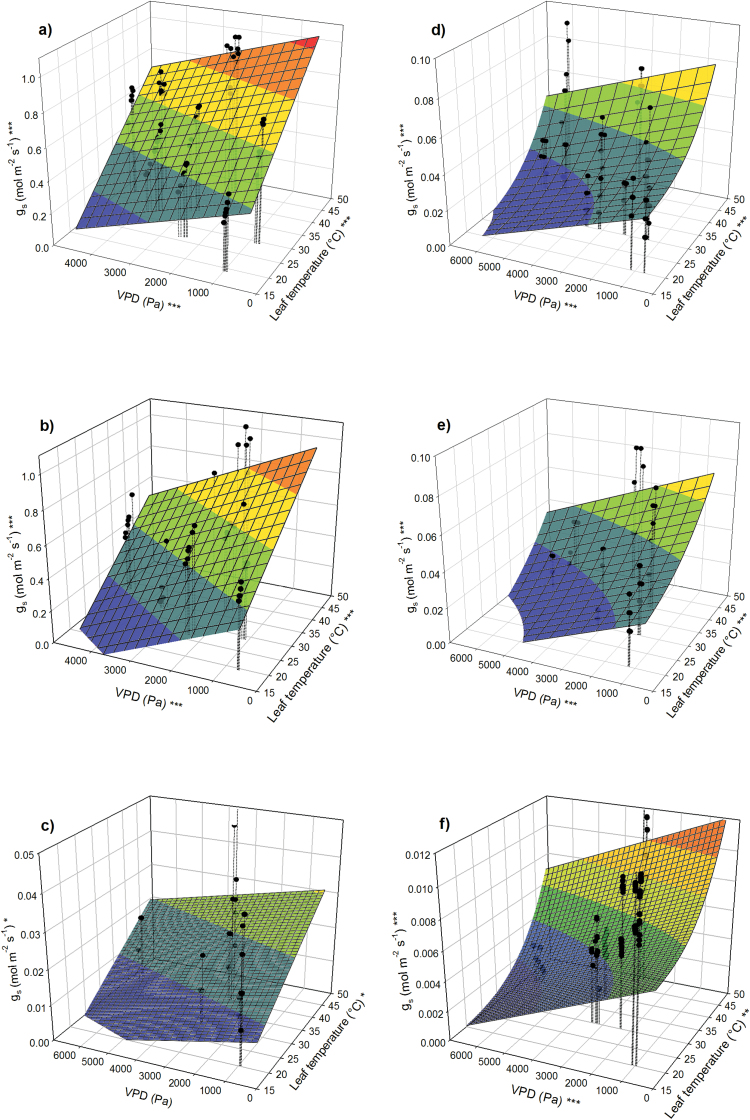
The gs increased with increasing Tl and Ta in both species in all tested environmental conditions (Fig. 1 and Supplementary Fig. S1, available at JXB online). Under unlimited soil water availability and a VPD of 1 kPa and [CO2] of 400 μmol mol−1, an increase in Tl from 30 to 40°C led to an increase in gs of 42% in poplar and 40% in loblolly pine (Fig. 1a, d; Supplementary Table S1; P < 0.001). The rate of increase in gs with temperature was linear in poplar, but gs increased more at high than at low Tl in loblolly pine. Increasing the [CO2] from 400 to 800 μmol mol−1 caused partial stomatal closure, which was more pronounced in poplar (mean decrease of 21% at VPD 3.5 kPa, P < 0.001) than in loblolly pine (mean decrease of 12% at the same VPD, P = 0.030). However, similar to results in ambient [CO2], gs increased with increasing Tl in both species under elevated [CO2] (Fig. 1b, e; P < 0.001). Soil water deficit significantly reduced gs in both species, but more so in poplar than pine (Fig. 1c, f; P < 0.001). Even though gs was reduced in drought conditions, gs of both species still increased with increasing Tl (P = 0.040 for poplar and P < 0.001 for loblolly pine).
Fig. 1.
Stomatal conductance (gs) of poplar (left panels) and loblolly pine (right panels) and its dependence on Tl and VPD. Plants were measured in high soil moisture conditions and (a, d) ambient [CO2] or (b, e) elevated [CO2]. (c, f) Measurements made on drought-stressed trees at ambient [CO2]. Linear regression was used to fit the data for poplar and non-linear regression was used for loblolly pine. Asterisks at the z-axis label indicate overall significance of the model; asterisks at the x-and y-axes indicate significance of the respective parameters (*P < 0.05; **P < 0.01; ***P < 0.001).
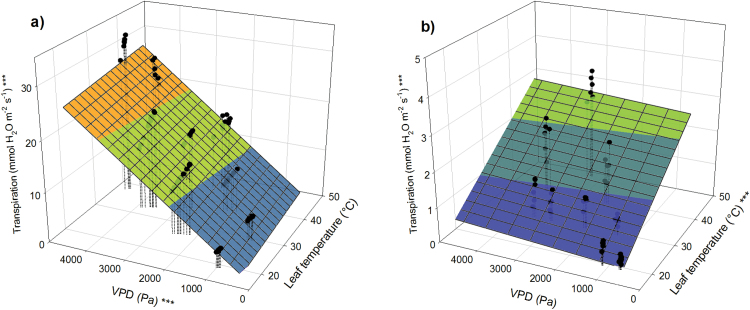
Transpiration (E) increased significantly with increasing Tl (and Ta) or VPD in both species under unlimited soil water availability and ambient [CO2] (Fig. 2a, b and Supplementary Fig. S2a, b, available at JXB online). However, the relationships between E and environmental variables differed substantially between poplar and loblolly pine. Transpiration of poplar increased with VPD (P < 0.001) but not with Tl (P = 0.06). Conversely, in loblolly pine, E increased only with Tl (P < 0.001) but not with VPD (P = 0.15).
Fig. 2.
Response of E to VPD in (a) poplar and (b) loblolly pine at varying Tl and VPD. Asterisks at the z-axis label indicate overall significance of the model; asterisks at the x- and y-axes indicate significance of the respective parameters (*P < 0.05; **P < 0.01; ***P < 0.001).
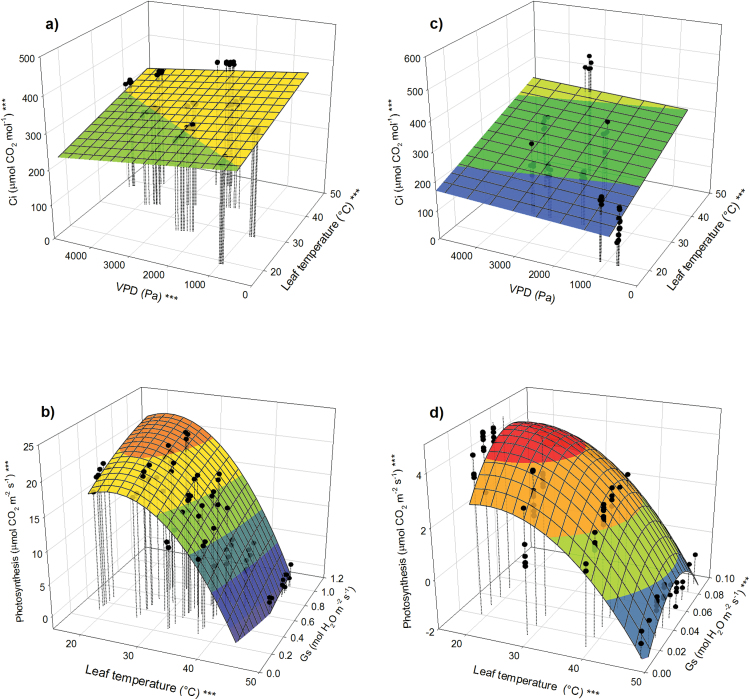
Under well-watered conditions, Ci increased with increasing temperature in both species (Fig. 3a, c, P < 0.001, and Supplementary Fig. S2c, d). A decrease in Ci with increasing VPD was observed in poplar (P < 0.001) but not in loblolly pine (P = 0.15). In addition, the range of Ci was smaller in poplar than in loblolly pine. Tl (and Ta) had an effect on A in both species (Fig. 3b, d, P < 0.001, and Supplementary Fig. S3, available at JXB online). In both species, at a given Tl there was a specific relationship between Asat and gs. However, this relationship between Asat and gs changed with Tl (Fig. 3b, d, P < 0.001).
Fig. 3.
(a, c) Relationship between Ci, Tl, and VPD for poplar (left panels) and loblolly pine (right panels). (b, d) Relationship between Asat, Tl, and gs. Asterisks at the z-axis label indicate overall significance of the model; asterisks at the x- and y-axes indicate significance of the respective parameters (*P < 0.05; **P < 0.01; ***P < 0.001).
A/Ci curves and Ls to Asat at various Tl
Temperature had a large effect on the parameters of A/Ci curves in both poplar and loblolly pine (Table 1). Stomata of poplar imposed a smaller limitation on the diffusion of CO2 than stomata of loblolly pine. The relative Ls in poplar did not exceed 20% while in loblolly pine they were between 23 and 78%. Ls was directly comparable between 30 and 40°C because it was measured at the same VPD. While Ls in poplar did not change (P = 0.21) with a Tl increase from 30 to 40°C, Ls in loblolly pine declined under the same temperature increase (P < 0.001). The values of parameters related to biochemical processes of photosynthesis (Vcmax, Jmax, VTPU, and Rd*) consistently increased with Tl in both species, with the exception of VTPU in poplar.
Table 1.
Parameters related to biochemical processes of photosynthesis in poplar and loblolly pine plants measured at three leaf temperatures
| Species | T l (°C) | Vcmax | J max | V TPU | R d * | L s |
|---|---|---|---|---|---|---|
| Poplar | 20 | 66 | 132 | 10.05 | 2.10 | 0.19 |
| 30 | 165 | 151 | 11.11 | 1.9 | 0.16 | |
| 40 | 301 | 165 | 11.46 | 3.25 | 0.2 | |
| P-value | <0.001 | <0.001 | 0.07 | <0.001 | 0.21 | |
| Loblolly pine | 20 | 21 | 45 | 3.62 | 1.55 | 0.41 |
| 30 | 67 | 71 | 4.57 | 2.73 | 0.78 | |
| 40 | 163 | 75 | 4.99 | 6.52 | 0.23 | |
| P-value | <0.000 | <0.001 | <0.001 | 0.011 | <0.001 |
Significant differences between measurements at different temperatures indicated in bold.
Effect of E on Tl
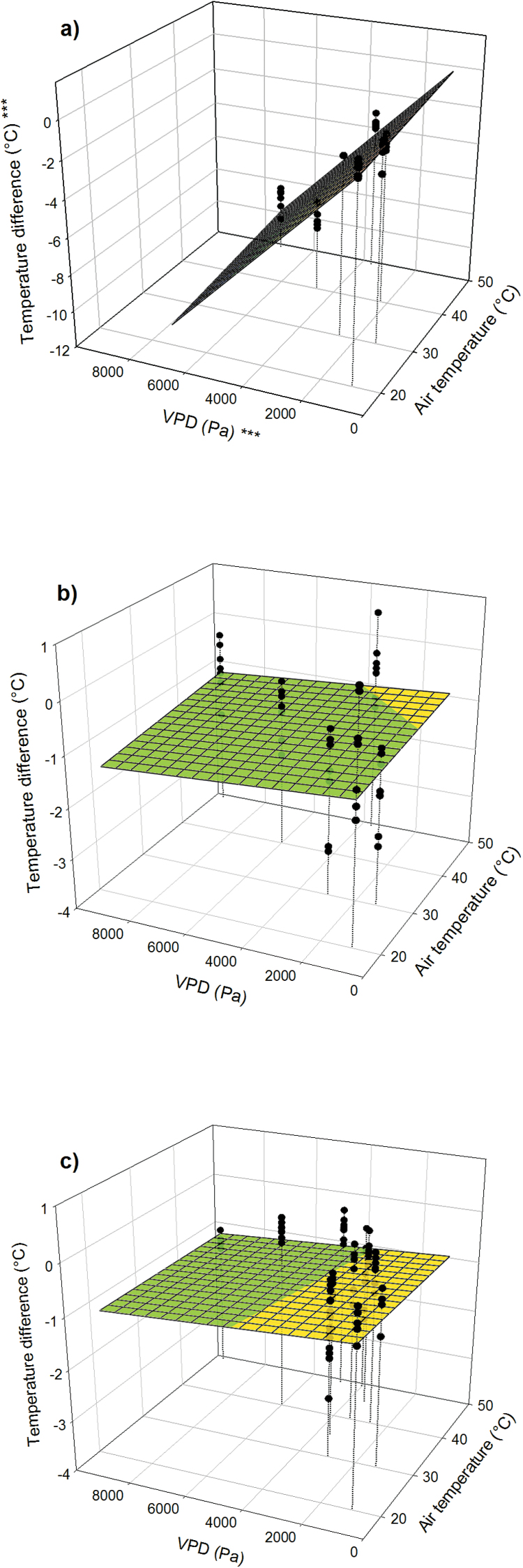
The temperature of transpiring leaves was lower than the temperature of foliage that did not transpire (Fig. 4). The magnitude of the temperature difference in poplars in wet soil reached up to 9.0°C and scaled with VPD (P < 0.001) but not with Ta (Fig. 4a). Transpiring leaves of poplar in dry soil were an average of 1.1°C cooler than non-transpiring leaves (P = 0.02) and the magnitude of the cooling effect depended on neither temperature nor VPD (Fig. 4b). In loblolly pine, transpiring needles were an average of 0.9°C cooler than those that did not transpire (P = 0.002). There was no effect of soil water availability and the magnitude of the cooling did not depend on temperature or VPD (Fig. 4c).
Fig. 4.
Evaporative cooling effect (temperature difference) of transpiration on (a) well-watered poplar, (b) drought-stressed poplar, and (c) loblolly pine at varying Ta and VPD. Asterisks at the z-axis label indicate overall significance of the model; asterisks at the x- and y-axes indicate significance of the respective parameters (*P < 0.05; **P < 0.01; ***P < 0.001).
Leaf water potential
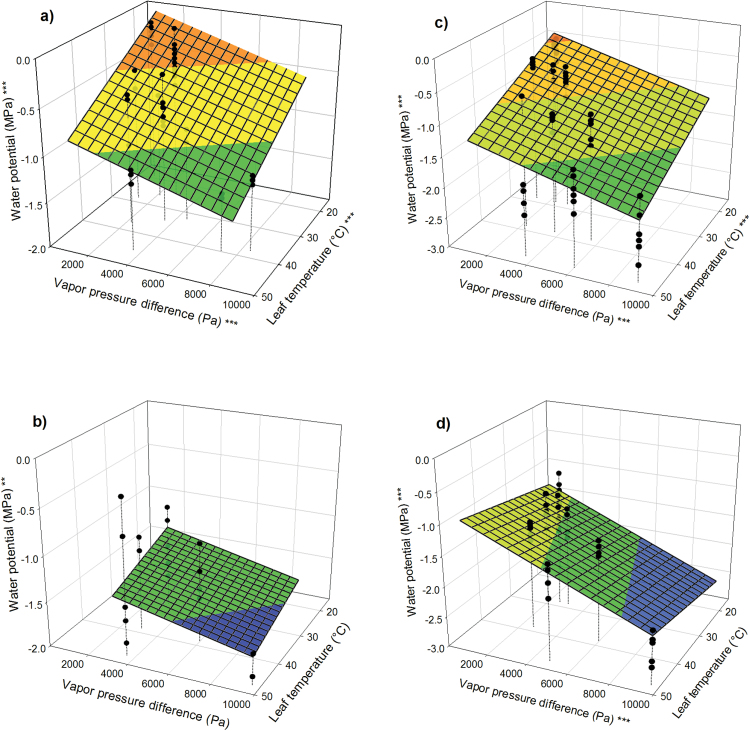
Leaf water potential decreased with increasing Tl and VPD in both species when the soil was wet (Fig. 5a, c). When soil was dry, leaf water potential scaled with both Tl and VPD in poplar, but in loblolly pine only VPD had an effect on water potential (Fig. 5b, d). At the same Tl and VPD, poplar maintained higher water potential than loblolly pine.
Fig. 5.
Leaf water potential of (a, b) poplar and (c, d) loblolly pine in wet soil (a, c) and dry soil (b, d). Asterisks at the z-axis label indicate overall significance of the model; asterisks at the x- and y-axes indicate significance of the respective parameters (*P < 0.05; **P < 0.01; ***P < 0.001).
Discussion
Stomatal conductance, stomatal limitations, and photosynthesis
Stomata play a key role in regulating fluxes of water and carbon dioxide between plant and atmosphere. They regulate both plant growth and cycles of mass and energy. Therefore, much attention has been focused on principles of stomatal regulation and several regulatory mechanisms have been identified. Most research has centred on the stomatal responses to various indices of water status and carbon balance (Farquhar and Sharkey, 1982; Jones, 1998; Buckley et al., 2003). Surprisingly little attention has been paid to the responses of gs to temperature, even though it is one of the most variable environmental factors. A few previous studies suggested a dependence of gs on temperature. However, these studies have often provided conflicting results. While some evidence suggested that gs increased with increasing temperature (Schulze et al., 1974; Lu et al., 2000; Mott and Peak, 2010), other studies found that temperature had no effect on stomata (Teskey et al., 1986; Sage and Sharkey, 1987; Cerasoli et al., 2014; von Caemmerer and Evans, 2015), or that increased temperature triggered stomatal closure (Weston and Bauerle, 2007; Lahr et al., 2015). One explanation for the conflicting results across these studies might be that the experiments were often conducted in uncontrolled environmental conditions in the field. The design of our experiment, in which the response of gs to Tl was separated from the effect of VPD and all measurements were made under constant illumination, allowed us to separate the effect of temperature from the effects of other factors.
Our results conclusively demonstrated that there is a strong direct positive response of gs to increasing Tl in two tree species. In well-watered trees, temperature and VPD had major effects on gs, as suggested by Freeden and Sage (1999). Elevated Ca caused a decline in gs but did not fully mitigate increased stomatal opening in response to increased temperature. The increase in gs with increased Tl was found in both species despite large differences in leaf morphology, xylem structure, and physiology. However, because of these differences, the magnitude of stomatal opening in response to Tl and closing in response to elevated [CO2], along with the effects on associated physiological processes (such as E and A), differed between the two species. The interplay between elevated Tl, which increased gs, and elevated [CO2], which decreased gs, differed between the two species, suggesting that it could contribute to differences in behaviour among species in the predicted future climate.
The two experimental species stand at opposite ends of the range of mechanisms for stomatal adjustment of water loss. E in poplar continuously increased with increasing VPD, while E of loblolly pine remained the same over a large range of VPD within a given Tl and increased with increases in Tl (Fig. 2, Supplementary Fig. S2). These results suggest that gs is regulated by more complex mechanisms than simply E (Mott and Parkhust, 1991), and that temperature changes affect the relationship between E and gs.
Leaf water potential declined with both increased temperature and increased VPD in both species (Fig. 5). Typically, gs declines with a decline in water potential across a wide range of both iso- and anisohydric species (Klein, 2014). But in our study, despite a decline in water potential, gs increased with temperature. Stomata may have opened with increasing temperature owing to, in part, changes in hydraulic conductivity. When temperature increases, the viscosity of water declines and mesophyll conductance (gm) increases, which may improve the supply of water to sites of evaporation and thus increase stomatal aperture (Cochard et al., 2000; von Caemmerer and Evans, 2015). However, this increase was not great enough to prevent a decline in leaf water potential. Therefore, it was proposed that resistance to water vapour and heat transfer among sites of evaporation and guard cells, which induce differences in temperature and VPD at these sites, may also regulate stomatal opening in response to transpiration and Tl (Mott and Peak, 2013). The general increase in overall tree hydraulic conductance due to water viscosity may be further modified by temperature-dependent variability in tree xylem hydraulic conductance, which, due to differences in vascular traits, may contribute to differences in the responses of conifers and angiosperm trees (Wolf et al., 2016). Changes in leaf gm may be further paired with xylem resistance to embolism and the safety margin against cavitation, which is higher in conifers than in angiosperms (Choat et al., 2012). Trees adjust their gs to maximize CO2 uptake (resulting in higher E) but still protect xylem against excessive cavitation (Brodribb et al., 2016). Loblolly pine strictly regulated transpiration such that it did not change with variation in VPD, thus protecting xylem against cavitation and maintaining a broad safety margin. However, when temperature increased, loblolly pine was not able to maintain this strict control over water loss, so E increased. This result may suggest that in the pine, overall resistance of the hydraulic pathway (including xylem and mesophyll resistance) significantly contributed to regulation of transpiration and that stomatal regulation was at least partly independent of E. In contrast, the broadleaf poplar exerted the same degree of stomatal control on E at all temperatures. The inability of loblolly pine to regulate E when temperature increases may negatively impact survival with climate change and may contribute to succession by angiosperm tree species (Carnicer et al., 2013).
Apart from plant water status, other mechanisms known to regulate gs are related to photosynthesis, to which stomata often present a large limitation. Ls in loblolly pine is usually lower than 65%. Higher Ls may occur but it is usually attributed to low soil water potential or low temperature (Teskey et al., 1986; Sasek and Richardson, 1989; Ellsworth, 2000). In this study, when VPD was high, Ls of 78% was observed at 30°C (Table 1), indicating strong stomatal control of carbon gain in the range of temperature which is optimal for photosynthesis. With increasing Tl, Ls declined. Therefore, photosynthesis of loblolly pine may partly benefit from the decline in Ls at increased temperature, even though the extremely high temperature will set biochemical limits to A and the resulting A may be the same or lower. In contrast to loblolly pine, Ls in poplar was unaffected by Tl and was generally lower than 20%. Low Ls in poplar in this study corresponded to low Ls in poplar observed previously; for example, Ls averaged 10% in two clones of Populus (Noormets and Sober, 2001). The lower Ls in poplar compared with loblolly pine may have been related to the ratio of gs to gm. Although we did not measure gm, it is generally lower in conifers than in angiosperm trees (Flexas et al., 2012), suggesting Ls should also be lower. However, because Ls was not lower, we speculate that the ratio of gs to gm also differed between the species. The high Asat in poplar might be related to high gs/gm, which could support increased photosynthesis by increasing Ci and keeping [CO2] at the chloroplasts high. It could also increase nutrient acquisition through increased E, which would enhance photosynthetic capacity. gm also increases with temperature in a wide range of species (von Caemmerer and Evans, 2015). However, this mechanism does not explain the increase in gs at supra-optimal temperatures at which Asat becomes low or negative.
Low Ls in poplar was linked to high gs, which results in low water use efficiency of photosynthesis. The advantage of low Ls, which favours fast-growing species under an unlimited soil water supply, may jeopardize their existence during heat waves when high E depletes available soil water, resulting in increased drought stress, especially under initial conditions of low soil moisture. The effect of variable Ls was further demonstrated by the alteration of Ci in loblolly pine. Normally the ratio of Ci:Ca is highly conserved (Liu and Teskey, 1995), as was observed in poplar when Ci consistently remained at ~300 μmol mol−1 at all temperatures (Fig. 3a). However, Ci in loblolly pine was highly variable, ranging from ~165 µmol mol−1 at 20°C to ~240 µmol mol−1 at 40°C (Fig. 3c), which corresponds with prior observations of high variability in Ci with changing environmental conditions in this species (Green and Mitchell, 1992).
Evaporative cooling
Evaporation of water from the leaf surface can significantly lower Tl (Monteith, 1981; Jones, 1999). As long as stomata remain open, evaporative cooling can mitigate the negative effect of supra-optimal Ta on A during heat waves and can positively affect photosynthesis, yield, and plant survival (Lu et al., 1994; Ameye et al., 2012). Maintaining Tl through regulation of E to minimize stress at high Ta was theoretically suggested (Mahan and Upchurch, 1988) and observations in Arabidopsis thaliana indicated that plants regulate water loss and even adjust their architecture to achieve the best cooling effect (Crawford et al., 2012). The magnitude of the cooling effect is often several degrees (Jones, 1999; Feller, 2006). In our study the maximum cooling, 9°C, was observed in poplar at high Ta and high VPD (Fig. 4). This rate of cooling lowered Tl from 49 to 40°C and positive A was observed at this extreme Ta. In contrast to poplar, gs of loblolly pine was roughly 10 times lower and therefore the maximum cooling effect was only 0.9°C. Consequently, at Ta of 49°C, poplar had positive A and loblolly pine did not. The cooling effect due to stomatal opening at high temperature (under well-watered conditions) is likely to be much more beneficial in species with high gs than those with low gs.
Evaporative cooling may help plants survive heat waves, especially when the air is dry. However, this mechanism requires sufficient soil water supply, which relies on high soil water capacity and sufficient hydraulic conductivity. With a long-duration heat wave, high E may result in the depletion of soil water storage and plants will no longer be able to utilize this mechanism to minimize heat stress. This effect was observed in our study: only a very small cooling effect (1.1°C) was observed in drought-stressed trees (Fig. 4b). Nevertheless, evaporative cooling proved to have a significant effect on A and may play an important role in the diurnal regulation of Tl during short-duration heat waves. In addition to soil water availability, elevated [CO2] affects gs. Stomatal closure resulting from elevated [CO2] will to some degree counteract the opening effect of elevated temperature. Results of this study, demonstrating that stomata of poplar are more sensitive to [CO2] than stomata of loblolly pine, were similar to previous findings on broadleaf and conifer species in general (Medlyn et al., 2001). Therefore, if stomata in broadleaf species close in response to future predicted increases in [CO2], the difference in the rate of evaporative cooling between broadleaf and conifer species may shrink.
Relationships among gs, Ci, and A
In both species we found that the positive relationship between Asat and gs observed at lower temperatures was not present at extremely high temperatures. The most obvious impairment occurred at a Tl >40°C, when Asat became negative and yet the stomata remained open (Fig. 3). Ci at this temperature increased and approached the ambient [CO2] of 400 µmol mol−1. Under these conditions a reduction in gs would be expected (Hashimoto et al., 2006), but instead the stomata opened even more. These results do not imply that stomata do not react to Ci. Rather, it appears that there was a direct stomatal response to supra-optimal temperature that overrode the response to Ci.
Many models of gs assume a fixed relationship between A and gs regardless of temperature (Ball et al., 1987; Leuning, 1995; Buckley et al., 2003). These models have been widely used and, in a comparison with other models of gs, provided the best results (Way et al., 2011). Our study also provided evidence of a stable relationship between Asat and gs at low temperatures (Fig. 3). However, that stability did not hold true at high temperature. As an extreme example, when Asat became negative at temperatures over ~40°C, the ratio A:Ci also became negative in both species. In such a case, the Ball–Berry–Leuning model, which uses that ratio to predict gs, would provide negative values of gs. Correctly predicting gs from photosynthesis and vice versa, especially at extreme temperatures during heat waves, will require detailed study of the interplay among A, Ci, VPD, Tl, and possibly other factors driving stomatal regulation, which, when applied simultaneously, can have complex effects (Merilo et al., 2014).
Conclusions
We conclude that Tl has a direct effect on stomatal opening in the two tree species we examined. For accurate predictions of gs and plant water use this temperature dependency should be taken into account, especially at high temperatures. Elevated [CO2] reduced gs of both species but general trends of increasing gs with increasing Tl remained similar regardless of [CO2]. Along with changes in gs, Tl also affected Ls to photosynthesis, Ci, and corresponding A. A became negative in both species at extremely high Tl. However, the effect of evaporative cooling, which lowered Tl in the rapidly transpiring poplar, significantly increased A. gs was decoupled from A at high Tl in both species, which is an indication that substantial changes are likely in gas exchange physiology at high temperatures. Further research should focus on verifying results of this laboratory study in the field, as well as discovering the principles of temperature dependency of stomatal regulation and implementing temperature functions into the models of gs.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Gs of poplar and loblolly pine and its dependence on Ta and VPD.
Fig. S2. E and Ci of poplar and loblolly pine and their dependence on Ta and VPD.
Fig. S3. Asat of poplar and loblolly pine and its dependence on gs at Ta 20–49°C.
Table S1. Regression equations and parameters of models used in Figs 1–5.
Supplementary Material
Acknowledgements
This research was supported by project MSMT COST LD 13017 financed by the Czech Republic under the framework of the COST FP1106 network STReESS and by project 5-100 financed by the Russian government. We thank ArborGen, Inc. for supplying loblolly pine clonal material.
Glossary
Abbreviations:
- ΨP
pre-light water potential (Pa)
- A
rate of net photosynthesis (μmol m−2 s−1)
- Asat
light-saturated net photosynthesis (μmol m−2 s−1)
- Ca
atmospheric concentration of CO2 (μmol mol−1)
- Ci
intercellular concentration of CO2 (μmol mol−1)
- E
rate of transpiration (mol H2O m−2 s−1)
- gm
mesophyll conductance (mol m−2 s−1)
- gs
stomatal conductance (mol m−2 s−1)
- Jmax
maximum rate of photosynthetic electron transport (μmol m−2 s−1)
- Ls
stomatal limitation to photosynthesis (%)
- PAR
photosynthetically active radiation
- Rd*
day respiration (μmol m−2 s−1)
- Ta
air temperature (°C)
- Tl
leaf temperature (°C)
- Vcmax
maximum rate of Rubisco carboxylation (μmol m−2 s−1)
- VPD
vapour pressure difference (Pa)
- VTPU
maximum rate of triose-phosphate utilization (μmol m−2 s−1).
References
- Ameye M, Wertin TM, Bauweraerts I, McGuire MA, Teskey RO, Steppe K. 2012. The effect of induced heat waves on Pinus taeda and Quercus rubra seedlings in ambient and elevated CO2 atmospheres. The New Phytologist 196, 448–461. [DOI] [PubMed] [Google Scholar]
- Ball J, Woodrow I, Berry J. 1987. A model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions. Progress in Photosynthesis Research IV, 221–224. [Google Scholar]
- Berry JA. 2012. There ought to be an equation for that. Annual Review of Plant Biology 63, 1–17. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SA, Carins Murphy MR. 2016. Xylem and stomata, coordinated through time and space. Plant, Cell & Environment [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Buckley TNT, Mott KAK, Farquhar GDG. 2003. A hydromechanical and biochemical model of stomatal conductance. Plant, Cell & Environment 26, 1767–1785. [Google Scholar]
- Carnicer J, Barbeta A, Sperlich D, Coll M, Peñuelas J. 2013. Contrasting trait syndromes in angiosperms and conifers are associated with different responses of tree growth to temperature on a large scale. Frontiers in Plant Science 4, 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerasoli S, Wertin T, McGuire MA, Rodrigues A, Aubrey DP, Pereira JS, Teskey RO. 2014. Poplar saplings exposed to recurring temperature shifts of different amplitude exhibit differences in leaf gas exchange and growth despite equal mean temperature. AoB Plants 6, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choat B, Jansen S, Brodribb TJ, et al. 2012. Global convergence in the vulnerability of forests to drought. Nature 491, 752–755. [DOI] [PubMed] [Google Scholar]
- Ciais P, Reichstein M, Viovy N, et al. 2005. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 437, 529–533. [DOI] [PubMed] [Google Scholar]
- Cochard H, Martin R, Gross P, Bogeat-Triboulot MB. 2000. Temperature effects on hydraulic conductance and water relations of Quercus robur L. Journal of Experimental Botany 51, 1255–1259. [PubMed] [Google Scholar]
- Coumou D, Robinson A. 2013. Historic and future increase in the global land area affected by monthly heat extremes. Environmental Research Letters 8, 34018. [Google Scholar]
- Crawford AJ, McLachlan DH, Hetherington AM, Franklin KA. 2012. High temperature exposure increases plant cooling capacity. Current Biology 22, R396–R397. [DOI] [PubMed] [Google Scholar]
- Ellsworth DS. 2000. Seasonal CO(2) assimilation and stomatal limitations in a Pinus taeda canopy. Tree Physiology 20, 435–445. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Sharkey TD. 1982. Stomatal conductance and photosynthesis. Annual Review of Plant Physiology 33, 317–345. [Google Scholar]
- Farquhar G, Wong S. 1984. An empirical model of stomatal conductance. Australian Journal of Plant Physiology 11, 191. [Google Scholar]
- Feller U. 2006. Stomatal opening at elevated temperature: an underestimated regulatory mechanism? General and Applied Plant Physiology Special issue, 19–31. [Google Scholar]
- Flexas J, Barbour MM, Brendel O, et al. 2012. Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Science 193–194, 70–84. [DOI] [PubMed] [Google Scholar]
- Freeden AL, Sage RF. 1999. Temperature and humidity effects on branchlet gas-exchange in white spruce: an explanation for the increase in transpiration with branchlet temperature. Trees 14, 161–168. [Google Scholar]
- Green TH, Mitchell RJ. 1992. Effects of nitrogen on the response of loblolly pine to water stress I. Photosynthesis and stomatal conductance. New Phytologist 122, 627–633. [Google Scholar]
- Hansen J, Sato M, Ruedy R. 2012. Perception of climate change. Proceedings of the National Academy of Sciences of the United States of America 109, E2415–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z, AghaKouchak A, Phillips TJ. 2013. Changes in concurrent monthly precipitation and temperature extremes. Environmental Research Letters 8, 34014. [Google Scholar]
- Hashimoto M, Negi J, Young J, Israelsson M, Schroeder JI, Iba K. 2006. Arabidopsis HT1 kinase controls stomatal movements in response to CO2. Nature Cell Biology 8, 391–397. [DOI] [PubMed] [Google Scholar]
- Hetherington AM, Woodward FI. 2003. The role of stomata in sensing and driving environmental change. Nature 424, 901–908. [DOI] [PubMed] [Google Scholar]
- Ingwers MW, Urban J, McGuire MA, Bhuiyan RA, Teskey RO. 2016. Physiological attributes of three- and four-needle fascicles of loblolly pine (Pinus taeda L.). Trees 30, 1923–1933. [Google Scholar]
- Jarvis AJ, Davies WJ. 1998. The coupled response of stomatal conductance to photosynthesis and transpiration. Journal of Experimental Botany 49, 399–406. [Google Scholar]
- Jones H. 1998. Stomatal control of photosynthesis and transpiration. Journal of Experimental Botany 49, 387–398. [Google Scholar]
- Jones HG. 1999. Use of thermography for quantitative studies of spatial and temporal variation of stomatal conductance over leaf surfaces. Plant, Cell & Environment 22, 1043–1055. [Google Scholar]
- Jones HG, Stoll M, Santos T, de Sousa C, Chaves MM, Grant OM. 2002. Use of infrared thermography for monitoring stomatal closure in the field: application to grapevine. Journal of Experimental Botany 53, 2249–2260. [DOI] [PubMed] [Google Scholar]
- Klein T. 2014. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Functional Ecology 28, 1313–1320. [Google Scholar]
- Lahr EC, Schade GW, Crossett CC, Watson MR. 2015. Photosynthesis and isoprene emission from trees along an urban-rural gradient in Texas. Global Change Biology 21, 4221–4236. [DOI] [PubMed] [Google Scholar]
- Lawson T, von Caemmerer S, Baroli I. 2011. Photosynthesis and stomatal behaviour. In: Luttge U, ed. Progress in Botany 72 Berlin, Heidelberg: Springer, 265–304. [Google Scholar]
- Leuning R. 1995. A critical appraisal of a combined stomatal—photosynthesis model for C3 plants. Plant, Cell & Environment 18, 339–355. [Google Scholar]
- Leuning R, Kelliher FM, Pury DGG, De Schulze ED. 1995. Leaf nitrogen, photosynthesis, conductance and transpiration: scaling from leaves to canopies. Plant, Cell & Environment 18, 1183–1200. [Google Scholar]
- Lin Y-S, Medlyn BE, Duursma RA, et al. 2015. Optimal stomatal behaviour around the world. Nature Climate Change 5, 1–6. [Google Scholar]
- Liu S, Teskey RO. 1995. Responses of foliar gas exchange to long-term elevated CO2 concentrations in mature loblolly pine trees. Tree Physiology 15, 351–359. [DOI] [PubMed] [Google Scholar]
- Long SP, Bernacchi CJ. 2003. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. Journal of Experimental Botany 54, 2393–2401. [DOI] [PubMed] [Google Scholar]
- Lu Z, Quiñones M, Zeiger E. 2000. Temperature dependence of guard cell respiration and stomatal conductance co-segregate in an F2 population of Pima cotton. Functional Plant Biology 27, 457–462. [Google Scholar]
- Lu Z, Radin JW, Turcotte EL, Percy R, Zeiger E. 1994. High yields in advanced lines of Pima cotton are associated with higher stomatal conductance, reduced leaf area and lower leaf temperature. Physiologia Plantarum 92, 266–272. [Google Scholar]
- Lynn BH, Healy R, Druyan LM. 2007. An analysis of the potential for extreme temperature change based on observations and model simulations. Journal of Climate 20, 1539–1554. [Google Scholar]
- Mahan J, Upchurch D. 1988. Maintenance of constant leaf temperature by plants—I. Hypothesis-limited homeothermy. Environmental and Experimental Botany 28, 351–357. [Google Scholar]
- Medlyn BE, Barton CVM, Broadmeadow MSJ, et al. 2001. Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: a synthesis. New Phytologist 149, 247–264. [DOI] [PubMed] [Google Scholar]
- Meehl GA, Tebaldi C. 2004. More intense, more frequent, and longer lasting heat waves in the 21st century. Science 305, 994–7. [DOI] [PubMed] [Google Scholar]
- Merilo E, Jõesaar I, Brosché M, Kollist H. 2014. To open or to close: species-specific stomatal responses to simultaneously applied opposing environmental factors. The New Phytologist 202, 499–508. [DOI] [PubMed] [Google Scholar]
- Mirfenderesgi G, Bohrer G, Matheny AM, Fatichi S, de Moraes Frasson RP, Schäfer KVR. 2016. Tree-level hydrodynamic approach for modeling aboveground water storage and stomatal conductance illuminates the effects of tree hydraulic strategy. Journal of Geophysical Research: Biogeosciences 121, 1792–1813. [Google Scholar]
- Monteith J. 1981. Evaporation and surface temperature. Quarterly Journal of the Royal Meteorological Society 107, 1–27. [Google Scholar]
- Mott K, Parkhust D. 1991. Stomatal response to humidity in air and in helox. Plant, Cell & Environment 14, 509–515. [Google Scholar]
- Mott KA, Peak D. 2010. Stomatal responses to humidity and temperature in darkness. Plant, Cell & Environment 33, 1084–1090. [DOI] [PubMed] [Google Scholar]
- Mott KA, Peak D. 2013. Testing a vapour-phase model of stomatal responses to humidity. Plant, Cell & Environment 36, 936–944. [DOI] [PubMed] [Google Scholar]
- National Climatic Data Center 2016. Climate of the United States—extreme events. https://www.ncdc.noaa.gov/climate-information/extreme-events. [Google Scholar]
- Niyogi D, Alapaty K, Raman S, Chen F. 2009. Development and evaluation of a coupled photosynthesis-based gas exchange evapotranspiration model (GEM) for mesoscale weather forecasting applications. Journal of Applied Meteorology and Climatology 48, 349–368. [Google Scholar]
- Noormets A, Sober A. 2001. Stomatal and non-stomatal limitation to photosynthesis in two trembling aspen (Populus tremuloides Michx.) clones exposed to elevated CO2 and/or O3. Plant, Cell & Environment 3, 327–336. [Google Scholar]
- Perkins SE, Alexander LV, Nairn JR. 2012. Increasing frequency, intensity and duration of observed global heatwaves and warm spells. Geophysical Research Letters 39, 1–5. [Google Scholar]
- Raven PH, Evert RF, Eichhorn SE. 2005. Biology of plants. New York:W.H.Freeman & Co Ltd; p727. [Google Scholar]
- Sage RF, Sharkey TD. 1987. The effect of temperature on the occurrence of O2 and CO2 insensitive photosynthesis in field grown plants. Plant Physiology 84, 658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasek TW, Richardson CJ. 1989. Effects of chronic doses of ozone on loblolly pine: photosynthetic characteristics in the third growing season. Forest Science 35, 745–755. [Google Scholar]
- Schlesinger WH, Jasechko S. 2014. Transpiration in the global water cycle. Agricultural and Forest Meteorology 189–190, 115–117. [Google Scholar]
- Schulze E, Lange OL, Evenari M, Kappen L, Buschbom U. 1974. The role of air humidity and leaf temperature in controlling stomatal resistance of Prunus armeniaca L. under desert conditions. I. A simulation of the daily course of stomatal resistance. Oecologia 17, 159–170. [DOI] [PubMed] [Google Scholar]
- Slot M, Garcia MN, Winter K. 2016. Temperature response of CO2 exchange in three tropical tree species. Functional Plant Biology 43, 468–478. [DOI] [PubMed] [Google Scholar]
- Stéfanon M, Drobinski P, D’Andrea F, Lebeaupin-Brossier C, Bastin S. 2014. Soil moisture-temperature feedbacks at meso-scale during summer heat waves over Western Europe. Climate Dynamics 42, 1309–1324. [Google Scholar]
- Teskey RO, Fites JA, Samuelson LJ, Bongarten BC. 1986. Stomatal and nonstomatal limitations to net photosynthesis in Pinus taeda L. under different environmental conditions. Tree Physiology 2, 131–142. [DOI] [PubMed] [Google Scholar]
- Teskey R, Wertin T, Bauweraerts I, Ameye M, McGuire MA, Steppe K. 2015. Responses of tree species to heat waves and extreme heat events. Plant, Cell & Environment 38, 1699–1712. [DOI] [PubMed] [Google Scholar]
- Tuzet A, Perrier A, Leuning R. 2003. A coupled model of stomatal conductance, photosynthesis and transpiration. Plant, Cell & Environment 26, 1097–1116. [Google Scholar]
- Verhoef A, Egea G. 2014. Modeling plant transpiration under limited soil water: comparison of different plant and soil hydraulic parameterizations and preliminary implications for their use in land surface models. Agricultural and Forest Meteorology 191, 22–32. [Google Scholar]
- von Caemmerer S, Evans JR. 2015. Temperature responses of mesophyll conductance differ greatly between species. Plant, Cell & Environment 38, 629–637. [DOI] [PubMed] [Google Scholar]
- Way DA, Oren R, Kim H-S, Katul GG. 2011. How well do stomatal conductance models perform on closing plant carbon budgets? A test using seedlings grown under current and elevated air temperatures. Journal of Geophysical Research 116, G04031. [Google Scholar]
- Weston DJ, Bauerle WL. 2007. Inhibition and acclimation of C3 photosynthesis to moderate heat: a perspective from thermally contrasting genotypes of Acer rubrum (red maple). Tree Physiology 27, 1083–1092. [DOI] [PubMed] [Google Scholar]
- Wolf A, Anderegg WRL, Pacala SW. 2016. Optimal stomatal behavior with competition for water and risk of hydraulic impairment. Proceedings of the National Academy of Sciences of the United States of America 113, E7222–E7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SC, Cowan IR, Farquhar GD. 1979. Stomatal conductance correlates with photosynthetic capacity. Nature 282, 424–426. [Google Scholar]
- Xu X, Medvigy D, Powers JS, Becknell JM, Guan K. 2016. Diversity in plant hydraulic traits explains seasonal and inter-annual variations of vegetation dynamics in seasonally dry tropical forests. The New Phytologist 212, 80–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.