Abstract
Spin labels selectively attached to biomolecules allow high-accuracy nanoscale distance measurements using pulsed Electron Paramagnetic Resonance (EPR), in many cases providing the only access to structure of complex biosystems. Triarylmethyl (TAM) radicals have recently emerged as a new type of spin labels expanding the applicability of the method to physiological temperatures. Along with other factors, the accuracy of the obtained distances crucially relies on the understanding of interactions between biomolecules and spin labels. In this work we consider such crucial interactions and their impact on pulsed EPR distance measurements in TAM-labeled DNAs. Using orientation-selective high-frequency (94 GHz) Double Electron-Electron Resonance (DEER) we demonstrate strong specific interactions between DNA termini and TAM label, leading to a significant restriction of its conformational mobility. Understanding of such interactions guides the way to selecting optimum TAM-labeling strategies, thus refining nanoscale EPR distance measurements in nucleic acids and their complexes at physiological conditions.
Site-Directed Spin Labeling (SDSL) is nowadays a widely used tool in biochemistry and structural biology.1,2 SDSL allows application of Electron Paramagnetic Resonance (EPR), in particular methods of pulsed dipolar EPR spectroscopy (PDS), thus providing nanometer scale distance distributions between the attached labels.3–11 A huge effort has been made during the last decades to develop new experimental approaches, as well as new types of spin labels with improved properties. However, until recently, PDS distance measurements at physiological conditions remained a long-standing dream. The new opportunity arose due to the development of triarylmethyl (TAM) based labels and synthetic protocols of their incorporation into biopolymers.12–14 The most important advantage of TAMs is a long enough phase memory time (Tm) near physiological conditions (ambient temperatures, water solutions),12–15 which allows distances up to 4–5 nm to be precisely measured using PDS.12,13 A potential disadvantage arises from the relatively large size of TAM labels including a linker, which might decrease the resolution of the obtained distance distributions. This expectation, however, was recently disproved by experimental demonstration that the distance distribution between two TAM labels is much narrower than the one between two nitroxide labels attached to the same double-stranded (ds) DNA.16 The observations were rationalized assuming hydrophobic interactions between TAM and DNA termini. Such interactions are of general interest and might be important in numerous situations. Moreover, an understanding of these interactions is crucial for interpretation of experimental EPR data and derivation of spin-spin distances, since it dramatically affects the expected conformational distribution of the label relative to its attachment site. Therefore, TAM-DNA interactions need to be investigated and identified. Up to now, the only experimentally demonstrated method for TAM-labeling of DNA used terminal base pairs for label attachment13,16; therefore in this work we address this practically relevant case in detail.
TAM radicals have extremely narrow EPR spectra with g-tensor anisotropy being almost completely unresolved at the most common X- and Q-band microwave frequencies (9/34 GHz).15 Resolution of the g-tensor components improves at higher frequencies; e.g., a recent PDS study at G-band (180 GHz) demonstrated orientation-selective Double Electron-Electron Resonance (DEER, also known as PELDOR17) measurements on TAM spin labels.18 Here, we use orientation selectivity to investigate the preferred TAM orientations in a set of doubly TAM-labeled 10-mer dsDNAs shown in Figure 1, in order to assess on this basis the underlying TAM-DNA interactions. In two dsDNAs, TAMs were attached using a relatively long piperazine linker (I, II), whereas in the third one (III), a much shorter amine linker was implemented for comparison of conformational flexibility.
Figure 1.
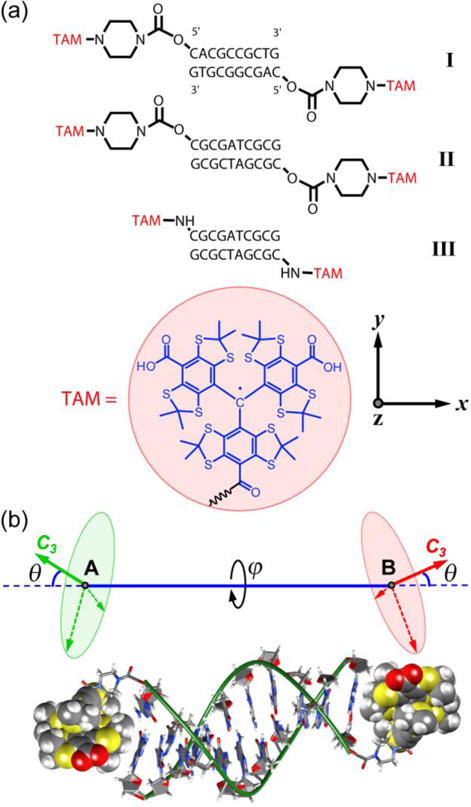
(a) Structures of studied spin-labeled dsDNAs I–III and TAM radical. Molecular frame (x,y,z) of TAM radical, where gz is parallel to the z axis, which is the threefold (C3) rotation symmetry axis of unlinked TAM. (b) Sketch of TAM-labeled dsDNA and corresponding theoretical model. Red and green solid arrows indicate z axes of TAM frame, θ is the angle between the z axes and the spin-spin direction AB, and ϕ is the torsion angle around the spin-spin axis AB.
Figure 2a shows W-band (94 GHz) echo-detected (ED) EPR spectra of the three studied spin-labeled dsDNAs. Evidently, the g-tensor anisotropy is partly resolved for I and II, and to a lesser extent for III. ED EPR spectra of I and II can be well simulated using an axially-symmetric g tensor with g⊥=2.00238 and g||=2.00168, whereas the spectrum of III is much narrower having g⊥=2.00233 and g||=2.00176. The g-tensor of III is noticeably less anisotropic compared to I-II, implying that the geometry of TAM is slightly different for the dsDNAs with the two different linkers. The ED EPR spectra may thus indicate that TAM interacts with dsDNA, which leads to the subtle changes of the g-tensor anisotropy depending on the length of the linker between TAM and DNA.
Figure 2.
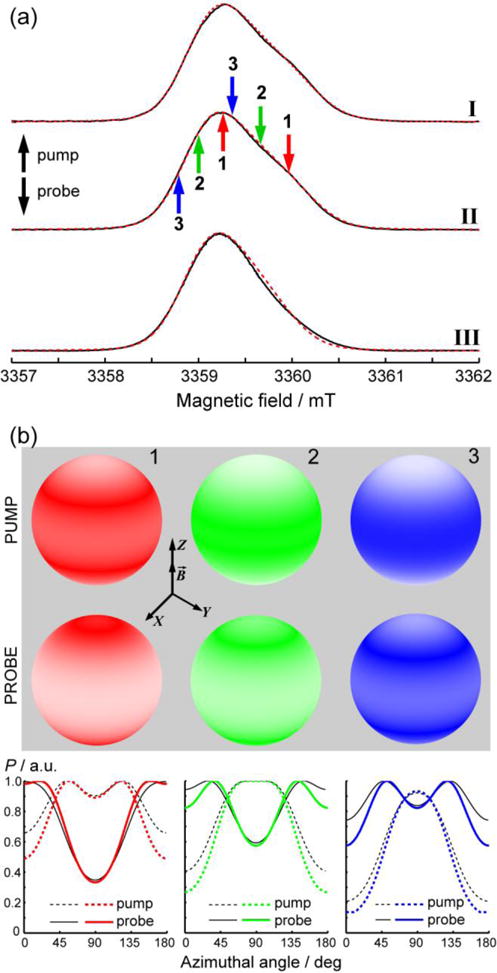
(a) Experimental (─) and simulated (---) two-pulse ED EPR spectra of dsDNAs I–III (indicated on the right) at 50 K. Up- and down-arrows indicate pump and probe positions at the spectrum, respectively. Each pair of pump/probe positions is indicated by a corresponding color and number (1–3). The color codes for pump/probe positions correspond to those used in Figures 3–4 for the obtained data. (b) Orientations of TAM radicals affected by pump and probe pulses at spectral positions 1–3. The magnetic field B is directed along the Z axis of the laboratory frame, and the color density on the unit sphere reflects the probability of the z axis of TAM (‖gz, see Fig.1) to coincide with this orientation. Axial symmetry of the g tensor of TAM leads to the axially symmetric distributions. For clarity, the graphs on the bottom show the probability function P (color density) vs. azimuthal angle (i.e. angle between z axis of TAM and the magnetic field). All data are calculated for II, except for the black (solid and dashed) lines at the bottom plots that were calculated for III and are shown for comparison.
Even though the g-tensor anisotropy is apparent at W-band, the overall width of the spectrum is relatively small (~2 mT, corresponding to 56 MHz) for PELDOR/DEER studies employing microwave pump/probe pulses at two frequencies. Following the strategy described in Ref. 19 for orientation-selective DEER on nitroxides, we selected three pairs or pump/probe positions (setups) indicated by arrows in Figure 2a. As will be shown below, this set of positions was sufficient for detecting the presence of orientation selection, and the setups 1–3 together covered nearly the whole range of TAM orientations.
Figure 3 shows the obtained four-pulse DEER traces and apparent distance distributions for dsDNAs I–III at setups 1–3, which correspond to the excited orientations shown in Figure 2b. Evidently, in case of dsDNAs I–II (Fig.3a,b) DEER data is strongly orientation-dependent. This is manifested by the appearance of an artefact peak at shorter distances (~3.6 nm), corresponding to the enhanced double dipolar frequency of the actual TAM-TAM distance ~4.5 nm.13,16 Such double-frequency peaks stem from orientations of the spin-spin vector AB parallel to the external magnetic field. In agreement with this, the frequency-domain dipolar spectra manifest enhanced parallel components of the distorted Pake patterns for setup 1 (Fig.3d). Essentially, apparent bimodal distributions were observed in setup 1, somewhat broader ones in setup 2, and essentially mono-modal distributions were obtained for setup 3.
Figure 3.
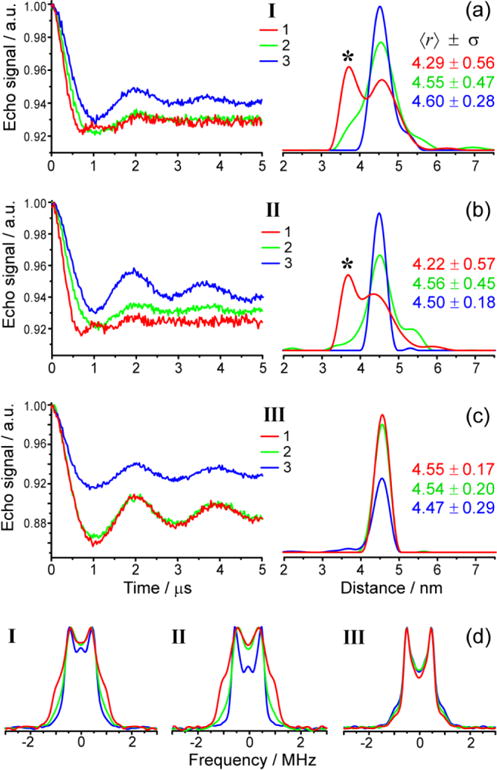
W-band DEER distance measurements on dsDNAs I (a), II (b) and III (c) obtained at the three pump/probe setups (1,2,3) shown in Figure 2a. Left plots show background-corrected DEER traces; right plots show the corresponding apparent distance distributions (Tikhonov regularization parameter 100). The color codes for all traces correspond to those for pump/probe setups shown in Figure 2. Asterisks mark the “double-frequency peak”, corresponding to the parallel component of the Pake doublet. The mean distances 〈r〉 and standard deviations σ are given in nm for each apparent distance distribution. (d) Frequency-domain dipolar spectra of I–III corresponding to data shown in (a)–(c).
The orientation dependence of DEER for dsDNAs I–II (Fig. 3a,b) has a clear qualitative interpretation. First, it implies the presence of preferred orientations of spin labels relative to the biomolecule and to each other. If orientation of TAM relative to the DNA helix was completely random, no orientation dependence would be anticipated. Second, mw pulses in setup 1 affect g|| orientations of TAM to a stronger extent compared to setup 2 and, even more, to setup 3. The observation of “double-frequency peak” in setup 1 means that the preferred orientations of TAMs have g|| and C3 axes closely parallel to the DNA helix, i.e. to the spin-spin vector AB (Fig.1b). Such orientations correspond to θ~0 and thus to TAM radicals capping the dsDNA base pairs, as is sketched in Fig.1b. The possibility of such conformations was theoretically rationalized recently.16,20
Note that the observed DEER orientation dependence for dsDNAs I–II is opposite to that recently found for a rigid TAM-TAM biradical at 180 GHz.18 In the cited study the double-frequency oscillations were observed at ~g⊥ orientations of ED EPR spectrum, meaning that the (x,y) plane of each TAM is closely parallel to the spin-spin vector AB. In agreement with this conclusion, the opposite DEER orientation dependence in our case implies opposite TAM orientations relative to the spin-spin vector AB: the (x,y) plane of TAM is perpendicular to AB and to the DNA helix axis. The alignment of the TAM radical in this orientation clearly implies its interaction with the terminal base pair, most likely having a hydrophobic origin.
Interestingly, despite clear manifestations of orientation selectivity for dsDNAs I–II, we did not observe any orientation-selective DEER for dsDNA III having shorter amine linker (Fig.3c). In all setups 1–3 the apparent distance distribution displays a single narrow peak at ~4.5 nm. It is plausible that the shorter amine linker in dsDNA III does not allow TAM to interact with the terminal base pair as efficiently as in the case of the longer piperazine linker in dsDNAs I–II. At the same time, the ED EPR spectrum of dsDNA III is noticeably narrower compared to dsDNAs I–II (Fig.2), therefore the orientation selectivity itself at W band might not be sufficient to resolve differences in spectral positions 1–3. The latter possibility was explored by numerical simulations.
Figure 2b shows the calculated orientations of the TAM radical (molecular z axis) with respect to the magnetic field B, which are excited in our experimental setups 1–3 (see Supporting Information (SI) for details). The probe pulse in setup 1 excites g|| orientations (the poles of the spheres, azimuthal angles near 0 and 180°) more efficiently than g⊥ orientations (the equators of the spheres, azimuthal angles near 90°). In setup 2 this trend becomes less obvious and it completely disappears in setup 3. These findings are in good agreement with the observed orientation selectivity for dsDNAs I–II. Although there are some differences in calculations for dsDNA I–II and dsDNA III (Fig.2b, bottom), they are too small to explain the absence of DEER orientation selectivity in dsDNA III. Our data thus indicates that TAM attached to DNA via the short amine linker exhibits much weaker interaction with the terminal base pair compared to TAM attached via the longer piperazine linker. Most probably this occurs due to steric constraints, which hinder “bending” of the short linker that would be required to cap the terminal base pair. Note that the distance distributions obtained for dsDNAs I–III at X-band are all very similar (〈r〉~4.5 nm and σ~0.2 nm 16) due to the non-selective excitation of all orientations, and therefore cannot reveal different TAM-DNA interactions in I–II vs. III. This indicates that there exist two ways for obtaining narrow distance distributions, either a short linker that is not flexible, or a more flexible, longer linker that allows for hydrophobic interaction of TAM with terminal base pairs.
To support the qualitative conclusion that TAM interacts with terminal base pairs of dsDNAs I–II and aligns with its z axis (C3, g‖) closely parallel to spin-spin vector AB, we considered two realistic models. Model 1 assumes that both TAM labels are rigidly fixed at dsDNA with the same angles θ and with torsion angle Δϕ=ϕA-ϕB. (see Fig.1b). Figure 4 shows the calculated probability density per unit solid angle PAB(ψ) of finding spin-spin vector AB at an inclination ψ with respect to the magnetic field for setups 1–3. A few representative combinations of θ and Δϕ are shown (see SI for more data with Δϕ≠0). The experimentally observed trend is well reproduced for θ=0–30°: PAB is enhanced near the poles (ψ=0 and 180°) in setup 1 and suppressed in setups 2 and 3. For θ=60° such trend becomes obscure, and for θ=90° it gets reversed. Thus, the values θ ~ 0–30° agree well with experimental observations.
Figure 4.
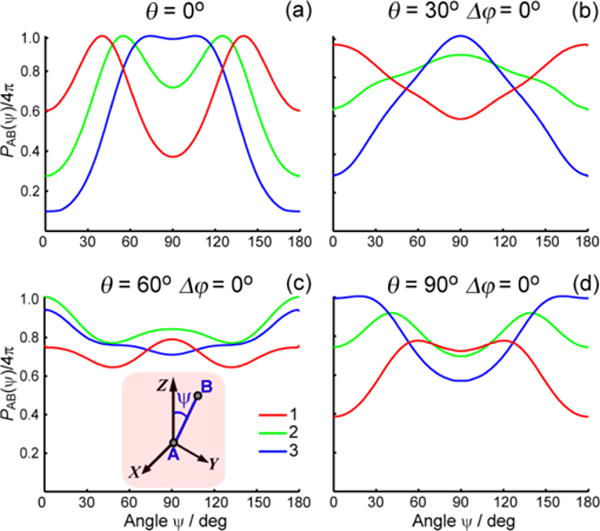
Calculated probability density PAB(ψ) of finding spin-spin vector AB at angle ψ toward the magnetic field (Z axis of laboratory frame). Model 1 (see text) for dsDNA II and three pump/probe setups indicated in Figure 2. The parameters of the model, angles θ and Δϕ, are indicated on the plots (a–d). Inset of (c): laboratory frame (B‖Z) and orientation of spin-spin vector AB (see also Figure 1).
Model 2 assumes that TAM labels are not fixed, but randomly distributed within a cone with opening angle θmax (Fig.1b). The results of Model 2 agree with Model 1, confirming that the best agreement with experiment is achieved for orientations of g‖ rather close to the direction of the spin-spin vector AB (SI). Higher-frequency DEER studies might allow for further refinement of these values.
In conclusion, we have demonstrated experimentally that TAM spin labels can interact with the dsDNA termini and have orientation preferences that are clearly manifest in orientation-selective DEER. Our simulations support these conclusions. Depending on flexibility of the linker between TAM and DNA, such interactions can be suppressed (dsDNA III) or enhanced (dsDNA I–II). TAM radicals nowadays find numerous applications in biosciences, including structural EPR,12–14,16,18 Dynamic Nuclear Polarization NMR,21–24 in vivo oxymetry and imaging25,26 etc.; therefore, general understanding of TAM-DNA interactions is of high urgency and importance. In addition, we stress that the great potential of TAMs in spin-labeling EPR is due to the possible application of such labels for distance measurements in physiological conditions.12,13 Such measurements should be performed at X band, where phase relaxation is slower15 and orientation selectivity for TAMs is negligible. Regarding the presence of specific interactions between label and biomolecule, this is unwelcome at the one hand, since it generally complicates the translation of the distance between labels into a distance between their attachment sites. On the other hand, this weakness could be turned into strength if such specific interactions were understood and taken into account. First, a suppression of conformational flexibility should lead to narrower label-to-label distance distributions, and might potentially improve resolution of TAM-based distance measurements. Second, specific interactions of spin labels with biomolecules are of special interest for physiological temperature experiments, since they might enhance phase relaxation times and prevent motional averaging of dipole-dipole interaction. In this regard, the TAM-DNA interactions highlighted in this work might be deliberately employed for designing optimum labels, linkers and labeling sites, thus providing an optimized methodology for structural EPR studies of nucleic acids and their protein complexes at physiological conditions.
Experimental
Doubly TAM-labeled dsDNAs were prepared using the procedure developed previously.16 All W-band DEER measurements were performed at T=50 K and used the standard four-pulse DEER sequence27 with π-pulses being 88–104 ns for probe and 84–112 ns for pump frequencies; the difference between pump and probe frequencies was 20 MHz. The probe frequency was 94.13–94.34 GHz in different experiments. ED EPR spectra used the standard two-pulse electron spin echo sequence with inter-pulse delay τ=200 ns. ED EPR spectra were simulated using EasySpin 5.0.2228; while DEER data were simulated using DeerAnalysis2013.29
Supplementary Material
Acknowledgments
This work was supported by Russian Science Foundation (no. 14-14-00922).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: …. I. Calculations of the excited orientations. II. Simulations using Model 1. III. Simulations using Model 2. IV. Original DEER data. (PDF)
References
- 1.Borbat PP, Costa-Filho AJ, Earle KA, Moscicki JK, Freed JH. Electron spin resonance in studies of membranes and proteins. Science. 2001;291:266. doi: 10.1126/science.291.5502.266. [DOI] [PubMed] [Google Scholar]
- 2.Jeschke G. DEER Distance Measurements on Proteins. Annu Rev Phys Chem. 2012;63:419. doi: 10.1146/annurev-physchem-032511-143716. [DOI] [PubMed] [Google Scholar]
- 3.Borbat PP, Mchaourab HS, Freed JH. Protein structure determination using long-distance constraints from double-quantum coherence ESR: Study of T4 lysozyme. J Am Chem Soc. 2002;124:5304. doi: 10.1021/ja020040y. [DOI] [PubMed] [Google Scholar]
- 4.Schiemann O, Weber A, Edwards TE, Prisner TF, Sigurdsson ST. Nanometer distance measurements on RNA using PELDOR. J Am Chem Soc. 2003;125:3434. doi: 10.1021/ja0274610. [DOI] [PubMed] [Google Scholar]
- 5.Borbat PP, Davis JH, Butcher SE, Freed JH. Measurement of Large Distances in Biomolecules using Double-Quantum Filtered Refocused Electron Spin-Echoes. J Am Chem Soc. 2004;126:7746–7747. doi: 10.1021/ja049372o. [DOI] [PubMed] [Google Scholar]
- 6.Schiemann O, Piton N, Mu YG, Stock G, Engels JW, Prisner TF. A PELDOR-based nanometer distance ruler for oligonucleotides. J Am Chem Soc. 2004;126:5722. doi: 10.1021/ja0393877. [DOI] [PubMed] [Google Scholar]
- 7.Cai Q, Kusnetzow AK, Hubbell WL, Haworth IS, Gacho GPC, Van Eps N, Hideg K, Chambers EJ, Qin PZ. Site-directed spin labeling measurements of nanometer distances in nucleic acids using a sequence-independent nitroxide probe. Nucleic Acids Res. 2006;34:4722. doi: 10.1093/nar/gkl546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiemann O, Prisner TF. Long-range Distance Determinations in Biomacromolecules by EPR spectroscopy. Q Rev Biophys. 2007;40:1–53. doi: 10.1017/S003358350700460X. [DOI] [PubMed] [Google Scholar]
- 9.Grohmann D, Klose D, Klare JP, Kay CWM, Steinhoff HJ, Werner F. RNA-Binding to Archaeal RNA Polymerase Subunits F/E: A DEER and FRET Study. J Am Chem Soc. 2010;132:5954. doi: 10.1021/ja101663d. [DOI] [PubMed] [Google Scholar]
- 10.Kim NK, Bowman MK, DeRose VJ. Precise Mapping of RNA Tertiary Structure via Nanometer Distance Measurements with Double Electron-Electron Resonance Spectroscopy. J Am Chem Soc. 2010;132:8882. doi: 10.1021/ja101317g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duss O, Michel E, Yulikov M, Schubert M, Jeschke G, Allain FH-T. Structural Basis of the non-Coding RNA RsmZ Acting as a Protein Sponge. Nature. 2014;509:588–592. doi: 10.1038/nature13271. [DOI] [PubMed] [Google Scholar]
- 12.Yang ZY, Liu YP, Borbat P, Zweier JL, Freed JH, Hubbell WL. Pulsed ESR Dipolar Spectroscopy for Distance Measurements in Immobilized Spin Labeled Proteins in Liquid Solution. J Am Chem Soc. 2012;134:9950–9952. doi: 10.1021/ja303791p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shevelev GY, Krumkacheva OA, Lomzov AA, Kuzhelev AA, Rogozhnikova OY, Trukhin DV, Troitskaya TI, Tormyshev VM, Fedin MV, Pyshnyi DV, Bagryanskaya EG. Physiological-Temperature Distance Measurement in Nucleic Acid using Triarylmethyl-Based Spin Labels and Pulsed Dipolar EPR Spectroscopy. J Am Chem Soc. 2014;136:9874–9877. doi: 10.1021/ja505122n. [DOI] [PubMed] [Google Scholar]
- 14.Yang Z, Bridges MD, López CJ, Rogozhnikova OYu, Trukhin DV, Brooks EK, Tormyshev V, Halpern HJ, Hubbel WL. A triarylmethyl spin label for long-range distance measurement at physiological temperatures using T1 relaxation enhancement. J Magn Reson. 2016;269:50–54. doi: 10.1016/j.jmr.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuzhelev AA, Trukhin DV, Krumkacheva OA, Strizhakov RK, Rogozhnikova OY, Troitskaya TI, Fedin MV, Tormyshev VM, Bagryanskaya EG. Room-Temperature Electron Spin Relaxation of Triarylmethyl Radicals at the X- and Q-Bands. J Phys Chem B. 2015;119:13630–13640. doi: 10.1021/acs.jpcb.5b03027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shevelev GY, Krumkacheva OA, Lomzov AA, Kuzhelev AA, Trukhin DV, Rogozhnikova OY, Tormyshev VM, Pyshnyi DV, Fedin MV, Bagryanskaya EG. Triarylmethyl Labels Toward Improving the Accuracy of EPR Nanoscale Distance Measurements in DNAs. J Phys Chem B. 2015;119:13641–13648. doi: 10.1021/acs.jpcb.5b03026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milov AD, Salikhov KM, Shirov MD. Application of PELDOR in Electron-Spin Echo for Paramagnetic Center Space Distribution in Solids. Fiz Tverd Tela. 1981;23:975–982. [Google Scholar]
- 18.Akhmetzyanov D, Schöps P, Marko A, Kunjir N, Sigurdsson ST, Prisner T. Pulsed EPR DipolarSpectroscopy at Q and G-Band on a Trityl Biradical. Phys Chem Chem Phys. 2015;17:24446–24451. doi: 10.1039/c5cp03671b. [DOI] [PubMed] [Google Scholar]
- 19.Polyhach Y, Bordignon E, Tschaggelar R, Gandra S, Godt A, Jeschke G. High sensitivity and versatility of the DEER experiment on nitroxide radical pairs at Q-band frequencies. Phys Chem Chem Phys. 2012;14:10762–10773. doi: 10.1039/c2cp41520h. [DOI] [PubMed] [Google Scholar]
- 20.Lomzov AA, Sviridov EA, Shernuykov AV, Shevelev GYu, Pyshnyi DV, Bagryanskaya EG. Study of a DNA Duplex by Nuclear Magnetic Resonance and Molecular Dynamics Simulations. Validation of Pulsed Dipolar Electron Paramagnetic Resonance Distance Measurements Using Triarylmethyl-Based Spin Labels. J Phys Chem B. 2016;120:5125–5133. doi: 10.1021/acs.jpcb.6b03193. [DOI] [PubMed] [Google Scholar]
- 21.Mathies G, Jain S, Reese M, Griffin RG. enPulsed Dynamic Nuclear Polarization with Trityl Radicals. J Phys Chem Lett. 2016;7:111–116. doi: 10.1021/acs.jpclett.5b02720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathies G, Caporini MA, Michaelis VK, Liu YP, Hu KN, Mance D, Zweier JL, Rosay M, Baldus M, Griffin RG. Efficient Dynamic Nuclear Polarization at 800 MHz/527 GHz with Trityl-Nitroxide Biradicals. Angew Chem Int Ed. 2015;45:11770–11774. doi: 10.1002/anie.201504292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lumata L, Kovacs Z, Sherry AD, Malloy C, Hill S, van Tol J, Yu L, Song LK, Merritt ME. Electron spin resonance studies of trityl OX063 at a concentration optimal for DNP. Phys Chem Chem Phys. 2013;15:9800–9807. doi: 10.1039/c3cp50186h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macholl S, Johannesson H, Ardenkjaer-Larsen JH. Trityl biradicals and C-13 dynamic nuclear polarization. Phys Chem Chem Phys. 2010;12:5804–5817. doi: 10.1039/c002699a. [DOI] [PubMed] [Google Scholar]
- 25.Mailer C, Sundramoorthy SV, Pelizzari CA, Halpern HJ. Spin echo spectroscopic electron paramagnetic resonance imaging. Magn Reson Med. 2006;55:904–912. doi: 10.1002/mrm.20849. [DOI] [PubMed] [Google Scholar]
- 26.Bobko AA, Dhimitruka I, Eubank TD, Marsh CB, Zweier JL, Khramtsov VV. Trityl-based EPR probe with enhanced sensitivity to oxygen. Free Rad Biol Med. 2009;47:654–658. doi: 10.1016/j.freeradbiomed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pannier M, Veit S, Godt A, Jeschke G, Spiess HW. Deadtime Free Measurement of Dipole-Dipole Interactions between Electron Spins. J Magn Reson. 2000;142:331–340. doi: 10.1006/jmre.1999.1944. [DOI] [PubMed] [Google Scholar]
- 28.Stoll S, Schweiger A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J Magn Reson. 2006;178:42–55. doi: 10.1016/j.jmr.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 29.Jeschke G, Chechik V, Ionita P, Godt A, Zimmermann H, Banham J, Timmel CR, Hilger D, Jung H. DeerAnalysis2006 – a Comprehensive Software Package for Analyzing Pulsed ELDOR Data. Appl Magn Reson. 2006;30:473–498. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


