Abstract
Tissue-specific autoimmune diseases such as type 1 diabetes (T1D) are characterized by T cell-driven pathology. Administration of autoantigenic peptides provides a strategy to selectively target the pathogenic T cell response. Indeed, treatment with β cell peptides effectively prevents T1D in NOD mice. However, the efficacy of peptide immunotherapy generally wanes as β cell autoimmunity progresses and islet inflammation increases. With the goal of enhancing the efficacy of peptide immunotherapy, soluble (s)IAg7-Ig dimers covalently linked to β cell autoantigen-derived peptides were tested for the capacity to suppress late preclinical T1D. NOD female mice with established β cell autoimmunity were vaccinated i.v. with a short course of sIAg7-Ig dimers tethered to peptides derived from glutamic acid decarboxylase (GAD)65 (sIAg7-pGAD65). Treatment with sIAg7-pGAD65 dimers and the equivalent of only ~7 μg of native peptide effectively blocked the progression of insulitis and the development of diabetes. Furthermore, suppression of T1D was dependent on β cell-specific IL-10-secreting CD4+ T cells, although the frequency of GAD65-specific FoxP3-expressing CD4+ T cells was also increased in sIAg7-pGAD65 dimer vaccinated NOD mice. These results demonstrate that MHC class II-Ig dimer vaccination is a robust approach to suppress ongoing T cell-mediated autoimmunity, and may provide a superior strategy of adjuvant-free peptide-based immunotherapy to induce immunoregulatory T cells.
Various tissue-specific autoimmune diseases such as type 1 diabetes (T1D)3 are mediated by pathogenic T cells (1–3). Considerable effort has been devoted to developing therapeutic approaches to target autoreactive T cells, and prevent or suppress tissue-specific autoimmunity. Strategies based on administration of immunosuppressant drugs, and Abs specific for T cells have been successfully used in experimental models, and in some instances the clinic (4–7). However, these approaches fail to discriminate between T cells specific for self- and foreign Ags, and compromise the normal function of the immune system to varying degrees. Peptide-based immunotherapies offer an approach to selectively target autoreactive T cells, leaving the remainder of the immune system intact (8). Approaches of peptide immunotherapy that induce IL-4- or IL-10-secreting adaptive immunoregulatory CD4+ T (aTreg) cells have proven to be effective for autoimmune diseases in which multiple autoantigens are targeted by T cells (9–11). Once established aTreg cells traffic to the relevant tissues and suppress, via cytokine secretion, the differentiation or function of pathogenic T effector cells in an Ag-independent manner (12).
Studies in the NOD mouse, a spontaneous model of T1D, demonstrate that administration of β cell peptides induces aTreg cells, and suppresses differentiation of type 1 T effector cells that mediate destruction of the insulin-producing β cells (9, 10, 13). Peptide immunotherapy is effective at early stages of disease progression but efficacy is generally limited at late preclinical stages of T1D when the frequency of pathogenic type 1 T effectors is high, and the proinflammatory milieu is well established in the β cell containing islets (14, 15). In addition to the stage of disease progression at which treatment is initiated, other factors influence the efficacy of peptide immunotherapy including dose and route of administration, the use of adjuvant, the binding affinity of peptides to MHC molecules, and in vivo peptide stability. For instance, peptides are rapidly cleared from the circulation and inefficiently presented by APC in vivo, which limits therapeutic efficacy (16–18).
One approach to overcome these limitations has been the engineering of peptide-soluble MHC class II-Ig (peptide-sMHCII-Ig) fusion proteins (19–23). These recombinants consist of the extra-cellular domains of the MHCII α- and β-chains supported by an Ig scaffold. A peptide is tethered to the sMHCII β-chain ensuring that each bivalent fusion molecule presents a peptide, which binds T cells directly independent of APC. Studies by Casares et al. (24) using a monoclonal TCR transgenic model targeting the neo-β cell autoantigen hemagglutinin (HA), provided initial evidence that peptide-sMHCII-Ig vaccination can be effective in treating autoimmunity. Administration of sIEd-Ig dimers linked to a HA peptide was found to delete HA-specific T effectors and reverse diabetes in treated mice expressing HA in β cells. sIEd-Ig dimer vaccination also induced HA-specific aTreg cells (24). Nevertheless, whether peptide-sMHCII-Ig vaccination can block autoimmunity mediated by pathogenic effector T cells with multiple specificities has yet to be established. Accordingly, we tested whether administration of sIAg7-Ig dimers covalently linked to β cell-derived peptides could suppress a late preclinical stage of T1D in NOD mice.
Materials and Methods
Mice
NOD/LtJ, NOD.IL-10null, and NOD.CB17.Prkdcscid/J (NOD.scid) mice were maintained and bred under specific-pathogen free conditions. Mice were diagnosed as diabetic with blood glucose measurements ≥250 mg/dl on three successive days as determined by an Autokit Glucose CII assay (WAKO). In our colony NOD female mice 12 wk of age typically exhibit elevated blood glucose levels (e.g., ~180–200 mg/dl). All procedures were reviewed and approved by the University of North Carolina Institutional Animal Care and Use Committee.
sIAg7-Ig dimer expression, purification, and vaccination
sIAg7-Ig dimers were engineered as previously described (25, 26). Briefly, IAg7 α- and β-chain extracellular domains were attached to fos and jun leucine zippers, respectively. The IAd α-chain was further modified with a murine IgG2a Fc domain to establish a divalent structure. Leucine residues at positions 234 and 235 in the IgG2a hinge region were substituted with alanines to prevent binding to FcγRI and FcγRII and activation of APC (27, 28). Peptide epitopes were covalently linked to the N terminus of the IAg7 β-chain by a flexible thrombin-GGGGS linker. cDNAs encoding the sIAg7-Ig chains were subcloned into the pMT-Bip vector (Invitrogen) and transgene expression driven by a metallothionein-inducible promoter. Expression vectors were cotransfected via calcium phosphate into Drosophila S2 cells with pHygro, and transfectants selected in hygromycin-containing Schneider’s medium. sIAg7-Ig dimer protein expression was induced by 500 μM CuSO4 for 7–10 days and purified by affinity chromatography on a protein A column (GE Bioscience).
Twelve-wk-old NOD female mice were i.v. immunized with 50 μg of sIAg7-Ig dimers prepared in 200 μl of PBS on three consecutive days. Three weeks later, a second course of three injections of sIAg7-Ig dimer was administered. In some experiments, 12-wk-old NOD female mice received three i.p. injections of 200 μg of peptide emulsified in 0.1 ml of IFA over a 3-wk period. Mixtures of peptides were also prepared in 0.1 ml of IFA.
FACS analysis
sIAg7-Ig dimers were multimerized using Alexa Fluor 647-coupled protein A (Molecular Probes and Invitrogen) for FACS. Cells were incubated with sIAg7-Ig multimers at room temperature for 1 h, followed by anti-CD3 (FITC), and CD4 (PacBlue) Abs (eBioscience) staining on ice for 30 min. Cells were then fixed, permeabilized, and intracellularly stained with anti-FoxP3 (PE) or anti–IL-10 (FITC) Abs (eBioscience).
In some experiments single cell suspensions were cultured with plate-bound anti-CD3 (2 μg/ml) and soluble anti-CD28 (2 μg/ml) Abs in the presence of recombinant murine IL-2 (10 ng/ml) for 2 days, and then stimulated with PMA (5 ng/ml) and ionomycin (500 ng/ml) in the presence of brefeldin A (10 μg/ml) for 5 h. Cells were stained with sIAg7-Ig multimers at room temperature for 1 h, followed by anti-CD3 (PeCy7), anti-CD4 (PacBlue), and anti-TGF-β (PerCp) Abs on ice for 30 min, and then fixed, permeabilized, and intracellularly stained with anti-IL-10 (FITC) and anti-FoxP3 (PE) Abs (eBioscience). Data were acquired on a Cyan flow cytometer (DakoCytomation) and analyzed using Summit software (DakoCytomation).
Islet isolation
Pancreas samples were perfused with 0.2 mg/ml Liberase (Roche) and digested for 30 min at 37°C. Islets were purified via Ficoll gradient, hand-picked and counted. For FACS analyses of islet infiltrating T cells, isolated islets were dissociated into a single cell suspension using enzyme-free cell dissociation solution (Sigma-Aldrich). Lymphocytes infiltrating the islets were collected and cellular debris removed by 70-μm nylon filters.
ELISPOT and ELISA
ELISPOT was conducted as described (29). Briefly, splenocytes (5 × 105/well) or cells from the pancreatic lymph nodes (PLN; 2 × 105/well) were resuspended in HL-1 medium (BioWhittaker) and cultured in 96-well ELISPOT plates (Millipore) coated with anti-IFN-γ, anti-IL-4, or anti-IL-10 Abs (BD Pharmingen) for 48 h at 37°C. Peptides were added at a final concentration of 20 μg/ml. Plates were washed, incubated with the appropriate biotinylated anti-mouse cytokine Abs and streptavidin-HRP (BD Pharmingen), and spot forming units developed with 100 mM sodium acetate buffer containing 0.3 mg/ml 3-amino-9-ethylcarbazole (Sigma-Aldrich) and 0.015% hydrogen peroxide. An ImmunoSpot plate reader (Cellular Technology) was used to count spot forming unit per well. For ELISA, cells were cultured in 96-well microtiter plates, stimulated with peptide as described, and culture supernatant was harvested after 48 h. TGF-β was measured via ELISA per the manufacturer’s instructions (R&D Systems).
T cell adoptive transfers and histopathology
Splenocytes prepared from diabetic NOD donors (5 × 106) were injected i.p. into 5- to 8-wk-old NOD.scid mice either: 1) alone, 2) with splenocytes (5 × 106), or 3) CD4+ T cells (5 × 105) purified by negative selection from sIAg7-Ig dimer treated NOD female mice. In some experiments NOD.scid recipients were injected twice weekly with 300 μg of anti-TGF-β Ab (1D11.16.8) over a period of 4 wk. In our hands this protocol effectively neutralizes TGF-β in vivo. Splenocytes (5 × 106) from diabetes-free sIAg7-Ig dimer treated NOD female mice alone were also i.p. injected into NOD.scid mice. Pancreases were harvested, and fixed with 10% formalin. Serial cross-sections separated by 150 μm were cut and stained with H&E.
Statistical analyses
Statistical analyses were performed using GraphPad Prism software. Incidence of diabetes was compared by Kaplan-Meier log-rank test. One-way ANOVA test, χ-square test, and Student’s t test were used. Findings were considered significant with values for p ≤ 0.05.
Results
sIAg7-GADp217 or sIAg7-GADp290 dimers suppress ongoing β cell autoimmunity and prevent diabetes in NOD female mice
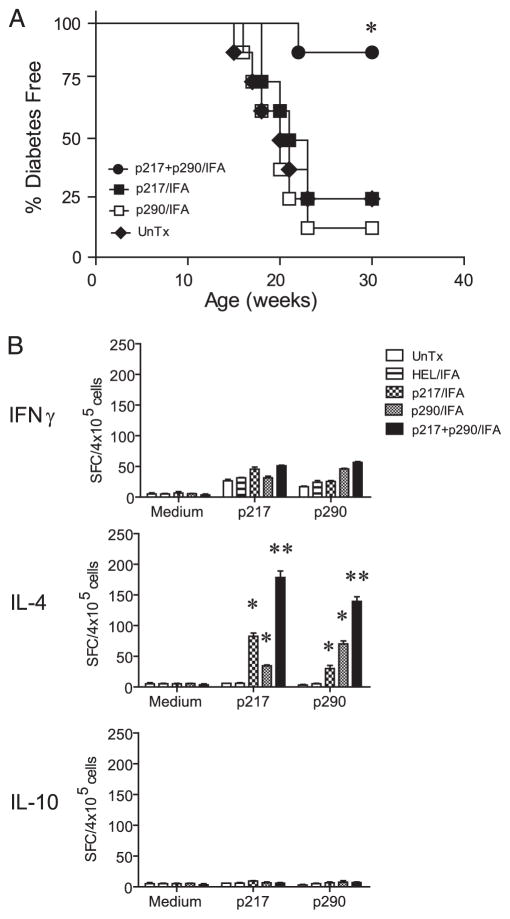
Previous work by our group (9) and a more recent analysis (Fig. 1A) demonstrated that coadministration of glutamic acid decarboxylase (GAD)65-specific peptides spanning amino acid residues 217 to 236 (GADp217) and residues 290 to 309 (GADp290) prepared in IFA suppressed β cell autoimmunity in NOD female mice at a late preclinical stage of T1D and prevented diabetes. Lack of diabetes in vaccinated NOD mice correlated with an increased frequency of GADp217- and GADp290-specific T cells secreting IL-4 but not IL-10 (Fig. 1B). Nevertheless, protection was induced only after multiple injections with high doses (e.g., 200 μg each injection) of the respective peptides in IFA. Furthermore, administration of either GADp217 or GADp290 alone failed to prevent diabetes (Fig. 1B) (9). With this in mind, we investigated whether sIAg7-Ig dimer vaccination is a more efficient strategy of peptide-based immunotherapy.
FIGURE 1.
Diabetes is prevented in NOD mice at a late preclinical stage of T1D with multiple and high doses of “native” GAD65 peptides in adjuvant. A, Groups of n = 8 NOD female mice 12 wk of age received a total of three i.p. injections of 200 μg of peptide in IFA over a 20-day period. Diabetes was monitored. *, p < 10−3 by Kaplan-Meier log-rank test for NOD mice coinjected with GADp217 and GADp290 vs NOD mice receiving only GADp217 or GADp290 or left untreated (UnTx). B, PLN were harvested from individual NOD female mice (n = 4) 3 wk after the last injection of peptide in IFA as in A, and the frequency of IFN-γ-, IL-4-, and IL-10-secreting T cells in response to 20 μg/ml peptide was measured by ELISPOT. Error bar represents mean ± SEM. *, p < 10−3, NOD mice treated with GADp217 or GADp290 alone vs HEL-treated or untreated animals. **, p < 10−3 by Student’s t test, for NOD mice coinjected with GADp217 and GADp290 vs NOD mice treated with GADp217 or GADp290 only.
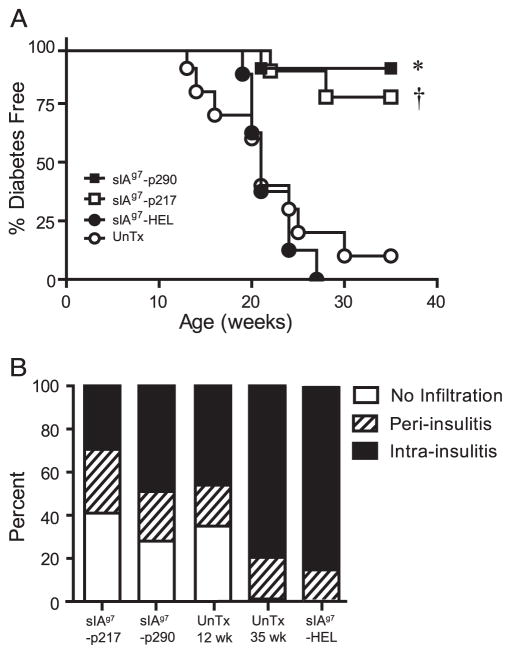
sIAg7-Ig dimers were tested which contained covalently linked GADp217 and GADp290, and the non-self hen egg lysozyme (HEL) epitope 12–26. T cell binding and stimulation by the respective sIAg7-Ig dimers were verified (data not shown). Twelve-wk-old NOD female mice, representing a late preclinical stage of T1D, received three i.v. injections of 50 μg of sIAg7-Ig in PBS over 3 days, followed by another three injections 3 wk later, and diabetes monitored up to 35 wk of age. Each 50-μg injection of sIAg7-Ig was equivalent to 1.1 μg of native peptide. No significant difference in the time of onset or frequency of diabetes was detected in NOD mice left untreated or receiving sIAg7-HEL (Fig. 2A). In contrast, the majority of NOD mice treated with sIAg7-GADp217 (8/10; p = 0.0017 vs untreated mice χ-square) or sIAg7-GADp290 (9/10; p = 0.0003 vs untreated mice χ-square) dimers remained diabetes-free (Fig. 2A). These results demonstrate that administration of sIAg7-GADp217 or sIAg7-GADp290, but not sIAg7-HEL, efficiently prevents diabetes at a late preclinical stage of T1D.
FIGURE 2.
sIAg7-GADp217 and sIAg7-GADp290 treatment prevents diabetes and blocks the progression of insulitis at a late preclinical stage of T1D. A, Twelve-wk-old NOD female mice were treated with sIAg7-GADp217 (n = 10), sIAg7-GADp290 (n = 10), sIAg7-HEL (n = 8), or left untreated (UnTx, n = 10), and diabetes was monitored. *, p ≤ 0.0015 by Kaplan-Meier log-rank test, for sIAg7-GADp217 vs sIAg7-HEL treated or untreated mice and †, p ≤ 0.0003, for sIAg7-GADp290 vs sIAg7-HEL treated or untreated mice. B, Insulitis in the pancreases of nondiabetic NOD female mice treated with sIAg7-GADp217 (n = 5) and sIAg7-GADp290 (n = 6) was evaluated at 35 wk by histological analysis. In addition, insulitis in the pancreases of untreated (UnTx) nondiabetic NOD female mice 12 wk (n = 4) and 35 wk (n = 5) of age were compared; and diabetic NOD female mice treated with sIAg7-HEL (n = 4). A minimum of 30 islets was examined for each mouse. p < 10−3 by one-way ANOVA test for frequency and severity of insulitis in sIAg7-GADp217 or sIAg7-GADp290 treated NOD mice vs 35-wk-old untreated NOD female mice.
Nondiabetic 35-wk-old NOD female mice treated with sIAg7-GADp217 or sIAg7-GADp290 were examined for islet infiltration. Histological analysis of pancreas samples showed a significantly reduced frequency and severity of insulitis in nondiabetic 35-wk-old NOD female mice treated with sIAg7-GADp217 or sIAg7-GADp290 compared with a group of untreated, 35-wk-old NOD female mice (Fig. 2B). Interestingly, the frequency and severity of insulitis in the 35-wk-old sIAg7-GAD65-treated NOD mice was analogous to that of untreated 12-wk-old NOD female mice (Fig. 2B). Therefore sIAg7-Ig dimer treatment prevents diabetes by suppressing the progression of islet infiltration in a β cell peptide-specific manner.
sIAg7-GADp217 and sIAg7-GADp290 vaccination blocks T1D progression by aTreg cell induction
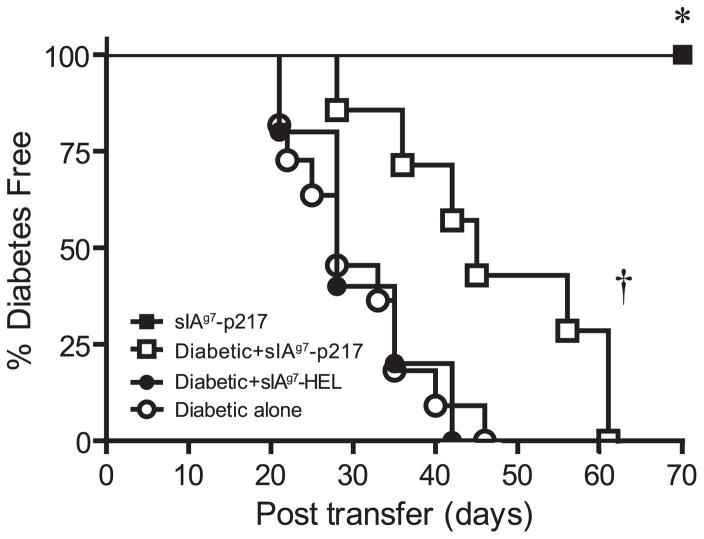
Treatment with self-peptide can mediate T cell tolerance by clonal anergy or deletion. Alternatively, self-peptide-specific aTreg cells can be induced that traffic to the site of inflammation, and suppress the differentiation or activity of pathogenic T effectors. To determine whether active immunoregulation was established by sIAg7-Ig dimer treatment, adoptive transfer experiments were conducted. Twelve-week-old NOD female mice were treated with sIAg7-GADp217 as described, and splenocytes harvested 3 wk after the last injection. Splenocytes from sIAg7-GADp217- or sIAg7-HEL treated groups were then mixed with splenocytes prepared from diabetic NOD donor mice and i.p. injected into NOD.scid recipients. Mice were monitored for diabetes. As expected, transfer of diabetogenic splenocytes alone or mixed with splenocytes from sIAg7-HEL treated NOD donors induced diabetes in all of the recipients (Fig. 3). In contrast, the onset of diabetes was significantly delayed in NOD.scid mice receiving an equal mixture of splenocytes from sIAg7-GADp217 treated and diabetic animals (p ≤ 0.002) (Fig. 3). Notably, transfer of splenocytes prepared from sIAg7-GADp217 treated mice alone failed to induce diabetes in NOD.scid recipients (0/6), indicating a lack of pathogenic T effectors (Fig. 3). The lack of diabetogenic activity and the suppressive effect of splenocytes suggested that the protection induced by sIAg7-GADp217 treatment was mediated by active immunoregulation.
FIGURE 3.
sIAg7-GADp217 vaccination induces active immunoregulation. NOD.scid mice received 5 × 106 splenocytes from diabetic (n = 11) or nondiabetic sIAg7-GADp217 treated (n = 6) NOD female mice alone, or a mixture of an equal number of splenocytes (5 × 106) from diabetic NOD plus sIAg7-GADp217 (n = 7) or sIAg7-HEL (n = 5) dimer treated NOD donors, and diabetes was monitored. *, p = 0.0005, by Kaplan-Meier log-rank test, for diabetogenic splenocytes alone vs sIAg7-GADp217 splenocytes alone and †, p ≤ 0.0015, for diabetogenic splenocytes alone or a mixture of diabetogenic plus sIAg7-HEL splenocytes vs a mixture of diabetogenic plus sIAg7-GADp217 splenocytes.
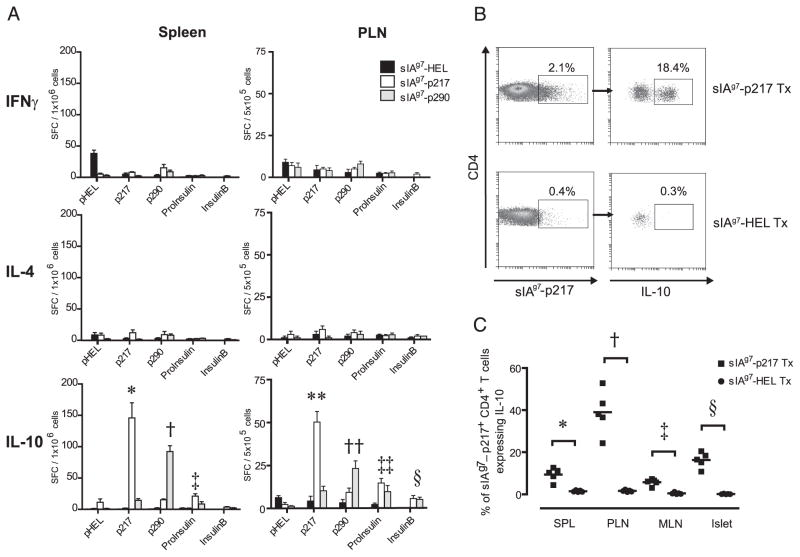
Next, the nature of the T cell response induced by the sIAg7-Ig dimers was studied. NOD female mice 12 wk of age were treated as above, and 3 wk after the last injection the frequency of IL-4-, IL-10-, and IFN-γ-secreting T cells in response to a panel of β cell peptides was measured via ELISPOT in the PLN and spleen. Cultures established from the sIAg7-GADp217 and sIAg7-GADp290 treated mice exhibited a significant increase in the frequency of p217- and p290-specific IL-10- but not IL-4-secreting T cells, respectively, compared with sIAg7-HEL-injected animals (Fig. 4A). In addition, IL-10-secreting T cells specific for proinsulin (B24-C36) and insulin B chain (p9-23) were increased in the PLN of sIAg7-GADp217 and sIAg7-GADp290 treated groups vs sIAg7-HEL treated mice (Fig. 4A), indicating epitope spread among IL-10-secreting aTreg cells. TGF-μ1 was not detected in supernatants of peptide-pulsed cultures established from the spleen and PLN of any of the treatment and control groups as measured by ELISA (data not shown). In addition, a significant increase in the frequency of CD4+ T cells that bind sIAg7-GADp217 multimer and expressed intracellular IL-10 was detected in the spleen, PLN, and islets of sIAg7-GADp217 treated but not sIAg7-HEL treated mice (Fig. 4, B and C). These results demonstrate that protection mediated by sIAg7-GADp217 and sIAg7-GADp290 treatment corresponds with the induction of IL-10- but not IL-4- or TGF-β1-secreting aTreg cells found in the spleen, PLN, and islets.
FIGURE 4.
sIAg7-GADp217 and sIAg7-GADp290 vaccination induces IL-10-secreting aTreg cells. Groups of n = 5 12-wk-old NOD female mice were treated with sIAg7-Ig and 3 wk after the second course of injections T cell reactivity assessed. A, Spleen and PLN suspensions were examined via ELISPOT, and the frequency of T cells secreting IFN-γ, IL-4, and IL-10 determined in response to 20 μg/ml peptide. For a given peptide used for in vitro stimulation, comparisons were made with cultures established from sIAg7-HEL dimer treated mice. *, p = 0.002; †, p = 0.005; ‡, p = 0.01; **, p = 0.0003; ††, p = 0.002; ‡‡, p = 0.005; and §, p = 0.005 by Student’s t test. Error bar represents mean ± SEM. B, Representative FACS data of the frequency of sIAg7-GADp217 multimer-staining CD4+ T cells expressing intracellular IL-10 cultured from the islets following anti-CD3 and anti-CD28 Ab stimulation of sIAg7-GADp217 and sIAg7-HEL dimer treated (Tx) mice. C, Frequency ± SD of sIAg7-GADp217 multimer-staining CD4+ T cells expressing intra-cellular IL-10 cultured from the spleen, PLN, MLN, and islets following anti-CD3 and anti-CD28 Ab stimulation of n = 5 individual NOD mice treated with sIAg7-GADp217 or sIAg7-HEL dimers. For a given tissue, comparisons were made between sIAg7-GADp217 vs sIAg7-HEL dimer treated mice. *, p = 0.048, spleen; †, p = 0.003, PLN; ‡, p = 0.01, MLN; and §, p = 0.005 by Student’s t Test. p = 0.02 by one-way ANOVA test, for frequency of sIAg7-GADp217 multimer-staining IL-10-expressing CD4+ T cells in sIAg7-GADp217 vs sIAg7-HEL dimer treated NOD mice.
sIAg7-GADp217 vaccination increases the frequency of GADp217-specific FoxP3-expressing Treg cells
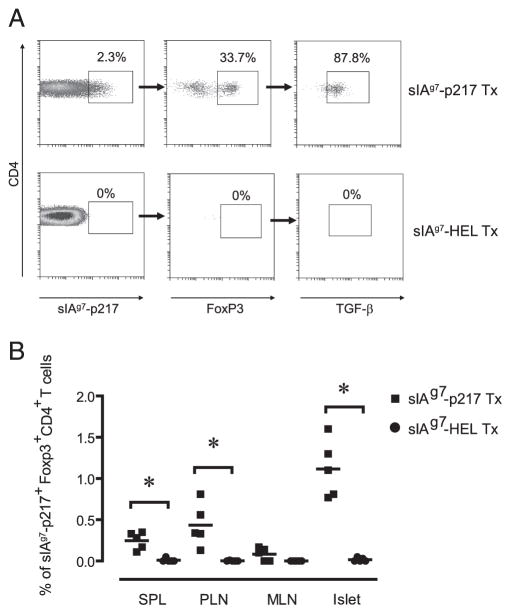
Because FoxP3-expressing Treg cells play a key role in regulating self-tolerance (30–32), whether sIAg7-Ig dimer treatment increased FoxP3-expressing Treg cells was investigated. The frequency of “bulk” FoxP3-expressing CD4+CD25+ T cells in the spleen, PLN, mesenteric lymph nodes (MLN) and islets and in vitro suppressor function of these Treg cells were similar in sIAg7-GADp217 and sIAg7-HEL treated NOD mice (see supplemental Fig. 1).4 No sIAg7-GADp217 multimer binding CD4+ T cells that expressed FoxP3 were detected in the spleen, PLN, MLN, and islets of sIAg7-HEL treated NOD mice (Fig. 5). Conversely, an increased frequency of sIAg7-GADp217 multimer-binding FoxP3-expressing CD4+ T cells was detected in the islets, and to a lesser extent the PLN and spleen but not the MLN of NOD mice treated with sIAg7-GADp217 (Fig. 5). Furthermore, the majority (~90%) of these FoxP3-expressing CD4+ T cells expressed surface TGF-β1 (Fig. 5A), whereas none expressed intra-cellular IL-10 (data not shown). These results demonstrate that sIAg7-Ig dimer vaccination induces or expands FoxP3-expressing Treg cells in a peptide-specific manner.
FIGURE 5.
An increased frequency of GADp217-specific FoxP3-expressing Treg cells is detected in sIAg7-GADp217 vaccinated NOD mice. A, Representative FACS data of sIAg7-GADp217 multimer-binding CD4+ T cells ex vivo expressing FoxP3 and surface TGF-β1 from the islets of sIAg7-GADp217 and sIAg7-HEL-treated (Tx) NOD female mice. B, Frequency ± SD of sIAg7-GADp217 multimer-staining CD4+ T cells ex vivo expressing FoxP3 in the spleen, PLN, MLN, and islets of n = 5 individual NOD female mice treated with sIAg7-GADp217 and sIAg7-HEL. *, p ≤ 0.004 by Student’s t test, for sIAg7-GADp217 vs sIAg7-HEL dimer treated animals for a given tissue. p < 10−3 by one-way ANOVA test for sIAg7-GADp217 vs sIAg7-HEL treated NOD mice.
IL-10 is necessary for protection induced by sIAg7-Ig dimer vaccination
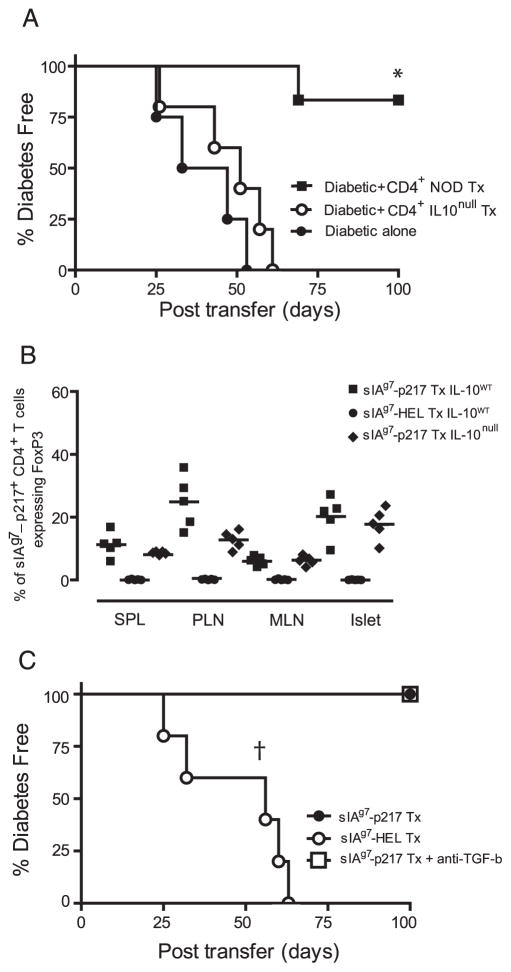
Because sIAg7-GADp217 treatment induced IL-10-expressing T cells, the relative contribution of these aTreg cells in mediating protection was examined. Twelve-week-old female NOD mice lacking IL-10 expression (NOD.IL-10null) or wild-type NOD mice were vaccinated with sIAg7-GADp217 as described. Three weeks after the last injection CD4+ T cells were purified via negative selection. CD4+ T cells were then mixed with splenocytes prepared from diabetic NOD donors, transferred into groups of NOD. scid recipients, and the onset of diabetes monitored. All of the NOD.scid mice receiving the diabetogenic splenocytes only developed diabetes (Fig. 6A). In marked contrast, CD4+ T cells from NOD mice treated with sIAg7-GADp217 effectively blocked the transfer of diabetes; 5 of 6 NOD.scid recipients remained diabetes-free (Fig. 6A). In contrast, the mixture containing CD4+ T cells from sIAg7-GADp217 treated NOD.IL-10null mice failed to prevent the transfer of diabetes (Fig. 6A). These results demonstrate that IL-10-secreting aTreg cells induced by sIAg7-Ig dimer vaccination play a key role in suppressing the function of established pathogenic T effectors.
FIGURE 6.
Protection mediated by sIAg7-GADp217 treatment is IL-10-dependent. A, Groups of NOD.scid mice (n = 4) received diabetogenic splenocytes alone or a mixture of purified splenic CD4+ T cells isolated from NOD (n = 6) or NOD.IL-10null (n = 5) female mice treated with sIAg7-GADp217, and diabetes was monitored. *, p = 0.001, by Kaplan-Meier log-rank test for CD4+ T cells from sIAg7-GADp217 treated (Tx) NOD vs NOD.IL10null mice or diabetogenic splenocytes alone. B, Frequency ± SD of sIAg7-GADp217 multimer-staining CD4+ T cells ex vivo expressing FoxP3 in the spleen, PLN, MLN, and islets of n = 5 individual NOD (IL-10wt) or NOD.IL-10null (IL-10null) female mice treated with sIAg7-GADp217 or sIAg7-HEL. C, CD4+ T cells (5 × 106) isolated from the PLN of NOD female mice vaccinated at 12 wk of age with sIAg7-GADp217 or sIAg7-HEL were mixed with splenocytes from diabetic NOD donors (5 × 106) and transferred into groups of n = 5 NOD.scid mice. One group of NOD.scid recipients of CD4+ T cells isolated from sIAg7-GADp217 vaccinated animals also received a TGF-β-neutralizing Ab. †, p = 0.0002, by Kaplan-Meier log-rank test, for recipients of CD4+ T cells from sIAg7-HEL vaccinated mice vs recipients of CD4+ T cells from sIAg7-GADp217 vaccinated mice with or without anti-TGF-β Ab.
Next, whether IL-10 was required for the induction or expansion of FoxP3-expressing Treg cells by sIAg7-Ig dimer vaccination was determined. Wild-type and NOD.IL-10null female mice received sIAg7-GADp217 injections as described, and 3 wk after the final injection, tissues were harvested and T cells stained with sIAg7-GADp217 multimer ex vivo. As demonstrated in Fig. 6B, no significant difference in the frequency of sIAg7-GADp217 multimer binding CD4+ T cells expressing FoxP3 was detected in the spleen, PLN, MLN, and islets of NOD and NOD.IL-10null female mice vaccinated with sIAg7-GADp217. These results indicated that induction/expansion of peptide-specific FoxP3-expressing Treg cells was independent of endogenous IL-10, and that these immunoregulatory effectors play only a limited role in sIAg7-GADp217-induced protection. To confirm the latter, a coadoptive transfer experiment was conducted. CD4+ T cells were isolated from the PLN of NOD female mice treated at 12 wk of age with sIAg7-GADp217 or sIAg7-HEL dimer, and then injected with diabetogenic splenocytes into NOD.scid recipients. One group of recipients was treated with a neutralizing anti-TGF-β Ab. As expected, CD4+ T cells from sIAg7-GADp217 but not sIAg7-HEL dimer treated NOD mice blocked the transfer of diabetes (Fig. 6C). Notably, administration of anti-TGF-β Ab had no effect on the protection mediated by Treg cells induced by sIAg7-GADp217 dimer vaccination (Fig. 6C). Altogether these results demonstrate that protection induced by sMHCII-Ig dimer treatment is primarily mediated by IL-10-secreting aTreg cells, and that Treg cells expressing TGF-β and FoxP3 have only a limited role.
Discussion
Administration of peptide-sMHCII recombinants has been shown to tolerize pathogenic T cells in mono-specific models of autoimmunity (24), including collagen induced arthritis (22), experimental autoimmune uveitis (33, 34), and experimental autoimmune encephalomyelitis (35). In these studies, prevention or suppression of induced autoimmunity has been associated with establishing hyporeactivity in the pathogenic peptide-specific T effector cells, or eliciting peptide-specific Treg cell reactivity. The current work provides evidence that sMHCII-Ig dimer vaccination effectively suppresses a diverse repertoire of established autoreactive T effector cells via induction of IL-10-secreting aTreg cells. The former is demonstrated by sIAg7-Ig dimer vaccination blocking β cell autoimmunity at a late preclinical stage of T1D in NOD mice (Figs. 1 and 2). Based on dose and relative efficacy of a given epitope to mediate protection, sIAg7-Ig dimer treatment was found to be more potent than administering the corresponding native GAD65 peptides (Fig. 1A) (9). Coinjection of a total of 600 μg of each native GADp217 and GADp290 prepared in IFA prevented diabetes in 12-wk-old NOD female mice, and when injected individually neither peptide was protective (Fig. 1A) (9). Furthermore, coinjection of up to 600 μg of each soluble GADp217 and GADp290 (e.g., in the absence of adjuvant) also had no protective effect in 12-wk-old NOD female mice (R. Tisch, unpublished results). In contrast, the equivalent of only ~7 μg total of native GAD65 peptide was sufficient to block diabetes using the sIAg7-Ig dimers, and either sIAg7-GADp217 or sIAg7-GADp290 alone did so equally well (Fig. 2). A single round of three injections of sIAg7-GADp217 or sIAg7-GADp290 had only a limited effect on the development of diabetes, indicating that the second set of sMHCII-Ig dimer injections is required to “boost” the immunoregulatory response (L. Li and R. Tisch, unpublished results).
The robust nature of sMHCII-Ig dimer vaccination is likely due to a variety of parameters. In vivo induction of aTreg cells is expected to be more efficient following treatment with sMHCII-Ig dimers relative to native peptide. For instance, a number of factors influence the efficacy of injected native peptide to stimulate aTreg cell induction/expansion including the binding affinity of the peptide for the MHCII molecule, the stability of the peptide-MHCII complex, and the type and number of APC presenting the peptide. However, these factors are negated by the use of sMHCII-Ig dimers because the peptide is covalently linked and the dimer complex directly binds T cells. Direct binding of sMHCII-Ig complexes would also be expected to enhance clonal anergy/deletion of CD4+ T cells at a sufficient dose (20, 22). Under the conditions used in this study, however, sIAg7-Ig-induced clonal anergy/deletion appears to have a minimal (if any) role in the tolergenic effect. For instance, significant expansion of GADp217-specific CD4+ T cells (~5-fold) was detected in the islets of sIAg7-GADp217 vs sIAg7-HEL treated NOD mice (Fig. 4B).
Another likely parameter contributing to the potency of sMHCII-Ig dimer vaccination is the nature of Treg cells that are induced or expanded. For instance, protection induced in NOD mice by coinjection of native GADp217 and GADp290 in IFA correlated with an increased frequency of IL-4- but not IL-10-secreting CD4+ T cells (Fig. 1B) (9), which in turn was reflected by the inability of the native GAD65 peptides to prevent diabetes in NOD.IL-4null mice (9). In contrast, protection induced by sIAg7-GADp217 or sIAg7-GADp290 vaccination was dependent on IL-10-secreting CD4+ T cells. These results are consistent with findings made by Casares et al. (24) demonstrating that sIEd-HA dimer vaccination elicits IL-10-secreting aTreg cells in vivo. IL-10-secreting effector cells are a particularly potent subset of aTreg cells by regulating the responses of naive and memory T cells, and suppressing Th1 cell-mediated pathologies through bystander suppression mediated by local release of IL-10 (36–38). IL-10 also inhibits the activation and function of APC such as dendritic cells (39 – 41). IL-10-treated dendritic cells gain a “tolergenic” phenotype and preferentially promote the development of aTreg cells (40 – 42). These direct and indirect effects of IL-10 would be expected to amplify the immunoregulatory response induced by sIAg7-Ig dimer vaccination and may explain the abrupt block in the progression of insulitis in protected NOD mice (Fig. 2B). Indeed, sIAg7-p217 and sIAg7-GADp290 treatment induced not only GAD65-specific IL-10-secreting aTreg cells, but also aTreg-specific for other β cell peptides (Fig. 4A). The relative potency of IL-10-secreting aTreg cells may also explain why sIAg7-p217 or sIAg7-GADp290 alone suppressed ongoing β cell autoimmunity (Fig. 2A), whereas coinjection of native GADp217 and GADp290 in IFA was needed to similarly prevent diabetes onset (Fig. 1A). Injection of both native GAD65-derived peptides in IFA likely is required to induce a sufficient frequency of IL-4-secreting CD4+ T cells, which is limiting with either peptide alone (Fig. 1B). Interestingly, an increase in GADp217-specific FoxP3- and TGF-β1-expressing Treg cells was also detected in the PLN and islets of sIAg7-GADp217 vaccinated NOD mice (Fig. 5). Although protection mediated by sIAg7-Ig dimer was dependent on induction of IL-10-secreting aTreg cells, β cell-specific FoxP3-expressing Treg cells would also be expected to contribute to immunoregulation within the PLN and islets. However, the increase in GADp217-specific FoxP3-expressing Treg cells (Fig. 6B) was insufficient to suppress diabetogenic T effectors in the absence of IL-10-secreting aTreg cells (Fig. 6A), and neutralizing TGF-β had no effect on the regulatory function of CD4+ T cells from sIAg7-p217 vaccinated NOD mice (Fig. 6C).
Based on our findings and the findings of other studies (23, 24), properties intrinsic to sMHCII-Ig dimers favor differentiation of naive T precursors toward IL-10-secreting aTreg cells. sMHCII-Ig dimers may also preferentially expand established IL-10-expressing aTreg cells. For instance Liu and colleagues (43) have detected GAD65-specific IL-10-expressing CD4+ T cells in the spleens of unmanipulated 8-wk-old NOD mice. However, the identity of the peptide bound by sMHCII-Ig dimer also appears to be a key factor determining the efficacy of a given recombinant. For instance, the Bluestone group (23) reported that vaccinating young NOD mice with a sIAg7-Ig dimer complexed with a mimetic peptide recognized by TCR transgenic BDC2.5 CD4+ T cells (sIAg7-p31) failed to induce aTreg cells that prevent diabetes in an adoptive transfer model. Similarly, we found that 12-wk-old NOD female mice injected with sIAg7-Ig dimer containing a different BDC2.5 mimetic peptide continued to develop diabetes (L. Li and R. Tisch, unpublished results). Nevertheless, preliminary findings demonstrate that vaccination with sIAg7-Ig dimers complexed with other β cell-derived autoantigens such as proinsulin, induce IL-10-secreting aTreg cells and block β cell autoimmunity in 12-wk-old NOD female mice (R. Tisch, unpublished results). Investigation of the biochemical and transcriptional signaling events transduced in CD4+ T cells will provide important insight into the events that regulate aTreg differentiation by sIAg7-Ig dimers. It is noteworthy that induction of aTreg cells is also achieved by administration of soluble peptide-linked single chain recombinants consisting of the α1 and β1 domains of MHCII (34, 35, 44). These recombinant TCR ligands directly bind to TCR independent of CD4, and have been found to also induce T cell hyporeactivity. Similar to our findings, a recent report showed that vaccination with recombinant TCR ligands linked to peptides derived from proteolipid protein reversed in mice experimental autoimmune encephalomyelitis induced by a whole spinal cord homogenate via induction of proteolipid protein-specific IL-10- (and IL-13-) secreting aTreg cells (44). This finding further argues that soluble peptide-MHCII vaccination may in general prove to be a highly effective approach of peptide immunotherapy to preferentially promote aTreg cell reactivity and suppress ongoing autoimmunity.
In summary, our findings indicate that peptide-sMHCII-Ig dimer treatment is a robust approach to suppress late preclinical β cell autoimmunity via induction of immunoregulatory T effector cells. This work also provides rationale for using sMHCII-Ig dimer vaccination as a mode of adjuvant-free peptide immunotherapy in the clinic. For instance, HLA-DR4 or HLA-DQ8 recombinants covalently linked to known β cell-derived peptides restricted to the corresponding HLA molecules can be used to induce IL-10-secreting aTreg cells. The apparent intrinsic property of sMHCII-Ig dimers to induce IL-10-secreting aTreg cells is particularly relevant in view of work by Peakman and colleagues (45). This group demonstrated an increased frequency of HLA-DR4-restricted β cell peptide-specific CD4+ T cells secreting IL-10 in HLA-matched healthy control vs diabetic individuals. A role for IL-10-secreting Treg cells has also been implicated in other tissue specific autoimmune diseases such as multiple sclerosis (46). The relatively low dose of total native peptide required to induce protection also has important safety implications in view of murine and clinical studies reporting anaphylaxis following administration of high doses of soluble peptide (47–49). Currently, efforts are ongoing in the laboratory to assess the properties of such HLA sMHCII-Ig dimers.
Footnotes
This work was supported by Grant R01AI058014 from the National Institutes of Health, Grant 1-2008-452 from the Juvenile Diabetes Research Foundation, and Grant 7-04-RA-121 from the American Diabetes Association. B.W. was supported by the 1-04-CD-09 American Diabetes Association Career Development Award.
Abbreviations used in this paper: T1D, type 1 diabetes; HEL, hen egg lysozyme; MHCII, MHC class II; HA, hemagglutinin; GAD, glutamic acid decarboxylase; Treg, immunoregulatory T; aTreg; adaptive Treg; PLN, pancreatic lymph node; MLN, mesenteric lymph node.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Bach JF. Insulin-dependent diabetes mellitus as an autoimmune disease. Endocr Rev. 1994;15:516–542. doi: 10.1210/edrv-15-4-516. [DOI] [PubMed] [Google Scholar]
- 2.Tisch R, McDevitt HO. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–297. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 3.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 4.Cooke A, Phillips JM, Parish NM. Tolerogenic strategies to halt or prevent type 1 diabetes. Nat Immunol. 2001;2:810–815. doi: 10.1038/ni0901-810. [DOI] [PubMed] [Google Scholar]
- 5.Bougneres PF, Landais P, Boisson C, Carel JC, Frament N, Boitard C, Chaussain JL, Bach JF. Limited duration of remission of insulin dependency in children with recent overt type I diabetes treated with low-dose cyclosporin. Diabetes. 1990;39:1264–1272. doi: 10.2337/diab.39.10.1264. [DOI] [PubMed] [Google Scholar]
- 6.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 7.Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA. A single course of anti-CD3 monoclonal antibody hOKT3γ1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bach JF. Immunotherapy of insulin-dependent diabetes mellitus. Curr Opin Immunol. 2001;13:601–605. doi: 10.1016/s0952-7915(00)00267-3. [DOI] [PubMed] [Google Scholar]
- 9.Tisch R, Wang B, Serreze DV. Induction of glutamic acid decarboxylase 65-specific Th2 cells and suppression of autoimmune diabetes at late stages of disease is epitope dependent. J Immunol. 1999;163:1178–1187. [PubMed] [Google Scholar]
- 10.Jain R, Tartar DM, Gregg RK, Divekar RD, Bell JJ, Lee HH, Yu P, Ellis JS, Hoeman CM, Franklin CL, Zaghouani H. Innocuous IFNγ induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J Exp Med. 2008;205:207–218. doi: 10.1084/jem.20071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fife BT, Guleria I, Gubbels Bupp M, Eagar TN, Tang Q, Bour-Jordan H, Yagita H, Azuma M, Sayegh MH, Bluestone JA. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203:2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Z, Herman AE, Matos M, Mathis D, Benoist C. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J Exp Med. 2005;202:1387–1397. doi: 10.1084/jem.20051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez NR, Augstein P, Moustakas AK, Papadopoulos GK, Gregori S, Adorini L, Jackson DC, Harrison LC. Disabling an integral CTL epitope allows suppression of autoimmune diabetes by intranasal proinsulin peptide. J Clin Invest. 2003;111:1365–1371. doi: 10.1172/JCI17166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang B, Tisch R. Parameters influencing antigen-specific immuno-therapy for type 1 diabetes. Immunol Res. 2008;42:246–258. doi: 10.1007/s12026-008-8090-5. [DOI] [PubMed] [Google Scholar]
- 15.Seifarth C, Pop S, Liu B, Wong CP, Tisch R. More stringent conditions of plasmid DNA vaccination are required to protect grafted versus endogenous islets in nonobese diabetic mice. J Immunol. 2003;171:469–476. doi: 10.4049/jimmunol.171.1.469. [DOI] [PubMed] [Google Scholar]
- 16.Babbitt BP, Matsueda G, Haber E, Unanue ER, Allen PM. Antigenic competition at the level of peptide-Ia binding. Proc Natl Acad Sci USA. 1986;83:4509–4513. doi: 10.1073/pnas.83.12.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller S, Adorini L, Juretic A, Nagy ZA. Selective in vivo inhibition of T cell activation by class II MHC-binding peptides administered in soluble form. J Immunol. 1990;145:4006–4011. [PubMed] [Google Scholar]
- 18.Ishioka GY, Adorini L, Guery JC, Gaeta FC, LaFond R, Alexander J, Powell MF, Sette A, Grey HM. Failure to demonstrate long-lived MHC saturation both in vitro and in vivo: implications for therapeutic potential of MHC-blocking peptides. J Immunol. 1994;152:4310–4319. [PubMed] [Google Scholar]
- 19.Casares S, Zong CS, Radu DL, Miller A, Bona CA, Brumeanu TD. Antigen-specific signaling by a soluble, dimeric peptide/major histocompatibility complex class II/Fc chimera leading to T helper cell type 2 differentiation. J Exp Med. 1999;190:543–553. doi: 10.1084/jem.190.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Appel H, Seth NP, Gauthier L, Wucherpfennig KW. Anergy induction by dimeric TCR ligands. J Immunol. 2001;166:5279–5285. doi: 10.4049/jimmunol.166.8.5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casares S, Bona CA, Brumeanu TD. Engineering and characterization of a murine MHC class II-immunoglobulin chimera expressing an immunodominant CD4 T viral epitope. Protein Eng. 1997;10:1295–1301. doi: 10.1093/protein/10.11.1295. [DOI] [PubMed] [Google Scholar]
- 22.Zuo L, Cullen CM, DeLay ML, Thornton S, Myers LK, Rosloniec EF, Boivin GP, Hirsch R. A single-chain class II MHC-IgG3 fusion protein inhibits autoimmune arthritis by induction of antigen-specific hyporesponsiveness. J Immunol. 2002;168:2554–2559. doi: 10.4049/jimmunol.168.5.2554. [DOI] [PubMed] [Google Scholar]
- 23.Masteller EL, Warner MR, Ferlin W, Judkowski V, Wilson D, Glaichenhaus N, Bluestone JA. Peptide-MHC class II dimers as therapeutics to modulate antigen-specific T cell responses in autoimmune diabetes. J Immunol. 2003;171:5587–5595. doi: 10.4049/jimmunol.171.10.5587. [DOI] [PubMed] [Google Scholar]
- 24.Casares S, Hurtado A, McEvoy RC, Sarukhan A, von Boehmer H, Brumeanu TD. Down-regulation of diabetogenic CD4+ T cells by a soluble dimeric peptide-MHC class II chimera. Nat Immunol. 2002;3:383–391. doi: 10.1038/ni770. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Wang B, Frelinger JA, Tisch R. T cell promiscuity in autoimmune diabetes. Diabetes. 2008;57:2099–2106. doi: 10.2337/db08-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnold PY, Vignali KM, Miller TB, La Gruta NL, Cauley LS, Haynes L, Scott Adams P, Swain SL, Woodland DL, Vignali DA. Reliable generation and use of MHC class II:γ2aFc multimers for the identification of antigen-specific CD4+ T cells. J Immunol Methods. 2002;271:137–151. doi: 10.1016/s0022-1759(02)00343-5. [DOI] [PubMed] [Google Scholar]
- 27.Lund J, Winter G, Jones PT, Pound JD, Tanaka T, Walker MR, Artymiuk PJ, Arata Y, Burton DR, Jefferis R, Woof JM. Human FcγRI and FcγRII interact with distinct but overlapping sites on human IgG. J Immunol. 1991;147:2657–2662. [PubMed] [Google Scholar]
- 28.Wines BD, Powell MS, Parren PW, Barnes N, Hogarth PM. The IgG Fc contains distinct Fc receptor (FcR) binding sites: the leukocyte receptors FcγRI and FcγRIIa bind to a region in the Fc distinct from that recognized by neonatal FcR and protein A. J Immunol. 2000;164:5313–5318. doi: 10.4049/jimmunol.164.10.5313. [DOI] [PubMed] [Google Scholar]
- 29.Wong CP, Li L, Frelinger JA, Tisch R. Early autoimmune destruction of islet grafts is associated with a restricted repertoire of IGRP-specific CD8+ T cells in diabetic nonobese diabetic mice. J Immunol. 2006;176:1637–1644. doi: 10.4049/jimmunol.176.3.1637. [DOI] [PubMed] [Google Scholar]
- 30.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 31.Bluestone JA, Boehmer H. Regulatory T cells. Semin Immunol. 2006;18:77. doi: 10.1016/j.smim.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Haxhinasto S, Benoist C, Mathis D. Regulatory T-cell differentiation: Committed to control: a precocious choice? Immunol Cell Biol. 2007;85:175–176. doi: 10.1038/sj.icb.7100041. [DOI] [PubMed] [Google Scholar]
- 33.Karabekian Z, Lytton SD, Silver PB, Sergeev YV, Schneck JP, Caspi RR. Antigen/MHC class II/Ig dimers for the study of uveitogenic T cells: IRBP p161–180 presented by both IA and IE molecules. Invest Ophthalmol Vis Sci. 2005;46:3769–3776. doi: 10.1167/iovs.05-0187. [DOI] [PubMed] [Google Scholar]
- 34.Adamus G, Burrows GG, Vandenbark AA, Offner H. Treatment of autoimmune anterior uveitis with recombinant TCR ligands. Invest Ophthalmol Vis Sci. 2006;47:2555–2561. doi: 10.1167/iovs.05-1242. [DOI] [PubMed] [Google Scholar]
- 35.Offner H, Subramanian S, Wang C, Afentoulis M, Vandenbark AA, Huan J, Burrows GG. Treatment of passive experimental autoimmune encephalomyelitis in SJL mice with a recombinant TCR ligand induces IL-13 and prevents axonal injury. J Immunol. 2005;175:4103–4111. doi: 10.4049/jimmunol.175.6.4103. [DOI] [PubMed] [Google Scholar]
- 36.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 37.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 38.Sing A, Rost D, Tvardovskaia N, Roggenkamp A, Wiedemann A, Kirschning CJ, Aepfelbacher M, Heesemann J. Yersinia V-antigen exploits toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J Exp Med. 2002;196:1017–1024. doi: 10.1084/jem.20020908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGuirk P, McCann C, Mills KH. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J Exp Med. 2002;195:221–231. doi: 10.1084/jem.20011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–617. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 41.Kullberg MC, Jankovic D, Gorelick PL, Caspar P, Letterio JJ, Cheever AW, Sher A. Bacteria-triggered CD4+ T regulatory cells suppress Helicobacter hepaticus-induced colitis. J Exp Med. 2002;196:505–515. doi: 10.1084/jem.20020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 43.You S, Chen C, Lee WH, Brusko T, Atkinson M, Liu CP. Presence of diabetes-inhibiting glutamic acid decarboxylase-specific IL-10-dependent regulatory T cells in naïve nonobese diabetic mice. J Immunol. 2004;173:6777–6785. doi: 10.4049/jimmunol.173.11.6777. [DOI] [PubMed] [Google Scholar]
- 44.Sinha S, Subramanian S, Miller L, Proctor TM, Roberts C, Burrows GG, Vandenbark AA, Offner H. Cytokine switch and bystander suppression of autoimmune responses to multiple antigens in experimental autoimmune encephalomyelitis by a single recombinant T cell receptor ligand. J Neurosci. 2009;29:3816–3823. doi: 10.1523/JNEUROSCI.5812-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arif S, Tree TI, Astill TP, Tremble JM, Bishop AJ, Dayan CM, Roep BO, Peakman M. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest. 2004;113:451–463. doi: 10.1172/JCI19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Astier AL, Meiffren G, Freeman S, Hafler DA. Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. J Clin Invest. 2006;166:3252–3257. doi: 10.1172/JCI29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu E, Moriyama H, Abiru N, Miao D, Yu L, Taylor RM, Finkelman FD, Eisenbarth GS. Anti-peptide autoantibodies and fatal anaphylaxis in NOD mice in response to insulin self-peptides B:9–23 and B:13–23. J Clin Invest. 2002;110:1021–1027. doi: 10.1172/JCI15488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pedotti R, Sanna M, Tsai M, DeVoss J, Steinman L, McDevitt H, Galli SJ. Severe anaphylactic reactions to glutamic acid decarboxylase (GAD) self peptides in NOD mice that spontaneously develop autoimmune type 1 diabetes mellitus. BMC Immunol. 2003;4:2–10. doi: 10.1186/1471-2172-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kappos L, Comi G, Panitch H, Oger J, Antel J, Conlon P, Steinman L. Induction of a non-encephalitogenic type 2 T helper cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. Nat Med. 2000;6:1098–1100. doi: 10.1038/80525. [DOI] [PubMed] [Google Scholar]