Highlight
Increasing the ratio of red to far red light promotes axillary bud outgrowth and suppresses bud abscisic acid signalling within 3 h, before stem auxin signalling is affected.
Keywords: Abscisic acid, Arabidopsis, auxin, axillary bud, branch, competition, phytochrome, R:FR.
Abstract
Arabidopsis thaliana shoot branching is inhibited by a low red light to far red light ratio (R:FR, an indicator of competition), and by loss of phytochrome B function. Prior studies have shown that phytochrome B deficiency suppresses bud growth by elevating systemic auxin signalling, and that increasing the R:FR promotes the growth of buds suppressed by low R:FR by inhibiting bud abscisic acid (ABA) accumulation and signalling. Here, systemic auxin signalling and bud ABA signalling were examined in the context of rapid bud responses to an increased R:FR. Increasing the R:FR promoted the growth of buds inhibited by a low R:FR within 6 h. Relative to a low R:FR, bud ABA accumulation and signalling in plants given a high R:FR showed a sustained decline within 3 h, prior to increased growth. Main stem auxin levels and signalling showed a weak, transient response. Systemic effects and those localised to the bud were further examined by decapitating plants maintained either under a low R:FR or provided with a high R:FR. Increasing the R:FR promoted bud growth before decapitation, but decapitated plants eventually formed longer branches. The data suggest that rapid responses to an increased R:FR may be mediated by changes in bud ABA physiology, although systemic auxin signalling is necessary for sustained bud repression under a low R:FR.
Introduction
Shoot architecture is determined to a large extent by the growth and development of branches, which is usually a plastic trait regulated by genetics, the environment, and interactions between the two. Axillary buds formed in the leaf axils from axillary meristems can remain arrested or elongate into branches of variable sizes, generating a wide variety of plant forms. Branching confers adaptation to diverse ecological conditions and contributes to fitness. In crops, branching impacts yield and productivity, and thus has been an important trait in domestication and is often targeted by breeders when developing novel cultivars/varieties.
In Arabidopsis (Arabidopsis thaliana), axillary buds may remain small in a quasi-dormant state, or elongate and form a branch. The transition from quasi- dormancy to sustained growth is determined by many factors intrinsic and external to the bud (Waldie et al., 2010; Domagalska and Leyser, 2011; Janssen 2014; Rameau et al., 2015). In typical Arabidopsis accessions grown under long days, the buds formed in the upper leaf axils begin to elongate first, whereas lower buds show a sequential delay of elongation (Hempel and Feldman, 1994). The growth of lower buds is variable and is contextually controlled by developmentally derived signals, environmental factors, or the combined action of both (Finlayson, 2007; Finlayson et al., 2010; Reddy et al., 2013; Reddy and Finlayson, 2014; Reddy et al., 2014). In many species of both monocots and eudicots, light signals that indicate a competitive or shaded environment inhibit branching (Deregibus et al., 1983; Davis and Simmons, 1994; Robin et al., 1994; Donohue and Schmitt, 1999; Wan and Sosebee, 1998; Kebrom et al., 2006; Finlayson et al., 2010; Kebrom et al., 2010). The low red light to far red light ratio (R:FR) generated in these competitive and/or shaded environments is sensed by the phytochrome (phy) family of photoreceptors, including the major R:FR sensor phyB. Signals perceived by phyB evoke a suite of adaptive responses termed the shade avoidance syndrome (SAS), including reduced branching (Casal, 2012). Several studies have shown that the abundance of the natural auxin indole-3-acetic acid (IAA) increases rapidly in young Arabidopsis seedlings in response to a low R:FR and contributes to the shade avoidance syndrome (Tao et al., 2008, Hornitschek et al., 2012, Pacín et al., 2016). A low R:FR and phyB deficiency have been shown to inhibit branching in Arabidopsis by altering the expression of a variety of genes and pathways that operate both systemically and in a bud autonomous fashion (Finlayson et al., 2010, González-Grandío et al., 2013, Reddy et al., 2013, Reddy and Finlayson, 2014).
Hormonal pathways regulating axillary bud growth and branching have received considerable attention. Auxin has been implicated as a systemic regulator of branching. Auxin synthesized in the main shoot apex and upper branches is transported basipetally in the polar auxin transport stream and inhibits bud growth indirectly, without entering the bud. The inhibitory influence of superior shoots on the development of lower branches is a form of correlative inhibition known as apical dominance. The correlative inhibition of lower bud growth can be attributed to the inhibitory effects of auxin sourced from more apical organs, though other mechanisms may also be involved (Cline, 1997; Morris et al., 2005). The depletion of auxin (and possibly other factors) in the main shoot, either by decapitation or by impeding auxin transport with chemical inhibitors, can result in robust promotion of bud growth. The precise mechanism by which auxin exerts its indirect effects on bud growth remains unresolved although interesting models have been presented. One model proposes that auxin impacts secondary messengers (e.g. cytokinins and strigolactones) that move into the bud to promote or inhibit growth (Brewer et al., 2009; Dun et al., 2009; Brewer et al., 2015). Another model provides evidence that axillary buds and superior apices compete for auxin transport capacity in the main stem (Bennett et al., 2006; Prusinkiewicz et al., 2009; Balla et al., 2011; Bennett et al., 2016). Axillary buds grow only if they are capable of establishing an auxin efflux into the main shoot polar auxin transport stream. phyB-deficient Arabidopsis exhibits a constitutive shade avoidance syndrome that includes exaggerated apical dominance. This response was attributed to elevated auxin signalling in the main stem, independent of auxin abundance in this tissue (Reddy and Finlayson, 2014).
Many studies have associated elevated bud ABA abundance with the inhibition of branching (Tamas et al., 1979; Knox and Wareing, 1984; Gocal et al., 1991; Mader et al., 2003), including in the context of responses to the R:FR (Tucker and Mansfield, 1972; Tucker, 1977). Pharmaceutical approaches have shown that exogenous ABA treatment inhibits branching in a variety of species (Arney and Mitchell, 1969; Chatfield et al., 2000; Cline and Oh, 2006), whereas the ABA biosynthesis inhibitor fluridone promoted branching in rose (Rosa hybrida) (Le Bris et al., 1999). Likewise, in vitro explants of genetically modified Poplar (Populus X canescens [Ait.] Sm.) with reduced ABA sensitivity exhibited enhanced branching (Arend et al., 2009). Bud growth in sugarcane (Saccharum officinarum) has been associated with reduced bud ABA abundance and modification of ABA signalling by small RNAs (Ortiz-Morea et al., 2013). Buds of sorghum (Sorghum bicolor) deficient in phyB exhibited retarded growth relative to wild type, and overexpressed a set of ABA-related genes (Kebrom and Mullet, 2016). Exposing Arabidopsis grown under a high R:FR to a low R:FR suppressed branching and enhanced ABA signalling (González-Grandío et al., 2013). The opposite approach of growing Arabidopsis under a low R:FR inhibited the growth of specific lower buds, which could then be rapidly promoted to grow by increasing the R:FR (Reddy et al., 2013). ABA signalling in these buds was suppressed by the increased R:FR and bud ABA levels declined within 12 h. A role for ABA as a regulator of branching was demonstrated using mutants deficient in ABA biosynthesis that exhibited incomplete suppression of bud growth in a low R:FR (Reddy et al., 2013). ABA has now been shown to inhibit lower bud growth under both high and low R:FRs and may act in part by suppressing the expression of genes associated with the cell cycle (Yao and Finlayson, 2015). ABA also inhibited the expression of genes associated with the bud autonomous auxin pathway and inhibited the accumulation of IAA in the bud, which may be associated with the establishment of bud auxin efflux necessary for growth (Yao and Finlayson, 2015).
Various pathways regulating branch development, including auxin, strigolactones, cytokinins and sugars, have been shown to be integrated by the TEOSINTE BRANCHED1/CYCLOIDEA/PCF (TCP) domain transcription factor BRANCHED 1 (BRC1), or its homologs in other species (Aguilar-Martínez et al., 2007; Finlayson, 2007; Braun et al., 2012; Dun et al., 2012; Chen et al., 2013; Mason et al., 2014; Barbier et al., 2015). ABA is an exception, because it functions downstream of BRC1 (Yao and Finlayson, 2015). BRC1 may not only target ABA because it has also been shown to regulate the expression of a variety of cell cycle- and ribosome-related genes not previously identified as ABA responsive (González-Grandío et al., 2013).
Although roles for systemic auxin signalling in the main stem and for bud-localized ABA have been shown to contribute to axillary bud responses to competition signals mediated by phyB, much still remains unknown. The temporal kinetics of bud growth modification by the R:FR have not yet been determined. If the effects of the R:FR on bud development are transduced mainly by bud-localized mechanisms then a more rapid response may be anticipated, whereas systemic effects may require more time to manifest. Furthermore, the relative contributions of the systemic auxin and bud-localized ABA pathways have not been determined and nor have the potential interactions between the two. In the present study the timing of the bud growth response to an increased R:FR was defined, as were changes in the physiology associated with the two targeted hormone pathways. It was hypothesized that increasing the R:FR would promote bud growth within hours and that ABA and auxin pathways would exhibit altered behaviour prior to changes in bud growth. It was also hypothesized that an increased R:FR would alter bud-localized ABA homeostasis and signalling prior to changes in auxin signalling in the main stem.
Materials and methods
Plant growth, treatments, and bud elongation measurements
Plant growth and light conditions were as described in Reddy et al. (2013). Wild-type A. thaliana (Col-0, ABRC CS60000) was used throughout. Seeds were stratified at 4°C for 3 days in the dark, and then sown on Sunshine LC1 soil-less media in 50 mL conical tubes that were cut down to 30 mL. The plants were grown in a growth chamber modified with an overhead array of FR light-emitting diodes (735 nm). The chamber was split into two equal parts with a light-impermeable baffle. Photosynthetically active radiation was provided by fluorescent lamps at 180 μmoles·m−2·s−1 photosynthetic photon flux density. Plants were initially exposed to a high R:FR (3.5) for 1 day, and then given supplemental FR to reduce the R:FR to 0.09. Spectra of the light sources are provided in Supplementary Fig. 1 (available at JXB online). Plants received a photoperiod of 16/8 h light/dark and temperatures of 24/18°C day/night. At 3 days after anthesis, the FR light-emitting diodes were turned off 1 h after dawn to increase the R:FR to 3.5 in one side of the chamber without changing the photosynthetic photon flux density.
For bud elongation measurements, plants were matched for uniformity of rosette and cauline leaf numbers, maturity, height, and bud size. Decapitation was conducted by cutting the main stem approximately 5 mm above the rosette, but always below the lowest cauline leaf. Bud n-2 (the third bud from the top of the rosette) was imaged with a digital camera equipped with a macro lens at various times after initiating light and/or decapitation treatments. ImageJ software was used to process the images to determine bud lengths.
Analysis of ABA and IAA abundance
Bud n-2 and basal main stem sections (15 mm adjacent to the rosette) were harvested into liquid N2 at 0, 1, 3, 6 and 12 h after increasing the R:FR for ABA and IAA analyses. Masses were determined immediately after harvest. ABA and IAA were extracted and measured using isotope dilution selected ion monitoring gas chromatography-mass spectroscopy as described previously (Yao and Finlayson, 2015). Each measurement was derived from four biological replicates composed of approximately 15 buds or six stem segments.
Gene expression analysis
Bud n-2 and basal main stem sections were harvested at the times indicated after increasing the R:FR as described above. Total RNA was extracted, cDNA was synthesized, and quantitative PCR was conducted as previously described (Su et al., 2011), except that expression of UBC21 was used for normalization. Hormone-responsive genes for the quantification of ABA and IAA signal outputs were identified from the stringent set described by Goda et al. (2008). Specific gene targets were selected based on expression response to the appropriate stimulus (ABA or auxin) and demonstrated expression in buds (from Reddy et al. 2013). To determine the average signalling status, gene expression values were normalized to the mean expression of a particular gene, and this value was then averaged with others representing the same pathway. Expression values for genes exhibiting repressed expression in response to hormones were inverted before normalizing and averaging. Primers for BRC1 were taken from Aguilar-Martínez et al. (2007). Primers for INDOLE-3-ACETIC ACID INDUCIBLE 19 (IAA19) and GH3.5 were obtained from Effendi et al. (2011). Primers for PROLIFERATING CELL NUCLEAR ANTIGEN 1 (PCNA1) and TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS 1 (TAA1) are given in Finlayson et al. (2010). Primer sequences for IAA2, IAA3, IAA6, and IAA29 are given in Reddy and Finlayson (2014). Primer sequences for HISTONE H1-3 (HIS1-3) are provided in Su et al. (2011). Primer sequences for CYCLIN A2;1 (CYCA2;1) and PIN-FORMED 1 (PIN1) are given in Yao and Finlayson (2015). The sequences of other primers used are specified in Table 1. Each measurement was derived from four biological replicates composed of approximately 10 buds or six stem segments.
Table 1.
Sequences of primers used for quantitative PCR
| Target | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) |
|---|---|---|
| UBC21 (At5G25760) | CTGCGACTCAGGGAATCTTCTAA | TTGTGCCATTGAATTGAACCC |
| NAP (At1g69490) | CGTCTCCATGATTCACGTAAAGCA | TACTTCGTCCATGAAACCCTCTTG |
| RAP2.6 (At1g43160) | TGGACGATGGGTCATAAGAGAGAA | CTCCAAGGACATTGAGCTTTCACA |
| PP2-A5 (At1g65390) | GAGATCTTTCCATTGCATGGTCAG | TACCTTGTCCTCGGGGTCAAATAT |
Statistics
Statistical significance was determined by Student’s t-test (two-tailed), or ANOVA followed by Tukey’s honest significant difference test using R. Significance was determined at α = 0.05.
Results
Bud growth was promoted rapidly in response to an increased R:FR
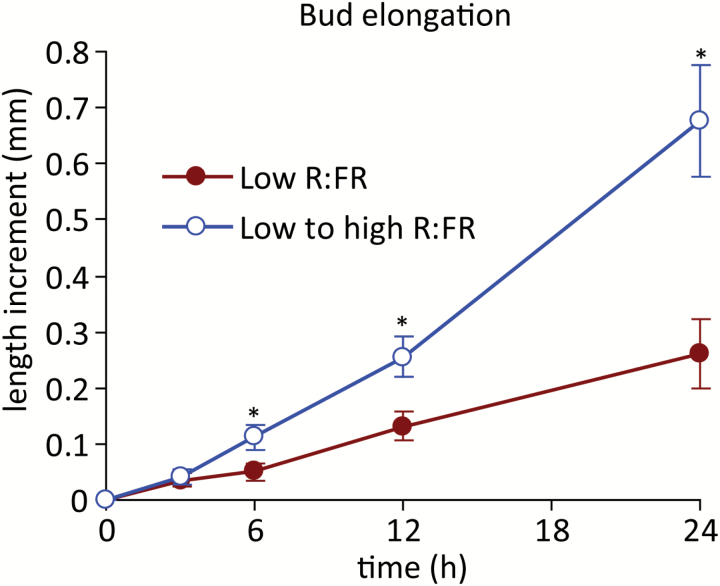
The R:FR was previously shown to regulate the growth of buds at specific rosette positions, with bud n-2 (the third bud from the top of the rosette) showing strong repression by low R:FR (Reddy et al., 2013). This prior work showed that the growth of bud n-2 was promoted within 24 h of increasing the R:FR. To further define the process, an experiment was conducted to determine the kinetics of the bud elongation response to the R:FR with greater temporal resolution than in the previous study. Plants were grown under a low R:FR to suppress bud elongation and, at 3 days after anthesis, one half of the plants were provided with a high R:FR (beginning 1 h after dawn) to promote the growth of bud n-2. The buds were not significantly different in size at the start of the experiment (low R:FR = 1.76 ± 0.10 mm, low to high R:FR = 1.69 ± 0.08 mm). An increased R:FR stimulated elongation within 6 h of initiation of the treatment, and by 24 h the bud growth increment of plants given a high R:FR was more than double that of plants maintained under a low R:FR (Fig. 1).
Fig. 1.
Elongation of bud n-2 under a low R:FR and at various times after increasing the R:FR. Data are means ± SE with n = 13. Asterisks indicate a significant difference between a low and low to high R:FR at α = 0.05. This figure is available in colour at JXB online.
BRC1 expression was rapidly suppressed in response to increased R:FR
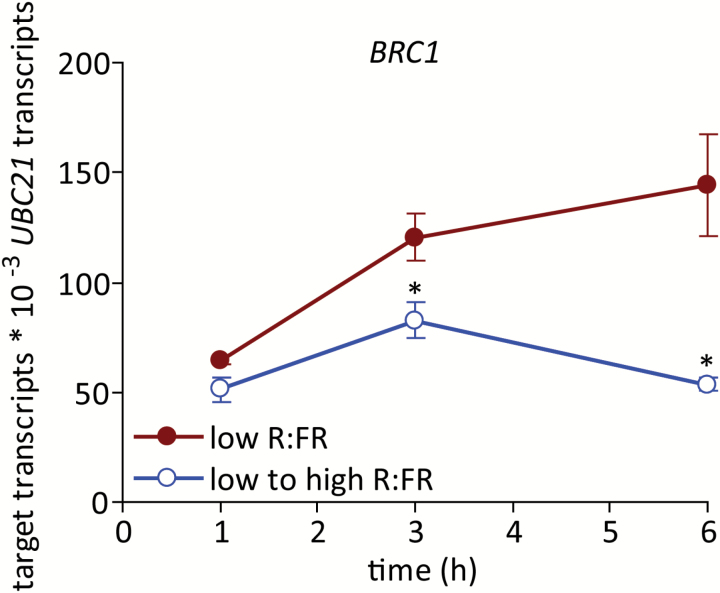
Relative to a low R:FR, expression of the bud growth inhibitor BRC1 was suppressed within 3 h of increasing the R:FR and declined to less than one half of the level in plants maintained under a low R:FR by 6 h (Fig. 2). Expression of BRC1 was dynamic even in buds of plants maintained under a low R:FR, with abundance displaying a steady increase over the duration of the experiment. This molecular probe of bud status thus indicates that a high R:FR alters the bud’s physiology within 3 h, before the measured increase in elongation.
Fig. 2.
Expression of BRC1 in bud n-2 under a low R:FR and at various times after increasing the R:FR. Data are means ± SE with n = 4. Asterisks indicate a significant difference between a low and low to high R:FR at α = 0.05. This figure is available in colour at JXB online.
ABA abundance and ABA signalling in buds declined in response to an increased R:FR prior to measured effects on bud elongation
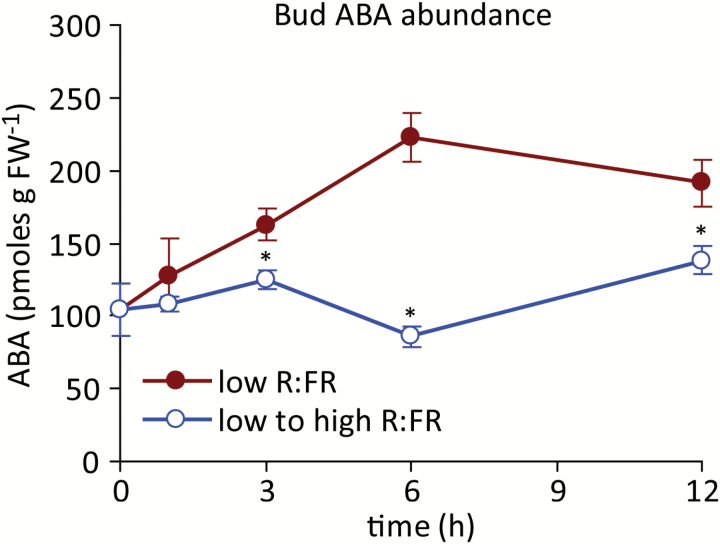
Bud ABA levels were previously shown to decline within 12 h of initiating the high R:FR treatment (Reddy et al., 2013). In the present study, ABA levels increased steadily to 6 h in buds of plants maintained under a low R:FR, then declined slightly (Fig. 3). Exposure to a high R:FR reduced bud ABA abundance relative to low R:FR within 3 h, and this reduction was maintained throughout the time course. A panel of ABA-responsive genes (Goda et al., 2008) was then surveyed for expression responses to provide a readout of ABA signalling status. The expression of ABA-induced genes (HIS1-3, ARABIDOPSIS NAC DOMAIN CONTAINING PROTEIN 29 [NAP] and RELATED TO AP2 6 [RAP2.6]), increased significantly up to 6 h in a low R:FR, whereas expression of the ABA-repressed gene PHLOEM PROTEIN 2 A5 (PP2-A5) was more stable (Fig. 4). An increased R:FR suppressed the expression of RAP2.6 within 3 h and of HIS1-3 and NAP within 6 h. PP2-A5 abundance was increased within 3 h. The abundance of each gene was normalized to the mean, the expression of PP2-A5 was inverted, and the values were averaged to provide an overall indicator of ABA signalling status (Fig. 4). Overall, bud ABA signalling was significantly suppressed by 3 h after increasing the R:FR.
Fig. 3.
ABA abundance in bud n-2 under a low R:FR and at various times after increasing the R:FR. Data are means ± SE with n = 4. Asterisks indicate a significant difference between a low and low to high R:FR at α = 0.05. This figure is available in colour at JXB online.
Fig. 4.
Expression of ABA-responsive genes in bud n-2 under a low R:FR and at various times after increasing the R:FR. (A) HIS1-3, (B) NAP, (C) RAP2.6, (D) PP2-A5, (E) average positive normalized expression. Data are means ± SE with n = 4. Asterisks indicate a significant difference between a low and low to high R:FR at α = 0.05. This figure is available in colour at JXB online.
Cell cycle- and auxin-related outputs of bud ABA signalling were altered in response to an increased R:FR
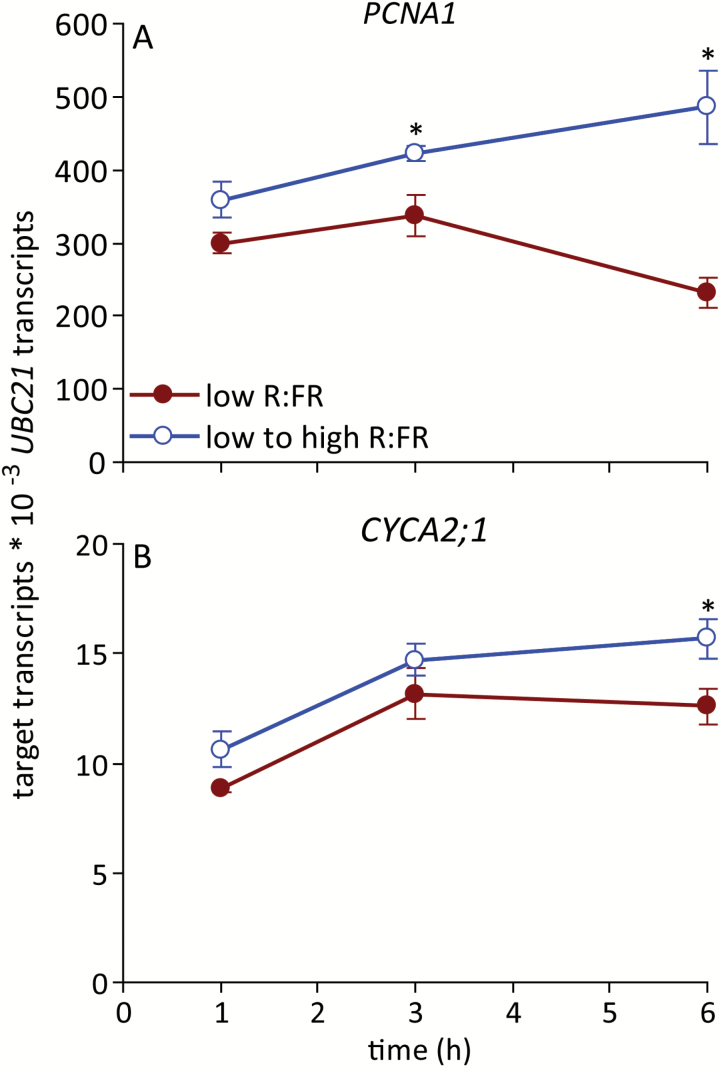
PCNA1 encodes a processivity factor necessary for the cell cycle that may also act as a regulatory component of the process (Strzalka and Ziemienowicz, 2011; Koundrioukoff et al., 2000). Bud PCNA1 expression was previously shown to be repressed by ABA (Yao and Finlayson, 2015). PCNA1 expression showed variation over the time course, declining at the 6 h time point in a low R:FR (Fig. 5). Like BRC1, PCNA1 expression was rapidly altered in response to an increased R:FR, increasing significantly relative to low R:FR by 3 h. Prior research indicated that expression of the cell cycle regulator CYCA2;1 may also be inhibited by ABA (Yao and Finlayson, 2015). Like PCNA1, CYCA2;1 expression was also promoted by an increased R:FR, but the effect was apparent slightly later, at 6 h (Fig. 5).
Fig. 5.
Expression of (A) PCNA1 and (B) CYCA2;1 in bud n-2 under a low R:FR and at various times after increasing the R:FR. Data are means ± SE with n = 4. Asterisks indicate a significant difference between a low and low to high R:FR at α = 0.05. This figure is available in colour at JXB online.
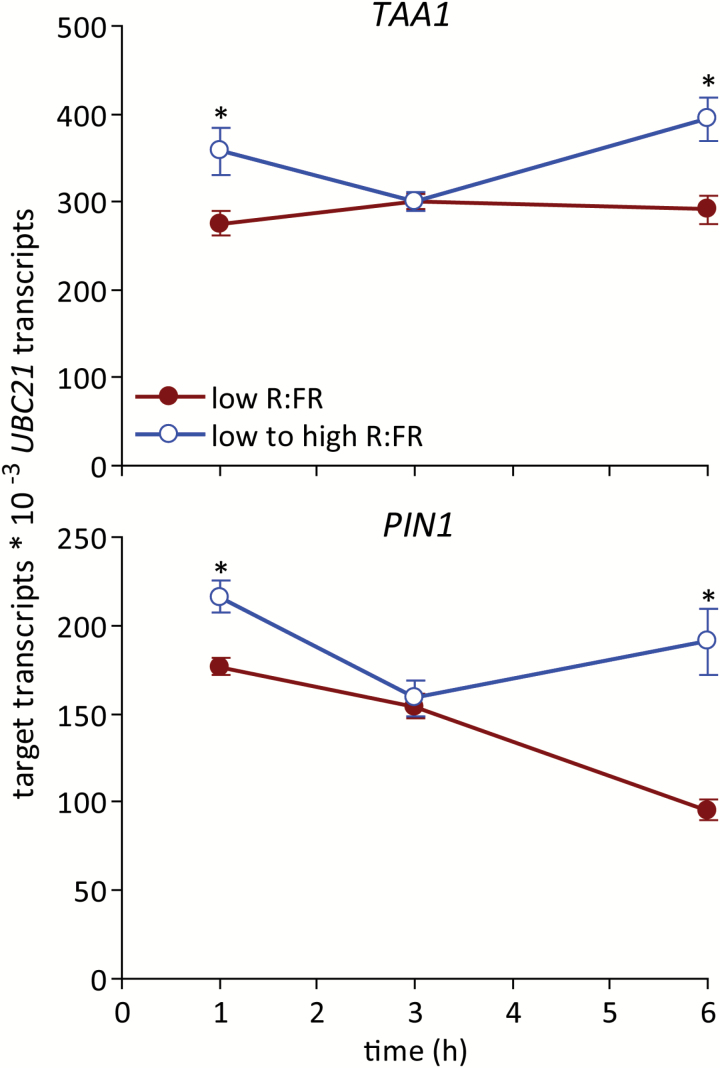
Bud autonomous expression of auxin biosynthesis and transport genes has been associated with ABA regulation of bud fate (Yao and Finlayson, 2015). The auxin biosynthesis gene TAA1 and the auxin transporter gene PIN1 showed similar expression patterns, with elevated abundance at 1 and 6 h after increasing the R:FR (Fig. 6). However, both genes showed equivalent accumulation under the different light treatments at 3 h.
Fig. 6.
Expression of (A) TAA1 and (B) PIN1 in bud n-2 under a low R:FR and at various times after increasing the R:FR. Data are means ± SE with n = 4. Asterisks indicate a significant difference between a low and low to high R:FR at α = 0.05. This figure is available in colour at JXB online.
The expression patterns of these genes are generally consistent with their potential functions regulating bud development downstream of ABA.
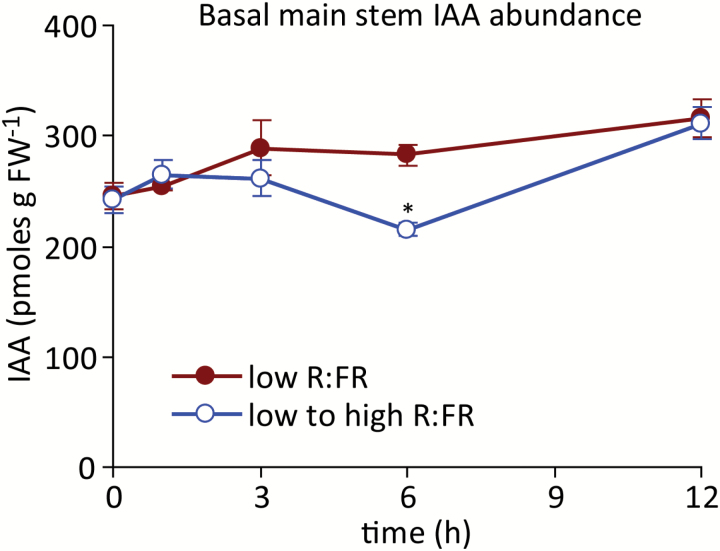
Stem IAA abundance declined transiently in response to an increased R:FR, but auxin signalling showed little effect
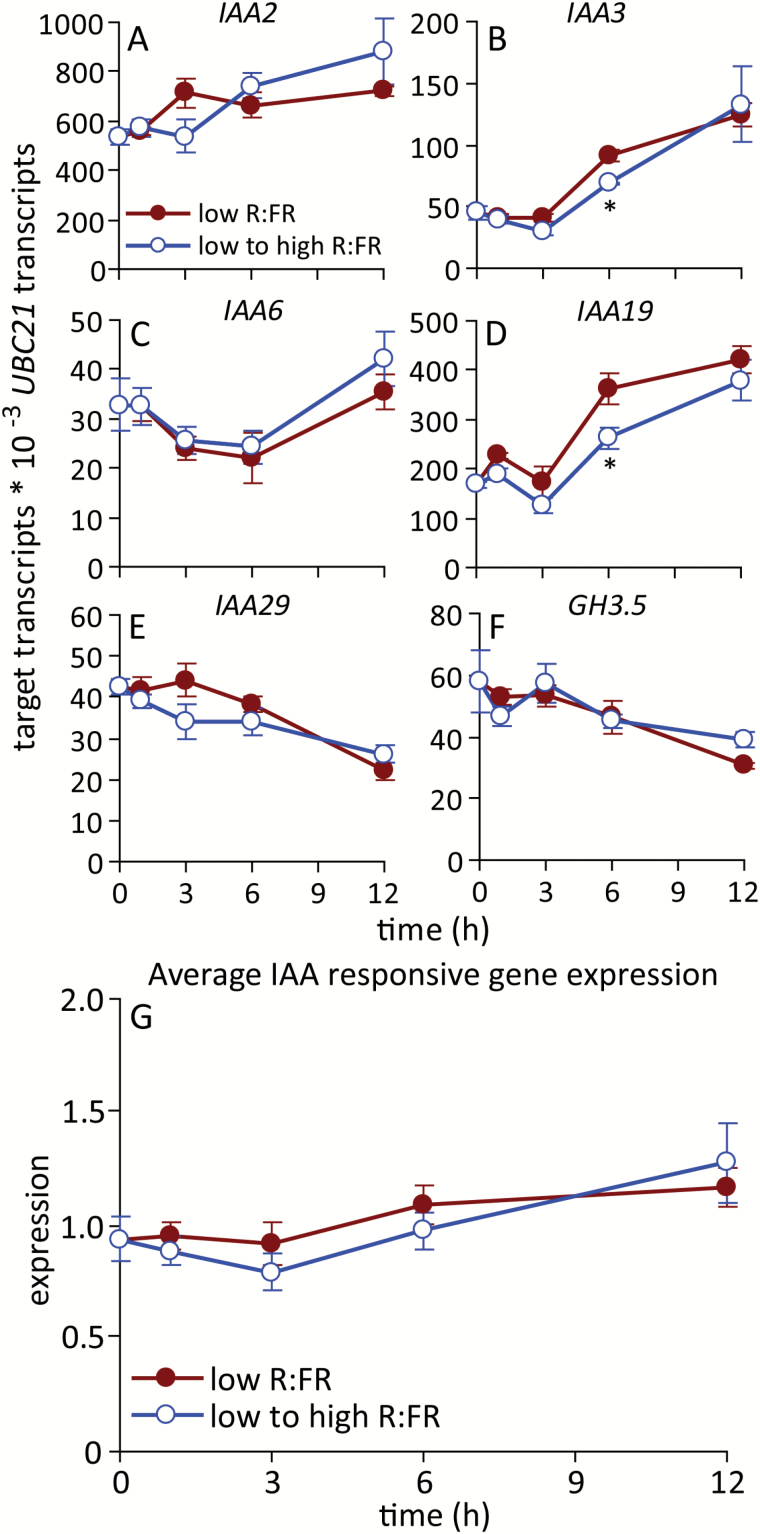
A low R:FR rapidly promotes the accumulation of IAA in young Arabidopsis seedlings (Tao et al., 2008, Hornitschek et al., 2012, Pacín et al., 2016). Elevated systemic auxin signalling in the main stem, independent of IAA accumulation, was previously found to contribute to the suppression of branching in phyB-deficient Arabidopsis (Reddy and Finlayson, 2014). The role of systemic auxin in the response to an increased R:FR was evaluated to determine if this pathway contributes to the modulation of bud growth by the R:FR. IAA abundance in basal main stem segments decreased transiently in response to an elevated R:FR at 6 h, but recovered to levels observed in plants maintained under a low R:FR at 12 h (Fig. 7). Auxin signalling status was assessed using a panel of auxin-inducible genes (Goda et al., 2008; Reddy and Finlayson, 2014), including IAA2, IAA3, IAA6, IAA19, IAA29 and GH3.5 (Fig. 8). Relative to a low R:FR, the expression of IAA3 and IAA19 was suppressed 6 h after increasing the R:FR, but there were no differences at any other times, or in the expression of the other four genes. The average expression of the auxin signalling panel was not altered by the R:FR (Fig. 8). Thus, while main stem IAA levels decreased transiently at 6 h, the molecular evidence indicated that there was only a brief and limited perturbation of specific auxin signalling components at 6 h.
Fig. 7.
IAA abundance in basal main stem segments under a low R:FR and at various times after increasing the R:FR. Data are means ± SE with n = 4. Asterisks indicate a significant difference between a low and low to high R:FR at α = 0.05. This figure is available in colour at JXB online.
Fig. 8.
Expression of IAA-responsive genes in basal main stem segments under a low R:FR and at various times after increasing the R:FR. (A) IAA2, (B) IAA3, (C) IAA6, (D) IAA19, (E) IAA29, (F) GH3.5, (G) average positive normalized expression. Data are means ± SE with n = 4. Asterisks indicate a significant difference between a low and low to high R:FR at α = 0.05. This figure is available in colour at JXB online.
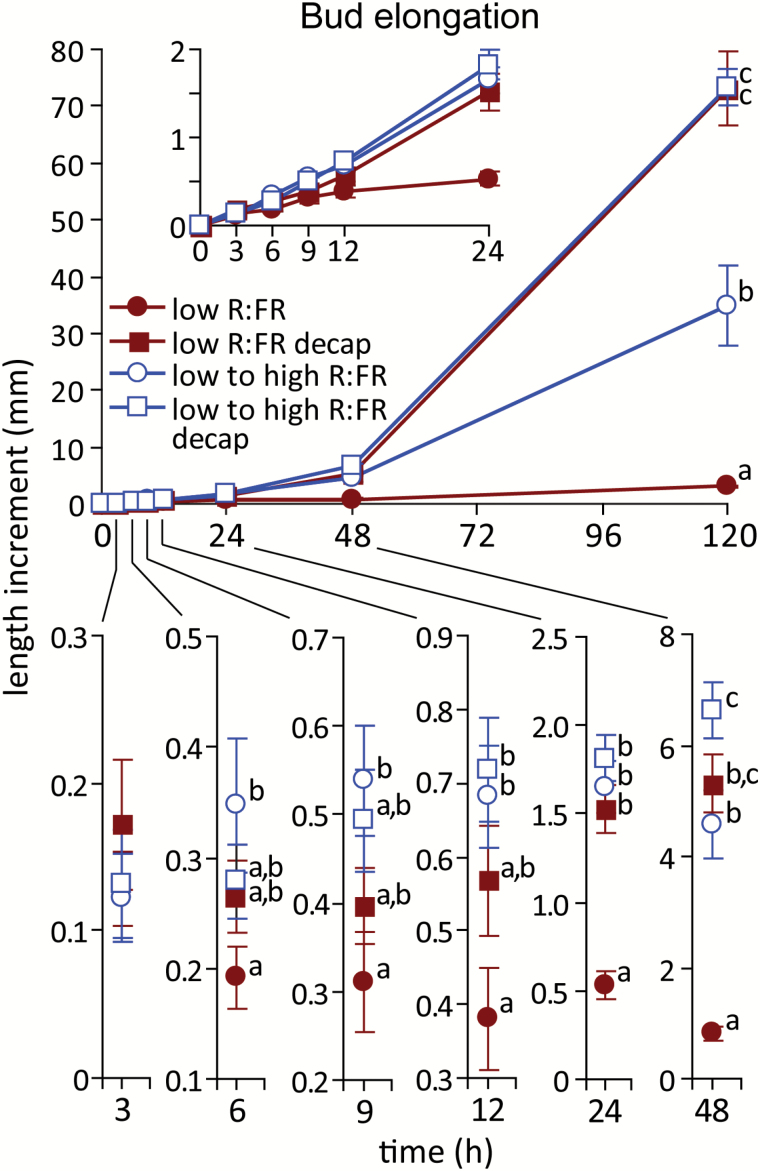
An increased R:FR promoted bud elongation more rapidly than decapitation
In most species tested, including Arabidopsis, decapitation of the main shoot results in rapid growth of otherwise repressed buds. While both decapitation of the main stem and increasing the R:FR promote bud growth, information regarding potential commonalities, differences, and interactions between these treatments is lacking. These issues were explored by decapitating plants grown and maintained under a low R:FR, and comparing the timing and extent of bud growth with counterparts decapitated and provided with an increased R:FR. Buds were not significantly different in size at the start of treatments (low R:FR = 2.30 ± 0.20 mm, decapitated low R:FR = 2.05 ± 0.12 mm, low to high R:FR = 2.33 ± 0.16 mm, decapitated low to high R:FR = 2.51 ± 0.12 mm). In agreement with the data presented in Fig. 1 above, increasing the R:FR promoted bud growth within 6 h (Fig. 9). In contrast, decapitation of plants maintained under a low R:FR resulted in increased bud growth after a lag of 24 h. Furthermore, the combination of an increased R:FR and decapitation delayed the growth response by 6 h, compared to plants provided only with increased R:FR. While the effect of increased R:FR was more rapid than that produced by decapitation, decapitation resulted in stronger branch elongation by the end of the experiment (120 h), with both decapitation treatments generating branches that were over twice as long as those from plants only given an increased R:FR. The combination of an increased R:FR and decapitation did not promote final branch elongation more than decapitation alone.
Fig. 9.
Elongation of bud n-2 under a low R:FR and at various times after decapitating the main stem and/or increasing the R:FR. Top panel shows full data set, inset magnifies the first 24 h, lower panel magnifies time points from 3 to 48 h. Data are means ± SE with n = 13. Values within time points with different letters are significantly different at α = 0.05. This figure is available in colour at JXB online.
Discussion
Rapid bud growth responses to an increased R:FR were preceded by the suppression of bud ABA accumulation and signalling relative to a low R:FR
The regulation of bud growth by R:FR signalling is a complex process involving at least two main pathways. Initial work indicated that an increased R:FR caused a reduction in bud-localized ABA, which allowed buds retarded by a low R:FR to grow more rapidly (Reddy et al., 2013). These results were supported by independent research associating the expression of ABA-related genes with bud responses to the R:FR (González-Grandío et al., 2013). A subsequent study demonstrated that the suppression of branching in phyB-deficient mutants resulted from systemic changes in auxin signalling in the main stem, independent of auxin abundance and transport (Reddy and Finlayson, 2014). Although the competition avoidance produced by a low R:FR may not exactly mirror the effects of phyB deficiency, the apparent similarities suggest that some of the effects of a low R:FR may also result from alterations in auxin abundance and/or signalling. In the current study, these two pathways were investigated in a time course analysis to determine how they might contribute to bud growth responses to the R:FR.
Bud growth was promoted very rapidly by increasing the R:FR. Both the abundance of ABA in buds and bud ABA signalling status decreased relative to a low R:FR prior (3 h post treatment) to the observed changes in bud growth. These changes may be directly related to bud function because ABA was necessary for the inhibition of lower bud elongation in a low R:FR (Reddy et al., 2013), and exogenous ABA inhibited the growth of buds under a high R:FR (Yao and Finlayson, 2015). Furthermore, the expression of the cell cycle components PCNA1 and CYCA2;1 increased commensurate with, or after, the decrease in ABA, suggesting possible targets for ABA function, as previously described (Yao and Finlayson, 2015). These potential targets also included genes involved in auxin biosynthesis (TAA1) and transport (PIN1) within the bud itself. TAA1 and PIN1 showed similar expression responses to an increased R:FR, with significantly increased expression relative to a low R:FR at 6 h, as might be expected if the changes resulted from the measured decrease in ABA. This conclusion is complicated by the observation that both genes also exhibited increased expression at 1 h, followed by a decline at 3 h to the levels observed in buds from plants maintained under a low R:FR. Such a pattern of accumulation could indicate a rapid, transient, ABA-independent promotion of their expression by the R:FR, possibly priming the bud for eventual release by more sustained signals. Assessing the rapid effects of cytokinin application to buds maintained under a low R:FR or provided with a high R:FR for one to several hours could help resolve the role of the initial transient increase in TAA1 and PIN1 expression.
Main stem IAA accumulation and signalling responses to an increased R:FR did not correlate with rapid bud growth responses
An increased R:FR also altered the abundance of IAA in the main stem, and impacted the expression of two of the six auxin signalling components surveyed. However, both phenomena were only transiently detected at 6 h, and thus occurred later than the changes observed in bud ABA physiology and at the same time that changes in bud elongation were measured. Decapitation was employed to determine the response of buds to complete removal of the apical auxin source. While increased bud elongation was observed 6 h after increasing the R:FR, decapitation promoted growth in plants maintained under a low R:FR only by 24 h after dissection. This severe and abrupt treatment would be expected to impact auxin levels and signalling in less than the 6 h required for the observed weak, transient auxin response to an increased R:FR, but decapitation still required more time to manifest in increased bud elongation than the light treatment. The combination of the delayed main stem auxin response to an altered R:FR and the delayed growth response to auxin depletion by decapitation indicate that auxin is unlikely to be the primary signal for bud release under an increased R:FR. Conversely, the long-term effects of decapitation were considerably stronger than those associated with an increased R:FR, and decapitation eventually overcame the low R:FR inhibition of bud elongation completely, indicating that R:FR signal perception and transduction events generated by the shoot apex or stem are necessary for persistent inhibition of bud growth. Thus, the primary early effects of an increased R:FR on bud growth can be attributed to alterations in bud ABA physiology, whereas later elongation responses may also involve altered systemic auxin physiology.
The decline in bud ABA abundance following exposure to a high R:FR occurred with a timing similar to changes in bud hormone status following decapitation in pea (Pisum sativum) and chickpea (Cicer arietinum). Decapitation promoted bud outgrowth in pea in 4–6 h (Morris et al., 2005). Decapitation also increased cytokinin abundance in pea buds within 3 h (Tanaka et al., 2006) and the buds of excised pea shoot sections showed an increased ability to transport auxin 2 h after removing the terminal bud (Balla et al., 2011). Rapid hormonal responses to decapitation were also observed in buds of chickpea, with cytokinin abundances increasing within 4 h, while ABA levels declined within 1 h (Mader et al., 2003). The delayed onset of bud outgrowth in Arabidopsis following decapitation compared to pea may reflect either an intrinsically slower response, or may result from the prior growth of these plants under a low R:FR.
It was interesting to note that the combination of decapitation and an increased R:FR promoted bud elongation less rapidly than increasing the R:FR alone, suggesting that the main stem may provide a positive regulator of early bud growth necessary for the rapid response. Alternatively, decapitation might differentially stimulate the growth of buds n and/or n-1, which could result in increased correlative inhibition of bud n-2 via the auxin transport competition theory (Bennett et al., 2006; Prusinkiewicz et al., 2009; Bennett et al., 2016). However, at the termination of the experiment, the combination of a high R:FR and decapitation did not significantly increase the length of the upper branches compared to a high R:FR alone (see Supplementary Fig. 2 at JXB online), making this seem unlikely.
A previous transcriptome profiling study using the same experimental system but only a single 3 h time point provided evidence that cytokinin signalling was upregulated in response to exposure to a high R:FR (Reddy et al., 2013). Gene ontology (GO) terms associated with cytokinin response were overrepresented and the expression of cytokinin-responsive type A RESPONSE REGULATOR (ARR) genes, including ARR4, ARR5, ARR6, ARR7, and ARR15, were induced from about 1.7 to 6-fold 3 h after increasing the R:FR. However, expression of the cytokinin biosynthesis ISOPENTENYLTRANSFERASE genes was not altered. GO analysis did not indicate overrepresentation of terms associated with the MORE AXILLARY BRANCHING (MAX) pathway, but MAX2 expression declined about 1.5-fold following exposure to a high R:FR. Thus, other hormonal pathways besides ABA and auxin are probably also rapidly modulated by the R:FR; defining the timing and how they interact is an objective for future research.
Bud BRC1 expression and ABA physiology were dynamically expressed
The abundance of ABA in buds maintained under a low R:FR was not static, but varied with time, reaching a maximum at 6 h (7 h after dawn). The expression of ABA-responsive genes and BRC1 in buds under a low R:FR was also dynamic and increased through the early part of the day. Even in a low R:FR, bud n-2 showed some elongation in spite of the increased expression of these negative regulators of bud growth, indicating that these factors are insufficient for complete arrest. As discussed previously (Yao and Finlayson, 2015), Arabidopsis buds do not appear to exhibit true dormancy, as in all cases where observations have been made, buds that superficially appeared dormant in fact showed some measurable growth (Finlayson et al., 2010, Su et al., 2011, Reddy et al., 2013). In the present study, bud growth in plants provided with a high R:FR may not be associated with an absolute reduction in the expression/accumulation of these negative regulators, but rather a lack of increase.
The apparent increase in expression/accumulation patterns of BRC1 and ABA may indicate that the bud growth response is gated to permit the process to initiate at a particular time of the day, presumably in the morning. There is considerable support for clock gating of ABA signalling (see Seung et al., 2012); however, less information is available regarding potential BRC1 expression rhythms. BRC1 is a member of the TCP protein family. Most TCP family genes show diurnal expression patterns (Giraud et al., 2010), although rhythmic expression of BRC1 was not tested due to its low abundance. Because ABA accumulation in lower buds is dependent on BRC1 function (Yao and Finlayson, 2015), it is tempting to speculate that increased diurnal expression of BRC1 results in the accumulation of ABA, which contributes to suppression of bud growth later in the day. Therefore, BRC1 expression could be the gated factor inhibiting bud growth, rather than, or perhaps in addition to, ABA signalling. Because the diurnal bud growth response was not assessed, this hypothesis currently remains untested.
Does the axillary bud autonomously sense and respond to the R:FR?
While BRC1 is necessary for maintaining elevated levels of ABA in lower buds (Yao and Finlayson, 2015), it is not known how ABA levels and signalling are suppressed by a high R:FR. The rapid modulation of ABA homeostasis by an increased R:FR could indicate that the bud itself is the primary site of perception and signal transduction for this response. In this scenario, changes in the R:FR detected by phytochromes within bud cells would potentially be transduced by PHYTOCHROME INTERACTING FACTORs (PIFs) to alter the expression of ABA biosynthesis and/or metabolism genes, either through PIF-mediated changes in BRC1 expression, or more directly. Phytochrome action was shown to suppress ABA accumulation in Lemna gibba (Weatherwax et al., 1996) and ABA accumulation in Arabidopsis seeds was suppressed by phyB regulation of both its biosynthesis and catabolism (Seo et al., 2006). The key ABA biosynthetic gene NINE-CIS-EPOXYCAROTENOID DIOXYGENASE 3 (NCED3) and the ABA hydroxylase gene CYTOCHROME P450, FAMILY 707, SUBFAMILY A, POLYPEPTIDE 4 (CYP707A4) were found to be regulated by PIF4/PIF5, which contribute to responses to the R:FR (Hornitschek et al., 2012). Additionally, CYP707A4 and its homolog CYP707A3, the NCED3 homolog NCED4, and genes encoding the ABA signalling transcription factors ABI5 and ABSCISIC ACID RESPONSIVE ELEMENTS-BINDING FACTOR 3 were also identified as binding targets for PIF5 (Hornitschek et al., 2012). Investigating potential links between canonical phyB signal transduction via PIFs and the expression of ABA biosynthesis, catabolism, and signalling genes in axillary buds may provide critical data to better understand the regulation of bud growth by the R:FR.
Supplementary Data
Supplementary data are available at JXB online.
Supplementary Fig. 1. Spectra of the light sources used for plant growth.
Supplementary Fig. 2. Lengths of upper rosette branches (n and n-1) of plants grown under a low R:FR, then provided with a high R:FR or maintained under a low R:FR, with and without decapitation at 120 h after initiating treatments.
Supplementary Material
Acknowledgments
This work was supported by Texas A&M University College of Agriculture and Life Sciences (SAF).
Glossary
Abbreviations:
- ABA
abscisic acid
- ARR
RESPONSE REGULATOR
- BRC1
BRANCHED 1
- CYCA2;1
CYCLIN A2;1
- CYP707A4
CYTOCHROME P450, FAMILY 707, SUBFAMILY A, POLYPEPTIDE 4
- GO
gene ontology
- HIS1-3
HISTONE H1-3
- IAA
indole-3-acetic acid
- IAA2
INDOLE-3-ACETIC ACID INDUCIBLE 2
- MAX
MORE AXILLARY BRANCHING
- NAP
ARABIDOPSIS NAC DOMAIN CONTAINING PROTEIN 29
- NCED
NINE-CIS-EPOXYCAROTENOID DIOXYGENASE
- PCNA1
PROLIFERATING CELL NUCLEAR ANTIGEN 1
- PIF
PHYTOCHROME INTERACTING FACTOR
- phy
phytochrome
- PIN1
PIN-FORMED 1
- PP2-A5
PHLOEM PROTEIN 2 A5
- RAP2.6
RELATED TO AP2 6
- R:FR
red light to far red light ratio
- TAA1
TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS 1
- TCP
TEOSINTE BRANCHED1/CYCLOIDEA/PCF.
References
- Aguilar-Martínez JA, Poza-Carrión C, Cubas P. 2007. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. The Plant Cell 19, 458–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend M, Schnitzler JP, Ehlting B, Hänsch R, Lange T, Rennenberg H, Himmelbach A, Grill E, Fromm J. 2009. Expression of the Arabidopsis mutant abi1 gene alters abscisic acid sensitivity, stomatal development, and growth morphology in gray poplars. Plant Physiology 151, 2110–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arney SE, Mitchell DL. 1969. The effect of abscisic acid on stem elongation and correlative inhibition. New Phytologist 68, 1001–1015 [Google Scholar]
- Balla J, Kalousek P, Reinöhl V, Friml J, Procházka S. 2011. Competitive canalization of PIN-dependent auxin flow from axillary buds controls pea bud outgrowth. The Plant Journal 65, 571–577. [DOI] [PubMed] [Google Scholar]
- Barbier F, Péron T, Lecerf M, et al. 2015. Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida. Journal of Experimental Botany 66, 2569–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T, Hines G, van Rongen M, Waldie T, Sawchuk MG, Scarpella E, Ljung K, Leyser O. 2016. Connective auxin transport in the shoot facilitates communication between shoot apices. PLoS Biology 14, e1002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O. 2006. The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Current Biology 16, 553–563. [DOI] [PubMed] [Google Scholar]
- Braun N, de Saint Germain A, Pillot JP, et al. 2012. The pea TCP transcription factor PsBRC1 acts downstream of strigolactones to control shoot branching. Plant Physiology 158, 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer PB, Dun EA, Ferguson BJ, Rameau C, Beveridge CA. 2009. Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiology 150, 482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer PB, Dun EA, Gui R, Mason MG, Beveridge CA. 2015. Strigolactone inhibition of branching independent of polar auxin transport. Plant Physiology 168, 1820–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ. 2012. Shade avoidance. The Arabidopsis Book 10, e0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield SP, Stirnberg P, Forde BG, Leyser O. 2000. The hormonal regulation of axillary bud growth in Arabidopsis. The Plant Journal 24, 159–169. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhou X, Xi L, Li J, Zhao R, Ma N, Zhao L. 2013. Roles of DgBRC1 in regulation of lateral branching in chrysanthemum (Dendranthema x grandiflora cv. Jinba). PLoS One 8, e61717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline M. 1997. Concepts and terminology of apical dominance. American Journal of Botany 84, 1064. [PubMed] [Google Scholar]
- Cline MG, Oh C. 2006. A reappraisal of the role of abscisic acid and its interaction with auxin in apical dominance. Annals of Botany 98, 891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MH, Simmons SR. 1994. Tillering response of barley to shifts in light quality caused by neighboring plants. Crop Science 34, 1604–1610 [Google Scholar]
- Deregibus VA, Sanchez RA, Casal JJ. 1983. Effects of light quality on tiller production in Lolium spp. Plant Physiology 72, 900–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagalska MA, Leyser O. 2011. Signal integration in the control of shoot branching. Nature Reviews. Molecular Cell Biology 12, 211–221. [DOI] [PubMed] [Google Scholar]
- Donohue K, Schmitt J. 1999. The genetic architecture of plasticity to density in Impatiens capensis. Evolution 53, 1377–1386 [DOI] [PubMed] [Google Scholar]
- Dun EA, Hanan J, Beveridge CA. 2009. Computational modeling and molecular physiology experiments reveal new insights into shoot branching in pea. The Plant Cell 21, 3459–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun EA, de Saint Germain A, Rameau C, Beveridge CA. 2012. Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiology 158, 487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effendi Y, Rietz S, Fischer U, Scherer GF. 2011. The heterozygous abp1/ABP1 insertional mutant has defects in functions requiring polar auxin transport and in regulation of early auxin-regulated genes. The Plant Journal 65, 282–294. [DOI] [PubMed] [Google Scholar]
- Finlayson SA. 2007. Arabidopsis Teosinte Branched1-like 1 regulates axillary bud outgrowth and is homologous to monocot Teosinte Branched1. Plant & Cell Physiology 48, 667–677. [DOI] [PubMed] [Google Scholar]
- Finlayson SA, Krishnareddy SR, Kebrom TH, Casal JJ. 2010. Phytochrome regulation of branching in Arabidopsis. Plant Physiology 152, 1914–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud E, Ng S, Carrie C, et al. 2010. TCP transcription factors link the regulation of genes encoding mitochondrial proteins with the circadian clock in Arabidopsis thaliana. The Plant Cell 22, 3921–3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocal GF, Pharis RP, Yeung EC, Pearce D. 1991. Changes after decapitation in concentrations of indole-3-acetic acid and abscisic acid in the larger axillary bud of Phaseolus vulgaris L. cv Tender Green. Plant Physiology 95, 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda H, Sasaki E, Akiyama K, et al. 2008. The AtGenExpress hormone and chemical treatment data set: experimental design, data evaluation, model data analysis and data access. The Plant Journal 55, 526–542. [DOI] [PubMed] [Google Scholar]
- González-Grandío E, Poza-Carrión C, Sorzano CO, Cubas P. 2013. BRANCHED1 promotes axillary bud dormancy in response to shade in Arabidopsis. The Plant Cell 25, 834–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel FD, Feldman LJ. 1994. Bi-directional inflorescence development in Arabidopsis thaliana: acropetal initiation of flowers and basipetal initiation of paraclades. Planta 192, 276–286 [Google Scholar]
- Hornitschek P, Kohnen MV, Lorrain S, et al. 2012. Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. The Plant Journal 71, 699–711. [DOI] [PubMed] [Google Scholar]
- Janssen BJ, Drummond RS, Snowden KC. 2014. Regulation of axillary shoot development. Current Opinion in Plant Biology 17, 28–35. [DOI] [PubMed] [Google Scholar]
- Kebrom TH, Brutnell TP, Finlayson SA. 2010. Suppression of sorghum axillary bud outgrowth by shade, phyB and defoliation signalling pathways. Plant, Cell & Environment 33, 48–58. [DOI] [PubMed] [Google Scholar]
- Kebrom TH, Burson BL, Finlayson SA. 2006. Phytochrome B represses Teosinte Branched1 expression and induces sorghum axillary bud outgrowth in response to light signals. Plant Physiology 140, 1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebrom TH, Mullet JE. 2016. Transcriptome profiling of tiller buds provides new insights into PhyB regulation of tillering and indeterminate growth in sorghum. Plant Physiology 170, 2232–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox JP, Wareing PF. 1984. Apical dominance in Phaseolus vulgaris L.: the possible roles of abscisic acid and indole-3-acetic acid. Journal of Experimental Botany 35, 239–244 [Google Scholar]
- Koundrioukoff S, Jónsson ZO, Hasan S, de Jong RN, van der Vliet PC, Hottiger MO, Hübscher U. 2000. A direct interaction between proliferating cell nuclear antigen (PCNA) and Cdk2 targets PCNA-interacting proteins for phosphorylation. The Journal of Biological Chemistry 275, 22882–22887. [DOI] [PubMed] [Google Scholar]
- Le Bris M, Michaux-Ferriere N, Jacob Y, Poupet A, Barthe P, Guigonis J-M, Le Page-Degivry M-T. 1999. Regulation of bud dormancy by manipulation of ABA in isolated buds of Rosa hybrida cultured in vitro. Australian Journal of Plant Physiology 26, 273–281 [Google Scholar]
- Mader JC, Emery RJN, Turnbull CGN. 2003. Spatial and temporal changes in multiple hormone groups during lateral bud release shortly following apex decapitation of chickpea (Cicer arietinum) seedlings. Physiologia Plantarum 119, 295–308 [Google Scholar]
- Mason MG, Ross JJ, Babst BA, Wienclaw BN, Beveridge CA. 2014. Sugar demand, not auxin, is the initial regulator of apical dominance. Proceedings of the National Academy of Sciences of the United States of America 111, 6092–6097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SE, Cox MC, Ross JJ, Krisantini S, Beveridge CA. 2005. Auxin dynamics after decapitation are not correlated with the initial growth of axillary buds. Plant Physiology 138, 1665–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Morea FA, Vicentini R, Silva GF, Silva EM, Carrer H, Rodrigues AP, Nogueira FT. 2013. Global analysis of the sugarcane microtranscriptome reveals a unique composition of small RNAs associated with axillary bud outgrowth. Journal of Experimental Botany 64, 2307–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacín M, Semmoloni M, Legris M, Finlayson SA, Casal JJ. 2016. Convergence of CONSTITUTIVE PHOTOMORPHOGENESIS 1 and PHYTOCHROME INTERACTING FACTOR signalling during shade avoidance. The New Phytologist 211, 967–979. [DOI] [PubMed] [Google Scholar]
- Prusinkiewicz P, Crawford S, Smith RS, Ljung K, Bennett T, Ongaro V, Leyser O. 2009. Control of bud activation by an auxin transport switch. Proceedings of the National Academy of Sciences of the United States of America 106, 17431–17436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameau C, Bertheloot J, Leduc N, Andrieu B, Foucher F, Sakr S. 2015. Multiple pathways regulate shoot branching. Frontiers in Plant Science 5, 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SK, Finlayson SA. 2014. Phytochrome B promotes branching in Arabidopsis by suppressing auxin signaling. Plant Physiology 164, 1542–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SK, Holalu SV, Casal JJ, Finlayson SA. 2013. Abscisic acid regulates Arabidopsis axillary bud outgrowth responses to the ratio of red to far-red light. Plant Physiology 163, 1047–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SK, Holalu SV, Casal JJ, Finlayson SA. 2014. The timing of low R:FR exposure profoundly affects Arabidopsis branching responses. Plant Signaling & Behavior 9, e28668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin CH, Hay MJM, Newton PCD, Greer DH. 1994. Effect of light quality (red:far-red ratio) at the apical bud of the main stolon on morphogenesis of Trifolium repens L. Annals of Botany 74, 119–123 [Google Scholar]
- Seo M, Hanada A, Kuwahara A, et al. 2006. Regulation of hormone metabolism in Arabidopsis seeds: phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. The Plant Journal 48, 354–366. [DOI] [PubMed] [Google Scholar]
- Seung D, Risopatron JP, Jones BJ, Marc J. 2012. Circadian clock-dependent gating in ABA signalling networks. Protoplasma 249, 445–457. [DOI] [PubMed] [Google Scholar]
- Strzalka W, Ziemienowicz A. 2011. Proliferating cell nuclear antigen (PCNA): a key factor in DNA replication and cell cycle regulation. Annals of Botany 107, 1127–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Abernathy SD, White RH, Finlayson SA. 2011. Photosynthetic photon flux density and phytochrome B interact to regulate branching in Arabidopsis. Plant, Cell & Environment 34, 1986–1998. [DOI] [PubMed] [Google Scholar]
- Tamas IA, Ozbun JL, Wallace DH. 1979. Effect of Fruits on dormancy and abscisic acid concentration in the axillary buds of Phaseolus vulgaris L. Plant Physiology 64, 615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H. 2006. Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. The Plant Journal 45, 1028–1036. [DOI] [PubMed] [Google Scholar]
- Tao Y, Ferrer JL, Ljung K, et al. 2008. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133, 164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker DJ. 1977. Effects of far-red light on lateral bud outgrowth in decapitated tomato plants and associated changes in levels of auxin and abscisic acid. Plant Science Letters 8, 339–344 [Google Scholar]
- Tucker DJ, Mansfield TA. 1972. Effects of light quality on apical dominance in Xanthium strumarium and the associated changes in endogenous levels of abscisic acid and cytokinins. Planta 102, 140–151. [DOI] [PubMed] [Google Scholar]
- Waldie T, Hayward A, Beveridge CA. 2010. Axillary bud outgrowth in herbaceous shoots: how do strigolactones fit into the picture? Plant Molecular Biology 73, 27–36. [DOI] [PubMed] [Google Scholar]
- Wan C, Sosebee RE. 1998. Tillering responses to red:far-red light ratio during different phenological stages in Eragrostis curvula. Environmental and Experimental Botany 40, 247–254 [Google Scholar]
- Weatherwax SC, Ong MS, Degenhardt J, Bray EA, Tobin EM. 1996. The interaction of light and abscisic acid in the regulation of plant gene expression. Plant Physiology 111, 363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Finlayson SA. 2015. Abscisic acid is a general negative regulator of Arabidopsis axillary bud growth. Plant Physiology 169, 611–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.