Highlight
Photosynthetic acclimation of Eucalyptus globulus leaves to elevated CO2 was underpinned by reduced leaf N and Rubisco contents; the opposite occurred under warming, coinciding with growth resumption in spring.
Keywords: Canopy position, elevated CO2 and temperature, Eucalyptus globulus, photosynthesis, Rubisco kinetics, Vcmax, whole-tree chambers.
Abstract
Leaf-level photosynthetic processes and their environmental dependencies are critical for estimating CO2 uptake from the atmosphere. These estimates use biochemical-based models of photosynthesis that require accurate Rubisco kinetics. We investigated the effects of canopy position, elevated atmospheric CO2 [eC; ambient CO2 (aC)+240 ppm] and elevated air temperature (eT; ambient temperature (aT)+3 °C) on Rubisco content and activity together with the relationship between leaf N and Vcmax (maximal Rubisco carboxylation rate) of 7 m tall, soil-grown Eucalyptus globulus trees. The kinetics of E. globulus and tobacco Rubisco at 25 °C were similar. In vitro estimates of Vcmax derived from measures of E. globulus Rubisco content and kinetics were consistent, although slightly lower, than the in vivo rates extrapolated from gas exchange. In E. globulus, the fraction of N invested in Rubisco was substantially lower than for crop species and varied with treatments. Photosynthetic acclimation of E. globulus leaves to eC was underpinned by reduced leaf N and Rubisco contents; the opposite occurred in response to eT coinciding with growth resumption in spring. Our findings highlight the adaptive capacity of this key forest species to allocate leaf N flexibly to Rubisco and other photosynthetic proteins across differing canopy positions in response to future, warmer and elevated [CO2] climates.
Introduction
Photosynthetic CO2 assimilation by the terrestrial biosphere constitutes the largest component of global CO2 fluxes. These photosynthetic processes and their responses to the environment are represented in the widely used Farquhar–von Caemmerer–Berry (FvCB) model (Farquhar et al., 1980), which is at the core of most global CO2 flux and vegetation productivity models (Zhang et al., 2012; Rezende et al., 2016). The accuracy of the FvCB model is heavily reliant on correct kinetic parameterization of the CO2-fixing enzyme Rubisco (ribulose 1,5-bisphosphate carboxylase, EC: 4.1.1.39) as well as knowledge of Jmax, the maximum rate of ribulose bisphosphate (RuBP) regeneration (Farquhar et al., 1980). Historically, kinetic surveys of vascular plant Rubisco have generally focused on those from crop and herbaceous species (Kapralov et al., 2011; Hermida-Carrera et al., 2016; Prins et al., 2016) and not for woody plants despite their dominant influence on global net primary production (McGuire et al., 2001; Sitch et al., 2003). Whether the kinetics of crop Rubisco can be used to simulate the rates of photosynthesis accurately in tree species via the FvCB model remains uncertain.
A key component of the FvCB model is the parameter Vcmax, the maximum rate of carboxylation by Rubisco. In particular, Vcmax is recognized as the most critical parameter for modelling global primary productivity and projecting future global change (Rogers, 2014). This importance stems from estimates of Jmax often being extrapolated from a linear function of Vcmax (Walker et al., 2014) and that many global carbon models estimate Vcmax as a fraction of leaf N content (Friend, 2010). Accordingly, the ability to determine Vcmax using biochemical and leaf photosynthetic measurements for a globally important woody plant genus such as Eucalyptus emerges as a key goal to be addressed. Eucalypts are important plants for both native forests and commercial plantations in Australia and worldwide.
According to the FvCB model (Farquhar et al., 1980), Vcmax is fitted using the initial slope of the rate of CO2 assimilation (A) versus intercellular [CO2] (Ci) response (A–Ci) curve, and can be expressed as:
| (1) |
where Vc is the CO2-limited photosynthetic rate, Cc is chloroplastic [CO2], Kc21%O2 is Rubisco’s apparent Michaelis–Menten constant for CO2 in air, Γ* is the CO2 compensation in the absence of mitochondrial respiration (Rd) calculated as 0.5×Oc/Sc/o, with Oc and Sc/o representing chloroplastic [O2] and Rubisco’s CO2/O2 specificity, respectively.
In most C3 photosynthesis gas exchange studies, A–Ci response curves are fitted with the FvCB model using ‘standard’ catalytic parameters measured for tobacco or spinach Rubisco (Wullschleger, 1993; Badger et al., 2000; Sharkey et al., 2007; Bernacchi et al., 2009). However, significant variation exists in Rubisco catalysis amongst C3 species (von Caemmerer and Quick, 2000; Galmes et al., 2005; Whitney et al., 2011a;Galmés et al., 2014; Hermida-Carrera et al., 2016; Orr et al., 2016; Prins et al., 2016), including differences in the temperature response of Rubisco between species (Walker et al., 2013; Hermida-Carrera et al., 2016; Prins et al., 2016). Therefore, questions arise about the accuracy of applying these ‘standard’ Rubisco parameters to universally model C3 photosynthesis and whether woody plants differ in these respects from crop and herbaceous plants. Consequently, the first objective of the current study was to compare the compatibility of Vcmax rates derived in vivo from the A–Ci curves with in vitro estimates of Vcmax derived from measures of Rubisco content together with assays of the kinetic properties of Eucalyptus globulus Rubisco at the standard temperature of 25 °C.
Nitrogen (N) is a major mineral resource limiting plant growth in many parts of the world. About 75% of leaf N is invested in the photosynthetic apparatus, with an average of 20% invested in Rubisco (Evans and Seemann, 1989). Partitioning of photosynthetic N is strongly influenced by the growth environment (Sage et al., 1987; Terashima and Evans, 1988; Evans and Seemann, 1989). It is well documented that elevated atmospheric [CO2] reduces leaf N content in many C3 species (Drake et al., 1997; Ainsworth and Rogers, 2007, while the effects of warming or CO2×warming responses on leaf N content and partitioning are less clear (Onoda et al., 2005a, b; Hikosaka et al., 2006; Wang et al., 2012). Given that most leaf N is associated with photosynthesis (Evans, 1989; Nakano et al., 1997), changes in leaf N in response to rising [CO2] and temperature will impact the photosynthetic biochemistry. To our knowledge, the question of how elevated [CO2] and temperature together will influence the underlying photosynthetic biochemistry and N partitioning has not been addressed in large, field-growing tree species. Hence, the second objective of this study was to establish whether Vcmax constitutes a constant fraction of leaf N under current and future climate conditions.
Only a few studies have investigated the effects of warming on photosynthetic biochemistry and leaf chemistry relative to the large body of work on the effects of elevated [CO2] alone. In addition, the interactive effects of climate factors with canopy position is under-represented in the literature (Crous and Ellsworth, 2004). Canopy position is known to influence leaf morphology and chemistry (Ellsworth and Reich, 1993; Kenzo et al., 2006). For example, upper canopy leaves can show the classical sun phenotype whereby a greater proportion of leaf N is allocated to soluble proteins, including Rubisco, and less to thylakoid complexes, including PSII (Boardman, 1977; Givnish, 1988). By addressing the two above-outlined objectives, the current study sought to elucidate the interactive effects of elevated [CO2], elevated temperature, and canopy position on determinants of Vcmax in large, soil-rooted eucalypt trees grown in whole-tree chambers (WTCs) at the Hawkesbury Forest Experiment (HFE) in Richmond, Sydney.
Materials and methods
Plant culture and growth conditions
Seedlings of Eucalyptus globulus Labill. ssp. were obtained from a commercial tree nursery (Elders Forestry Ltd, Albany, Vic., Australia). Seeds (No. 08-12-106M) were collected at 38°48'S and 143°37'E, ~700 km and five latitudinal degrees pole-ward relative to the experimental site (HFE) of this study (Crous et al., 2013). The HFE site is situated on the alluvial floodplain of the Hawkesbury River (33°36'40''S and 150°44'26.5''E). The soil is a loamy-sand with low organic matter content (0.7%) and low fertility (pH 5.5, N <1 mg kg–1, and P ~8 mg kg–1). Seedlings were exposed to their respective treatment conditions in the WTCs in August 2010. One tree seedling was transplanted into the ground of each WTC in December 2010 and supplied with an initial fertilization of 50 g of (NH4)2PO4 and 10 mm of water every third day to ensure good establishment. Thereafter, trees were watered every 3–7 d to maintain non-limiting water supply, and were harvested when 10 m tall at 15 months in November 2011. The chamber trees were grown in a continuous block of forest in order to simulate shading by neighbouring trees normally experienced in a canopy.
The WTCs used for this study were designed continuously to track outside (ambient) conditions of temperature, humidity, and [CO2] (Barton et al., 2010), and were equipped with improved temperature control (Crous et al., 2013). There were 12 WTCs maintained at four treatments (three WTCs per treatment): ambient [CO2] and ambient temperature (aCaT; tracking ambient CO2 and ambient temperature); elevated [CO2] (tracking ambient CO2+240 µl l–1) and ambient temperature (eCaT); ambient [CO2] and elevated temperature (tracking ambient temperature+3°C) (aCeT); and elevated [CO2] and elevated temperature (eCeT). Measurements were made in the early spring of 2011 (August–September 2011) on juvenile, lower and upper canopy leaves of ~7 m tall trees. Only two replicate trees remained in the eCaT treatment as a result of one tree dying following the heatwave of January 2011 (Crous et al., 2013). The other treatments had three healthy trees. During the study period, average growth conditions were: 453 µl l–1 and 634 µl l–1 for aC and eC treatments, respectively, and 21.4/5.8 °C and 24.3/8.7 °C (day/night) for aT and eT treatments, respectively.
Leaf gas exchange
A portable open gas exchange system (LI-6400XT, LI-COR, Lincoln, NE, USA) was used to measure the light-saturated photosynthetic rate (Asat) of upper and lower canopy leaves. Single-point measurements were taken in early spring between 10:00 h and 14:00 h at a leaf temperature of 25 °C, photosynthetic photon flux density of 1500 µmol m–2 s–1 (light saturating for E. globulus, KY Crous, unpublished data), and growth [CO2] (400 µl l–1 or 640 µl l–1). Leaf-to-air vapour pressure deficit (VPDl) varied between 0.8 kPa and 1.2 kPa. Each leaf was allowed to stabilize for 15–20 min in the LI-6400XT leaf chamber before measurements were taken. Upper and lower canopy leaves were measured on each of the 11 WTC trees.
Photosynthetic responses to intercellular [CO2] (A–Ci curves) were measured on upper canopy leaves only. Measurements were made using 12 CO2 steps at a leaf temperature of 25 °C, 1800 µmol photons m–2 s–1, and VPDl of 0.8–1.1 kPa. The biochemical model of Farquhar et al. (1980) was used to estimate Vcmax25 (maximal RuBP carboxylation-limited rate) and Jmax25 (maximal RuBP regeneration-limited rate) at 25 °C. The model was parameterized using Rubisco catalytic parameters shown in Table 1 for E. globulus and mesophyll conductance (gmes) for this species obtained from Crous et al. (2013).
Table 1.
Catalytic parameters for E. globulus and Nicotiana tabacum (tobacco) Rubisco at 25 °C
Values (±SD) are the average of the number of biological replicates (n) indicated (see the Materials and methods for more detail). The oxygention rate (kcato) was calculated using the equation Sc/o=(kcatc/Kc)/(kcato/Ko). Γ*, the CO2 compensation in the absence of mitochondrial respiration (Rd), was calculated as =0.5×[O2]c/Sc/o, where [O2]c is the O2 concentration in the chloroplast. Values used for fitting the A–Ci curves (Table 3; Fig. 2) were converted into the gas phase using the solubility constants for CO2 (0.0334 M M–1 bar–1) and O2 (0.00126 M M–1 bar–1) at 25 oC. CE and OE are the derived carboxylation and oxygenation efficiencies, respectively.
| Eucalyptus globulus | Nicotiana tabacum | |
|---|---|---|
| K m (CO2) at 0% O2, Kc0%O2 (µM) | 9.8 ± 0.3 (293 µbar) | 9.4 ± 0.1 |
| K m (CO2) at 21% O2, Kc21%O2 (µM) | 21 (629 µbar) | 19.5 |
| k cat c (s–1) | 3.0 ± 0.2 (n=6) | 3.1 ± 0.2 (n=19) |
| K i (O2), K o (µM) | 220 ± 18 (175 mbar) | 236 ± 9 |
| S c/o (M M–1) (n=2) | 80.4 ± 0.9 (2131 bar bar–1) | 82.3 ± 0.3 |
| Γ* (µM) | 1.57 (48.6 µbar) | 1.54 |
| k cat o (s-1) | 0.94 | 0.83 |
| CE21% O2, kccatc/Km (CO2)21% O2 (mM–1 s–1) | 143 | 159 |
| OE, kocato/Ki (O2) (mM–1 s–1) | 3.8 | 4.0 |
Content measurements of Rubisco, soluble protein, and chlorophyll
Following the single-point gas exchange measurements, replicate leaf discs (0.98 cm2) were rapidly frozen in liquid nitrogen then stored at –80 °C until analysed. High concentrations of secondary metabolites in eucalypt leaves are known to reduce the extraction yield of soluble proteins (Warren et al., 2000). Extraction yield from eucalypt leaves was improved by increasing the polyvinylpolypyrrolidone (PVPP) concentration and adding glycerol to the extraction buffer (see Supplementary Fig. S1A, B at JXB online). Each leaf disc was extracted in 1 ml of ice-cold eucalypt protein extraction buffer [50 mM EPPS-NaOH pH 8.0, 5 mM DTT, 15 mM NaHCO3, 20 mM MgCl2, 2 mM EDTA, 4% (v/v) protease inhibitor cocktail (Sigma), 4% (w/v) PVPP, and 20% (v/v) glycerol] using a 2 ml Potter–Elvehjem glass homogenizer kept on ice. Subsamples were taken from the total extract for chlorophyll determination (90 µl) in 80% acetone (Porra et al., 1989) and SDS–PAGE analysis (75 µl) of total leaf protein. The remaining extract was centrifuged at 16 100 g for 1 min and the supernatant used for extractable Rubisco and soluble protein determination. Extractable Rubisco content was quantified by the irreversible binding of [14C]carboxyarabinitol bisphosphate (CABP) to the fully carbamylated enzyme (Ruuska et al., 1998). Extractable soluble proteins were measured using the Coomassie Plus kit (Pierce) against BSA. To account for non-extractable soluble proteins (precipitated in the pellet due to high secondary metabolites, Supplementary Fig. S1A), an extraction yield was calculated based on the Coomassie stain intensity of Rubisco large subunit (LSu) detected in total leaf protein (i.e. homogenate before centrifugation) and extractable soluble leaf protein (i.e. supernatant) as described below (Supplementary Fig. S1C). There was a strong relationship between the Coomassie intensity of Rubisco LSu and the amount of Rubisco determined by the [14C]CABP assay (Supplementary Fig. S1D), indicating that intensity measurements were adequate for determining differences in Rubisco content and calculating the extraction yield. Total Rubisco and soluble protein concentrations were calculated by dividing extractable Rubisco or soluble proteins by the extraction yield:
| (2) |
The extraction yield obtained for Rubisco in the current study varied between 0.5 and 0.9.
Extraction yield and immunoblot of Rubisco and PSII
Subsamples of total and extractable soluble protein fractions were mixed with 0.25 vols of 4× LDS buffer (Invitrogen) containing 100 mM DTT, snap-frozen in liquid nitrogen, and stored at –20 °C until analysed. Protein samples were separated by SDS–PAGE at 200 V using TGX Any kD (Bio-Rad) pre-cast polyacrylamide gels in the Mini-Protean apparatus buffered with Tris-glycine SDS buffer (Bio-Rad). Proteins were visualized by staining with Bio-Safe Coomassie G-250 (Bio-Rad) and imaged using the VersaDoc imaging system (Bio-Rad). The extraction yield of leaf protein was determined from the relative band densitometry of the ~52 kDa Rubisco LSu in 4 µl of both the total and extractable soluble protein fractions according to the equation:
| (3) |
For immunoblot analysis, total extracts for each leaf sample were separated by SDS–PAGE as outlined above and transferred at 4 °C to nitrocellulose membranes (0.45 µm; Bio-Rad) using the Xcell Surelock western transfer module (Invitrogen) buffered with 1× Transfer buffer [20×: 25 mM Bicine, 25 mM Bis-Tris, 1 mM EDTA, 20% (v/v) methanol]. After 1 h transfer at 30 V, the membrane was placed in blocking solution [3% (w/v) skim milk powder in Tris-buffered saline (TBS; 50 mM Tris–HCl pH 8, 150 mM NaCl) for 1 h at room temperature with gentle agitation.
Primary antiserum raised against tobacco Rubisco was diluted 1:4000 in TBS, and antiserum raised against PSII-D1 protein was obtained from AgriSera (PsbA, Cat. AS05-084) and diluted with TBS 1:10 000. Primary antisera were incubated with membranes at room temperature for 1 h with gentle agitation before washing three times with TBS. Secondary goat anti-rabbit antiserum conjugated to horseradish peroxidase (HRP; Cat. NEF 812001EA, Perkin Elmer) was diluted 1:5000 in TBS and incubated with the membranes for 1 h at room temperature followed by three washes with TBS. Immunoreactive peptides were detected using the Immun-Star Western C kit (Cat. 170-5070, Bio-Rad) and imaged using the VersaDoc. Leaf PsbA contents were quantified from band densitometry comparison against 0.25–1 pmol of PsbA global protein standard (Cat. 125-10016, AgriSera) using VersaDoc software Quantity 1.
Measurements of in vitro Rubisco catalytic parameters
Rubisco substrate-saturated turnover rate kcatc and the Michaelis–Menten constants (Km) for CO2 (Kc) and O2 (Ki) were determined by 14CO2 fixation assays at 25 °C as described (Sharwood et al., 2008; Whitney et al., 2011b). Leaf samples were extracted in 1 ml of ice-cold eucalypt extraction buffer (no NaHCO3) and the soluble protein activated for 7 min at 25 °C in buffer containing 10 mM NaH14CO3 and 20 mM MgCl2 before adding 20 µl to start 0.5 ml assays in 7 ml septum-capped scintillation vials (Perkin Elmer). The assays contained buffer [50 mM HEPES-NaOH (pH 8.2), 10 mM MgCl2, 0.5 mM RuBP] and varying concentrations of NaH14CO3 (0–74 μM). Assays were equilibrated with 0, 10, 15, 20, 25, or 30% (v/v) O2 mixed with N2 using Wosthoff gas mixing pumps (Whitney et al., 2011b), and terminated after 1 min with 0.2 ml of 20% (v/v) formic acid. For CO2/O2 specificity (Sc/o) measurements, Rubisco was rapidly purified from ~5 g of young fresh leaves as described in Sharwood et al. (2008). Sc/o was measured using the method of Kane et al. (1994). Tobacco was used as a reference species for all Rubisco kinetic assays.
To determine the integrity of Rubisco holoenzyme used for activity assays and to confirm the accuracy of [14C]CABP Rubisco content measurements, soluble leaf protein was added to 5× native loading buffer [1 M Tris pH 6.8, 80% glycerol, 1% (w/v) bromophenol blue], separated by non-denaturing PAGE at 60 V for 16 h at 4 °C in 4–12% NuPAGE Tris-glycine gels and the ~520 kDa Rubisco holoenzyme visualized by Coomassie staining (Supplementary Fig. S1B).
Leaf mass per area, nitrogen, and carbohydrate analyses
Following gas exchange, adjacent leaves were sampled from the upper and lower canopy, their area determined using a leaf area meter (LI-3100A, LI-COR), then oven-dried at 70 °C for 48 h, weighed, and ground to a homogenous powder in a ball mill (MM-400, Retsch, Germany). Leaf mass per area (LMA) was calculated and N content was determined on subsamples using a CN analyser (LECO TruSpec, LECO Corporation, Michigan, USA). The allocation of N into Rubisco, PSII, and soluble proteins was calculated by assuming that proteins contain 16% N by mass, with a mol. wt. of 550 kDa and 417 kDa for Rubisco and PSII, respectively (Evans and Seemann, 1989; Ghannoum et al., 2005).
Another set of matching gas exchange leaves were sampled from the upper and lower canopy at dawn (04:00 h), midday (12:00 h), and dusk (16:00 h), frozen in liquid nitrogen before being stored at –80°C until they were freeze-dried, then ground in a ball mill. Total non-structural carbohydrate (TNC) concentration was calculated as the sum of total starch and soluble sugars, which were measured as described in Ghannoum and Conroy (1998).
Data analysis
Leaf [N], LMA, TNC, photosynthesis, Rubisco, soluble proteins, and chlorophyll were measured on individual leaf samples per WTC tree and canopy level. For all the parameters, three-way ANOVA was used to test the significance of canopy level, growth [CO2], and growth temperature. There were three biological replicates per treatment and canopy level (n=3 except for eCaT, where n=2 due to tree death). For the quantification of Rubisco, soluble proteins, and PSII proteins, two independent extractions were performed per leaf (two analytical replicates per biological replicate).
Results
Leaf gas exchange of E. globulus at growth CO2
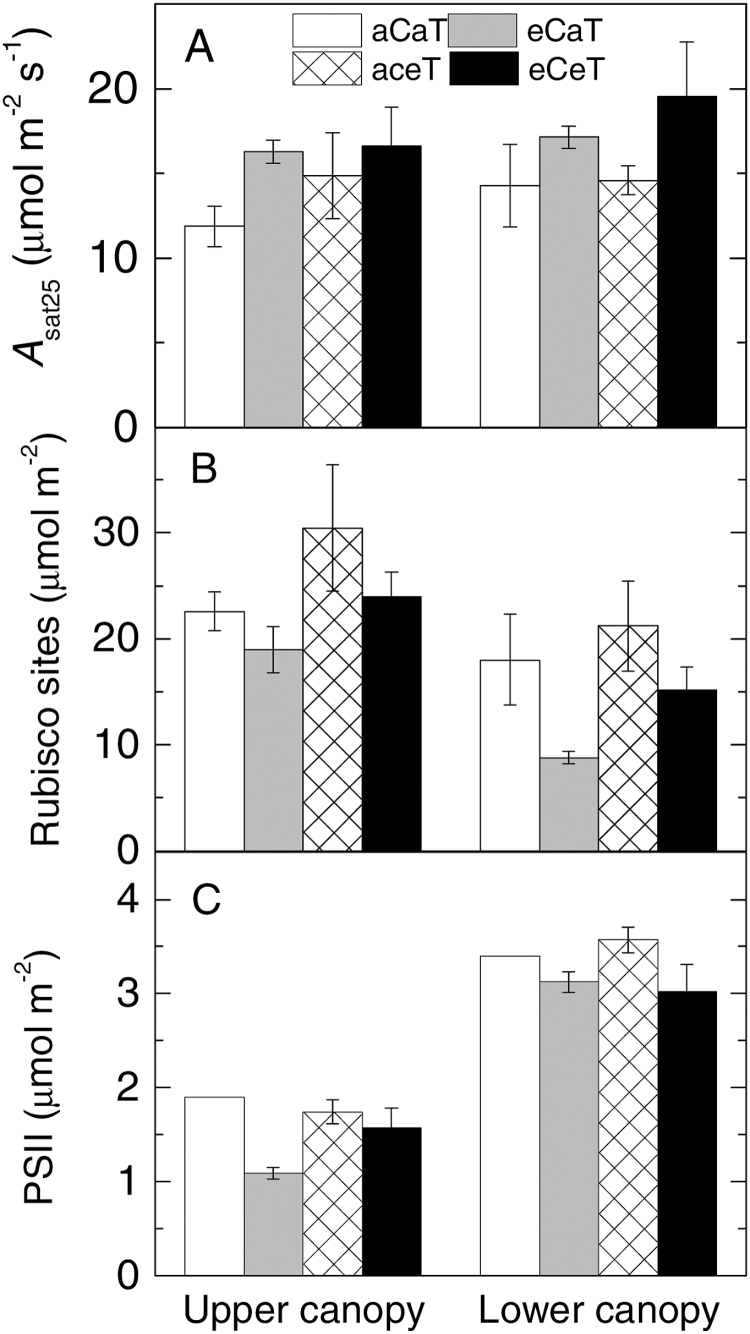
When measured at a common temperature of 25 °C and growth [CO2], leaf photosynthetic rates (Asat25) of E. globulus were stimulated (P=0.04) at elevated [CO2] by an average of 30% relative to ambient [CO2] across both temperatures and canopy positions. In contrast, warming and canopy position had no significant effects on Asat25 (Fig. 1A; Table 2).
Fig. 1.
Effect of elevated CO2 and temperature on main photosynthetic parameters. Light-saturated rates of photosynthesis, Asat25 (A), Rubisco content (B), and PSII-D1 protein content (C) in leaves of E. globulus trees (upper canopy on the left and lower canopy on the right) grown at ambient (aC, clear and checked columns) or elevated (eC, grey and black columns) atmospheric [CO2], and at ambient (aT, clear and grey columns), or elevated (eT, checked and black columns) air temperature. Values represent averages of 2–3 biological replicates ±SE (n=3 except for eCaT, where n=2). A statistical summary is shown in Table 2.
Table 2.
Summary of three-way ANOVA (canopy×growth [CO2]×growth temperature) for leaf parameters measured in E. globulus grown at two atmospheric [CO2] (ambient and ambient+240 µl l–1) and two air temperatures (ambient and ambient+3 oC)
Values are means ±SE. Analysis was done with 2–3 biological replicates per treatment and canopy level. There were no significant three-way interactions, and these were not shown. LMA-TNC and Leaf [N]M-TNC were expressed on a structural dry mass basis [i.e. dry mass corrected for TNC (total non-structural carbohydrate) accumulation].
| Parameter | Canopy | Treatments | Statistical significance, P | |||||
|---|---|---|---|---|---|---|---|---|
| aCaT (n=3) |
eCaT (n=2) |
aCeT (n=3) |
eCeT (n=3) |
Canopy Canopy×CO2 |
CO2 Canopy×temp |
Temp Temp×CO2 |
||
| A sat25 | Upper | 11.9 ± 1.2 | 16.3 ± 0.7 | 14.8 ± 2.5 | 16.6 ± 2.2 | 0.359 | 0.042 | 0.350 |
| (µmol m–2 s–1) | Lower | 14.3 ± 2.4 | 17.1 ± 0.7 | 14.6 ± 0.8 | 19.5 ± 3.2 | 0.797 | 0.916 | 0.935 |
| Leaf [N]M | Upper | 15.8 ± 1.9 | 12.2 ± 0.7 | 24.7 ± 3.5 | 18.0 ± 4.0 | 0.959 | 0.025 | 0.0020 |
| (mg g–1) | Lower | 17.4 ± 2.5 | 14.9 ± 0.5 | 22.2 ± 1.9 | 15.7 ± 2.0 | 0.866 | 0.258 | 0.376 |
| Leaf [N]A | Upper | 1.84 ± 0.12 | 1.85 ± 0.27 | 2.32 ± 0.22 | 1.82 ± 0.38 | 0.007 | 0.283 | 0.486 |
| (g m–2) | Lower | 1.47 ± 0.24 | 1.42 ± 0.10 | 1.55 ± 0.13 | 1.35 ± 0.18 | 0.725 | 0.520 | 0.331 |
| PNUE | Upper | 92 ± 15 | 126 ± 23 | 89 ± 11 | 133 ± 15 | 0.000 | 0.000 | 0.444 |
| [µmol (mol N)–1 s-1] | Lower | 136 ± 4 | 171 ± 19 | 133 ± 12 | 201 ± 8 | 0.531 | 0.544 | 0.280 |
| Rubisco sites | Upper | 22.5 ± 1.5 | 18.9 ± 2.2 | 30.5 ± 5.9 | 23.9 ± 2.3 | 0.145 | 0.026 | 0.046 |
| (µmol m–2) | Lower | 18.0 ± 4.3 | 8.8 ± 0.6 | 21.2 ± 4.3 | 15.1 ± 2.2 | 0.427 | 0.345 | 0.904 |
| Soluble proteins | Upper | 5.4 ± 0.8 | 5.7 ± 1.3 | 6.8 ± 0.3 | 6.7 ± 1.9 | 0.059 | 0.330 | 0.323 |
| (g m–2) | Lower | 5.2 ± 0.7 | 3.2 ± 1.0 | 5.1 ± 0.6 | 4.5 ± 0.8 | 0.466 | 0.851 | 0.903 |
| Chlorophyll | Upper | 0.75 ± 0.17 | 0.57 ± 0.05 | 0.88 ± 0.07 | 0.85 ± 0.17 | 0.222 | 0.400 | 0.157 |
| (mmol m–2) | Lower | 0.62 ± 0.15 | 0.63 ± 0.04 | 0.72 ± 0.03 | 0.63 ± 0.11 | 0.723 | 0.395 | 0.874 |
| LMA | Upper | 118 ± 7 | 152 ± 13 | 97 ± 11 | 102 ± 2 | 0.000 | 0.002 | 0.000 |
| (g m–2) | Lower | 84 ± 2 | 95 ± 4 | 70 ± 2 | 86 ± 3 | 0.490 | 0.015 | 0.210 |
| Average daily TNC | Upper | 30 ± 1 | 39 ± 2 | 20 ± 3 | 23 ± 1 | 0.000 | 0.003 | 0.000 |
| (g m–2) | Lower | 17 ± 1 | 23 ± 3 | 15 ± 1 | 16 ± 1 | 0.296 | 0.004 | 0.049 |
| LMA-TNC | Upper | 88 ± 5 | 113 ± 11 | 77 ± 7 | 80 ± 1 | 0.000 | 0.003 | 0.001 |
| (g DM-TNC m–2) | Lower | 66 ± 2 | 72 ± 1 | 54 ± 3 | 70 ± 2 | 0.615 | 0.037 | 0.381 |
| Leaf [N]M-TNC | Upper | 21 ± 3 | 16 ± 1 | 31 ± 4 | 23 ± 5 | 0.837 | 0.023 | 0.031 |
| [mg g (DM-TNC)–1] | Lower | 22 ± 3 | 20 ± 1 | 28 ± 2 | 19 ± 2 | 0.890 | 0.297 | 0.317 |
Contents of leaf Rubisco, PSII, soluble proteins, and chlorophyll
Leaf Rubisco contents were assayed using two independent techniques: (i) quantification of total Rubisco catalytic sites using tight stoichiometric [14C]CABP binding (Table 2) and (ii) confirmatory relative Rubisco content measured by immunoblot analysis of SDS–PAGE-separated Rubisco LSu (Supplementary Fig. S1). The two methods reconciled closely with each other (Supplementary Fig. S1D). Upper canopy leaves tended (P=0.14) to have more Rubisco content relative to the lower canopy (Fig. 1B). Elevated [CO2] (eC) reduced Rubisco content (P=0.03) by 16% and 51% in upper and lower leaves, respectively, while elevated temperature (eT) enhanced Rubisco content (P=0.046) by 36% and 18% in upper and lower leaves, respectively (Fig. 1B; Table 2). The effects of eC and eT were additive such that leaf Rubisco content was similar between the eCeT and aCaT treatments (Fig. 1B; Table 2). The leaf soluble protein content tended (P=0.059) to be lower in the lower relative to the upper canopy leaves; and was reduced by ~40% in eC in the lower canopy while it was increased by ~25% in eT in the upper canopy (Table 2).
The content of PSII, a key thylakoid protein involved in light harvesting and electron transport, was determined by measuring the abundance of the PsbA (D1) protein by immunoblot analysis using the Agrisera global D1 protein antibody designed to react equally with D1 protein from all plant species. Upper canopy leaves had about half of the PSII content compared with the lower canopy (Fig. 1C). The eC treatment reduced the D1 protein content in the upper canopy leaves but was not significantly affected by eT (Fig. 1C). Despite these changes in PSII, leaf chlorophyll content in E. globulus was not significantly affected by either canopy position, growth [CO2], or temperature (Table 2).
Catalytic comparison of E. globulus and tobacco Rubisco
The catalytic properties of E. globulus Rubisco measured at 25 °C are similar to those of tobacco Rubisco (Table 1). With regard to the parameters used in Equation 1, both Rubisco isoforms show comparable substrate-saturated carboxylation rates (kcatc) while the CO2/O2 specificity (Sc/o) and carboxylation efficiency [kcatc/Km (CO2)21% O2] of E. globulus Rubisco at 21% O2 were slightly lower than those of tobacco Rubisco (Table 1).
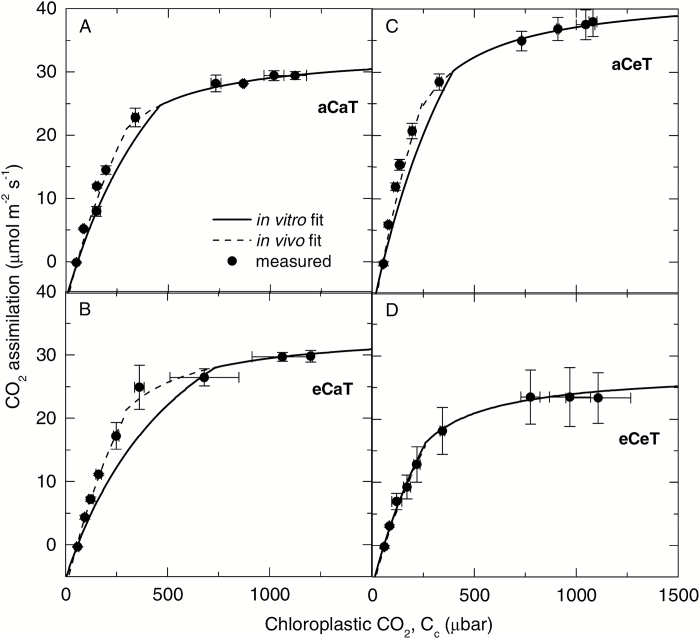
Analysis of the A–Ci curves at 25 oC
The CO2 response curves of photosynthesis were measured for upper canopy leaves (Fig. 2). Analysis of the A–Ci response curves using catalytic parameters for E. globulus Rubisco (Table 1) and gmes reported in Crous et al. (2013) revealed that elevated [CO2] had no significant effect on the in vivo gas exchange estimates of either Vcmax25 or Jmax25. In contrast, elevated temperature (aCeT) enhanced the in vivo Vcmax25 and Jmax25 by 26% relative to the aCaT treatment (Fig. 2; Table 3). The ratio J/V (1.5–1.7) was not significantly affected by any treatment (Table 3).
Fig. 2.
Effects of elevated CO2 and temperature on photosynthetic CO2 response curves. The response of photosynthetic rates to chloroplastic [CO2], Cc in the upper canopy leaves of E. globulus trees grown at ambient (aC) or elevated (eC) atmospheric [CO2], and at ambient (aT) or elevated (eT) air temperature. Data points (filled circles) are the average (±SE) A–Ci curve measured at 25 °C for 2–3 biological replicates (n=3 except for eCaT, where n=2). Lines represent theoretical A–Ci curves modelled using the in vitro (solid lines) or in vivo (dashed lines) estimates of Vcmax as described in Table 1.
Table 3.
Summary of in vitro and in vivo estimates of Vcmax and Jmax for upper canopy leaves of E. globulus
In vitro V cmax was calculated as [Rubisco sites]×kcatc. The biochemical model of Farquhar et al. (1980) was used to estimate in vivo Vcmax (maximal RuBP carboxylation-limited rate) and Jmax (maximal RuBP regeneration-limited rate) from the A–Ci curves measured at 25oC for upper canopy leaves. The model was parameterized using Rubisco catalytic parameters shown in Table 1 for E. globulus and mesophyll conductance (gmes) obtained from Crous et al. (2013). The equations used were: ; and where Cc is the chloroplastic [CO2]. Other parameters are defined in Table 1.
| Parameter | aCaT | eCaT | aCeT | eCeT |
|---|---|---|---|---|
| In vitro V cmax (μmol m–2 s–1) | 67 ± 5 | 57 ± 6 | 91 ± 18 | 72 ± 7 |
| In vivo V cmax (μmol m–2 s–1) | 81 ± 5 | 81 ± 15 | 115 ± 17 | 72 ± 21 |
| In vivo J max (μmol m–2 s–1) | 138 ± 5 | 140 ± 16 | 175 ± 17 | 115 ± 23 |
| In vivo J/V | 1.7 | 1.7 | 1.5 | 1.6 |
| V cmax (in vivo/in vitro ratio) | 0.8 | 0.7 | 0.8 | 1.0 |
In vitro and in vivo estimates of Vcmax were equal for the eCeT treatment, such that measured leaf Rubisco contents could account for the carboxylase-limited assimilation rates of the A–Ci curve (Fig. 2D; Table 3). For the other treatments, 20–30% more Rubisco sites were required to account fully for the CO2 assimilation rates measured in vivo under limiting Ci (Fig. 2A–C; Table 3). This demonstrates the difficulty of achieving high extraction yields for leaf soluble proteins in recalcitrant species, such as eucalypts.
Leaf nitrogen and carbohydrates
LMA and TNC changed in parallel with the various treatments (Table 2). LMA and TNC were generally greater in upper than in lower canopy leaves (P<0.001). In both canopies, LMA and TNC increased at elevated [CO2] (P=0.002) and decreased at elevated temperature (P<0.001) such that values were similar for the aCaT and eCeT treatments (Table 2). Changes in LMA mirrored those observed for TNC-corrected LMA (LMA-TNC), indicating that elevated [CO2] and temperature affected both structural and non-structural carbohydrates (Table 2).
Expressed on a dry mass basis, leaf nitrogen content ([N]M) was similar in both canopy positions (P=0.96). Relative to the aCaT treatment, leaf [N]M decreased by 23% at elevated [CO2] (P=0.03) and increased by 56% at elevated temperature (P=0.002); leaves grown at aCaT and eCeT had similar leaf [N]M (Table 2). Similar trends were observed for [N]M when dry mass was corrected for TNC, [N]M-TNC (Table 2). Expressed on an area basis, leaf nitrogen concentration ([N]A) was 25% greater in upper than in lower canopy leaves (P=0.008). Elevated [CO2] and elevated temperature had no significant effect on leaf [N]A either separately or jointly (Table 2).
Leaf nitrogen relations and its allocation
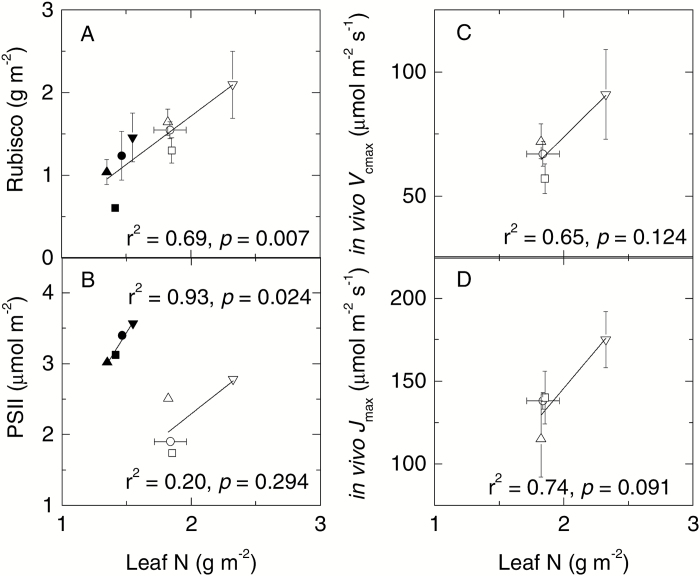
Leaf contents of Rubisco (Fig. 3A) and soluble proteins (r2=0.58, P=0.035) scaled strongly with leaf [N]A across the treatment combinations. In contrast, leaf PSII content and [N]A scaled better in lower relative to upper canopy leaves (Fig. 3B). In vivo Vcmax25 and Jmax25 correlated with leaf [N]A in upper canopy leaves across the [CO2] and temperature treatments in E. globulus (Fig. 3C, D).
Fig. 3.
Effects of elevated CO2 and temperature on the relationship between leaf photosynthesis and N. Relationships between leaf Rubisco (A) and PSII (B) contents and in vivo Vcmax (C) and Jmax (D) with leaf N of E. globulus trees grown in whole-tree chambers. Values represent the means ±SE of 2–3 biological replicates for upper (open symbols) and lower (filled symbols) canopy leaves grown at aCaT (circles), eCaT (squares), aCeT (inverted triangles), and eCeT (upright triangles). The solid lines are linear fits of the data points.
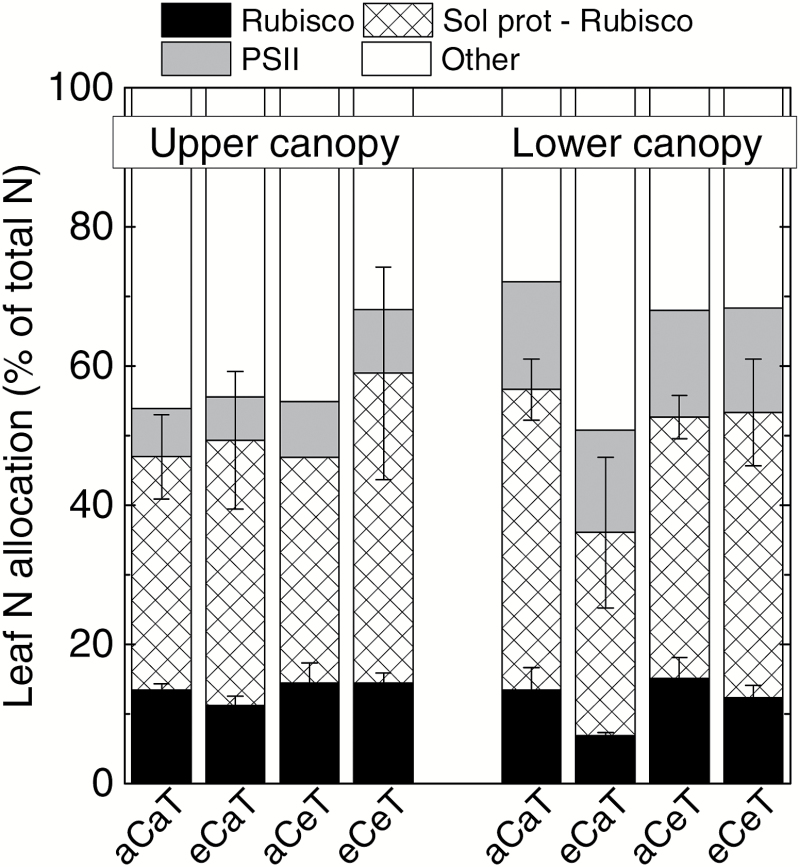
Rubisco constituted 19–31% of the leaf soluble proteins and 7–15% of leaf N in E. globulus (Table 2). Relative to the lower canopy, upper canopy leaves invested 2-fold more N in Rubisco, 1.5-fold more N in soluble proteins, and 2-fold less N in PSII (Fig. 4). Either separately or together, elevated [CO2] and temperature had no significant effect on the proportion of leaf N invested in soluble proteins, Rubisco, or PSII (Fig. 4).
Fig. 4.
Leaf nitrogen allocation of E. globulus in trees grown at ambient (aC) or elevated (eC) atmospheric [CO2], and at ambient (aT) or elevated (eT) air temperature. Values represent averages of 2–3 biological replicates ±SE (n=3 except for eCaT, where n=2). The N percentages were calculated using data in Table 2 and Fig. 1. Other details are as described in the Materials and Methods.
Upper canopy leaves had 30% lower photosynthetic nitrogen use efficiency, PNUE (P=0.0001) compared with the lower canopy (Table 2). Elevated [CO2] increased PNUE (P=0.0003) by ~30% in non-warmed trees and ~50% in warmed trees, while warming had no significant effect on PNUE (Table 2).
Discussion
Estimating Vcmax using Rubisco parameters
This study presents the first comprehensive attempt at estimating in vivo and in vitro Vcmax for a widely planted tree species (E. globulus) grown under varying atmospheric [CO2] and temperature using combined measurements of its leaf Rubisco content and inherent Rubisco kinetic properties together with leaf gas exchange. For trees, numerous studies have considered the response of leaf gas exchange to single or multiple climate change variables (Tjoelker et al., 1998; Teskey and Will, 1999; Ellsworth et al., 2004; Crous et al., 2008, 2013; Ghannoum et al., 2010b; Quentin et al., 2015). Studies examining the response of photosynthetic proteins or enzyme activity to climate change have largely focused on crop and other non-woody species, and mostly in response to elevated [CO2] without warming (Nakano et al., 1997; Rogers et al., 1998; Theobald et al., 1998; Adam et al., 2000). Only a few tree studies have measured changes in leaf Rubisco content (Tissue et al., 1993; Rogers and Ellsworth, 2002; Kosvancova et al., 2009; Urban et al., 2012) with accurate quantification in the leaves of trees such as eucalypts or pines particularly challenging due to high levels of protein-damaging, secondary metabolites (Rogers et al., 2001). Inclusion of glycerol, high amounts PVPP, and a plant protease inhibitor were found to be effective in establishing a method to meet our first objective, the extraction and quantification of active Rubisco from E. globulus leaves to derive in vitro estimates of Vcmax at 25 oC. The derived values were consistent, although slightly lower, than the in vivo measures of Vcmax extrapolated from gas exchange (Table 3). In agreement with our finding, similar differences between the in vitro and in vivo estimates of Vcmax were reported for loblolly pines (Rogers et al., 2001).
A critical aspect of photosynthesis modelling is the ability to link Vcmax to easily measured leaf traits such as leaf N content, as well as the relationship between Vcmax and Jmax (Walker et al., 2014). Data presented in this study demonstrated that Rubisco constituted 7–15% of leaf N across the various treatments. As shown in Table 3, these values are 20–30% lower based on in vivo Vcmax estimates. Hence, the true Rubisco fraction is expected to be 9–18% of leaf N, which is somewhat lower than the average of 20% generally observed for C3 species (Evans, 1989). Nevertheless, Vcmax values observed for E. globulus (57–115 µmol m–2 s–1) were generally higher than values (<70 µmol m–2 s–1) recorded for broad-leaf evergreen tree species (Rogers, 2014), suggesting differences in the Rubisco content and/or catalytic properties between these tree species. This is an area worthy of further investigation. Importantly, there was a strong linear relationship between leaf N and Rubisco content (Fig. 3A) across canopy positions. Vcmax was also well correlated with leaf N in the upper canopy (Fig. 3C), while the Jmax/Vcmax ratio was constant across the various treatments (Table 3). Taken together, these results indicate that in E. globulus Rubisco largely remained a constant fraction of leaf N across the elevated CO2, warming, and canopy position treatments, and that Vcmax in this species can be predicted from leaf N and the Rubisco fraction, while Jmax can also be estimated from Vcmax. These are important relationships for whole-tree and canopy scale modelling of CO2 fixation (Rogers, 2014; Walker et al., 2014).
Acclimation in response to elevated [CO2] and temperature
In the soil-grown E. globulus trees, elevated [CO2] increased LMA and reduced leaf [N] expressed on both a total dry mass and structural dry mass basis by the same proportion. In contrast, elevated temperature had the opposite effects on LMA and leaf [N], respectively. In response to eC and eT, leaf N expressed on an area basis was unchanged. These findings correlate with those observed for many other C3 species exposed to elevated [CO2] in controlled environments or in the field (Tjoelker et al., 1998; Ellsworth et al., 2004; Crous et al., 2008; Ghannoum et al., 2010a), and refute carbohydrate dilution as an explanation for reduced leaf [N]M at eC (Taub and Wang, 2008). The effects of eT on leaf [N] in tree species are less well documented and more variable, with reports of an increase for Scots pine (Kellomaki and Wang, 1997) compared with small decreases for sugar maple (Gunderson et al., 2010) and eucalypts (Ghannoum et al., 2010a; Sherwin et al., 2013). Similar trends for LMA and leaf TNC have been reported for various tree species in response to warming (Tjoelker et al., 1998; Gunderson et al., 2010). Importantly, in E. globulus, changes in leaf N and Rubisco content were linearly related across the various treatments (Fig. 1A) with the leaf D1 content (an indicator of the amount of the thylakoid PSII complex) also shown to scale with leaf N within each canopy position (Fig. 1B). Consequently, changes in Rubisco and PSII contents in response to eC and eT were underpinned by generic shifts in leaf N.
Our findings indicate that the leaf CO2 assimilation rates in E. globulus grown under both aC and eC remained predominantly RuBP carboxylation limiting (Bowes, 1991; Sage, 1994; Moore et al., 1999; Rogers and Humphries, 2000; Rogers and Ellsworth, 2002). Under eC, leaf Rubisco content was reduced without compromising Vcmax25 (derived in vivo from gas exchange) or the short-term stimulation of photosynthetic rates in response to increased [CO2] determined at a common temperature (Asat25). The maintenance of Vcmax25 with less leaf Rubisco content may be due to increased Rubisco activation (i.e. the proportion of active/total Rubisco sites) at eC as noted in other studies using C3 species (Salvucci et al., 1986; Sage et al., 1989; Tissue et al., 1993). This finding is in line with the strong biomass stimulations observed for the E. globulus trees grown at eC (Quentin et al., 2015). In contrast, eT led to increased leaf [N] of upper canopy E. globulus leaves as well as a large increase in Vcmax25 (+40%) and Jmax25 (+30%), while Asat25 did not significantly vary between the temperature treatments. Hence, thermal acclimation expressed as up-regulation of photosynthetic proteins served to sustain photosynthetic rates of E. globulus at elevated temperature. This finding concurs with the lack of biomass stimulation observed in this species when grown under eT (Quentin et al., 2015). Moreover, in early spring when the current study was undertaken, leaf photosynthesis was operating near its thermal optimum, ~21 °C (Crous et al., 2013), which is much higher compared with its winter thermal optimum in its native range, ~16.5 °C (Battaglia et al., 1996). Consequently, short-term warming of +3 °C (in the absence of acclimation) may have reduced photosynthetic rates at ambient [CO2] by shifting the operating range beyond the thermal optimum. This type of acclimation which elicits a generic up-regulation of leaf N and photosynthetic proteins is described as ‘quantitative’ by Way and Sage (2008) and is contingent on the availability of N resources for increased investment in the photosynthetic apparatus.
The interactive effects of elevated [CO2] and temperature
The effects of eC and eT on photosynthetic components were additive and offsetting such that most measured parameters were similar between the aCaT and eCeT treatments, with the exception of Asat. Up-regulation of Vcmax and Jmax in response to eT served to alleviate the negative effects of short-term increases in temperature on photosynthesis when operating close to the thermal optimum. Elevated [CO2] shifted the temperature response of photosynthesis upwards and increased its thermal optimum due to increased RuBP carboxylation and decreased RuBP oxygenation (Long, 1991; also reported in Crous et al., 2013). In so doing, eC eliminated the need for the resource-expensive ‘quantitative’ acclimation response under eCeT; while allowing photosynthesis to respond positively to warming. Accordingly, the extent to which the effects of eC and eT on photosynthetic capacity cancel each other out depends on whether warming occurs towards or away from the photosynthetic thermal optimum, in addition to the plasticity of the plant to adjust their thermal optimum seasonally or in response to changes in growth conditions.
The influence of canopy position
Eucalyptus globulus trees examined in the current study were 1.5 years old, ~7 m tall, and with extensive amounts of juvenile foliage. Canopy position influenced leaf morphology and photosynthetic N allocation rather than leaf [N]M or photosynthetic rates. Relative to the lower canopy, the upper canopy in E. globulus allocated a greater proportion of their leaf N to soluble proteins, including Rubisco, and less to PSII (Table 2; Figs 1, 4). These differences are in line with the classical sun versus shade phenotypes observed across plant species (Boardman, 1977; Givnish, 1988). Similar results have been observed for other tree species whereby the positional effect is expressed at the level of photosynthetic N allocation to achieve similar photosynthetic rates (Ellsworth and Reich, 1993; Crous and Ellsworth, 2004; Kenzo et al., 2006; Turnbull et al., 2007; Ellsworth et al., 2012; Mao et al., 2012; Weerasinghe et al., 2014). Hence, clear positional effects on leaf traits were observed in E. globulus despite the fact that light distribution may be more diffuse within the WTC relative to the open air field (Medhurst et al., 2006; Barton et al., 2010).
One of the objectives of our study was to establish whether the photosynthetic response to eC and eT in E. globulus depended on canopy position. It has been hypothesized that within-canopy differences in the light environment may influence the response to eC (Kubiske et al., 2002) as a result of light-driven differences in photosynthetic N allocation (Evans, 1993; Hikosaka and Terashima, 1995). Although lower canopy leaves showed weaker responses to eC and eT relative to upper canopy leaves, there were no significant canopy×treatment interactions for any of the measured parameters (except for a significant canopy×temperature effect on LMA), probably due to the open crown structure in Eucalyptus species. In other experiments where there were large positional trends in leaf [N], the response to eC differed with canopy position (Herrick and Thomas, 1999; Crous and Ellsworth, 2004).
Conclusions
Rubisco catalytic parameters for E. globulus measured at 25 °C were similar to the widely used tobacco kinetics in C3 photosynthesis, and may provide a model for evergreen plantation trees, although caution is needed in the general applicability of these parameters across different taxa and temperatures (Sharwood et al., 2016). Characterization of Rubisco protein content and catalytic parameters enabled in vitro estimates of Vcmax that were consistent, although slightly lower, than in vivo rates extrapolated from gas exchange. In E. globulus, Vcmax can be predicted from leaf N and the Rubisco contents, while Jmax can also be estimated from Vcmax.
In response to eC, the leaves of E. globulus trees underwent a photosynthetic acclimation underpinned by down-regulation of leaf N and Rubisco contents that improved PNUE. In contrast, there was a generic up-regulation of photosynthetic proteins in eT via increased leaf [N]; this response could be key to the resumption of growth in spring, albeit at an added N cost. The ability of E. globulus leaves to allocate leaf N flexibly in response to environmental cues led to opposite and offsetting effects of eC and eT on photosynthetic capacity. Consequently, the biochemical balance of E. globulus leaves in the warmer elevated [CO2] treatment was not markedly different from that in the current climate. In contrast to the CO2 and temperature treatments, canopy position affected the allocation of leaf N to Rubisco and PSII proteins.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Determination of Rubisco integrity and extraction yield in E. globulus using Coomassie blue staining.
Fig. S2. Relative content per leaf area of the thylakoid complex PSII.
Supplementary Material
Acknowledgements
This is a contribution from the Hawkesbury Forest Experiment. We thank Burhan Amiji and Dr Craig Barton for their assistance in undertaking gas exchange. This research was supported by funding from ARC grant DP160102452, the Forest Industries Climate Change Research Fund from the Australian Department of Agriculture, and the Commonwealth Government through the Education Investment Fund. The loan of the whole-tree chambers from Sweden by Professor Sune Linder on behalf of SLU is greatly appreciated. KC and RS greatly acknowledge DECRA funding from the Australian Research Council.
References
- Adam NR, Wall GW, Kimball BA, et al. 2000. Acclimation response of spring wheat in a free-air CO(2) enrichment (FACE) atmosphere with variable soil nitrogen regimes. 1. Leaf position and phenology determine acclimation response. Photosynthesis Research 66, 65–77. [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Rogers A. 2007. The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant, Cell and Environment 30, 258–270. [DOI] [PubMed] [Google Scholar]
- Badger MR, von Caemmerer S, Ruuska S, Nakano H. 2000. Electron flow to oxygen in higher plants and algae: rates and control of direct photoreduction (Mehler reaction) and rubisco oxygenase. Philosophical Transactions of the Royal Society B: Biological Sciences 355, 1433–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton CVM, Ellsworth DS, Medlyn BE, et al. 2010. Whole-tree chambers for elevated atmospheric CO2 experimentation and tree scale flux measurements in south-eastern Australia: the Hawkesbury Forest Experiment. Agricultural and Forest Meteorology 150, 941–951. [Google Scholar]
- Battaglia M, Beadle C, Loughhead S. 1996. Photosynthetic temperature responses of Eucalyptus globulus and Eucalyptus nitens. Tree Physiology 16, 81–89. [DOI] [PubMed] [Google Scholar]
- Bernacchi CJ, Rosenthal DM, Pimentel C, Long SP, Farquhar GD. 2009. Modeling the temperature dependence of C3 photosynthesis. In: Laisk A, Nedbal L, Govindjee, eds. Photosynthesis in silico: understanding complexity from molecules to ecosystems. Berlin: Springer, 231–246. [Google Scholar]
- Boardman NK. 1977. Comparative photosynthesis of sun and shade plants. Annual Review of Plant Physiology and Plant Molecular Biology 28, 355–377. [Google Scholar]
- Bowes G. 1991. Growth at elevated CO2—photosynthetic responses mediated through Rubisco. Plant, Cell and Environment 14, 795–806. [Google Scholar]
- Crous KY, Ellsworth DS. 2004. Canopy position affects photosynthetic adjustments to long-term elevated CO2 concentration (FACE) in aging needles in a mature Pinus taeda forest. Tree Physiology 24, 961–970. [DOI] [PubMed] [Google Scholar]
- Crous KY, Quentin AG, Lin YS, Medlyn BE, Williams DG, Barton CVM, Ellsworth DS. 2013. Photosynthesis of temperate Eucalyptus globulus trees outside their native range has limited adjustment to elevated CO2 and climate warming. Global Change Biology 19, 3790–3807. [DOI] [PubMed] [Google Scholar]
- Crous KY, Walters MB, Ellsworth DS. 2008. Elevated CO(2) concentration affects leaf photosynthesis–nitrogen relationships in Pinus taeda over nine years in FACE. Tree Physiology 28, 607–614. [DOI] [PubMed] [Google Scholar]
- Drake BG, Gonzalez-Meler MA, Long SP. 1997. More efficient plants: a consequence of rising atmospheric CO2? Annual Review of Plant Physiology and Plant Molecular Biology 48, 609–639. [DOI] [PubMed] [Google Scholar]
- Ellsworth DS, Reich PB. 1993. Canopy structure and vertical patterns of photosynthesis and related leaf traits in a deciduous forest. Oecologia 96, 169–178. [DOI] [PubMed] [Google Scholar]
- Ellsworth DS, Reich PB, Naumburg ES, Koch GW, Kubiske ME, Smith SD. 2004. Photosynthesis, carboxylation and leaf nitrogen responses of 16 species to elevated pCO2 across four free-air CO2 enrichment experiments in forest, grassland and desert. Global Change Biology 10, 2121–2138. [Google Scholar]
- Ellsworth DS, Thomas R, Crous KY, Palmroth S, Ward E, Maier C, Delucia E, Oren R. 2012. Elevated CO2 affects photosynthetic responses in canopy pine and subcanopy deciduous trees over 10 years: a synthesis from Duke FACE. Global Change Biology 18, 223–242. [Google Scholar]
- Evans JR. 1989. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78, 9–19. [DOI] [PubMed] [Google Scholar]
- Evans JR. 1993. Photosynthetic acclimation and nitrogen partitioning within a lucerne canopy. 1. Canopy characteristics. Australian Journal of Plant Physiology 20, 55–67. [Google Scholar]
- Evans JR, Seemann JR. 1989. The allocation of nitrogen in the photosynthetic apparatus: costs, consequences and control. In: Briggs WR, ed. Photosynthesis. New York: Alan R. Liss Inc, 183–205. [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. 1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90. [DOI] [PubMed] [Google Scholar]
- Friend AD. 2010. Terrestrial plant production and climate change. Journal of Experimental Botany 61, 1293–1309. [DOI] [PubMed] [Google Scholar]
- Galmés J, Kapralov MV, Andralojc PJ, Conesa MÀ, Keys AJ, Parry MA, Flexas J. 2014. Expanding knowledge of the Rubisco kinetics variability in plant species: environmental and evolutionary trends. Plant, Cell and Environment 37, 1989–2001. [DOI] [PubMed] [Google Scholar]
- Galmes J, Flexas J, Keys AJ, Cifre J, Mitchell RAC, Madgwick PJ, Haslam RP, Medrano H, Parry MAJ. 2005. Rubisco specificity factor tends to be larger in plant species from drier habitats and in species with persistent leaves. Plant, Cell and Environment 28, 571–579. [Google Scholar]
- Ghannoum O, Conroy JP. 1998. Nitrogen deficiency precludes a growth response to CO2 enrichment in C3 and C4 Panicum grasses. Australian Journal of Plant Physiology 25, 627–636. [Google Scholar]
- Ghannoum O, Evans JR, Chow WS, Andrews TJ, Conroy JP, von Caemmerer S. 2005. Faster Rubisco is the key to superior nitrogen-use efficiency in NADP-malic enzyme relative to NAD-malic enzyme C4 grasses. Plant Physiology 137, 638–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannoum O, Phillips NG, Conroy JP, Smith RA, Attard RD, Woodfield R, Logan BA, Lewis JD, Tissue DT. 2010. a. Exposure to preindustrial, current and future atmospheric CO2 and temperature differentially affects growth and photosynthesis in Eucalyptus. Global Change Biology 16, 303–319. [Google Scholar]
- Ghannoum O, Phillips NG, Sears MA, Logan BA, Lewis JD, Conroy JP, Tissue DT. 2010. b. Photosynthetic responses of two eucalypts to industrial-age changes in atmospheric [CO2] and temperature. Plant, Cell and Environment 33, 1671–1681. [DOI] [PubMed] [Google Scholar]
- Givnish TJ. 1988. Adaptation to sun and shade—a whole plant perspective. Australian Journal of Plant Physiology 15, 63–92. [Google Scholar]
- Gunderson CA, O’Hara KH, Campion CM, Walker AV, Edwards NT. 2010. Thermal plasticity of photosynthesis: the role of acclimation in forest responses to a warming climate. Global Change Biology 16, 2272–2286. [Google Scholar]
- Hermida-Carrera C, Kapralov MV, Galmés J. 2016. Rubisco catalytic properties and temperature response in crops. Plant Physiology 171, 2549–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick JD, Thomas RB. 1999. Effects of CO2 enrichment on the photosynthetic light response of sun and shade leaves of canopy sweetgum (Liquidambar styraciflua) in a forest ecosystem. Tree Physiology 19, 779–786. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Ishikawa K, Borjigidai A, Muller O, Onoda Y. 2006. Temperature acclimation of photosynthesis: mechanisms involved in the changes in temperature dependence of photosynthetic rate. Journal of Experimental Botany 57, 291–302. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Terashima I. 1995. A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant, Cell and Environment 18, 605–618. [Google Scholar]
- Kane HJ, Viil J, Entsch B, Paul K, Morell MK, Andrews TJ. 1994. An improved method for measuring the CO2/O2 specificity of Ribulose bisphosphate carboxylase-oxygenase. Australian Journal of Plant Physiology 21, 449–461. [Google Scholar]
- Kapralov MV, Kubien DS, Andersson I, Filatov DA. 2011. Changes in Rubisco kinetics during the evolution of C4 photosynthesis in Flaveria (Asteraceae) are associated with positive selection on genes encoding the enzyme. Molecular Biology and Evolution 28, 1491–1503. [DOI] [PubMed] [Google Scholar]
- Kellomaki S, Wang KY. 1997. Effects of long-term CO2 and temperature elevation on crown nitrogen distribution and daily photosynthetic performance of Scots pine. Forest Ecology and Management 99, 309–326. [Google Scholar]
- Kenzo T, Ichie T, Watanabe Y, Yoneda R, Ninomiya I, Koike T. 2006. Changes in photosynthesis and leaf characteristics with tree height in five dipterocarp species in a tropical rain forest. Tree Physiology 26, 865–873. [DOI] [PubMed] [Google Scholar]
- Kosvancova M, Urban O, Sprtova M, Hrstka M, Kalina J, Tomaskova I, Spunda V, Marek MV. 2009. Photosynthetic induction in broadleaved Fagus sylvatica and coniferous Picea abies cultivated under ambient and elevated CO2 concentrations. Plant Science 177, 123–130. [Google Scholar]
- Kubiske ME, Zak DR, Pregitzer KS, Takeuchi Y. 2002. Photosynthetic acclimation of overstory Populus tremuloides and understory Acer saccharum to elevated atmospheric CO2 concentration: interactions with shade and soil nitrogen. Tree Physiology 22, 321–329. [DOI] [PubMed] [Google Scholar]
- Long SP. 1991. Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations—has its importance been underestimated. Plant, Cell and Environment 14, 729–739. [Google Scholar]
- Mao QZ, Watanabe M, Imori M, Kim YS, Kita K, Koike T. 2012. Photosynthesis and nitrogen allocation in needles in the sun and shade crowns of hybrid larch saplings: effect of nitrogen application. Photosynthetica 50, 422–428. [Google Scholar]
- McGuire AD, Sitch S, Clein JS, et al. 2001. Carbon balance of the terrestrial biosphere in the twentieth century: analyses of CO2, climate and land use effects with four process-based ecosystem models. Global Biogeochemical Cycles 15, 183–206. [Google Scholar]
- Medhurst J, Parsby J, Linder S, Wallin G, Ceschia E, Slaney M. 2006. A whole-tree chamber system for examining tree-level physiological responses of field-grown trees to environmental variation and climate change. Plant, Cell and Environment 29, 1853–1869. [DOI] [PubMed] [Google Scholar]
- Moore BD, Cheng SH, Sims D, Seemann JR. 1999. The biochemical and molecular basis for photosynthetic acclimation to elevated atmospheric CO2. Plant, Cell and Environment 22, 567–582. [Google Scholar]
- Nakano H, Makino A, Mae T. 1997. The effect of elevated partial pressures of CO2 on the relationship between photosynthetic capacity and N content in rice leaves. Plant Physiology 115, 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda Y, Hikosaka K, Hirose T. 2005a The balance between RuBP carboxylation and RuBP regeneration: a mechanism underlying the interspecific variation in acclimation of photosynthesis to seasonal change in temperature. Functional Plant Biology 32, 903–910. [DOI] [PubMed] [Google Scholar]
- Onoda Y, Hikosaka K, Hirose T. 2005b Seasonal change in the balance between capacities of RuBP carboxylation and RuBP regeneration affects CO2 response of photosynthesis in Polygonum cuspidatum. Journal of Experimental Botany 56, 755–763. [DOI] [PubMed] [Google Scholar]
- Orr DJ, Alcântara A, Kapralov MV, Andralojc PJ, Carmo-Silva E, Parry MA. 2016. Surveying Rubisco diversity and temperature response to improve crop photosynthetic efficiency. Plant Physiology 172, 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE. 1989. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophyll a and chlorophyll b extracted with 4 different solvents—verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochimica et Biophysica Acta 975, 384–394. [Google Scholar]
- Prins A, Orr DJ, Andralojc PJ, Reynolds MP, Carmo-Silva E, Parry MA. 2016. Rubisco catalytic properties of wild and domesticated relatives provide scope for improving wheat photosynthesis. Journal of Experimental Botany 67, 1827–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quentin AG, Crous KY, Barton CV, Ellsworth DS. 2015. Photosynthetic enhancement by elevated CO2 depends on seasonal temperatures for warmed and non-warmed Eucalyptus globulus trees. Tree Physiology 35, 1249–1263. [DOI] [PubMed] [Google Scholar]
- Rezende LF, Arenque BC, Aidar ST, Moura MS, Von Randow C, Tourigny E, Menezes RS, Ometto JP. 2016. Evolution and challenges of dynamic global vegetation models for some aspects of plant physiology and elevated atmospheric CO2. International Journal of Biometeorology 60, 945–955. [DOI] [PubMed] [Google Scholar]
- Rogers A. 2014. The use and misuse of Vcmax in earth system models. Photosynthesis Research 119, 15–29. [DOI] [PubMed] [Google Scholar]
- Rogers A, Ellsworth DS. 2002. Photosynthetic acclimation of Pinus taeda (loblolly pine) to long-term growth in elevated pCO2 (FACE). Plant, Cell and Environment 25, 851–858. [Google Scholar]
- Rogers A, Ellsworth DS, Humphries SW. 2001. Possible explanation of the disparity between the in vitro and in vivo measurements of Rubisco activity: a study in loblolly pine grown in elevated pCO2. Journal of Experimental Botany 52, 1555–1561. [DOI] [PubMed] [Google Scholar]
- Rogers A, Fischer BU, Bryant J, Frehner M, Blum H, Raines CA, Long SP. 1998. Acclimation of photosynthesis to elevated CO2 under low-nitrogen nutrition is affected by the capacity for assimilate utilization. Perennial ryegrass under free-air CO2 enrichment. Plant Physiology 118, 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A, Humphries SW. 2000. A mechanistic evaluation of photosynthetic acclimation at elevated CO2. Global Change Biology 6, 1005–1011. [Google Scholar]
- Ruuska S, Andrews TJ, Badger MR, Hudson GS, Laisk A, Price GD, von Caemmerer S. 1998. The interplay between limiting processes in C3 photosynthesis studied by rapid-response gas exchange using transgenic tobacco impaired in photosynthesis. Australian Journal of Plant Physiology 25, 859–870. [Google Scholar]
- Sage RF. 1994. Acclimation of photosynthesis to increasing atmospheric CO2: the gas exchange perspective. Photosynthesis Research 39, 351–368. [DOI] [PubMed] [Google Scholar]
- Sage RF, Pearcy RW, Seemann JR. 1987. The nitrogen use efficiency of C3 and C4 plants: III. Leaf nitrogen effects on the activity of carboxylating enzymes in Chenopodium album (L.) and Amaranthus retroflexus (L.). Plant Physiology 85, 355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Sharkey TD, Seemann JR. 1989. Acclimation of photosynthesis to elevated CO2 in five C3 species. Plant Physiology 89, 590–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci ME, Portis AR, Ogren WL. 1986. Light and CO2 response of ribulose-1,5-bisphosphate carboxylase/oxygenase activation in Arabidopsis leaves. Plant Physiology 80, 655–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL. 2007. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant, Cell and Environment 30, 1035–1040. [DOI] [PubMed] [Google Scholar]
- Sharwood RE, Ghannoum O, Kapralov MV, Gunn LH, Whitney SM. 2016. Temperature responses of Rubisco from Paniceae grasses provide opportunities for improving C3 photosynthesis. Nature Plants 2, 16186. [DOI] [PubMed] [Google Scholar]
- Sharwood RE, von Caemmerer S, Maliga P, Whitney SM. 2008. The catalytic properties of hybrid Rubisco comprising tobacco small and sunflower large subunits mirror the kinetically equivalent source Rubiscos and can support tobacco growth. Plant Physiology 146, 83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin GL, George L, Kannangara K, Tissue DT, Ghannoum O. 2013. Impact of industrial-age climate change on the relationship between water uptake and tissue nitrogen in eucalypt seedlings. Functional Plant Biology 40, 201–212. [DOI] [PubMed] [Google Scholar]
- Sitch S, Smith B, Prentice IC, et al. 2003. Evaluation of ecosystem dynamics, plant geography and terrestrial carbon cycling in the LPJ dynamic global vegetation model. Global Change Biology 9, 161–185. [Google Scholar]
- Taub DR, Wang X. 2008. Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. Journal of Integrative Plant Biology 50, 1365–1374. [DOI] [PubMed] [Google Scholar]
- Terashima I, Evans JR. 1988. Effects of light and nitrogen nutrition on the organization of the photosynthetic apparatus in spinach. Plant and Cell Physiology 29, 143–155. [DOI] [PubMed] [Google Scholar]
- Teskey RO, Will RE. 1999. Acclimation of loblolly pine (Pinus taeda) seedlings to high temperatures. Tree Physiology 19, 519–525. [DOI] [PubMed] [Google Scholar]
- Theobald JC, Mitchell RA, Parry MA, Lawlor DW. 1998. Estimating the excess investment in ribulose-1,5-bisphosphate carboxylase/oxygenase in leaves of spring wheat grown under elevated CO2. Plant Physiology 118, 945–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissue DT, Thomas RB, Strain BR. 1993. Long-term effects of elevated CO2 and nutrients on photosynthesis and rubisco in loblolly pine seedlings. Plant, Cell and Environment 16, 859–865. [Google Scholar]
- Tjoelker MG, Oleksyn J, Reich PB. 1998. Seedlings of five boreal tree species differ in acclimation of net photosynthesis to elevated CO(2) and temperature. Tree Physiology 18, 715–726. [DOI] [PubMed] [Google Scholar]
- Turnbull TL, Kelly N, Adams MA, Warren CR. 2007. Within-canopy nitrogen and photosynthetic gradients are unaffected by soil fertility in field-grown Eucalyptus globulus. Tree Physiology 27, 1607–1617. [DOI] [PubMed] [Google Scholar]
- Urban O, Hrstka M, Zitová M, Holišová P, Sprtová M, Klem K, Calfapietra C, De Angelis P, Marek MV. 2012. Effect of season, needle age and elevated CO2 concentration on photosynthesis and Rubisco acclimation in Picea abies. Plant Physiology and Biochemistry 58, 135–141. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Quick WP. 2000. Rubisco: physiology in vivo. In: Leegood RC, Sharkey DT, von Caemmerer S, eds. Photosynthesis: physiology and metabolism. Dordrecht: Kluwer Academic Publishers, 85–113. [Google Scholar]
- Walker AP, Beckerman AP, Gu L, et al. 2014. The relationship of leaf photosynthetic traits—Vcmax and Jmax—to leaf nitrogen, leaf phosphorus, and specific leaf area: a meta-analysis and modeling study. Ecology and Evolution 4, 3218–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B, Ariza LS, Kaines S, Badger MR, Cousins AB. 2013. Temperature response of in vivo Rubisco kinetics and mesophyll conductance in Arabidopsis thaliana: comparisons to Nicotiana tabacum. Plant, Cell and Environment 36, 2108–2119. [DOI] [PubMed] [Google Scholar]
- Wang D, Heckathorn SA, Wang X, Philpott SM. 2012. A meta-analysis of plant physiological and growth responses to temperature and elevated CO2. Oecologia 169, 1–13. [DOI] [PubMed] [Google Scholar]
- Warren CR, Chen Z-L, Adams MA. 2000. Effect of N source on concentration of Rubisco in Eucalyptus diversicolor, as measured by capillary electrophoresis. Physiologia Plantarum 110, 52–58. [Google Scholar]
- Way DA, Sage RF. 2008. Thermal acclimation of photosynthesis in black spruce [Picea mariana (Mill.) B.S.P.]. Plant, Cell and Environment 31, 1250–1262. [DOI] [PubMed] [Google Scholar]
- Weerasinghe LK, Creek D, Crous KY, Xiang S, Liddell MJ, Turnbull MH, Atkin OK. 2014. Canopy position affects the relationships between leaf respiration and associated traits in a tropical rainforest in Far North Queensland. Tree Physiology 34, 564–584. [DOI] [PubMed] [Google Scholar]
- Whitney SM, Houtz RL, Alonso H. 2011a Advancing our understanding and capacity to engineer nature’s CO2-sequestering enzyme, Rubisco. Plant Physiology 155, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney SM, Sharwood RE, Orr D, White SJ, Alonso H, Galmes J. 2011b Isoleucine 309 acts as a C4 catalytic switch that increases ribulose-1,5-bisphosphate carboxylase/oxygenase (rubisco) carboxylation rate in Flaveria. Proceedings of the National Academy of Sciences, USA 108, 14688–14693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger SD. 1993. Biochemical limitations to carbon assimilation in C3 plants—a retrospective analysis of the A/Ci curves from 109 species. Journal of Experimental Botany 44, 907–920. [Google Scholar]
- Zhang F, Chen JM, Chen J, Gough CM, Martin TA, Dragoni D. 2012. Evaluating spatial and temporal patterns of MODIS GPP over the conterminous U.S. against flux measurements and a process model. Remote Sensing of Environment 124, 717–729. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.