Evolutionary developmental biology (evo-devo) deals with the identification of genetic events underlying the evolution of morphological diversity – a complex task. A new paper in Journal of Experimental Botany by Tai et al. (pages 403–414) provides evidence that evolutionarily young non-syntenic genes were involved in the appearance of seminal roots in the maize evolutionary lineage.
From a strictly developmental perspective, and ignoring environmental effects, a morphological feature (such as an organ) is the result of developmental events that initially occur at the level of one or a few cells. These cells integrate external and endogenous signals, and then the first step on the developmental pathway is a change in gene expression. This is followed by division, growth, differentiation, and/or programmed cell death. Resolving the appearance of a morphological trait from an evolutionary developmental (evo-devo) perspective remains a more elusive proposition (Carroll, 2008; de Bruijn et al., 2012). In this case, the genetic events (mutations) which occurred in the evolutionary lineages and led to large developmental changes have to be identified and arranged in temporal order. The same events should also be considered in terms of natural selection and genetic drift in order to explain their diffusion into the population. In any case, it is well recognized that the molecular genetics of the developmental processes under investigation should first be fully deciphered (Carroll, 2008).
The development of a specific root-type in maize – the seminal roots (Box 1) – is a useful model in an evo-devo context for a number of reasons. First, seminal roots evolved in the teosinte (the wild ancestor of cultivated maize)/maize lineage quite recently and after divergence from sorghum, which is closely related but lacks this type of root (Singh et al., 2010). Second, high-quality genome sequences (Paterson et al., 2009; Schnable et al., 2009) and well-described synteny relationships (Wei et al., 2007) are available for both species, which makes it possible to use comparative genomics to identify mutations of all types that occurred after the two species diverged.
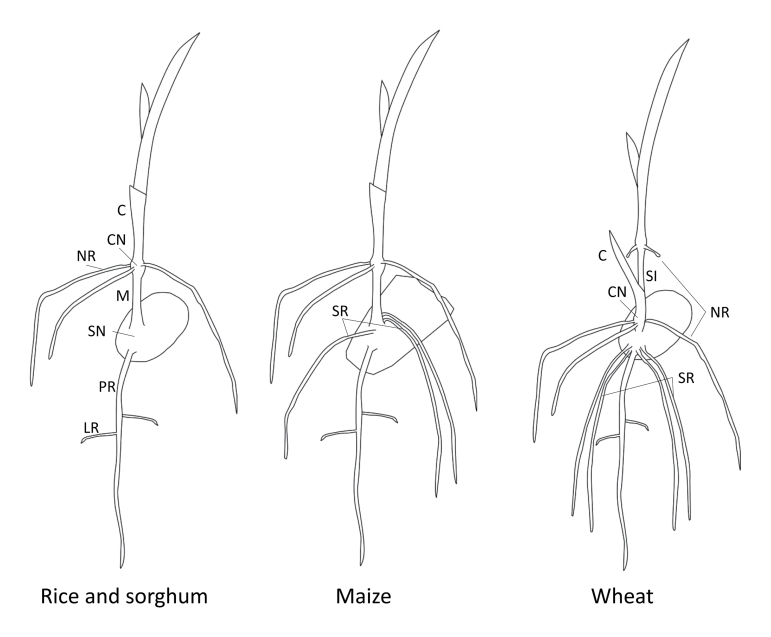
Box 1. Schematic representation of embryonic and early post-embryonic root systems of maize, rice, sorghum and wheat
In the four species, a distinguishable primary root (PR) is the first root to emerge at the base of the embryo. Overall, rice and sorghum have a similar architecture with the first nodal roots (NR) or crown roots (also called ‘embryonic’ crown roots in rice; Rebouillat et al., 2009) developing at the coleoptilar node (CN). Maize shares the same architecture but develops a variable number (0–13; Hochholdinger, 2009) of seminal roots (SR), which emerge from the scutellar node (SN) both dorsally and ventrally relative to the shoot (Salvi et al., 2016). Four to five seminal roots also develop in wheat, next to its primary root. Rice, sorghum and maize share a variably elongated mesocotyl (M), which is anatomically a root–stem hybrid structure connecting the scutellum with the coleoptilar node. In these species, mesocotyl elongation pushes the young shoot tip to emerge from the soil. In wheat, instead, it is the first internode (also called the subcrown internode, SI) which usually elongates. For all species, a large number of nodal (crown) roots develop at the basal nodes of the stem later in development (not shown, with the exception of the first emerging nodal roots in wheat). All types of root discussed above develop lateral roots (LR). C, coleoptile.
The teosinte/maize and sorghum lineages diverged about 12 million years ago from a ten-chromosome ancestor, and between 12 and 5 million years ago a tetraploidization – a whole-genome duplication either by auto or allopolyploidy (Schnable et al., 2009) – occurred in the teosinte/maize lineage only. This event was followed by a massive genome rearrangement which eventually resulted, again, in a ten-chromosome genome where two subgenomes can still be distinguished (Wei et al., 2007). At the same time, the genome also underwent a significant expansion caused by massive transposon activity (SanMiguel et al., 1998). Altogether, this provides the perfect scenario for an evo-devo type of investigation.
Developmental genetics of the maize root system
Maize has a complex root system including a primary root (or radicle) that develops directly from the basal end of the embryo, seminal roots which are initiated from the scutellar node, and nodal (or shoot-borne) roots (called crown or brace, if below or above the soil level, respectively; see Box 1 in Hochholdinger, 2016) developing from the lower nodes of the stem. All these roots also carry lateral roots. Depending on the genotype and growing conditions, primary and seminal roots may persist throughout the life cycle of the plant or die as the seedling develops shoot-borne roots (Hochholdinger and Tuberosa, 2009). The maize mutants rootless concerning crown and seminal roots (rtcs) and rootless with undetectable meristems 1 (rum1) (both involved in auxin signalling) lack seminal root initiation but not in an organ-specific fashion: rtcs also lacks shoot-borne roots, whereas rum1 does not show any lateral root development from the primary root in addition to the seminal root defect (Hochholdinger, 2009). Quantitative trait loci (QTLs) for seminal root number and architecture have been mapped in correspondence with these two loci and in other genome locations (Kumar et al., 2012; Zhang et al., 2013; Salvi et al., 2016).
Variation for seminal root traits has adaptive potential. Different seminal root architectures appear to affect early crop establishment and P acquisition (Zhu et al., 2005; Lynch, 2013; Mi et al., 2016) (see also Gao and Lynch, 2016; Hochholdinger, 2016). Seminal roots also have unique anatomical features which are suggestive of higher absorption efficiency by comparison with other root types (Tai et al., 2016). Thus, it is not surprising that there may have been positive selection for number of seminal roots during maize domestication and improvement (Burton et al., 2013).
Evo-devo mechanisms behind the appearance of seminal roots in maize
An important observation presented by Tai et al. (2017) sheds light on the genetic and regulatory mechanisms involved in the evolution of seminal roots in maize. The authors ran a transcriptome analysis (RNA-seq) on histological samples of wild-type and rtcs-mutant (deficient in seminal roots) embryos about a month after pollination (thus before seed dormancy). It is at this stage that seminal root primordia are initiated, right in the centre of the embryo, on the axis that connects the shoot apical and primary root meristems, in the scutellar node region (Box 1). More than 3000 differentially expressed genes were identified between wild-type and rtcs samples. Quite unexpectedly, these genes were significantly enriched with evolutionarily young genes. Therefore, given that seminal roots are a recent and specific acquisition of the teosinte/maize evolutionary lineage, Tai et al. (2017) suggest that there is a causal connection between the young genes and the presence of seminal roots.
But what exactly are evolutionarily young genes? In this context, these are non-syntenic genes, namely genes that lie away from their expected chromosome positions in the reconstructed gene order of the evolutionary ancestor. In the comparison between the two maize subgenomes and sorghum, non-syntenic genes amount to 40–50% of the maize transposon-filtered gene set (Schnable et al., 2011; Schnable, 2015). The most likely explanation for their current position is that they were inserted recently (after the divergence of the teosinte/maize lineage from the sorghum one and after the maize-specific whole-genome duplication), by a copy-and-paste mechanism linked with transposon movement (Schnable, 2015). Tai et al. (2017) suggest that the genes currently acting downstream of the key root development transcription factor RTCS were recruited to the RTCS regulatory network from such non-syntenic young genes. At these genes, imperfect duplication events and other mutations probably caused sufficient reshuffling of regulatory elements (including RTCS binding sites) and/or coding properties that led to novel expression patterns and eventually to the initiation of seminal roots at the scutellum node. All this was probably soon reinforced by positive natural selection, given the adaptive value of seminal roots. The same authors proposed a similar hypothesis in a different paper in 2016 (Tai et al., 2016), where they had observed that an Aux/IAA gene (ZmIAA33), a non-syntenic homologue of the seminal root gene rum1, was highly and specifically expressed in seminal roots.
In order to test this hypothesis, additional evidence could be gathered through genomic and morphological investigations in Tripsacum, which is the only other genus in the teosinte/maize (Zea) lineage. Tripsacum and Zea shared the same whole-genome duplication but subsequently had different histories of genomic rearrangement (Schnable, 2015). It would also be interesting to clone QTLs for number of seminal roots in maize (natural variation for this trait goes from none to more than ten: Hochholdinger, 2009). This should tell us if the variation within species (maize) and between species (maize vs sorghum) shares the same molecular basis.
The relationship between non-syntenic genes and QTLs
The hypothesis proposed by Tai and co-authors is intriguing because most non-syntenic genes have been suggested to have little or no biological function (Schnable, 2015). Indeed, Schnable and Freeling (2011) observed that non-syntenic genes are nine times less likely to represent the causal loci behind mutations identified by classical genetics than syntenic genes. The only case in which allelic variation at non-syntenic genes has been shown to cause strong phenotypes is in relation to disease resistance. Disease resistance genes are overrepresented among genes found at non-syntenic locations among plants, as shown in a re-sequencing project of multiple rice genomes (Xu et al., 2011). Notably, the Fusarium-resistance gene recently cloned in wheat is non-syntenic (Rawat et al., 2016).
These observations could also contribute to the interpretation of the molecular nature of QTLs. Most QTLs in a QTLome (the whole set of QTLs for a given trait in a given species; Salvi and Tuberosa, 2015) have subtle quantitative effects on phenotypes. The prevailing interpretation is that strong unfavourable QTL alleles are quickly purged by selection (natural or artificial), or quickly fixed if they have favourable effects (discussed in Salvi and Tuberosa, 2015). Thus, strong-effect QTLs are rare. When found, they have often been shown to correspond to genes already known based on classical mutations. This connection is also known as Robertson’s hypothesis, from its initial proposer (Robertson, 1985). The combination of the observation of Schnable and Freeling (2011) with Robertson’s hypothesis (1985) suggests that strong-effect QTLs should most frequently correspond to syntenic genes, whereas weaker-effect QTLs should correspond to non-syntenic ones, such as the genes identified in Tai et al. (2017). This is a testable hypothesis in the current era of high-throughput, high-resolution QTLome mapping, and the result could be useful in cloning genes of agronomic value.
References
- Burton AL, Brown KM, Lynch JP. 2013. Phenotypic diversity of root anatomical and architectural traits in Zea species. Crop Science 53, 1042–1055. [Google Scholar]
- Carroll SB. 2008. Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell 134, 25–36. [DOI] [PubMed] [Google Scholar]
- de Bruijn S, Angenent GC, Kaufmann K. 2012. Plant ‘evo-devo’ goes genomic: from candidate genes to regulatory networks. Trends in Plant Science 17, 441–447. [DOI] [PubMed] [Google Scholar]
- Gao Y, Lynch JP. 2016. Reduced crown root number improves water acquisition under water deficit stress in maize (Zea mays L.). Journal of Experimental Botany 67, 4545–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochholdinger F. 2009. The maize root system: morphology, anatomy, and genetics. In: Bennetzen J, Hake S, eds, Handbook of maize: its biology, Springer: New York, 145–160. [Google Scholar]
- Hochholdinger F. 2016. Untapping root system architecture for crop improvement. Journal of Experimental Botany 67, 4431–4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochholdinger F, Tuberosa R. 2009. Genetic and genomic dissection of maize root development and architecture. Current Opinion in Plant Biology 12, 172–177. [DOI] [PubMed] [Google Scholar]
- Kumar B, Abdel-Ghani AH, Reyes-Matamoros J, Hochholdinger F, Luebberstedt T. 2012. Genotypic variation for root architecture traits in seedlings of maize (Zea mays L.) inbred lines. Plant Breeding 131, 465–478. [Google Scholar]
- Lynch JP. 2013. Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Annals of Botany 112, 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi G, Chen F, Yuan L, Zhang F. 2016. Ideotype root system architecture for maize to achieve high yield and resource use efficiency in intensive cropping systems. Advances in Agronomy 139, 73–97. [Google Scholar]
- Paterson AH, Bowers JE, Bruggmann R, et al. 2009. The Sorghum bicolor genome and the diversification of grasses. Nature 457, 551–556. [DOI] [PubMed] [Google Scholar]
- Rawat N, Pumphrey MO, Liu S, et al. 2016. Wheat Fhb1 encodes a chimeric lectin with agglutinin domains and a pore-forming toxin-like domain conferring resistance to Fusarium head blight. Nature Genetics 48, 1576–1580. [DOI] [PubMed] [Google Scholar]
- Rebouillat J, Dievart A, Verdeil JL, Escoute J, Giese G, Breitler JC, Gantet P, Espeout S, Guiderdoni E, Périn C. 2009. Molecular genetics of rice root development. Rice 2, 15–34. [Google Scholar]
- Robertson DS. 1985. A possible technique for isolating genic DNA for quantitative traits in plants. Journal of Theoretical Biology 117, 1–10. [Google Scholar]
- Salvi S, Giuliani S, Ricciolini C, Carraro N, Maccaferri M, Presterl T, Ouzunova M, Tuberosa R. 2016. Two major quantitative trait loci controlling the number of seminal roots in maize co-map with the root developmental genes rtcs and rum1 . Journal of Experimental Botany 67, 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi S, Tuberosa R. 2015. The crop QTLome comes of age. Current Opinion in Biotechnology 32, 179–185. [DOI] [PubMed] [Google Scholar]
- SanMiguel P, Gaut BS, Tikhonov A, Nakajima Y, Bennetzen JL. 1998. The paleontology of intergene retrotransposons of maize. Nature Genetics 20, 43–45. [DOI] [PubMed] [Google Scholar]
- Schnable JC. 2015. Genome evolution in maize: from genomes back to genes. Annual Review of Plant Biology 66, 329–343. [DOI] [PubMed] [Google Scholar]
- Schnable JC, Freeling M. 2011. Genes identified by visible mutant phenotypes show increased bias toward one of two subgenomes of maize. PLOS ONE 6, e17855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnable JC, Springer NM, Freeling M. 2011. Differentiation of the maize subgenomes by genome dominance and both ancient and ongoing gene loss. Proceedings of the National Academy of Sciences, USA 108, 4069–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnable PS, Ware D, et al. 2009. The B73 maize genome: complexity, diversity, and dynamics. Science 326, 1112–1115. [DOI] [PubMed] [Google Scholar]
- Singh V, van Oosterom EJ, Jordan DR, Messina CD, Cooper M, Hammer GL. 2010. Morphological and architectural development of root systems in sorghum and maize. Plant and Soil 333, 287–299. [Google Scholar]
- Tai H, Lu X, Opitz N, Marcon C, Paschold A, Lithio A, Nettleton D, Hochholdinger F. 2016. Transcriptomic and anatomical complexity of primary, seminal, and crown roots highlight root type-specific functional diversity in maize (Zea mays L.). Journal of Experimental Botany 67, 1123–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai H, Opitz N, Lithio A, Lu X, Nettleton D, Hochholdinger F. 2017. Non-syntenic genes drive RTCS-dependent regulation of the embryo transcriptome during formation of seminal root primordia in maize (Zea mays L.). Journal of Experimental Botany 68, 403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Coe ED, Nelson W, et al. 2007. Physical and genetic structure of the maize genome reflects its complex evolutionary history. PLoS Genetics 3, e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Liu X, Ge S, et al. 2011. Resequencing 50 accessions of cultivated and wild rice yields markers for identifying agronomically important genes. Nature Biotechnology 30, 105–111. [DOI] [PubMed] [Google Scholar]
- Zhang E, Yang Z, Wang Y, Hu Y, Song X, Xu C. 2013. Nucleotide polymorphisms and haplotype diversity of RTCS gene in China elite maize inbred lines. PLoS One 8, e56495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Kaeppler SM, Lynch JP. 2005. Topsoil foraging and phosphorus acquisition efficiency in maize (Zea mays). Functional Plant Biology 32, 749–762. [DOI] [PubMed] [Google Scholar]