Abstract
T cells engineered to express CD19-specific chimeric antigen receptors (CARs) have shown breakthrough clinical successes in patients with B-cell lymphoid malignancies. However, similar therapeutic efficacy of CAR T cells in solid tumors is yet to be achieved. In this study we systematically evaluated a series of CAR constructs targeting glypican-3 (GPC3), which is selectively expressed on several solid tumors. We compared GPC3-specific CARs that encoded CD3ζ (Gz) alone or with costimulatory domains derived from CD28 (G28z), 4-1BB (GBBz), or CD28 and 4-1BB (G28BBz). All GPC3-CARs rendered T cells highly cytotoxic to GPC3-positive hepatocellular carcinoma, hepatoblastoma, and malignant rhabdoid tumor cell lines in vitro. GBBz induced the preferential production of Th1 cytokines (interferon γ/granulocyte macrophage colony-stimulating factor) while G28z preferentially induced Th2 cytokines (interleukin-4/interleukin-10). Inclusion of 4-1BB in G28BBz could only partially ameliorate the Th2-polarizing effect of CD28. 4-1BB induced superior expansion of CAR T cells in vitro and in vivo. T cells expressing GPC3-CARs incorporating CD28, 4-1BB, or both induced sustained tumor regressions in two xenogeneic tumor models. Thus, GBBz CAR endows T cells with superior proliferative potential, potent antitumor activity, and a Th1-biased cytokine profile, justifying further clinical development of GBBz CAR for immunotherapy of GPC3-positive solid tumors.
Keywords: : glypican-3, chimeric antigen receptor, rhabdoid tumor, hepatoblastoma, hepatocellular carcinoma, T-cell therapy, immunotherapy
Introduction
Chimeric antigen receptors (CARs) are hybrid molecules that combine antigen-binding properties of monoclonal antibodies (mAb) and signaling elements of lymphocyte activating molecules. CARs commonly consist of an antigen-specific single chain variable fragment (scFv) derived from a mAb, a spacer, a transmembrane domain, and an endodomain containing signaling domains derived from CD3 zeta (CD3ζ) and costimulatory molecules such as CD28 or/and 4-1BB.1,2 T cells genetically engineered to express CARs specific for CD19 have shown breakthrough clinical successes in patients with B-cell lymphoid malignancies in recent years.3–9 The technology holds great promise for other types of cancer, including solid tumors for which conventional cytoreductive therapies often fail.10 However, the early clinical testing of CAR T cells against solid tumors has thus far benefited only a small fraction of patients,11–18 highlighting the need to explore novel antigens and to optimize antigen-specific CAR design.
Glypican-3 (GPC3), a membrane-bound proteoglycan, is expressed in several solid tumors including hepatocellular carcinoma (HCC), hepatoblastoma, embryonal sarcoma, malignant rhabdoid tumor (MRT), yolk sac tumor, Wilm's tumor, squamous cell carcinoma of the lung, and liposarcoma.19–29 GPC3 is an attractive target for immunotherapy since it is not expressed at detectable levels in nonmalignant tissues including normal or cirrhotic liver.19–22 In addition, GPC3 expression in HCC is associated with significantly worse prognosis even after complete tumor removal, likely due to GPC3’s ability to enhance wnt signaling and stimulate tumor growth.30–37 Two recent early phase clinical trials tested a GPC3-specific GC33 mAb and demonstrated that targeting GPC3 is safe, well tolerated and, depending on the density of GPC3 expression on tumor cells, can achieve significant antitumor responses in patients with advanced HCC.38,39
Unlike clinically tested CD19-CARs, which contained either CD28 or 4-1BB co-stimulatory endodomains, the only GPC3-CAR construct reported contained both CD28 and 4-1BB endodomains.19,40 The inclusion of two endodomains in GPC3-CAR may be necessary, as in contrast to B-cell malignancies, solid tumors generally express little or no costimulatory ligands for T cells.41 However, relative contribution of CD28 and 4-1BB in the effector functionality of GPC3-CAR T cells remains unknown and the clear benefit of their combined expression has not been experimentally established.42–45 In this study, we systematically evaluated the antitumor properties of T cells expressing GPC3-CARs encoding CD28, 4-1BB, either endodomain, or none. In addition to using a well-established HCC tumor model for evaluating GPC3-CARs, for the first time we tested the antitumor potential of GPC3-CAR T cells against MRT. We found that the costimulatory endodomain composition of the CARs has significant effect on the cytokine polarization in T cells and 4-1BB induces a Th1 polarized profile. We show that the inclusion of 4-1BB endodomain alone in GPC3-CAR is sufficient to generate T cells with enhanced proliferation, in vivo persistence, and potent therapeutic activity in xenogeneic tumor models.
Materials and Methods
Cell lines
HepG2, Hep3B, A549, G401, and HEK 293T cell lines were purchased from the American Type Culture Collection (Manassas, VA). Huh-7 was a kind gift from Dr. Xiao-Tong Song (Baylor College of Medicine, Houston, TX), and its identity was confirmed at the Characterized Cell Line Core Facility at MD Anderson Cancer Center (Houston, TX) by short-tandem repeat method. Cell lines were maintained according to the manufacturer's manual. Huh-7 and G-401 cells expressing an enhanced green fluorescent protein/firefly luciferase fusion gene (eGFP.Ffluc)46 were generated by single cell cloning after transduction with a retrovirus encoding eGFP.Ffluc. A549 cells expressing GPC3 (A549.GPC3) were generated by transducing wild type A549 with a retrovirus encoding GPC3.
Generation of retroviral constructs
A codon-optimized gene was synthesized by GeneArt® (Thermo Fisher Scientific, Waltham, MA) encoding the GPC3-specific scFv from GC33,47 and subcloned in frame into retroviral vectors containing expression cassettes encoding an IgG1 short hinge, a CD28 transmembrane domain, and CD3ζ, CD28ζ, 4-1BBζ, or CD28.4-1BBζ signaling domains (Fig. 1). The sequence of each cloned CAR was verified by sequencing (Seqwright, Houston, TX). In addition to nontransduced (NT) T cells, disialoganglioside (GD2)-specific CAR derived from 14g2a.scFv and CD19-specific CAR from FMC63.scFv containing CD3ζ and CD28.4-1BB costimulatory endodomains were used as negative controls.48,49
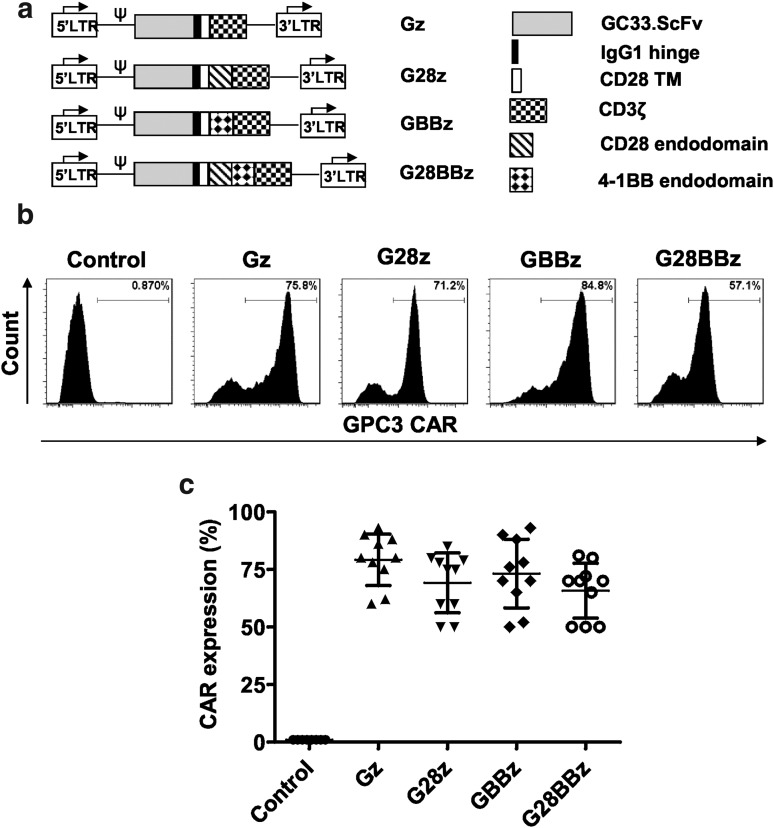
Figure 1.
Generation of glypican-3 chimeric antigen receptor (GPC3-CAR) T cells. The GPC3-specific single chain variable fragment (scFv) derived from GC33 monoclonal antibodies was cloned in frame into retroviral vectors encoding the immunoglobin 1 (IgG1) short hinge, the CD28 transmembrane domain and a CD3ζ signaling domain with or without the costimulatory endodomains derived from CD28, 4-1BB, or their combination. (a) Schematic map of CAR constructs. (b, c) GPC3-CAR cell surface expression determined by flow cytometry for 1 representative donor and summary data for 10 independent donors (mean and standard deviation [SD]: Gz, 79.2 ± 11.13; G28z, 69.2 ± 12.97; GBBz, 73.2 ± 14.9; G28BBz, 65.8 ± 11.9). Parental T cells are shown as controls. No difference was detected between the expression levels of GPC3 CARs (analysis of variance [ANOVA]).
Generation of CAR T cells
Retroviral supernatants were produced by transient transfection of HEK 293T cells with plasmids containing one of GPC3-CARs, RDF plasmid encoding the RD114 envelope and PegPam3 plasmid encoding the MoMLV gag-pol as previously described.50 Human peripheral blood mononuclear cells isolated from healthy volunteer donors (Gulf Coast Regional Blood Center, Houston, TX) were stimulated with OKT-3/CD28 mAb–coated plates for 48 h in complete RPMI medium (HyClone RPMI 1640, 10% heat inactivated fetal bovine serum and 2 mM Glutamax) with interleukin 7 (IL-7; 10 ng/mL) (PeproTech, Rocky Hill, NJ), and IL-15 (5 ng/mL) (Peprotech). IL-7 and IL-15 were used to optimize CAR T cell expansion.51 After 48 h of stimulation, cells were transduced on Retronectin (Kusatsu, Japan)–coated and retroviral particle–loaded plates and after 48 h cells were removed, washed, and cultured in IL-7 and IL-15 containing complete RPMI media for further expansion.
Flow cytometry
For all flow cytometry analyses, FACSArray or LSR-II instruments were used (BD Biosciences, Franklin Lakes, NJ). Results were analyzed by FlowJo (FlowJo, LLC; Ashland, OR). GPC3-CAR expression was detected by anti-F(ab)2 Alexa Fluor 647–conjugated antibody (Jackson ImmunoResearch, Cat No. 115-605-006) and anti-goat IgG1 isotype control (Jackson ImmunoResearch, Cat #: 115-605-006). GPC3 expression of tumor cell lines was detected with YP7 mAb 52 provided by Mitchell Ho (National Institutes of Health, Bethesda, MD).
Cytotoxicity assay
The ability of GPC3-CAR T cells to kill GPC3-positive tumor cells in vitro was tested in a standard 4-h chromium 51 (51Cr) release assay as previously described.53 In brief, target cells were loaded with 51Cr for 1 h, washed three times, and mixed with effector cells in 96-well plates at various effector to target ratios. Supernatant was collected after 4 h of incubation and radioactivity was measured to determine specific cytotoxicity.
Multiplex cytokine quantification
Resting GPC3-CAR T cells were co-cultured at a 1:1 ratio with target cells (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/hum) and supernatant was collected at 24 h. Samples were analyzed with Human Cytokine/Chemokine Immunoassay Kit (Millipore, Billerica, MA) according to the manufacturer's manual.
In vitro serial killing and CAR T cell proliferation assay
Tumor cells and effector cells were cocultured at a 1:1 ratio in complete RPMI without cytokines. Effector cells were counted and re-plated with fresh tumor cells every 3 days in fresh media. Carboxyfluorescein succinimidyl ester (Invitrogen, Carlsbad CA) dilution was measured on day 3 after first stimulation, as described by Hawkins et al.54, and 7-amino-actynomycin (BD Biosciences) staining was performed on day 7 according to the manufacturer's manual.
Animal models
NOD/SCID/IL2γnull (NSG mice, The Jackson Laboratory) were maintained at the Small Animal Core Facility of Texas Children's Hospital and were treated according to the protocols approved by Baylor College of Medicine's Institutional Biosafety Committee and Institutional Animal Care and Use Committee. Twelve-week-old, gender-matched NSG mice were injected with different tumor cell lines intraperitoneally (IP) respectively to generate the tumor-bearing model. To determine the in vivo proliferation of GPC3-CAR T cells and controls, T cells were cotransduced with CAR and eGFP.Ffluc46 constructs and intravenously (IV) injected into Huh-7 tumor–bearing mice followed by bioluminescence imaging every other day. To determine the antitumor potential of GPC3-CAR T cells, mice were injected with eGFP.Ffluc expressing Huh-7 (2 × 106 per mouse), G401 (5 × 106 per mouse), A54955 (2 × 106 per mouse) tumor cells followed by the IV injection of T cells 5 days (A549), 2 weeks (Huh-7) or 3 weeks (G401) later, respectively. Tumor bioluminescence was measured by weekly imaging and survival was documented for each group.
Statistical analyses
Descriptive analysis was performed to summarize cytokine production and CAR expression on T cells. Plots of growth curves were generated to visually illustrate patterns of survival and expansion of CAR T cells. Area under the curve (AUC) values for CAR T-cell frequencies were calculated using trapezoidal rule for each treatment group. The normality assumption was examined, and if the assumption was in doubt, the data were transformed when necessary. Statistical comparisons were analyzed using ANOVA and t-test with Bonferroni adjustment as appropriate. Antitumor efficacy was analyzed using the Kaplan–Meier method and compared using the Gehan–Breslow—Wilcoxon test. Statistical analyses were carried out using GraphPad Prism 5.0 (GraphPad Software) and SAS 9.4. A p value <0.05 was considered statistically significant.
Results
GPC3-CARs are stably expressed on T cells and costimulatory endodomains do not alter expression levels
The scFv of GC33 mAb was cloned in frame into SFG gamma-retroviral vectors containing CAR expression cassettes with CD3ζ (Gz), CD28.CD3ζ (G28z), 4-1BB (GBBz), or CD28.4-1BB.CD3ζ (G28BBz) endodomains (Fig. 1a). CD3/CD28-activated T cells were transduced with RD114-pseudotyped retroviral vectors encoding the indicated constructs. Cell surface expression of CARs was measured by flow cytometry (Fig. 1b, c). All 4 CARs were stably expressed on the cell surface of T cells with no significant difference between constructs. The median CAR expression was 79.2% (range 60–93%) for Gz, 80% (range 50–85%) for G28z, 80% (range 52–93%) for GBBz and 70% (range 50–81%) for G28BBz (Fig. 1c). The generated CAR T-cell lines contained >95% CD3-positive T cells, which were comprised of CD4- and CD8-positive T-cell subsets with the same ratio as in NT T cells (Supplementary Fig. S1).
GPC3-CAR T cells recognize and kill GPC3-positive tumor cells
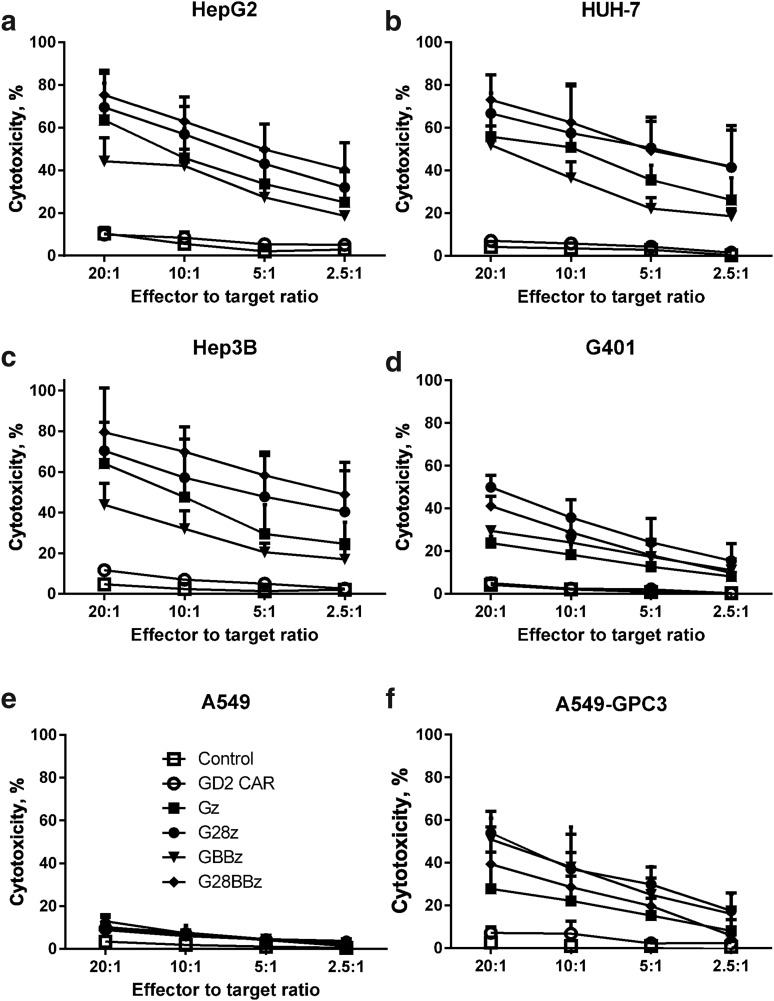
To test whether transgenic expression of GPC3-CARs renders T cells cytotoxic toward GPC3-positive targets, we performed standard 51Cr-release cytotoxicity assays at the indicated effector to target ratios using GPC3-positive [HepG2 (hepatoblastoma), Huh-7 (HCC), Hep3B (HCC), G401 (MRT)], GPC3-negative, unmodified A549 (lung cancer) cells, and A549 that were genetically modified to express GPC3 (A549.GPC3, Supplementary Fig. S2, Supplementary Table S1). NT- and GD2-specific CAR-expressing (CAR-GD2) T cells served as negative effector controls. GPC3-CAR T cells specifically killed A549.GPC3 but not unmodified A549 cells, confirming the specificity of the generated CARs. Furthermore, GPC3-CAR T cells effectively killed all other tumor cells (HepG2, Huh-7, Hep3B, G401) that were GPC3 positive (Fig. 2). CAR-GD2 and NT T cells did not kill any of the target cells, demonstrating that the cytolytic activity of T cells depends on the expression of GPC3 on target cells and GPC3-CARs on T cells.
Figure 2.
GPC3-CAR T cells recognize and kill GPC3-positive tumor cells. Tumor cell lysis was measured with standard 4 h chromium 51 (51Cr) release assay at indicated effector to target ratios against GPC3-positive solid tumor cell lines HepG2, Huh-7, Hep3B, and G401. GPC3-negative A549 cell line served as negative control, and A549, genetically modified to express GPC3 (A549.GPC3), as positive control. (a–f) Combined results of sux independent experiments. Nontransduced T cells and T cells expressing CAR-GD2 were used as controls. GPC3-CAR T cells recognized and killed GPC3-positive cell lines (GPC3-CAR T cells vs. controls: p < 0.001 for all GPC3-positive targets) but did not lyse GPC3-negative A549.
Costimulation by 4-1BB and CD28 have opposing effects on Th1 and Th2 cytokine production by GPC3-CAR T cells
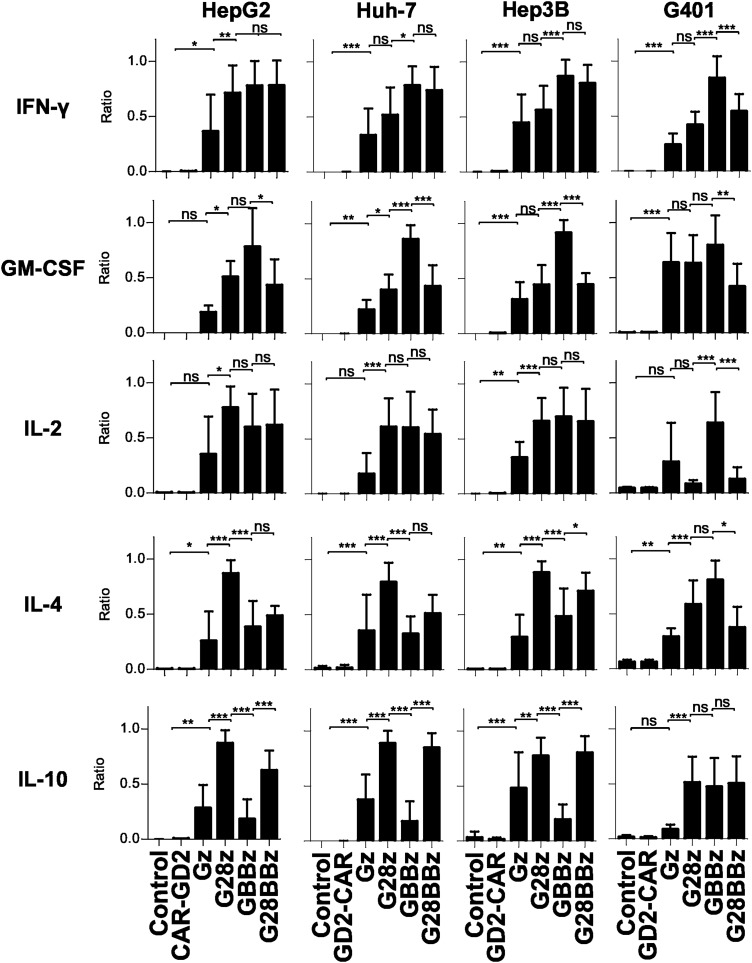
Having estiablished that GPC3-CAR T cells recognize GPC3-positive target cells in an antigen-specific manner, we next determined in coculture assays the ability of GPC3-CAR T cells to secrete Th1 (interferon gamma [IFNγ], granulocyte macrophage colony-stimulating factor [GM-CSF], IL-2) and Th2 (IL-4, IL-10) cytokines. We used the same panel of GPC3-positive and GPC3-negative tumor cells, and effector controls as for the cytotoxicity assays. GPC3-positive target cells induced cytokine production of GPC3-CAR T cells and not of NT or CAR-GD2 T cells confirming specificity (Supplementary Fig. S3a, b), and striking differences were noted in cytokine release pattern dependent on the costimulatory endodomains of GPC3-CARs. After controlling for donor-to-donor variation of absolute amount of cytokine release we found that 4-1BB costimulation induced higher levels of IFN-γ and GM-CSF production compared with CD28 costimulation compared to Gz (p < 0.05 in 3 of 4 and 2 of 4 tested cell lines, respectively, Fig. 3). In regard to Th2 cytokines, CD28 costimulation induced significantly more IL-4 and IL-10 production than 4-1BB costimulation (p < 0.05 in 3 of 4 tested cell lines for both cytokines, Fig. 3). Thus, CD28 costimulation skews cytokine productions of T cells toward a Th2 pattern, whereas 4-1BB costimulation enhances Th1 cytokine production. Introducing 4-1BB costimulation into G28z T cells could not overcome the Th2 bias of CD28 costimulation, and G28BBz T cells produced more IL-10 than Gz T cells for all cell lines tested (Fig. 3).
Figure 3.
Differential cytokine production of GPC3-CAR T cells dependent on costimulatory endodomains. GPC3-positive HepG2, Huh-7, Hep3B, and G401 cells were cocultured with GPC3-CAR T cells for 24 h at 1:1 ratio and indicated cytokine levels in tissue culture supernatant were measured using Luminex. To correct for donor to donor variability of absolute cytokine concentrations, the data were normalized as a ratio relative to the maximum in each experiment, with the maximal cytokine level in each experiment being 1 (formula: sample value / maximum value of the experiment for the tested cytokine; 3–5 independent donors for all GPC3 CAR constructs). Mean and SD are shown. *p < 0.05; **p < 0.01; ***p < 0.001, one-way ANOVA with Bonferoni post-test analysis. ns, not significant (p ≥ 0.05).
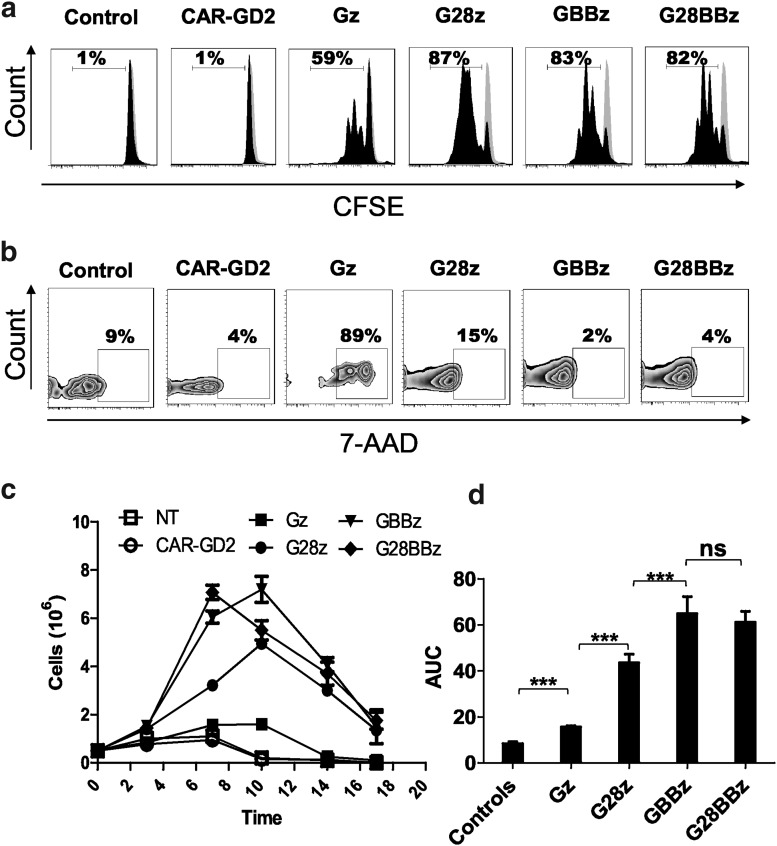
Costimulation by 4-1BB results in improved GPC3-CAR T cell survival during serial killing of tumor cells in vitro
To determine the effect of costimulatory endodomains on GPC3-CAR T cell expansion in vitro, we performed a 7-day proliferation assay using carboxyfluorescein succinimidyl ester–labeled CAR T cells that were stimulated with GPC3-positive tumor cells. NT and CAR-GD2 T cells were used as controls. G28z, GBBz, and G28BBz induced greater T cell proliferation than did Gz (Fig. 4a) and increased their viability as judged by 7-amino-actynomycin staining (Fig. 4b). Next, we performed co-cultures in which GPC3-CAR T cells were restimulated every 3 days with fresh GPC3-positive tumor cells in the absence of cytokines. G28z, GBBz, and G28BBz outperformed Gz as judged by fold-increase of absolute number of CAR T cells at the indicated timepoints (Fig. 4c, d; AUC: Gz vs. controls p < 0.001, Gz vs. G28z or GBBz or G28BBz p < 0.001). GBBz T cells expanded faster and to a greater extent than G28z T cells (day 7: G28z mean 3.2, SD 0.021, GBBz mean 6.0, SD 0.25, G28z vs. GBBz p < 0.001; day 10: G28z mean 4.9, SD 0.19, GBBz mean 7.2, SD 0.54, G28z vs. GBBz p < 0.001). No difference was observed between GBBz and G28BBz T cells. Thus, inclusion of the 4-1BB endodomain in GPC3-CARs results in enhanced T-cell proliferation and improved survival of T cells after serial killing of GPC3-positive tumor cells in vitro.
Figure 4.
4-1BB improves survival of GPC3-CAR T cells after repeated stimulation by GPC3-positive target in vitro. GPC3-positive Huh-7 was cocultured at 1:1 ratio with GPC3-CAR T cells and cells were replated every 3 days with fresh tumor cells without the addition of cytokines. (a) Carboxyfluorescein succinimidyl ester dilution of proliferating GPC3 CAR T cells on day 3. (b) Staining of GPC3-CAR T cells with 7-amino-actynomycin on day 7. (c) Combined results from four experiments measuring the absolute number of CAR-positive T cells at indicated time points. For nontransduced T cells, absolute T cell number is shown. Day 7: G28z mean 3.2, SD 0.021; GBBz mean 6.0, SD 0.25; G28z vs. GBBz p < 0.001. Day 10: G28z mean 4.9, SD 0.19; GBBz mean 7.2, SD 0.54; G28z vs. GBBz p < 0.001). (d) Area under the curve (AUC) of cell number of GPC3 CAR T cells and controls are shown (mean and SD): Gz vs. GBBz p < 0.001; Gz vs. G28BBz p < 0.001; G28z vs. GBBz p = 0.002; G28z vs. G28BBz p = 0.001.
Costimulation by 4-1BB results in enhanced antigen-specific GPC3-CAR T-cell expansion in vivo
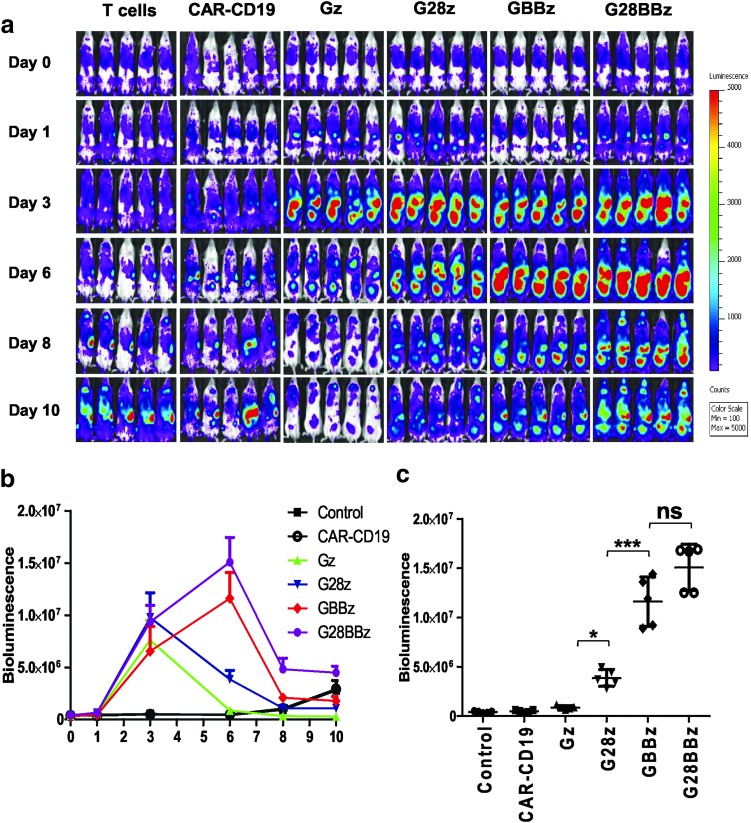
Having established that 4-1BB costimulation results in superior T-cell proliferation in vitro after repeated stimulation, we next determined the ability of GPC3-CAR T cells to expand in vivo. NSG mice were IP injected with 2 × 106 Huh-7 cells followed by the IV injection on day 14 of 5 × 106 GPC3-CAR T cells that were genetically modified to coexpress eGFP/Ffluc. The eGFP.Ffluc construct encodes an enhanced Ffluc, which is optimal to track small number of engineered cells in vivo.46 Ffluc-expressing T cells and CD19-CAR T cells served as controls. Gz, G28z, GBBz, and G28BBz T cells expanded in comparison to control T cells within 3 days post T-cell injection (p < 0.001; Fig. 5a, b). After day 3, Gz and G28z T-cell populations contracted, as judged by a decrease in bioluminescence signal, whereas GBBz and G28BBz T cells continued to expand peaking on day 6 (GBBz vs. G28z: p < 0.001; G28BBz vs. G28z: p < 0.001) (Fig. 5c). These results demonstrate that 4-1BB costimulation provides superior GPC3-CAR T-cell in vivo expansion.
Figure 5.
4-1BB improves the expansion of GPC3-CAR T cells induced by GPC3-positive target in vivo. NOD/SCID/IL2γnull (NSG) mice were injected with 2 × 106 GPC3-positive Huh-7 tumor cells intraperitoneally (IP) followed by injection of 5 × 107 green fluorescent protein/firefly luciferase fusion gene (eGFP.Ffluc)–expressing GPC3-CAR T cells intravenously (IV) on day 21. Parental T cells and CAR-CD19 T cells coexpressing eGFP.Ffluc served as controls. (a) Serial bioluminescence images of mice at indicated time points. (b) Mean photon count with SDs of mice groups shown at indicated time points. (c) Bioluminescence on day 6 of indicated treatment groups. Bioluminescence from each mouse (n = 5 per group) with SD from the mean is shown (Control, 4.01 × 105 ± 90.41 × 104; CD19-CAR, 4.81 × 105 ± 1.11 × 105; Gz, 8.63 × 105 ± 1.86 × 105; G28z, 3.86 × 106 ± 8.41 × 105; GBBz, 1.16 × 107 ± 2.5 × 106; and G28BBz, 1.5 × 107 ± 2.56 × 106). *p < 0.05; **p < 0.01; ***p < 0.001, one-way ANOVA with Bonferoni post-test analysis.
GPC3-positive tumor xenografts can be completely eliminated by GPC3-CAR T cells in vivo
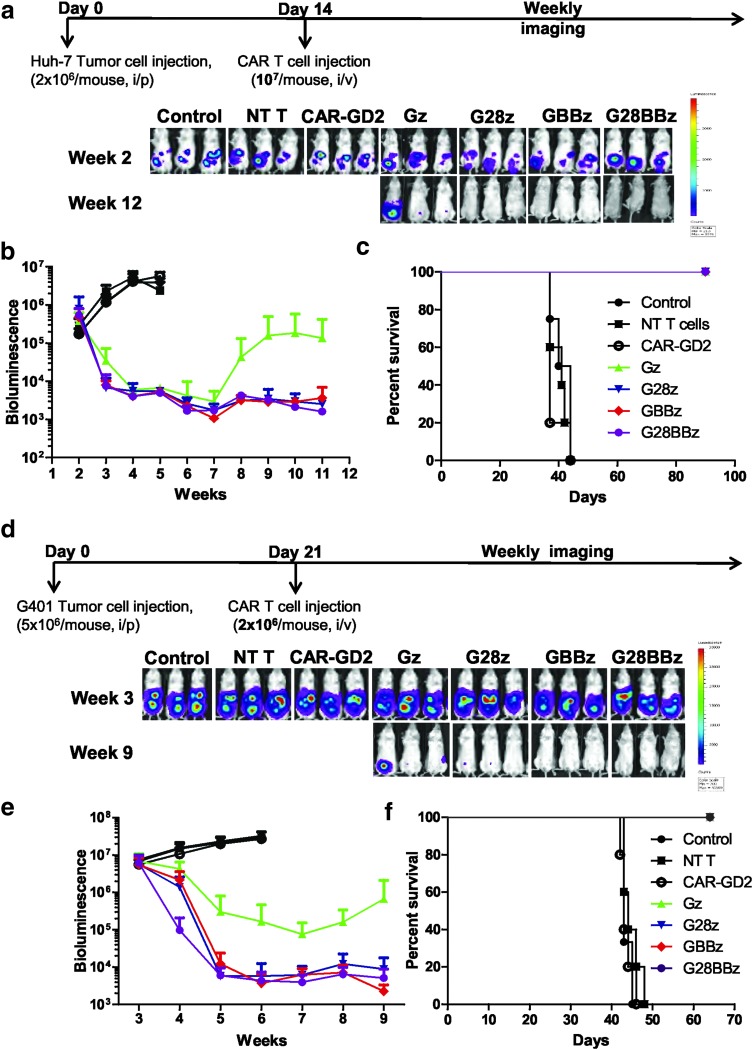
To evaluate the in vivo therapeutic potential of GPC3-CAR T cells, NSG mice were injected with 2 × 106 Huh-7.Ffluc cells IP followed by the IV injection of 1 × 107 Gz, G28z, GBBz, or G28BBz T cells on day 14. Mice injected with phosphate-buffered saline, NT or CAR-GD2 T cells served as controls. GPC3-CAR T cells produced sustained tumor regression as judged by bioluminescence imaging resulting in a survival advantage in comparison to control groups (controls vs. GPC3-CAR T cells p < 0.001; Fig. 6a–c). Since we could not find significant differences between GPC3-CAR constructs, we decreased the dose of GPC3-CAR T cells 10-fold. Mice that had received T cells expressing GPC3-CARs with costimulatory endodomains had significant survival advantage compared with control groups or Gz (p < 0.001, p < 0.001, respectively, Supplementary Fig. S4). However, there were no significant differences among treatment groups of G28z, GBBz or G28BBz T cells judged by tumor bioluminescence or survival of tumor-bearing mice. To confirm that the observed antitumor activity depends on the expression of GPC3 on tumor cells, NSG mice were injected with 2 × 106 A549.Ffluc cells IV followed by the IV injection of 1 × 107 GBBz, or NT T cells on day 5. After injection of GPC3-CAR or NT T cells, tumors continued to grow as judged by bioluminescence imaging, with no survival difference between both groups (Supplementary Fig. S5).
Figure 6.
GPC3-CAR T cells eliminate HCC and MRT xenografts in vivo. (a) NSG mice (n = 5 per group) were injected with 2 × 106 GPC3-positive Huh-7.Ffluc tumor cells IP followed by injection of 1 × 107 GPC3-CAR T cells and controls (parental T cells and CAR-GD2 T cells) IV on day 14. Serial tumor bioluminescence imaging of mice at indicated time points. (b) Tumor bioluminescence as mean photon count with standard deviations of mice groups. Control groups vs. CAR T cell groups p < 0.001 in weeks 4 and 5. (c) Kaplan–Meier survival curve of tumor-bearing mice after treatment with GPC3-CAR T cells. Control groups vs. GPC3-CAR T cell groups p < 0.001 by Gehan-Breslow-Wilcoxon test. (d) NSG mice (n = 4–5 per group) were injected with 5 × 106 GPC3-positive G401.Ffluc tumor cells IP followed by IV injection of GPC3-CAR T cells and controls (parental T cells and CAR-GD2 T) cells on day 21. Serial tumor bioluminescence imaging of mice at indicated time points. (e) Mean photon count with standard deviations of mice groups shown at indicated time points. G28z, GBBz, and G28BBz vs. Gz p = 0.0083 (AUC). (f) Survival of tumor-bearing mice after GPC3 CAR T cell treatment. Control groups vs. GPC3-CAR T cell groups p < 0.001 by Gehan-Breslow-Wilcoxon test.
To test the ability of GPC3-CAR T cells to eliminate another GPC3-positive tumor we focused on G-401, since compared with other tested tumor cells, it was the least sensitive to cell-mediated cytotoxicity and induced the lowest amount of cytokine production in GPC3-CAR T cells in our in vitro experiments (Figs. 2, 3). On day 21, 5 × 106 G-401.Ffluc cells were injected IP, followed by the IV injection of 2 × 106 Gz, G28z, GBBz, or G28BBz T cells (Fig. 6d). Mice injected with phosphate-buffered saline, NT, or CAR-GD2 T cells served as controls. All GPC3-CAR T cell treated mice had a survival advantage compared to controls (p < 0.001) (Fig. 6f). Injection of G28z, GBBz, and G28BBz T cells resulted in significantly greater reduction in tumor burden compared with Gz T cells and as judged by tumor bioluminescence over time (AUC p = 0.0083) (Fig. 6e). Similar to the Huh-7 xenograft model, we did not find significant differences between G28z, 4-1BBz, and G28BBz T cells in regards to antitumor activity and overall survival (Fig. 6f).
Discussion
The early phase testing of CAR T cells in patients with solid tumors have generated modest results compared to results obtained testing CD19-specific CAR T cells in patients with B-cell leukemias and lymphomas, highlighting the need for systematic testing and optimization of this technology for solid tumors. We generated a set of GPC3-CARs and compared their antitumor potential against GPC3-positive solid tumors (hepatoblastoma, HCC, MRT) in vitro and in vivo with the goal of defining the CARs with the most promising antitumor properties for further clinical development. We found that the inclusion of 4-1BB in GPC3-CARs induced Th1-like polarization of T-cell cytokine production and stimulated higher rates of in vitro proliferation and in vivo expansion compared with CARs with CD28 endodomain or without costimulation. In contrast, the inclusion of CD28 induced high levels of IL-10. We show that GPC3-CAR T cells have robust antitumor activity in two xenogeneic tumor models. In addition to hepatoblastoma and HCC, we demonstrate for the first time that malignant rhabdoid tumors can be effectively targeted with CAR T cells.
Since GPC3 is expressed on several solid tumors but not expressed on mature healthy tissues, it is an attractive immunotherapeutic target.19–25,36,37,56 We tested the specificity and antitumor potential of T cells expressing GPC3-CARs with or without CD28 and/or 4-1BB costimulatory endodomains. The generated CAR T cells specifically killed GPC3-positive tumor cells.
Studies have shown that the inclusion of costimulatory endodomains in CARs can enhance T-cell IFN-γ and IL-2 production.44,49,50,57,58 We noticed that antigen dependent stimulation through GPC3-CARs with costimulatory endodomains did not always result in higher production of cytokines compared with the first generation GPC3-CAR and that cytokine production was differentially affected by CD28 and 4-1BB costimulatory endodomains within GPC3-CARs. Indeed, the inclusion of 4-1BB induced significantly higher levels of Th1 cytokines IFN-γ and GM-CSF, whereas the inclusion of CD28 resulted in higher levels of IL-10 and IL-4. Our group has recently described a similar pattern of Th1/Th2 polarization of natural killer T (NKT) cells expressing CAR-GD2s with 4-1BB- or CD28 endodomains.48 However, the preferential induction of Th2 cytokines by CD28ζ CARs has not been previously reported in T cells. When targeting CD19 or mesothelin with CAR T cells, others have found that the inclusion of CD28 costimulation in CARs induced T cells to produce higher amounts of both Th1 (IFN-γ, IL-2, TNF-α, IL-6) and Th2 (IL-4, IL-10) cytokines compared with 4-1BB.44,49,58 This suggest that the balance of Th1/Th2 cytokine production by T cells after CD28ζ CAR stimulation depends on the targeted antigen. Gao et al. reported promising antitumor activity of a third generation GPC3-CAR with a CD28 and 41BB costimulatory endodomains in HCC models.19 Our systematic testing of GPC3-CARs showed that the inclusion of 4-1BB costimulatory endodomain alone is sufficient to induce similar antitumor responses; however, it produces a more favorable, Th1 cytokine release pattern. Thus, to find the construct for clinical testing, it is necessary to evaluate a panel of CARs with different costimulatory endodomains for new target antigens since Th2-biased cytokine production may suppress antitumor immune responses.59–62
Consistent with other published studies,63,64 we found improved proliferation and expansion of T cells with the inclusion of costimulatory endodomains in GPC3-CARs. We observed that GBBz and G28BBz induced superior T-cell expansion both in vitro and in vivo compared to Gz and G28z. Clinical testing of CAR T cells have shown that longer T-cell persistence is associated with better antitumor effect and overall outcome.6,13 However, in our xenograft models the improved T-cell expansion and/or Th1 cytokine profile endowed by GBBz and G28BBz did not translate into a survival benefit compared with G28z in treated mice. This is most likely due to the limitations of NSG mouse models—foremost, the induction of xenogeneic GvHD by mature human T cells and the lack of an immunosuppressive tumor microenvironment.63,65 Thus, novel models, preferentially immune competent animal models, are needed to study CAR T-cell/tumor cell interactions in vivo. However, even immune competent animal models do not adequately recapitulate “human tumors.” Thus, the definitive answer for choosing GBBz or G28BBz for later stage clinical testing may have to come from a clinical trial in which both CARs are compared within individual patients.66
In conclusion, GPC3-CARs with 4-1BB endodomains endow T cells with a Th1-biased cytokine profile, superior proliferative potential, and potent therapeutic activity in vivo, justifying further clinical development.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Malcolm Brenner (Center for Cell and Gene Therapy, Baylor College of Medicine, Houston, TX) and Dr. Milton Finegold (Department of Pathology, Baylor College of Medicine, Houston, TX) for constructive discussions. We also thank Dr. Mitchell Ho (National Institutes of Health, Bethesda, MD) for providing YP7 mAb. This work was supported by National Institutes of Health/National Cancer Institute grants (K12 CA090433 to A.H.; RO1 CA116548 to L.M.) Pablove Foundation Research Award (A.H.), and the Pediatric Pilot Award of Baylor College of Medicine (A.H.).
Author Disclosure
The Center for Cell and Gene Therapy has a research collaboration with Cell Medica and Bluebird Bio. S.G., L.M., G.D., W.L., and A.H. have patent applications in the field of T-cell and gene-modified T-cell therapy for cancer.
References
- 1.Savoldo B, Dotti G. Chimeric antigen receptors (CARs) from bench-to-bedside. Immunol Lett 2013;155:40–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curran KJ, Pegram HJ, Brentjens RJ. Chimeric antigen receptors for T cell immunotherapy: current understanding and future directions. J Gene Med 2012;14:405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368:1509–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 2011;3:95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011;365:725–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015;33:540–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014;6:224ra225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385:517–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohler BA, Sherman RL, Howlader N, et al. Annual Report to the Nation on the Status of Cancer, 1975–2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J Natl Cancer Inst 2015;107:djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed N, Brawley VS, Hegde M, et al. Human epidermal growth factor receptor 2 (HER2) -specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol 2015;33:1688–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med 2008;14:1264–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louis CU, Savoldo B, Dotti G, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 2011;118:6050–6056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beatty GL, Haas AR, Maus MV, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce antitumor activity in solid malignancies. Cancer Immunol Res 2014;2:112–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abken H. Adoptive therapy with CAR redirected T cells: the challenges in targeting solid tumors. Immunotherapy 2015;7:535–544 [DOI] [PubMed] [Google Scholar]
- 16.Gilham DE, Debets R, Pule M, Hawkins RE, Abken H. CAR–T cells and solid tumors: tuning T cells to challenge an inveterate foe. Trends Mol Med 2012;18:377–384 [DOI] [PubMed] [Google Scholar]
- 17.Lamers CH, Sleijfer S, Vulto AG, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol 2006;24:e20-e22 [DOI] [PubMed] [Google Scholar]
- 18.Lamers CHJ, Sleijfer S, van Steenbergen S, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther 2013;21:904–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao H, Li K, Tu H, et al. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res 2014;20:6418–6428 [DOI] [PubMed] [Google Scholar]
- 20.Wang XY, Degos Fo, Dubois S, et al. Glypican-3 expression in hepatocellular tumors: diagnostic value for preneoplastic lesions and hepatocellular carcinomas. Hum Pathol 2006;37:1435–1441 [DOI] [PubMed] [Google Scholar]
- 21.Yamauchi N, Watanabe A, Hishinuma M, et al. The glypican 3 oncofetal protein is a promising diagnostic marker for hepatocellular carcinoma. Mod Pathol 2005;18:1591–1598 [DOI] [PubMed] [Google Scholar]
- 22.Enan ET, El-Hawary AK, El-Tantawy DAE-A, Abu-Hashim MM, Helal NM. Diagnostic role of glypican 3 and CD34 for differentiating hepatocellular carcinoma from nonmalignant hepatocellular lesions. Ann Diagn Pathol 2013;17:490–493 [DOI] [PubMed] [Google Scholar]
- 23.Coston WM, Loera S, Lau SK, et al. Distinction of hepatocellular carcinoma from benign hepatic mimickers using Glypican-3 and CD34 immunohistochemistry. Am J Surg Pathol 2008;32:433–444 [DOI] [PubMed] [Google Scholar]
- 24.Baumhoer D, Tornillo L, Stadlmann S, Roncalli M, Diamantis EK, Terracciano LM. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: a tissue microarray analysis of 4,387 tissue samples. Am J Clin Pathol 2008;129:899–906 [DOI] [PubMed] [Google Scholar]
- 25.Chan ES, Pawel BR, Corao DA, et al. Immunohistochemical expression of glypican-3 in pediatric tumors: an analysis of 414 cases. Pediatr Dev Pathol 2013;16:272–277 [DOI] [PubMed] [Google Scholar]
- 26.Levy M, Trivedi A, Zhang J, et al. Expression of glypican-3 in undifferentiated embryonal sarcoma and mesenchymal hamartoma of the liver. Hum Pathol 2012;43:695–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tretiakova M, Zynger DL, Luan C, et al. Glypican 3 overexpression in primary and metastatic Wilms tumors. Virchows Arch 2015;466:67–76 [DOI] [PubMed] [Google Scholar]
- 28.Zynger DL, Gupta A, Luan C, Chou PM, Yang GY, Yang XJ. Expression of glypican 3 in hepatoblastoma: an immunohistochemical study of 65 cases. Hum Pathol 2008;39:224–230 [DOI] [PubMed] [Google Scholar]
- 29.Zynger DL, Dimov ND, Luan C, Teh BT, Yang XJ. Glypican 3: a novel marker in testicular germ cell tumors. Am J Surg Pathol 2006;30:1570–1575 [DOI] [PubMed] [Google Scholar]
- 30.Yorita K, Takahashi N, Takai H, et al. Prognostic significance of circumferential cell surface immunoreactivity of glypican-3 in hepatocellular carcinoma. Liver Int 2011;31:120–131 [DOI] [PubMed] [Google Scholar]
- 31.Shirakawa H, Suzuki H, Shimomura M, et al. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci 2009;100:1403–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ning S, Bin C, Na H, et al. Glypican-3, a novel prognostic marker of hepatocellular cancer, is related with postoperative metastasis and recurrence in hepatocellular cancer patients. Mol Biol Rep 2012;39:351–357 [DOI] [PubMed] [Google Scholar]
- 33.Yu MC, Lee YS, Lin SE, et al. Recurrence and poor prognosis following resection of small hepatitis B-related hepatocellular carcinoma lesions are associated with aberrant tumor expression profiles of glypican 3 and osteopontin. Ann Surg Oncol 2012;19:S455–463 [DOI] [PubMed] [Google Scholar]
- 34.Wang YL, Zhu ZJ, Teng DH, Yao Z, Gao W, Shen ZY. Glypican-3 expression and its relationship with recurrence of HCC after liver transplantation. World J Gastroenterol 2012;18:2408–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu SJ, Qi CY, Xiao WK, Li SQ, Peng BG, Liang LJ. Glypican-3 is a potential prognostic biomarker for hepatocellular carcinoma after curative resection. Surgery 2013;154:536–544 [DOI] [PubMed] [Google Scholar]
- 36.Capurro M, Martin T, Shi W, Filmus J. Glypican-3 binds to Frizzled and plays a direct role in the stimulation of canonical Wnt signaling. J Cell Sci 2014;127:1565–1575 [DOI] [PubMed] [Google Scholar]
- 37.Filmus J, Capurro M. Glypican-3: a marker and a therapeutic target in hepatocellular carcinoma. FEBS J 2013;280:2471–2476 [DOI] [PubMed] [Google Scholar]
- 38.Zhu AX, Gold PJ, El-Khoueiry AB, et al. First-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clin Cancer Res 2013;19:920–928 [DOI] [PubMed] [Google Scholar]
- 39.Ikeda M, Ohkawa S, Okusaka T, et al. Japanese phase I study of GC33, a humanized antibody against glypican-3 for advanced hepatocellular carcinoma. Cancer Sci 2014;105:455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li K, Pan X, Bi Y, et al. Adoptive immunotherapy using T lymphocytes redirected to glypican-3 for the treatment of lung squamous cell carcinoma. Oncotarget 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abken H, Hombach A, Heuser C, Kronfeld K, Seliger B. Tuning tumor-specific T-cell activation: a matter of costimulation? Trends Immunol 23:240–245 [DOI] [PubMed] [Google Scholar]
- 42.Kunkele A, Johnson AJ, Rolczynski LS, et al. Functional Tuning of CARs Reveals Signaling Threshold above Which CD8+ CTL Antitumor Potency Is Attenuated due to Cell Fas-FasL-Dependent AICD. Cancer Immunol Res 2015;3:368–379 [DOI] [PubMed] [Google Scholar]
- 43.Krenciute G, Krebs S, Torres D, et al. Characterization and functional analysis of scFv-based cars to redirect T cells to IL13Rα2-positive glioma. Mol Ther 2015;24:354–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci USA 2009;106:3360–3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther 2010;18:413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabinovich BA, Ye Y, Etto T, et al. Visualizing fewer than 10 mouse T cells with an enhanced firefly luciferase in immunocompetent mouse models of cancer. Proc Natl Acad Sci USA 2008;105:14342–14346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishiguro T, Sugimoto M, Kinoshita Y, et al. Anti-glypican 3 antibody as a potential antitumor agent for human liver cancer. Cancer Res 2008;68:9832–9838 [DOI] [PubMed] [Google Scholar]
- 48.Heczey A, Liu D, Tian G, et al. Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood 2014;124:2824–2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther 2009;17:1453–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pule MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther 2005;12:933–941 [DOI] [PubMed] [Google Scholar]
- 51.Xu Y, Zhang M, Ramos CA, et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood 2014;123:3750–3759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phung Y, Gao W, Man YG, Nagata S, Ho M. High-affinity monoclonal antibodies to cell surface tumor antigen glypican-3 generated through a combination of peptide immunization and flow cytometry screening. MAbs 2012;4:592–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rooney CM, Rickinson AB, Moss DJ, Lenoir GM, Epstein MA. Paired Epstein-Barr virus-carrying lymphoma and lymphoblastoid cell lines from Burkitt's lymphoma patients: comparative sensitivity to non-specific and to allo-specific cytotoxic responses in vitro. International journal of cancer. Int J Cancer 1984;34:339–348 [DOI] [PubMed] [Google Scholar]
- 54.Hawkins ED, Hommel M, Turner ML, Battye FL, Markham JF, Hodgkin PD. Measuring lymphocyte proliferation, survival and differentiation using CFSE time-series data. Nat Protoc 2007;2:2057–2067 [DOI] [PubMed] [Google Scholar]
- 55.Kakarla S, Chow KK, Mata M, et al. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol Ther 2013;21:1611–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Filmus J, Selleck SB. Glypicans: proteoglycans with a surprise. J Clin Invest 2001;108:497–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vera J, Savoldo B, Vigouroux S, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood 2006;108:3890–3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brentjens RJ, Santos E, Nikhamin Y, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res 2007;13:5426–5435 [DOI] [PubMed] [Google Scholar]
- 59.Sato T, Terai M, Tamura Y, Alexeev V, Mastrangelo M, Selvan S. Interleukin 10 in the tumor microenvironment: a target for anticancer immunotherapy. Immunol Res 2011;51:170–182 [DOI] [PubMed] [Google Scholar]
- 60.Mannino MH, Zhu Z, Xiao H, Bai Q, Wakefield MR, Fang Y. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett 2015;367:103–107 [DOI] [PubMed] [Google Scholar]
- 61.Wu AA, Drake V, Huang H-S, Chiu S, Zheng L. Reprogramming the tumor microenvironment: tumor-induced immunosuppressive factors paralyze T cells. OncoImmunology 2015;4:e1016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dennis KL, Blatner NR, Gounari F, Khazaie K. Current status of IL-10 and regulatory T-cells in cancer. Curr Opin Oncol 2013;25:637–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev 2014;257:107–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dotti G, Savoldo B, Brenner M. Fifteen years of gene therapy based on chimeric antigen receptors: “are we nearly there yet?”. Hum Gene Ther 2009;20:1229–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ali N, Flutter B, Sanchez Rodriguez R, et al. Xenogeneic graft-versus-host-disease in NOD-IL-2Rγnull mice display a T-effector memory phenotype. PLoS One 2012;7:e44219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest 2011;121:1822–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.