Abstract
Purpose
To relate balance measures to visual field (VF) damage from glaucoma.
Methods
The OPAL kinematic system measured balance, as root mean square (RMS) sway, on 236 patients with suspect/diagnosed glaucoma. Balance was measured with feet shoulder width apart while standing on a firm/foam surface with eyes opened/closed (Instrumental Clinical Test of Sensory Integration and Balance [ICTSIB] conditions), and eyes open on a firm surface under feet together, semi-tandem, or tandem positions (standing balance conditions). Integrated VF (IVF) sensitivities were calculated by merging right and left eye 24-2 VF data.
Results
Mean age was 71 years (range, 57–93) and mean IVF sensitivity was 27.1 dB (normal = 31 dB). Lower IVF sensitivity was associated with greater RMS sway during eyes-open foam-surface testing (β = 0.23 z-score units/5 dB IVF sensitivity decrement, P = 0.001), but not during other ICTSIB conditions. Lower IVF sensitivity also was associated with greater RMS sway during feet together standing balance testing (0.10 z-score units/5 dB IVF sensitivity decrement, P = 0.049), but not during other standing balance conditions. Visual dependence of balance was lower in patients with worse IVF sensitivity (β = −21%/5 dB IVF sensitivity decrement, P < 0.001). Neither superior nor inferior IVF sensitivity consistently predicted balance measures better than measures of overall VF sensitivity.
Conclusions
Balance was worse in glaucoma patients with greater VF damage under foam surface testing (designed to inhibit proprioceptive contributions to balance) as well as feet-together firm-surface conditions when somatosensory inputs were available.
Translational Relevance
Good balance is essential to avoid unnecessary falls and patients with VF loss from glaucoma may be at higher risk of falls because of poor balance.
Keywords: quality of life, vision and function, low vision
Introduction
Balance is integral to all components of walking, including straight-ahead walking,1 initiation and termination of gait, and changing directions.2 Balance impairment severely limits one's ability to perform activities of daily living,3 and is associated with reduced participation in social activities.4 Numerous studies have shown that patients with balance problems as a result of lower extremity weakness, sensory loss, or Parkinson's disease have a significantly greater risk of falling attributable to their poor balance.5–7 Poor balance also is associated with a greater fear of falling,8,9 and improving balance through directed training programs can reduce fear of falling independent of one's fall history.10,11
Vision is recognized as an important sensory input enabling balance,12,13 and glaucoma patients in some studies have been shown to suffer from balance deficits as a result of their loss of side vision.14,15 However, few studies have assessed balance deficits under conditions encountered commonly in daily life, such as standing on a firm surface as opposed to a foam surface meant to eliminate proprioceptive inputs to balance.15,16 Indeed, some studies have found balance not to be associated with glaucoma-related visual field (VF) loss at all,17 or reported worse stability only in the context of stimuli presented through virtual reality goggles,18 possibly reflecting compensatory proprioceptive inputs.19,20 Thus, it remains unclear whether glaucoma-related VF damage affects balance under physiologic (i.e., firm surface) conditions or only under artificial conditions in which other sensory inputs are removed.
A growing body of evidence suggests that the location of VF loss may differentially impact quality of life and performance-based measures.21–23 In studies specifically evaluating VF loss and balance, increased postural sway was associated with VF loss in the inferior hemifield.15,24 However, substantial numbers of patients are required to identify the independent effects of correlated visual measures (such as superior and inferior VF loss), and prior studies have been limited by the number of patients examined (less than 100 for all studies).15,24 Furthermore, prior studies examining the importance of specific VF locations have largely focused on different patterns of VF loss within the central 24° to 30°, ignoring the potential impact of more peripheral VF loss, which may be particularly important for falls.25
In this study, we aimed to understand the relationship between balance and VF loss secondary to glaucomatous damage in a large sample of glaucoma patients with a broad spectrum of VF loss. Innovative aspects of the study included the evaluation of balance using a set of lightweight motion sensors that directly measure acceleration in various spatial planes in real time. Additionally, sensors were used to quantify balance under a broad range of conditions shown to be associated with health outcomes,26,27 including the Instrumental Clinical Test of Sensory Integration and Balance (ICTSIB) test series28 (standing on a firm or foam surface with one's eyes open or closed) and the standing balance test series29 (feet together, semi-tandem, and tandem positions) on a firm surface.
Methods
Study Design and Study Population
Participants were recruited between September 2013 and March 2015 from the Glaucoma Center of Excellence at the Wilmer Eye Institute, Johns Hopkins University to participate in the Falls in Glaucoma Study (FIGS). The research followed the tenants of the Declaration of Helsinki. Informed consent was obtained from study participants after an explanation of the nature and potential consequences of the study, and the research was approved by the Institutional Review Board at Johns Hopkins Hospital. Inclusion criteria were: (1) age 57 or older; (2) glaucoma suspect or diagnosis of primary open angle glaucoma, primary angle closure glaucoma, pseudoexfoliation glaucoma, or pigmentary glaucoma; (3) residence within a 60-mile radius from the Wilmer Eye Institute; (4) ability to perform VF testing; and (5) ability to complete at least 1 balance test. Glaucoma suspects, in addition to patients with a glaucoma diagnosis, were included in the study to determine the relationship between VF damage and balance over the full spectrum of VF loss (with suspects capturing balance in persons with little to no VF loss). Glaucoma diagnoses were determined by a glaucoma specialist following a comprehensive eye exam, including best corrected visual acuity, slit-lamp biomicroscopy, dilated fundus exam, VF analysis (Humphrey Field Analyzer II; Carl Zeiss Meditec, Inc., Dublin CA), and optical coherence tomography (Cirrus HD-OCT; Carl Zeiss Meditec). We reported baseline data obtained before collection of falls assessment data.
Balance Evaluation
Balance data were collected using the Opal kinematic system (APDM, Inc., Portland, OR). The Opal system measures postural sway via body-worn accelerometers placed around patients' wrists, shins, and waist, and on the chest.
Two different sets of balance tests were performed. The first set of tests, the ICTSIB, consisted of 4 test conditions: standing on a hard surface with eyes open or closed, and standing on a foam surface with eyes open or closed.30 Patients were instructed to maintain an upright standing position with their arms crossed and feet approximately shoulder width apart for 30 seconds, with foot distance standardized by a wooden block placed between the patient's feet. In the second set of tests, the standing balance tests,29 patients stood on a hard surface with their arms by their sides or extended laterally to aid their balance, and were asked not to move for 30 seconds under three conditions: feet together (heels and toes touching), feet in a semi-tandem position (heel of one foot touching the medial edge of the large toe of the other foot), and feet in a tandem position (heel of one foot touching the tip of the toe of the other foot).16
Overall sway, as well as sway in the anterior-posterior (AP) and medial-lateral (ML) directions, were evaluated during the various test conditions given prior work suggesting that glaucoma patients sway more in the AP than the ML direction.14
Balance Outcome Measures
Three parameters were analyzed to quantify balance for each test condition as these parameters have high test–retest variability and distinguish control subjects from those with known balance deficits:31,32
Root mean square (RMS) sway (m/s2) – RMS of the acceleration vector length. Larger sway values reflect worse balance.
Jerk (m2/s5) – Time derivative of acceleration, a measure of dynamic stability reflecting the amount of active postural corrections. Jerk values were analyzed as a log transformation of the raw jerk values to achieve a normal distribution. Higher jerk values reflect worse balance.
Visual dependence (VD) – Ratio between eyes closed (EC) ellipse sway to eyes open (EO) ellipse sway14 under foam testing conditions based on the Romberg Quotient,28,33 where ellipse sway is the 95% confidence ellipse encompassing the sway trajectory that correlates with the movement of the center of pressure in force plate testing.34 Higher values indicate that balance is more dependent on visual input.
Visual Assessment
Central and peripheral visual field tests were performed on the Humphrey Field Analyzer II (Carl Zeiss Meditec) as described by Odden et al.35 Tests were obtained at the baseline study visit or within 6 months before this baseline visit.
For the central VF, the 24-2 Swedish interactive threshold algorithm (SITA) standard test was used and data from the right and left eyes were merged to calculate sensitivity in the integrated VF (IVF) by using the maximum sensitivity of spatially corresponding points. As previously described, average sensitivities over the full IVF, or over specific regions of the IVF (i.e., the superior and inferior hemifields) were calculated per the following method: (1) each sensitivity value in dB was divided by 10 and exponentiated, (2) the resulting values were averaged, and (3) the log10 of this sensitivity value was taken and multiplied by 10 to derive an average IVF sensitivity in dB.36
To evaluate the peripheral VF, the suprathreshold peripheral 60 screening test pattern was used and the number of missed points (possible range = 0–60) in the better eye was chosen to summarize peripheral VF damage.35
Evaluation of Covariates
Age, sex, and race were collected using standardized questionnaires. Information on the participants' prescription medication was obtained by direct observation of bottles when provided or by patient report, and classified as polypharmacy if 5 or more non eye drop prescription medications were used.37 Patients were questioned about 15 comorbid medical conditions known to affect mobility (arthritis, broken or fractured hip, back problems, history of heart attack, history of angina/chest pain, congestive heart failure, peripheral vascular disease, high blood pressure, diabetes, emphysema, asthma, stroke, Parkinson's disease, cancer other than the skin cancer, and history of vertigo or Meniere's disease) using a standardized questionnaire.38 Comorbidity was quantified as the total number of comorbid conditions. A small number of participants with more than 5 comorbidities (n = 9) were reclassified to have 5 comorbidities given the lack of linearity of comorbid disease on balance observed beyond 5 comorbid illnesses.
Statistical Analysis
Balance outcome variables were treated as continuous variables. Distribution plots, as well as Shapiro-Wilks testing were performed for each balance measure, and measures that were normally distributed were used in the regression models. Log transformation was required to obtain normal distributions for the jerk and visual dependence variables.
Multivariable regression models were used to determine predictors of poor balance. In these models, the dependent variable was the balance parameter, while the independent variable was IVF sensitivity or peripheral points missed. In orthogonal regression models, each 5 dB of IVF sensitivity was associated with 14 additional peripheral points missed; therefore, regression models were constructed to show the effects of a 5 dB decrement in IVF sensitivity or 14 peripheral points missed (reflecting a comparable degree of VF damage in each region). Additional analyses were conducted to examine the effects of lower sensitivity in the inferior or superior IVF or more missed points in the inferior or superior peripheral VF.
Each balance measure was described in z-score units, with the mean value for the study population set at 0, and persons 1 standard deviation (SD) above and below the population mean given a value of 1 and −1, respectively, with conversions between z-score and actual values provided in the Appendix. Age, sex, race, polypharmacy, and number of medical comorbidities were included as covariates in multivariable models based on previous literature that suggested that these covariates were relevant to balance.39–42 All analyses were conducted using STATA 14.0 (StataCorp, College Station, TX).
Results
Recruitment
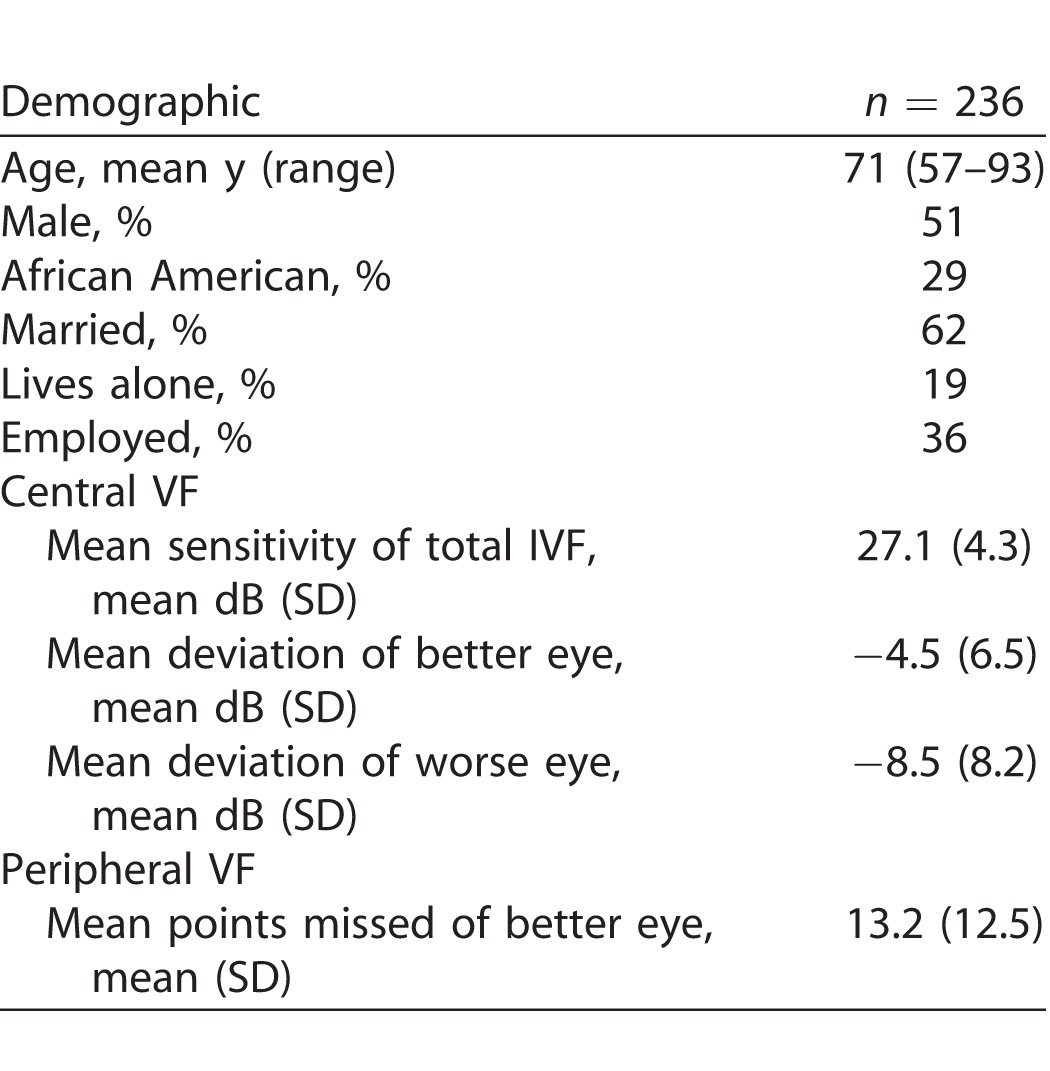
A total of 236 patients with suspect (n = 52) or manifest (n = 184) glaucoma completed visual testing and at least 1 balance test. Mean age was 71 years (range, 57–93), with an approximately equal number of males and females (Table 1). Mean sensitivity over the total IVF was 27.1 dB (normal value = 31), while average mean deviation in the better eye was −4.5 dB (IQR −5.4 to −0.67) and average mean deviation of the worse eye was −8.5 dB (interquartile ratio [IQR] −12.4 to −2.6). Of the study patients, 51 and 25 (22% and 11% of the study population, respectively) had a better-eye mean deviation worse than −6 and −12 dB, respectively.
Table 1.
Characteristics of Subjects Completing Balance and VF Testing
Eyes Open/Closed, Foam/Firm Surface Testing (ICTSIB Tests)
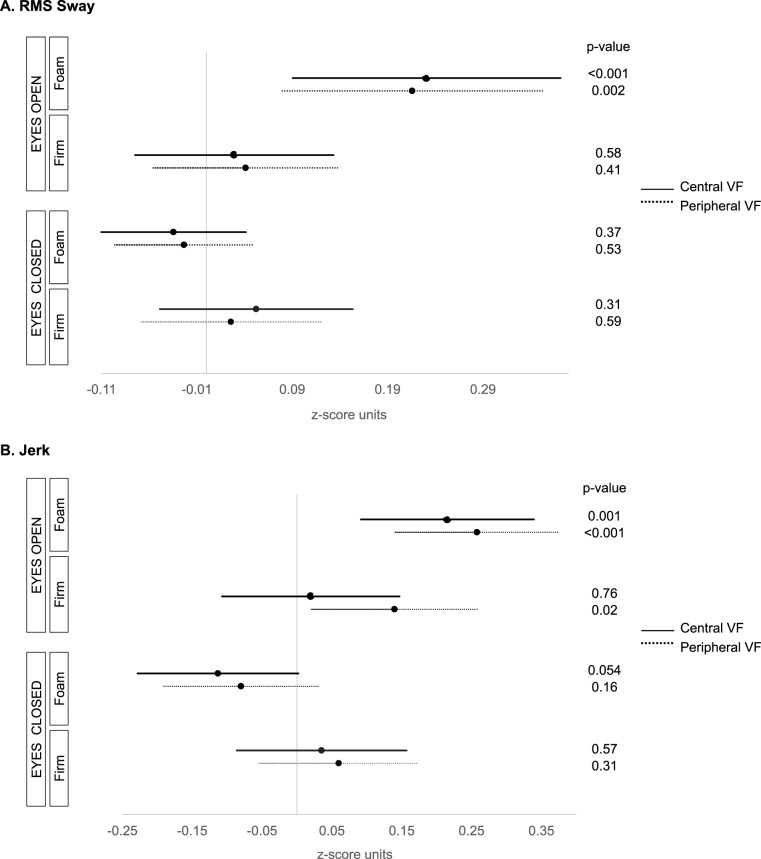
Under foam surface eyes-open conditions, greater RMS sway was observed with worse IVF sensitivity (β = 0.23 z-score units per 5 dB decrement in IVF sensitivity; 95% confidence interval [CI], 0.09–0.37; P = 0.001) and more peripheral points missed (β = 0.22 z-score units per 14 pts missed; 95% CI, 0.08–0.35; P = 0.002). Neither IVF sensitivity nor peripheral missed points were associated with RMS sway during eyes-open firm surface, or eyes-closed foam or firm surface testing (P > 0.31 for all, Fig. 1A).
Figure 1.
Association between balance and visual field damage during eyes open/closed, foam/firm surface testing (ICTSIB tests). (A) RMS sway. (B) Jerk. Figures show the effects of a 5 dB decrement in IVF sensitivity for central VF testing, or 14 missed points on peripheral VF testing. Jerk values were log transformed.
Under foam surface eyes-open conditions, greater jerk was observed with worse IVF sensitivity (β = 0.22 z-score units per 5 dB decrement in IVF sensitivity; 95% CI, 0.10–0.34; P = 0.001) and more peripheral points missed (β = 0.26 z-score units per 14 pts missed; 95% CI, 0.14 to 0.38; P < 0.001; Fig. 1B). Greater jerk also was associated with more peripheral points missed under the eyes-open firm surface condition (β = 0.14 z-score per 14 pts missed; 95% CI, 0.02–0.26; P = 0.02), though no significant association was noted between jerk and IVF sensitivity (P = 0.76). Neither IVF sensitivity nor peripheral missed points were associated with jerk during eyes-closed firm or foam conditions (P > 0.05 for all).
We performed an additional analysis for RMS sway/jerk and IVF sensitivity under ICTSIB test conditions where patients were stratified by glaucoma diagnosis (suspect versus manifest). The relationships between RMS sway/jerk and IVF sensitivity were the same as described above, specifically that worse IVF sensitivity was associated with greater RMS sway and jerk for suspect and manifest groups under the eyes-open foam surface condition (P < 0.03 for both), but not for other test conditions (P > 0.06 for both groups).
AP/ML Balance During Eyes-Open Foam Surface Testing
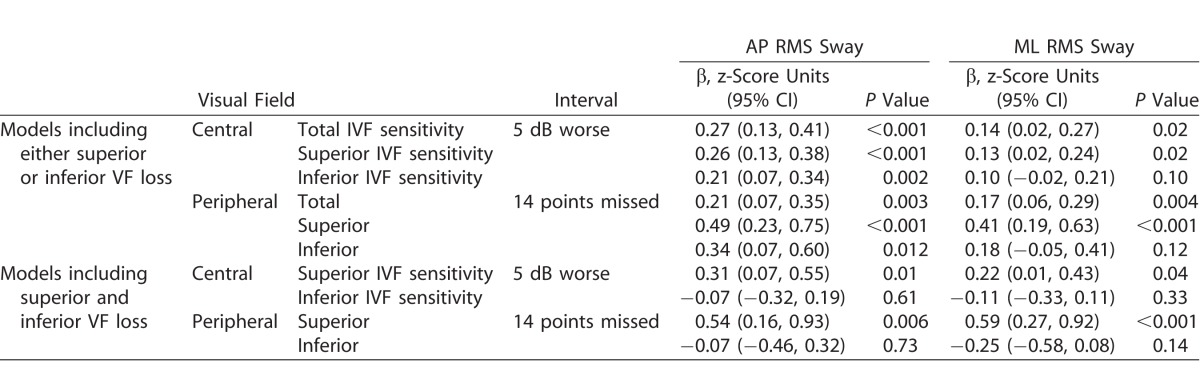
Additional analyses evaluated the impact of VF damage on RMS sway, with sway values during eyes open on a foam surface taken as the primary measure given the strength of the association with this balance measure. Greater RMS sway was noted in patients with worse IVF sensitivity in the AP direction (β = 0.27 z-score units per 5 dB decrement in IVF sensitivity; 95% CI, 0.13–0.41; P < 0.001) and ML direction (β = 0.14 z-score units per 5 dB decrement in IVF sensitivity; 95% CI, 0.02–0.27; P = 0.02). AP and ML RMS sway also were greater in patients with more peripheral points missed (Table 2).
Table 2.
Association between VF Damage and AP/ML RMS Sway
Greater AP and ML RMS sway were observed with worse superior and worse inferior IVF sensitivity, as well as more superior and more inferior peripheral VF points missed, when each measure was analyzed as the sole visual predictor. When the effects of superior and inferior VF damage were assessed in models where both were included, superior VF damage (worse superior IVF sensitivity or more superior peripheral points missed) remained associated with worse balance while inferior VF damage was not (Table 2). However, high variance inflation factors (4.2–4.3 for models including superior and inferior IVF sensitivity, and 2.3–2.4 for models including superior and inferior peripheral points missed) were noted. An additional model was run with the total IVF and difference between the superior and inferior IVF, and the total IVF was significant (P < 0.001) while the difference was not (P = 0.12).
Feet Together, Tandem, and Semi-Tandem Stands During Eyes-Open Firm Surface Testing (Standing Balance Tests)
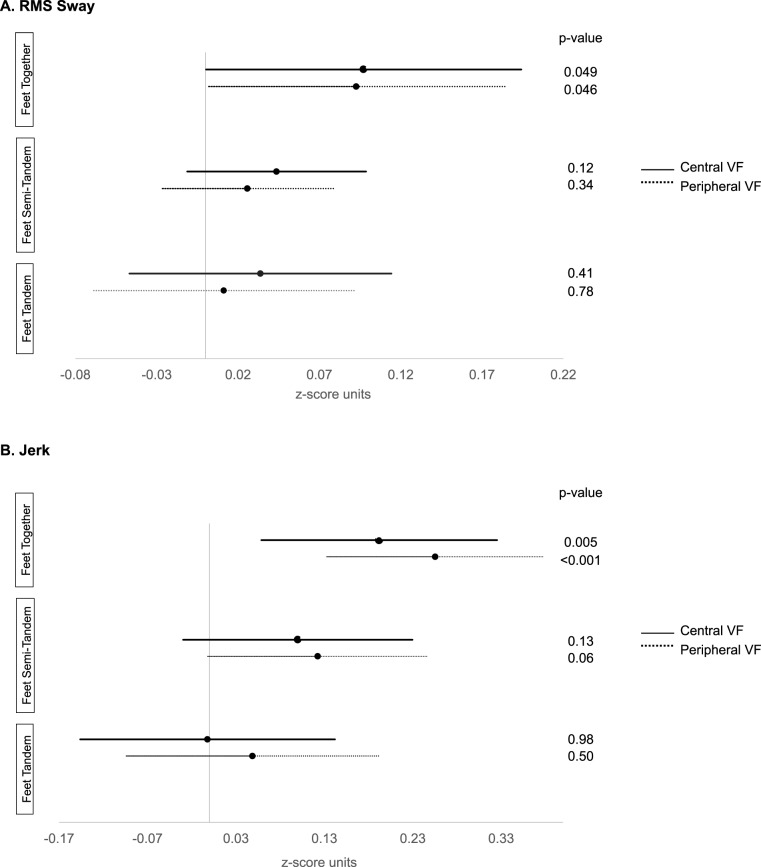
During feet together testing, worse IVF sensitivity was associated with greater RMS sway (0.10 z-score units per 5 dB decrement in IVF sensitivity; 95% CI, 0.0004–0.20; P = 0.049). Greater RMS sway also was observed with more peripheral points missed (β = 0.10 z-score units per 14 pts missed; 95% CI, 0.002–0.18; P = 0.046). Greater RMS sway was not associated with IVF sensitivity or peripheral points missed under tandem or semi-tandem conditions (P > 0.12 for all; Fig. 2A).
Figure 2.
Association between balance parameters and visual field damage during feet together, tandem, and semi-tandem testing (standing balance tests). (A) RMS sway. (B) Jerk. Figures show the effects of a 5 dB decrement in IVF sensitivity for central VF testing, or 14 missed points on peripheral VF testing. Jerk values were log transformed.
During feet together testing, greater jerk was associated with worse IVF sensitivity (β = 0.20 z-score units per 5 dB decrement in IVF sensitivity; 95% CI, 0.06–0.33; P = 0.005) and peripheral points missed (β = 0.26 z-score units per 14 pts missed; 95% CI, 0.13–0.38; P < 0.001). Greater jerk was not associated with IVF sensitivity or peripheral points missed under tandem or semi-tandem conditions (P > 0.06 for all; Fig. 2B).
Visual Dependence of Balance
Visual dependence of balance was lower in patients with worse IVF sensitivity (β = −21% per 5 dB decrement in IVF sensitivity, 95% CI, −30 to −12, P < 0.001). When evaluated in separate regression models, worse IVF sensitivity in the superior and inferior hemifields also were associated with lower visual dependence (superior IVF, β = −19% per 5 dB decrement in sensitivity; 95% CI, −26 to −11, P < 0.001; inferior IVF β = −19% per 5 dB decrement in sensitivity; 95% CI, −27 to −10; P < 0.001; Table 3). When superior and inferior IVF sensitivity were evaluated in the same regression model, neither was associated with visual dependence (P > 0.10 for both). The variance inflation factor (VIF) for the superior and inferior VFs were 4.4 and 4.3, respectively, and, because of these high values, an additional model was run with the total IVF sensitivity and difference between the superior and inferior IVF sensitivities as predictors of visual dependence. In this model, worse total IVF sensitivity was significantly associated with lower visual dependence (P < 0.001) while the difference between the superior and inferior IVF sensitivity was not (P = 0.53).
Table 3.
Percent Decrease in Visual Dependence of Balance for a 5 dB Decrement in IVF Sensitivity for Central VF Testing, or 14 Missed Points on Peripheral VF Testing
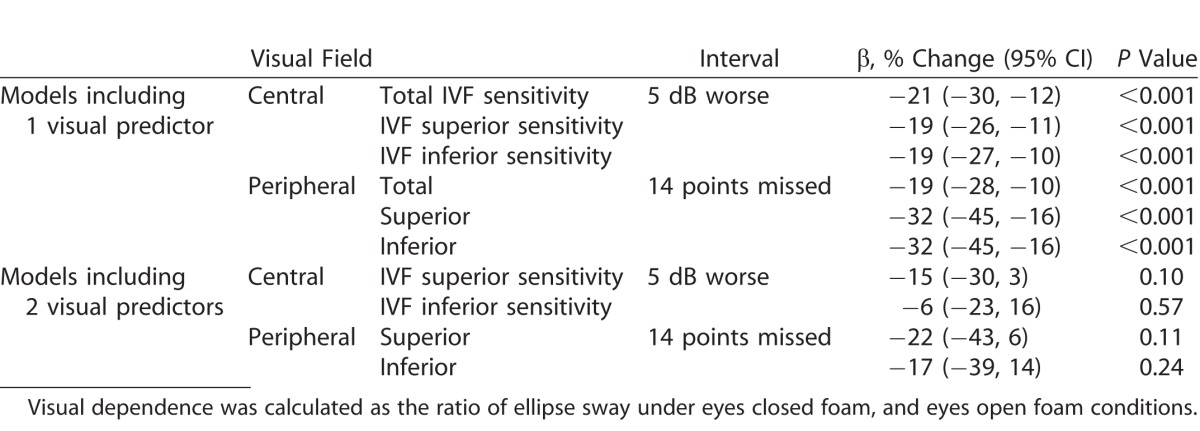
Visual dependence of balance was lower in patients with more points missed for the total peripheral VF (β = −19% per 5 dB decrement in IVF sensitivity; 95% CI, −28 to −10; P < 0.001). When evaluated in separate regression models, more points missed in the superior and inferior peripheral visual fields also were associated with lower visual dependence (superior VF, β = −32% per 14 pts missed; 95% CI, −45 to −16; P < 0.001; inferior VF, β = −32% per 14 pts missed; 95% CI; −45 to −16; P < 0.001). When superior and inferior peripheral points missed were evaluated in the same regression model, neither was significantly correlated with visual dependence (P > 0.11 for both). In this model, the VIF for superior and inferior points missed was 2.4 for both.
Other Factors Associated with Balance
Age was associated with greater RMS sway and jerk during all ICTSIB test and standing balance test conditions (P < 0.03 for all). No other studied variable (sex, race, polypharmacy, or number of comorbidities) was associated with either RMS sway or jerk during any of the ICTSIB or standing balance test conditions (P > 0.06).
Discussion
Patients with glaucoma had balance difficulties, and these difficulties increased linearly with increasing amounts of VF loss. This finding was observed across a wide range of testing conditions and balance measures, and was seen consistently during foam surface testing, which removes proprioceptive inputs to balance. Balance difficulties also were noted for some balance measures/test conditions during firm surface standing, suggesting that balance is affected by poor vision even when other proprioceptive compensatory mechanisms are available, which corroborates previous work.15 Additionally, in this study, location of VF loss was not a major contributor to balance impairment, which differs from findings in prior work.23,24,43 Together, these results indicated that one's field of vision has an important role in maintaining balance under conditions corresponding to real-world situations (i.e., firm surface standing), and to an even greater degree when other sensory inputs are compromised.
Balance was worse with more severe VF defects when measured by RMS sway and jerk. RMS sway and jerk were chosen as metrics of balance given that both have high test–retest variability, and distinguish control subjects from patients with known balance deficits as a result of conditions, such as Parkinson's disease.32 Additionally, RMS sway measures of body acceleration obtained in the current study correlated well with sway measures derived from force plates, which determine displacement of the center of pressure. Jerk, the time derivative of acceleration measured by the body sensors used in the current study, is not available on force place testing but has been one of the most discriminative measures for separating controls from individuals with balance disorders, such as Parkinson's patients.31 Higher jerk measures represent uneven acceleration/deceleration, corresponding with what one would call jerk/surge in real life.
Worse balance was correlated with more severe VF loss under foam and firm surface conditions. Several prior reports have demonstrated an association between VF damage and balance under foam-surface, eyes-open conditions. Under these conditions compensatory proprioceptive inputs for balance are suppressed.14,15 The practical implications of foam surface testing are unclear given the paucity of real world situations where proprioceptive inputs are not available. While one previous study found that glaucoma patients compensate for VF loss when standing on a firm surface by using other senses,17 another reported that firm surface sway increased with the severity of VF damage.15 Additionally, Friedman et al.16 found differences in the likelihood of completing various firm surface balance stances when comparing patients with no, unilateral, and bilateral glaucoma. We found that greater VF damage was associated with worse balance under firm surface conditions, particularly feet together testing in which balance is challenged by a narrow stance. Of note, jerk was the balance measure most strongly associated with VF damage severity under these conditions, with each increment of VF damage causing a greater decrement in jerk compared to RMS sway (in z-score units). For example, greater peripheral VF damage was significantly associated with higher jerk values, but not RMS sway, during broad stance eyes-open firm-surface standing. Jerk reflects active postural corrections made during testing, suggesting that greater VF damage is associated more strongly with active postural corrections (defined by jerk) as opposed to directional changes in movement (captured by RMS sway). More broadly, these findings additionally suggest that other senses cannot compensate sufficiently for the impact of VF damage on standing balance.
The location of VF damage did not clearly influence the degree of balance impairment in our study. When considering visual dependence of balance as an outcome, worse IVF sensitivity or more peripheral points missed in the superior and inferior VFs were each associated with lower visual dependence when evaluated in separate models, though neither stood out as uniquely important in models designed to test for the independent importance of damage in each region. With regards to RMS sway, superior VF damage, but not inferior VF damage, was associated with AP and ML sway when both were considered in a single model. However, high collinearity was noted in these models (VIFs of 4.2–4.3 and 2.3–2.4 for models evaluating central and peripheral VF loss, respectively), suggesting that the derived regression coefficients may not be meaningful. Furthermore, when the difference between superior and inferior VF damage was considered in models with overall damage included as a covariate, no association was noted with sway measures. Our findings are in contrast to those of Black et al.15 who suggested that an excess of inferior VF damage (assessed using a hemifield difference score) was associated with greater levels of postural sway. These differences may be explained by the greater proportion of patients without VF damage in the study by Black et al.15 (56% with no VF loss per AGIS score), or the fact that the two studies used slightly different measures of balance (measures of actual body displacement in the study of Black et al.15 versus body acceleration in the current study). Given these contradictory findings, and the challenges involved in assessing the importance of location of VF damage on balance, it remains unclear if the location of VF damage meaningfully contributes to balance.
VF damage in the present study impacted the AP and ML components of sway. This finding lies in contrast to previous studies that found a statistically significant difference in AP versus ML sway.14,44 One explanation for this finding may relate to differences in the specific balance measures used (i.e., jerk versus sway) or the different techniques used to measure sway (body accelerometers versus force plates).14 A further difference in study findings is that previous work decreased somatosensory contributions to balance in the AP direction through the use of a tilted surface, but did not adequately reduce somatosensory contributions to balance in the lateral direction, so an association was found between VF loss and sway in the AP direction but not in the lateral direction.44 We also found very little difference with regards to the degree to which central and peripheral VF damage impacted balance in models designed to assess a comparable amount of central and peripheral VF damage. Our findings contradicted a prior study showing that the peripheral VF has a greater contribution to standing balance,45 but were consistent with a different study examining the function of the central versus peripheral retina on body sway,46 suggesting the need for further studies on this topic.
Balance metrics (RMS sway and jerk) were converted to a z-score to compare the impact of VF damage on multiple disparate measures and to simplify the interpretation of our data. For example, under eyes-open foam testing conditions, a 5 dB increase in IVF damage resulted in a 0.23 z-score change in RMS sway and a 0.22 z-score change for jerk. Therefore, compared to a patient with no VF damage and an average (50th percentile) level of RMS sway or jerk, an otherwise similar patient with a 5 dB lower IVF sensitivity would be in the 60th and 59th percentiles for RMS sway and jerk, respectively. Similarly, under feet together firm surface testing, every 5 dB decrement in IVF sensitivity corresponded to a 0.10 and 0.20 z-score difference in RMS sway and jerk. Therefore, compared to a patient with no VF damage and an average (50th percentile) level of RMS sway or jerk, an otherwise-similar patient with a 5 dB lower IVF sensitivity would be in the 54th and 58th percentiles for RMS sway and jerk, respectively. These findings illustrated that, while statistically significant, the contributions of VF damage to balance were modest, likely because of the multiple factors contributing to balance in the population.
Limitations of this study included the fact that patients were barefoot, which is not necessarily representative of the conditions they encounter on a daily basis. Further, patients were tested on a single firm surface even though many types of surfaces, such as tile and grass, have been suggested as common surfaces where patients experience poor balance and falls. We also only examined static balance instead of dynamic balance, which may be particularly important to falls. Finally, there is possible recruitment bias into the study, with persons more or less inclined to fall participating, or with more sick individuals deciding not to participate. We do not believe that selection bias would dramatically affect our findings.
Good balance is essential to avoid unnecessary falls. Previous research has suggested that glaucoma patients have higher rates of falling and are more afraid of falling47 independent of age, sex, and other systemic conditions.25,43 Given previous work showing associations between glaucomatous visual field loss and balance limitations as well as the current findings, future research should specifically assess to what extent balance mediates the association between VF loss and falls, or if this association is the result of other difficulties, such as, gait or poor hazard perception.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health (Bethesda, MD, USA) Grant EY022976 and Research to Prevent Blindness. The authors alone are responsible for the content and writing of this paper.
Disclosure: R.A. de Luna, None; A. Mihailovic, None; A.M. Nguyen, None; D.S. Friedman, None; L.N. Gitlin, None; P.Y. Ramulu, None
References
- 1. Rantanen T,, Guralnik JM,, Ferrucci L,, Leveille S,, Fried LP. Coimpairments: strength and balance as predictors of severe walking disability. J Gerontol A Biol Sci Med Sci. 1999; 54: M172–M176. [DOI] [PubMed] [Google Scholar]
- 2. Winter DA. Human blance and posture control during standing and walking. Gait Posture. 1995; 3: 193–214. [Google Scholar]
- 3. Era P,, Avlund K,, Jokela J,, et al. Postural balance and self-reported functional ability in 75-year-old men and women: a cross-national comparative study. J Am Geriatr Soc. 1997; 45: 21–29. [DOI] [PubMed] [Google Scholar]
- 4. Miller WC,, Deathe AB,, Speechley M,, Koval J. The influence of falling, fear of falling, and balance confidence on prosthetic mobility and social activity among individuals with a lower extremity amputation. Arch Phys Med Rehabil. 2001; 82: 1238–1244. [DOI] [PubMed] [Google Scholar]
- 5. Wolfson L,, Judge J,, Whipple R,, King M. Strength is a major factor in balance, gait, and the occurrence of falls. J Gerontol A Biol Sci Med Sci. 1995; 50: 64–67. [DOI] [PubMed] [Google Scholar]
- 6. Thurman DJ,, Stevens JA,, Rao JK. Practice parameter: assessing patients in a neurology practice for risk of falls (an evidence-based review): report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2008; 70 (6): 473–479. [DOI] [PubMed] [Google Scholar]
- 7. Alghwiri AA,, Whitney SL. Balance and falls. In: Guccione A, Wong R, Avers D, eds. Geriatric Physical Therapy. 3rd ed. St. Louis, MO: Elsevier; 2012:331–353.
- 8. Rosén E,, Sunnerhagen KS,, Kreuter M,, Rosen E. Fear of falling, balance, and gait velocity in patients with stroke. Physiother Theory Pract. 2005; 21: 113–121. [DOI] [PubMed] [Google Scholar]
- 9. Vellas BJ,, Wayne SJ,, Romero LJ,, Baumgartner RN,, Garry PJ. Fear of falling and restriction of mobility in elderly fallers. Age Ageing. 1997; 26: 189–193. [DOI] [PubMed] [Google Scholar]
- 10. Gusi N, Carmelo Adsuar J, Corzo H, del Pozo-Cruz B, Olivares PR, Parraca JA. Balance training reduces fear of falling and improves dynamic balance and isometric strength in institutionalised older people: a randomised trial. J Physiother. 2012; 58: 97–104. [DOI] [PubMed] [Google Scholar]
- 11. Hagedorn DK,, Holm E. Effects of traditional physical training and visual computer feedback training in frail elderly patients. A randomized intervention study. Eur J Phys Rehabil Med. 2010; 46: 159–168. [PubMed] [Google Scholar]
- 12. Redfern MS,, Yardley L,, Bronstein AM. Visual influences on balance. J Anxiety Disord. 2001; 15: 81–94. [DOI] [PubMed] [Google Scholar]
- 13. Massion J. Postural control system. Curr Opin Neurobiol. 1994; 4: 877–887. [DOI] [PubMed] [Google Scholar]
- 14. Kotecha A,, Richardson G,, Chopra R,, Fahy RTA,, Garway-Heath DF,, Rubin GS. Balance control in glaucoma. Invest Ophthalmol Vis Sci. 2012; 53: 7795–7801. [DOI] [PubMed] [Google Scholar]
- 15. Black AA,, Wood JM,, Lovie-Kitchin JE,, Newman BM. Visual impairment and postural sway among older adults with glaucoma. Optom Vis Sci. 2008; 85: 489–497. [DOI] [PubMed] [Google Scholar]
- 16. Friedman DS,, Freeman E,, Munoz B,, Jampel HD,, West SK. Glaucoma and mobility performance. The Salisbury Eye Evaluation Project. Ophthalmology. 2007; 114: 2232–2238. [DOI] [PubMed] [Google Scholar]
- 17. Shabana N,, Cornilleau-Peres V,, Droulez J,, Goh JCH,, Lee GSM,, Chew PTK. Postural stability in primary open angle glaucoma. Clin Exp Ophthalmol. 2005; 33: 264–273. [DOI] [PubMed] [Google Scholar]
- 18. Diniz-Filho A,, Boer ER,, Gracitelli CPB,, et al. Evaluation of postural control in patients with glaucoma using a virtual reality environment. Ophthalmology. 2015; 122: 1131–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patel M,, Fransson PA,, Lush D,, et al. The effects of foam surface properties on standing body movement. Acta Otolaryngol. 2008; 128: 952–960. [DOI] [PubMed] [Google Scholar]
- 20. Blackburn T,, Guskiewicz KM,, Petshauer MA,, Prentice WE. Balance and joint stability: the relative contributions of proprioception and muscular strength. J Sport Rehabil. 2000; 9: 315–328. [Google Scholar]
- 21. van Gestel a,, Webers C a B,, Beckers HJM,, et al. The relationship between visual field loss in glaucoma and health-related quality-of-life. Eye (Lond). 2010; 24: 1759–1769. [DOI] [PubMed] [Google Scholar]
- 22. Cheng H-C,, Guo C-Y,, Chen M-J,, Ko Y-C,, Huang N,, Liu CJ. Patient-reported vision-related quality of life differences between superior and inferior hemifield visual field defects in primary open-angle glaucoma. JAMA Ophthalmol. 2015; 133: 269–275. [DOI] [PubMed] [Google Scholar]
- 23. Black AA,, Wood JM,, Lovie-Kitchin JE. Inferior visual field reductions are associated with poorer functional status among older adults with glaucoma. Ophthalmic Physiol Opt. 2011; 31: 283–291. [DOI] [PubMed] [Google Scholar]
- 24. Wood JM,, Lacherez PF,, Black AA,, Cole MH,, Boon MY,, Kerr GK. Postural stability and gait among older adults with age-related maculopathy. Invest Ophthalmol Vis Sci. 2009; 50: 482–487. [DOI] [PubMed] [Google Scholar]
- 25. Freeman EE,, Mun∼oz B,, Rubin G,, West SK. Visual field loss increases the risk of falls in older adults: The Salisbury Eye Evaluation. Invest Opthalmology Vis Sci. 2007; 48: 4445. [DOI] [PubMed] [Google Scholar]
- 26. Boulgarides LK,, McGinty SM,, Willett JA,, Barnes CW. Use of clinical and impairment-based tests to predict falls by community-dwelling older adults. Phys Ther. 2003; 83: 328–339. [PubMed] [Google Scholar]
- 27. Chu LW,, Chi I,, Chiu AYY. Incidence and predictors of falls in the Chinese elderly. Ann Acad Med Singapore. 2005; 34: 60–72. [PubMed] [Google Scholar]
- 28. Fling BW,, Dutta GG,, Schlueter H,, Cameron MH,, Horak FB. Associations between proprioceptive neural pathway structural connectivity and balance in people with multiple sclerosis. Front Hum Neurosci. 2014; 8: 814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peel C, Sawyer Baker P, Roth DL, Brown CJ, Brodner E V, Allman RM. Assessing mobility in older adults: the UAB Study of Aging Life-Space Assessment. Phys Ther. 2005; 85: 1008–1119. [PubMed] [Google Scholar]
- 30. Willis JR,, Vitale SE,, Agrawal Y,, Ramulu PY. Visual impairment, uncorrected refractive error, and objectively measured balance in the United States. JAMA Ophthalmol. 2013; 131: 1049–1056. [DOI] [PubMed] [Google Scholar]
- 31. Mancini M,, Horak FB,, Zampieri C,, Carlson-Kuhta P,, Nutt JG,, Chiari L. Trunk accelerometry reveals postural instability in untreated Parkinson's disease. Park Relat Disord. 2011; 17: 557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mancini M,, Salarian A,, Carlson-Kuhta P,, et al. ISway: a sensitive, valid and reliable measure of postural control. J Neuroeng Rehabil. 2012; 9: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bronstein AM,, Hood JD,, Gresty MA,, Panagi C. Visual control of balance in cerebellar and parkinsonian syndromes. Brain. 1990; 113: 767–779. [DOI] [PubMed] [Google Scholar]
- 34. Kinzey SJ,, Armstrong CW. The reliability of the star-excursion test in assessing dynamic balance. J Orthop Sports Phys Ther. 1998; 27: 356–360. [DOI] [PubMed] [Google Scholar]
- 35. Odden JL,, Mihailovic A,, Boland M V,, Friedman DS,, West SK,, Ramulu PY. Evaluation of central and peripheral visual field concordance in glaucoma. Invest Ophthalmol Vis Sci. 2016; 57: 2797–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yonge A V,, Swenor BK,, Miller R,, et al. Quantifying fall-related hazards in the homes of persons with glaucoma. Ophthalmology. 2017; 124: 562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gnjidic D,, Hilmer SN,, Blyth FM,, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012; 65: 989–995. [DOI] [PubMed] [Google Scholar]
- 38. Lord SR,, Dayhew J. Visual risk factors for falls in older people. J Am Geriatr Soc. 2001; 49: 508–515. [DOI] [PubMed] [Google Scholar]
- 39. Quach L,, Galica AM,, Jones RN,, et al. The nonlinear relationship between gait speed and falls: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Geriatr Soc. 2011; 59: 1069–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Salzman B. Gait and balance disorders in older adults. Am Fam Physician. 2010; 82: 61–68. [PubMed] [Google Scholar]
- 41. Schultz AB,, Ashton-Miller JA,, Alexander NB. What leads to age and sex differences in balance maintenance and recovery? Muscle Nerve Suppl. 1997; 5: S60–S64. [PubMed] [Google Scholar]
- 42. Roberts DS,, Lin HW,, Bhattacharyya N. Health care practice patterns for balance disorders in the elderly. Laryngoscope. 2013; 123: 2539–2543. [DOI] [PubMed] [Google Scholar]
- 43. Black AA,, Wood JM,, Lovie-Kitchin JE. Inferior field loss increases rate of falls in older adults with glaucoma. Optom Vis Sci. 2011; 88: 1275–1282. [DOI] [PubMed] [Google Scholar]
- 44. Turano KA,, Dagnelie G,, Herdman SJ. Visual stabilization of posture in persons with central visual field loss. Invest Ophthalmol Vis Sci. 1996; 37: 1483–1491. [PubMed] [Google Scholar]
- 45. Berencsi A,, Ishihara M,, Imanaka K. The functional role of central and peripheral vision in the control of posture. Hum Mov Sci. 2005; 24: 689–709. [DOI] [PubMed] [Google Scholar]
- 46. Straube A,, Krafczyk S,, Paulus W,, Brandt T. Dependence of visual stabilization of postural sway on the cortical magnification factor of restricted visual fields. Exp Brain Res. 1994; 99: 501–506. [DOI] [PubMed] [Google Scholar]
- 47. Ramulu PY,, Van Landingham SW,, Massof RW,, Chan ES,, Ferrucci L,, Friedman DS. Fear of falling and visual field loss from glaucoma. Ophthalmology. 2012; 119: 1352–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.