Introduction
The costs of managing diabetes are growing at an unsustainable rate. According to the ADA website, the cost (direct and indirect) in 2012 was $245 billion and continues to grow.1 As clinicians, we fully appreciate that lowering the A1c is one of the keys to lowering the costs associated with diabetes complications. It has been estimated that lowering the A1c across all patients with diabetes by 1% could lower the cost of care by $55 billion annually.1–3 It is also well established that intensive diabetes management to lower the A1c is accompanied by the increased risks associated with glycemic variability. Indeed, emergency room visits for hypoglycemia in 2011 were ∼282,000 with an estimated $1.2 billion in hospitalization costs.1,4,5
There is abundant evidence that a necessary intervention to prevent and reduce glycemic variability is the inclusion of glucose monitoring in the treatment plan, with optimization of patient engagement in this process. The current state of glucose monitoring includes the use of A1c, self-monitoring of blood glucose (SMBG), and continuous glucose monitoring (CGM), each with its own strengths and limitations. The A1c reigns as the current standard of care and represents the average glucose over approximately a 12-week period. As an average, it has limitations in detecting hypoglycemia and glycemic variability. An example is that an average glucose of 154 mg/dL can be achieved by blood glucose levels fluctuating between 120 and 188 mg/dL, whereas a similar average glucose of 154 mg/dL can be achieved by blood glucose levels fluctuating between 50 and 258 mg/dL. Both glycemic patterns would be approximated by an A1c of 7.0% but reflecting very different treatment responses. The SMBG provides information only for points in time and is limited by the frequency of testing and the accuracy of reporting. Overnight data are very difficult to obtain and hypoglycemic episodes are often missed. SMBG is also limited by the accuracy of reporting. Langer and Mazze6 reported that among 34 patients with gestational diabetes who were instructed to do and record SMBG four times daily for 2 weeks, 33 (97%) omitted from their logbooks values that were recorded in their meter memory. Professional CGM provides a significant amount of glucose data over time that can be useful in informing therapeutic decisions for the clinician and patients. Professional CGM with the ambulatory glucose profile (AGP) has the ability to translate large amounts of glucose data into clinically relevant, useful, and actionable information. However, professional CGM has not been widely adopted in type 2 diabetes mellitus (DM) in large part because of reimbursement issues, high startup costs, and nonstandardized reporting of glycemic data and patterns.
We have seen from previous studies that glucose dynamics and glycemic variability may contribute to the progression of micro- and macrovascular disease in individuals with DM. An illustrative example for macrovascular disease has been shown between glycemic variability and carotid intima media thickening.7,8
It is postulated that glycemic variability causes an overproduction of potentially harmful reactive oxygen species and increases oxidative stress resulting in worsened endothelial function.9,10 Evidence also exists for glycemic variability as a strong predictor of hypoglycemia and poor glycemic control, irrespective of the baseline A1c.11–13 These observations make a compelling argument for the importance of glycemic variability and for minimizing it as a key treatment goal, irrespective of the form of diabetes.
The A1c is still considered the gold standard indicator of glycemic control in diabetes management in large part because of its demonstrated correlation with microvascular complications. However, the A1c represents the average blood glucose over 8 to 12 weeks and cannot characterize glucose fluctuations during the day or glycemic variability. Although beyond the scope of this report, there are other influences that can affect the A1c in ways that make this metric less reflective of true blood sugar levels over time, such as hematocrit, hemoglobinopathies, or concomitant use of certain medications, to name a few.14 Thus, for many reasons, the A1c alone is frequently insufficient to make adjustments in glucose lowering therapy. Additional information is needed to avoid extremes of hypo- and hyperglycemia. This gap in information has traditionally been filled by SMBG. However, the unreliability of SMBG, as noted previously, makes the task of adjustments unnecessarily difficult. The advent of CGM has improved our ability to make the necessary adjustments safely and effectively. Professional CGM can provide retrospective data and information that provides more accurate views of glucose patterns over a period of time compared with SMBG, facilitating better management decisions. In a recent study, the use of 5-day blinded CGM in 108 type 2 DM patients on glucose lowering therapy revealed that 49% had ≥1 hypoglycemic episode and that 75% of these episodes were asymptomatic. In addition, it was noted that glycemic variability was 28% higher in those patients using insulin versus oral agents.15 Despite such findings, the use of CGM has been limited in these patients who potentially would greatly benefit from this intervention. Use of professional CGM has been shown to reveal unrecognized hypoglycemic episodes in patients with type 2 DM. As demonstrated in the 2-week blinded sensor wear in the REPLACE Study, 1.3 h per day of hypoglycemia <70 mg/dL was detected with 0.5 h of hypoglycemia occurring during sleep.16 Of particular note, in the REPLACE Study, the average A1c was 8.6%.16
Thus there is growing and compelling evidence for the clinical utility of glucose monitoring and the more limited advantages of the “point in time” measurements, including average blood glucose values like the A1c. The demonstrated importance of glycemic variability now makes greater use of accurate, reliable continuous forms of glucose monitoring an important component of diabetes management for many patients with diabetes. In the report, we present representative cases that demonstrate the clinical value of professional CGM using the newly approved FreeStyle Libre Pro.
Case 1
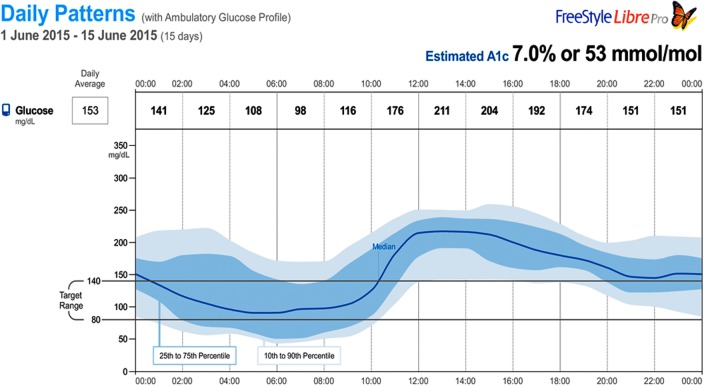
The first case is a 75 years old woman with a 17-year history of type 2 DM. She also had hypothyroidism, anxiety, and insomnia. Her medications were levothyroxine 25 μg once daily and glipizide 10 mg once daily. She was intolerant to metformin and unable to afford sitagliptin and ended up on glipizide that had been titrated up to 10 mg twice daily. Approximately 3 months earlier, she reduced her dose of glipizide to 10 mg once daily after the unexpected loss of her husband, and a couple of episodes of hypoglycemia were noted and recorded during her bereavement period. With this change in her dose, her “spells” decreased and she felt better. She performed SMBG occasionally, usually once or twice a week, primarily in the mornings. Her reported readings over the 3-month period before her visit were between 60 and 258 mg/dL and her A1c was 7.0%. Because of her history of symptomatic hypoglycemia and the wide swings recorded in her blood sugars, she had a professional CGM wear with the FreeStyle Libre Pro for 2 weeks and was encouraged to continue her SMBG as she had been doing. Upon her return and review of her CGM data, significant glycemic variability was noted accompanied by significant hypoglycemia (<80 mg/dL) and hyperglycemia (>180 mg/dL). Over the two-week wear period, 15% of the time she was hypoglycemic and 60% of the time she was hyperglycemic. In addition, her AGP (Fig. 1) revealed that most of her hypoglycemic readings occurred overnight and a significant number of these readings were <60 mg/dL. Of note, she was asymptomatic throughout this time.
FIG. 1.
AGP of 2 weeks of professional CGM: Case 1. AGP, ambulatory glucose profile; CGM, continuous glucose monitoring.
Case 2
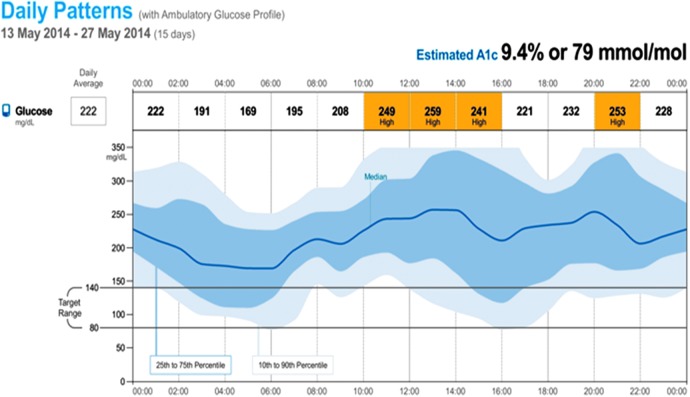
The second case is a 44-year-old male with a four-year history of type 2 DM. The patient is referred to a diabetologist for evaluation and treatment recommendations. He had been started on insulin previously because of high A1c levels and inability to achieve control on other therapies. His current insulin regimen is glargine 16 U at bedtime with a range of AM blood sugars of 80–150 mg/dL and rapid acting insulin 5 U before breakfast, 9 U before lunch, and 10 U before supper. He has no reported history of hypoglycemia and his laboratory measured A1c is 12.0%. He has been trained in diabetes self-management training, carbohydrate counting training, and an insulin adjustment based on meal content and current glucose levels. He has not always been reliable with his SMBG testing at home. He has pain in his feet and takes gabapentin 100 mg three times a day. He is a smoker and has a history of hyperlipidemia and is currently treated with simvastatin 20 mg daily. He has no history of cardiovascular, renal, or retinal complications. A 2-week professional CGM was performed with the FreeStyle Libre Pro. Upon return and review of the AGP (Fig. 2), the glycemic pattern hereunder revealed hyperglycemia with significant glycemic variability and hypoglycemic risk overnight and during the day. With the details from this additional information, the diabetologist can now formulate appropriate individualized management recommendations to address the glycemic variability, including the risks for hypoglycemia and the frequent hyperglycemic excursions.
FIG. 2.
AGP of 2 weeks of professional CGM: Case 2.
Discussion and Conclusions
The achievement of reduced time-averaged levels of blood glucose remains an important goal in diabetes management, given the demonstrated causal relationship between poor glycemic control and development of microvascular complications.17,18 In addition, the growing body of evidence demonstrating the association between hyperglycemic excursions and macrovascular complications further heightens the urgency for reducing glycemic variability as a central goal of diabetes treatment.19,20 The achievement of such glucose targets to date has depended heavily on the judicious application of SMBG. It is now clear that SMBG is an essential tool for optimal control of glycemia in diabetes management, regardless of the type of diabetes.21 Accordingly, the A1c has remained the gold standard for overall or long-term glycemic control. However, a recognized limitation of the A1c is that it does not provide information about moment-to-moment, day-to-day, or intraday changes in glucose, the essential metrics that allow assessment of the influence of lifestyle changes (nutrition, physical activity, illness, etc.), the impact of medications, and the extent of variability in glucose levels. Although many of these limitations can be addressed by appropriate use of interventions like SMBG pattern analysis, such an approach suffers the disadvantage of limitations in sampling frequency, sensitivity to user technique dependence, and a low likelihood of data capture during the overnight or early morning periods, times when hypoglycemia or rebound episodes of hyperglycemia may be encountered.22–24 Thus, the emergence of CGM technology has been an important development in the area of blood glucose assessment as a component of diabetes management. This technology eliminates the limitations of “point-in-time” measures of glucose, as reflected in FPG and PPG. It also overcomes the inadequacy of the A1c in assessing moment-to-moment, day-to-day, and overall glycemic variability. In addition, CGM effectively overcomes the shortcomings of frequent SMBG, including pattern analysis, and allows for comprehensive assessment of blood glucose at all times of day during the period of observation with minimal intervention required by the patient. As demonstrated in the cases summarized in this report, the AGP provides for a comprehensive assess of the dynamics of glucose control in a patient, including the periods of time spent in a prespecified “euglycemic” range (time in range). The graphic display of the data allows for rapid assessment of the presence, frequency, and severity of what might be previously unsuspected or unconfirmed episodes of hypoglycemia. It also reveals the timing, extent, duration, and severity of hyperglycemic excursions. Both sets of observations enable important treatment decisions designed to mitigate glycemic variability. Importantly, in the first case discussed in this report, the use of professional CGM made it possible to document that previously ambiguous and poorly defined episodes (“spells”) experienced by the patient were indeed periods of hypoglycemia sustained under circumstances where the patient's A1c reflected excellent control, and she was largely devoid of symptoms indicative of underlying hypoglycemia.
The data captured by the use of professional CGM such as the FreeStyle Libre Pro offer the promise of vastly improved treatment outcomes and likely substantial savings in the management of acute and chronic complications of diabetes. Elimination or significant reduction in glycemic excursions can reduce or prevent glycemic variability, resulting in reduced rates of micro- and macrovascular events with significant reductions of associated healthcare costs. Significant additional benefits such as improved quality of life would also be expected from access to the ability to unmask extremes of glucose excursions across the spectrum of diabetes. The emergence of and more widespread use of this technology will have tremendous impact on the improvement in design of diabetes treatment plans and subsequent improvement in outcomes.
Author Disclosure Statement
Dr. E.E.W. and Dr. J.R.G. disclose that they are advisors, consultants, and speakers for Abbott Diabetes Care.
References
- 1.ADA website Statistics About Diabetes (accessed February2016)
- 2.US Diabetes 2015 Patient Study: Market Overview. Gfk Roper Diabetes. November 2015
- 3.Aagren M, Luo W: Association between glycemic control and short-term healthcare costs among commercially insured diabetes patients in the United States. J Med Econ 2011;14:108–114 [DOI] [PubMed] [Google Scholar]
- 4.Ginde AA, Espinola JA, Camargo Jr. CA: Trends and disparities in U.S. Emergency Department Visits for Hypoglycemia, 1993–2005. Diabetes Care 2008;31:511–513 [DOI] [PubMed] [Google Scholar]
- 5.Quilliam BJ, Simeone JC, Ozbay AB, Kogut SJ: The incidence and costs of hypoglycemia in type 2 diabetes. Am J Manag Care 2011;17:673–680 [PubMed] [Google Scholar]
- 6.Langer O, Mazze R: Diabetes in pregnancy: evaluating self-monitoring performance and glycemic control with memory-based reflectance meters. Am J Obstet Gynecol 1986;155:635–637 [DOI] [PubMed] [Google Scholar]
- 7.Esposito K, Ciotola M, Carleo D, et al. : Post-meal glucose peaks at home associated with carotid intima-media thickness in type 2 diabetes. J Clin Endocrinol Metab 2008;93:1345–1350 [DOI] [PubMed] [Google Scholar]
- 8.Monnier L, Colette C, Leiter L, et al. : The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care 2007;30:185–186 [DOI] [PubMed] [Google Scholar]
- 9.Monnier L, Mas E, Ginet C, et al. : Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006;295:1681–1687 [DOI] [PubMed] [Google Scholar]
- 10.Ceriello A, Esposito K, Piconi L, et al. : Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008;57:1349–1354 [DOI] [PubMed] [Google Scholar]
- 11.Monnier L, Wojtusciszyn A, Colette C, Owens D: The contribution of glucose variability to asymptomatic hypoglycemia in persons with type 2 diabetes. Diabetes Technol Ther 2011;13:813–818 [DOI] [PubMed] [Google Scholar]
- 12.Testa MA, Gill J, Su M, et al. : Comparative effectiveness of basal-bolus versus premix analog insulin on glycemic variability and patient-centered outcomes during insulin intensification in type 1 and type 2 diabetes: a randomized, controlled, crossover trial. J Clin Endocrinol Metab 2012;97:3504–3514 [DOI] [PubMed] [Google Scholar]
- 13.Weinstock RS, Xing D, Maahs DM, et al. : Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange Clinic Registry. J Clin Endocrinol Metab 2013:98:3411–3419 [DOI] [PubMed] [Google Scholar]
- 14.Hinzmann R, Schlaeger C, Tran CT: What do we need beyond hemoglobin A1c to get the complete picture of glycemia in people with diabetes? Int J Med Sci 2012;9:665–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gehlaut RR, Dogbey GY, Schwartz FL, et al. : Hypoglycemia in type 2 diabetes – More common than you think: A continuous glucose monitoring study. J Diabetes Sci Technol 2015;9:999–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.An Evaluation of a Novel Glucose Sensing Technology in Type 2 Diabetes (REPLACE). [Clinical Trial Identifier: NCT02082184] Presented at annual Advanced Technologies and Treatment for Diabetes, Milan, Italy, February 2016 [Google Scholar]
- 17.Diabetes Control and Complications Trial (DCCT) Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 18.Klein R: Hyperglycemia and microvascular and macrovascular disease in diabetes. Diabetes Care 1995;18:258–268 [DOI] [PubMed] [Google Scholar]
- 19.Hanefeld M, Koehler C, Schaper F, et al. : Postprandial plasma glucose is an independent risk factor for increased carotid intima-media thickness in non-diabetic individuals. Atherosclerosis 1999;144:229–235 [DOI] [PubMed] [Google Scholar]
- 20.Cieriello A, Esposito K, Piconi L, et al. : Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008;57:1349–1354 [DOI] [PubMed] [Google Scholar]
- 21.Parkin CG, Davidson JA: Value of self-monitoring blood glucose pattern analysis in improving diabetes outcomes. J Diabetes Sci Technol 2009;3:500–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber KK, Lohmann T, Busch K, et al. : High frequency of unrecognized hypoglycaemias in patients with type 2 diabetes is discovered by continuous glucose monitoring. Exp Clin Endocrinol Diabetes 2007;115:491–494 [DOI] [PubMed] [Google Scholar]
- 23.Boland E, Monsod T, Delucia M, et al. : Limitations of conventional methods of self-monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with type 1 diabetes. Diabetes Care 2001;24:1858–1862 [DOI] [PubMed] [Google Scholar]
- 24.MacGowan K, Thomas W, Moran A: Spurious reporting of nocturnal hypoglycemia by CGMS in patients with tightly controlled type 1 diabetes. Diabetes Care 2002;25:1499–1503 [DOI] [PubMed] [Google Scholar]