Abstract
Herpesviral pathogenesis stems from infection of multiple cell types including the site of latency and cells that support lytic replication. Herpesviruses utilize distinct cellular pathways, including low pH endocytic pathways, to enter different pathophysiologically relevant target cells. This review details the impact of the mildly acidic mileu of endosomes on the entry of herpesviruses, with particular emphasis on herpes simplex virus 1 (HSV-1). Epithelial cells, the portal of primary HSV-1 infection, support entry via low pH endocytosis mechanisms. Mildly acidic pH triggers reversible conformational changes in the HSV-1 class III fusion protein glycoprotein B (gB). In vitro treatment of herpes simplex virions with a similar pH range inactivates infectivity, likely by prematurely activating the viral entry machinery in the absence of a target membrane. How a given herpesvirus mediates both low pH and pH-independent entry events is a key unresolved question.
Keywords: Viral entry, herpesviruses, endosomes, low pH, herpes simplex viruses, endocytosis, gB
Introduction
The fundamental cellular activity of endocytosis is commandeered by the majority of both enveloped and non-enveloped animal virus families to introduce their genetic material to the cell interior (1). Endocytic processes offer the incoming virus the acidic environment of endosomal compartments, which is often needed to mediate penetration into the cytosol. Entry of enveloped viruses into cells requires the fusion of viral and cellular membranes. Membrane fusion is driven by conformational changes in viral fusion proteins, most commonly triggered by endosomal low pH, although sometimes by receptor binding alone (2).
Herpesviruses
Herpesviruses are large enveloped DNA viruses that cause characteristic lifelong latent infections. Upon successful entry into a host cell, herpesviral genomes are deposited to the nucleus. Depending on the cell type, herpesviruses produce progeny virions (lytic infection) and/or establishment of a latent infection. A given herpesvirus can enter via more than a single cellular pathway, also dependent on the target cell. Low pH-dependent herpesviral entry is the subject of this review with a focus on herpes simplex virus 1 (HSV-1), the prototype alphaherpesvirus. HSVs remain major pathogens worldwide causing cold sores, ocular and genital infections, and rare but often fatal encephalitis (3). In cell culture, HSV-1 can enter myriad cell lines derived from multiple species and tissues. In the immune-competent host, HSV infection is typically confined to the mucosal epithelia and neurons of the peripheral nervous system. However, in cases of disseminated infection of the immune-naive and immune-compromised HSV can infect diverse organ systems including the respiratory tract, gastrointestinal tract, and the central nervous system. This broad tropism may be explained in part by HSV’s ability to utilize multiple entry routes, including low pH pathways.
Mildly acidic pH of vesicular compartments
Intracellular low pH is generated and maintained by large, membrane-associated multi-subunit vacuolar H+-ATPases (V-ATPases) (4). A rotary mechanism powered by ATP hydrolysis transports protons from the cytosol to the interior of intracellular compartments. Regulation of V-ATPase activity, and subsequently intravesicular pH, occurs in several ways. For example, increased assembly of the V1 and V0 domains of the V-ATPase correlates with a decrease in pH (5). V-ATPase phosphorylation and membrane trafficking also regulate pump activity (6). Intracellular acidification is critical for many cellular processes. Early endosome acidification triggers the dissociation of internalized ligands (e.g., low density lipoprotein or transferrin) from their receptors and is also required for endosome maturation. Acid-activated, hydrolytic enzymes resident in lysosomes are required for degradation of proteins, lipids and nucleic acids. For the majority of both enveloped and non-enveloped viruses, a decrease in pH triggers viral surface proteins to initiate viral penetration of an internal host cell membrane (7). A drop in pH may serve as a cue for the virus to escape the endocytic pathway prior to arrival in the degradative lysosome.
pH-altering lysosomotropic agents
Viral entry via a low pH route is suggested when treatment of cells with a lysosomotropic agent (Table 1) blocks infection as demonstrated for Semliki Forest virus in the seminal paper by Helenius et al. (8). In the extracellular space, ammonium chloride exists predominantly in a protonated form that is membrane-impermeable. However, the non-protonated fraction enters the cell. Once inside a low pH compartment, ammonium chloride becomes protonated in a manner inversely proportional to pH. Ammonium chloride becomes trapped and concentrated in the compartment, elevating the luminal pH. Other weak bases such as chloroquine and methylamine operate in a similar manner (9). Bafilomycin A1 is a macrolide antibiotic from Streptomyces griseus that affects intracellular pH by inhibiting the function of all the vacuolar ATPases (4, 10). It is less effective at inhibiting E1E2 ATPases and is ineffective against F1F0 ATPases found in bacteria and mitochondria (11). Bafilomycin binds to the membrane-bound V0 domain, which mediates proton translocation (12).
Table 1.
Toolbox of lysosomotropic agents that inhibit low pH viral entry.
| Agent | Class | Target | Inhibitory range |
|---|---|---|---|
| Ammonium chloride | weak base | Lumen of acidic compartment | mM |
| Bafilomycin A1 | macrolide | V0 domain of V-ATPases | nM |
| Chloroquine | weak base | Lumen of acidic compartment | mM |
| Methylamine | weak base | Lumen of acidic compartment | mM |
| Monensin | polyether | Monovalent cations | μM |
Inhibition of virus infection by these pH-altering agents may suggest an endocytosis mode of entry, but this should be demonstrated directly and independently by microscopic approaches coupled with perturbation of specific endocytosis pathways. Importantly, lysosomotropic agents also alter the pH of secretory compartments such as trans-Golgi network vesicles. Thus, when ascribing the importance of a low pH environment to viral entry it is important to distinguish between effects on endosomal compartments during entry and downstream effects such as viral protein maturation and processing. This can be achieved experimentally by specifically assaying entry events at appropriately early times post-infection. Another indirect effect of modifying intravesicular pH on virus entry might be the disruption of the normal cellular distribution of host receptors.
HSV-1 entry
The promiscuous entry activity of HSV may be explained at least partly by HSV’s ability to use multiple entry pathways, including low pH-dependent and-independent routes. Recent excellent reviews describe the current understanding of HSV receptor binding and membrane fusion in depth (13–15). Entry of HSV-1 can be initiated by attachment of virions to cell surface glycosaminoglycans, principally heparan sulfate (16). Attachment is not essential for subsequent fusion and entry. It is mediated by HSV-1 gC and to a lesser extent gB. This is followed by a requisite binding to one of several host cell entry receptors mediated by gD, including nectin-1, a calcium-independent, immunoglobulin-like cellular adhesion molecule (17–19) and HVEM, a member of the tumor necrosis factor receptor family (20). HSV-1s with individually deleted gB, gD, gH or gL fail to fuse with target cells and are incompetent for entry (21–25). Therefore, gB, gD, and gH/gL are required for virus-cell fusion and entry, but it has not been formally shown whether they are sufficient. The viral envelope contains > 10 additional viral proteins that may have roles in entry. Cell-to-cell spread of HSV is additionally facilitated by gE and gI (26). In the case of cell-cell fusion assays, gB, gD, and gH/gL expressed on the transfected cell surface in the absence of other viral gene products are both necessary and sufficient for fusion (27).
Early studies of HSV-1 entry routes
It is increasingly appreciated that herpesviruses utilize diverse entry pathways, including low pH endocytic routes to enter their pathophysiologically relevant cell targets. However, for decades HSV-1 along with HIV was considered a prototype virus that enters via fusion with the plasma membrane in a pH-independent manner (28–34). This conclusion was based on early electron microscopic images of direct entry of HSV-1 capsids at the cell surface (28, 29) and functional studies of a small subset of susceptible cells, including the African green monkey kidney cell line Vero. For many years Vero has been a workhorse for HSV researchers. Vero cells are easily propagated in culture and yield high HSV titers. HSV entry does indeed proceed by direct pH-independent penetration at the Vero plasma membrane. Virus-neutralizing antibodies block HSV-1 fusion with the surface of Vero cells (30), and HSV-1 entry into Vero and HEp-2 cells is not blocked by lysosomotropic agents (31, 32). In addition to Vero cells, human neurons, human foreskin fibroblasts, HaCaT and rat-kangaroo kidney (PtK2) cells are proposed to support HSV-1 entry by plasma membrane fusion (35–38).
Low pH-dependent, endocytic entry of HSV-1
In 2003, HeLa cells and CHO cells expressing human receptors that bind to HSV-1 gD, were shown to support HSV-1 and HSV-2 entry via a low pH, endocytic pathway (34). Over a short period of time the notion that herpesviruses utilize acid-dependent endocytosis in a cell-type dependent manner became a conventional idea. This was due to analysis of HSV entry into additional cell types, increased characterization of the pathway, and identification of similar low pH routes for other herpesviruses including Kaposi’s sarcoma-associated herpesvirus (39), human cytomegalovirus (40), varicella zoster virus (41), and equine herpesvirus 1 (42). Table 2 documents the low pH-dependent entry of different herpesvirus-cell combinations.
Table 2.
Herpesviral entry into host cells inhibited by lysosomotropic agents.
| Herpesvirus (strain, isolate) | Cell type | Agent | References |
|---|---|---|---|
| Alphaherpesvirus subfamily | |||
| HSV-1 (KOS, F) | CHO-nectin-1, CHO-HVEM cells | A, B, C, M | (34, 50, 94) |
| HSV-1 (KOS, F) | CHO-HVEM cells | A, B, M | (34, 50) |
| HSV-1 (KOSrid1) | CHO-nectin-1, CHO-nectin-2 cells, HeLa | A, M | (34, 95) |
| HSV-1 (KOS, F) | HeLa | A, B, M | (34, 50) |
| HSV-1 (MP) | CHO-nectin-1 cells | A, M | (34) |
| HSV-1 (F) | J-nectin-1-EGFR1, J-nectin1-GPI cells | A, B | (50) |
| HSV-1 | HaCaT | A, M | (36, 38) |
| HSV-1 (KOS) | Normal human epidermal keratinocytes | A, M | (36, 38) |
| HSV-1 (KOS) | Human squamous cell carcinoma (SCC13) keratinocytes | B, M | (36) |
| HSV-1 (KOS) | Primary human corneal fibroblasts | A, B, C | (94) |
| HSV-1 (KOS) | Human retinal pigment epithelial cells | A, B, C | (96) |
| HSV-1 (KOS) | Human corneal epithelial, conjunctival epithelial cells | B, C | (97, 98) |
| HSV-1 (ANG, ANGpath) | CHO-nectin-1, CHO-HVEM cells | A, M | (68, 95) |
| HSV-1 (F) | 293T-integrin αVβ3, J-nectin-1-integrin αVβ3 | B | (99) |
| HSV-2 (G) | CHO-nectin-1 cells, CHO-HVEM cells | A, M | (34) |
| VZV (POka) | CHO-K1 | A | (41) |
| EHV-1 (RacL11) | CHO-K1 | B | (42) |
| EHV-1 (HH1) | E. Derm cells | A, B | (100) |
| Betaherpesvirus subfamily | |||
| HCMV (TR) | Retinal pigment epithelial cell line ARPE-19 | A, B, C | (40) |
| HCMV (TR) | Transformed human umbilical vein endothelial cells | A, B, C | (40) |
| HCMV (fibroBADrUL131) | ARPE-19 | A, B | (101) |
| HCMV (fibroBFXwt) | ARPE-19 | A, B | (101) |
| MCMV (m74-null) | NIH 3T3 cells | A, B | (102) |
| Gammaherpesvirus subfamily | |||
| KSHV | Human foreskin fibroblasts | A, B | (39) |
| KSHV | 293T (T1H6) cells | A, B, M | (103) |
| KSHV | Human umbilical vein endothelial cells | A, B, M | (104, 105) |
| KSHV | Human dermal microvascular endothelial cells | A, B | (104) |
| KSHV | Human THP-1 monocytes | A, B | (106) |
| RRV (26–95) | Rhesus fibroblasts | A, B, M | (107) |
| MuHV-4 (G2.4) | Mouse mammary (NMuMG) epithelial cells | B, Con | (64) |
A, ammonium chloride
B, bafilomycin A1
C, chloroquine
Con, concanamycin A
M, monensin
HSV-1 entry via a low pH endocytic pathway is rapid. During an infection synchronized by first binding to the cell surface at 4 C, HSV-1 is internalized from the plasma membrane with a t½ of 8–9 min. Trafficking of the enveloped virus followed by fusion with an acidic compartment occurs by 30 minutes post-infection (25). The intracellular site of penetration is not known. Its identification is complicated given that many entering particles visualized by fluorescence microscopy are non-infectious. The pH threshold of HSV-1 gB conformational changes is ~ 6.2 to 6.4 (43), which broadly corresponds to early endosomal pH.
Despite ample evidence of herpesviral inhibition by lysosomotropic inhibitors, direct activation of membrane fusion by acidic pH remains to be demonstrated. The application of mildly acid pH to cell-bound HSV-1 does not promote membrane fusion with the plasma membrane, as it does for other viruses (44) (45–47). Cells transfected with HSV-1 gB, gD, and gH/gL will fuse with target cells without a requirement for low pH (27). However, the transfected or infected cell plasma membranes are quite different effectors of fusion than actual viral particles involved in entry. All herpesviruses acquire their envelopes from interior membranes. Thus, the viral envelope may differ from infected and transfected plasma membranes in ways that render it capable of both pH-dependent and pH-independent fusion. Entry into cells that support HSV low pH entry requires gB, gD, and gH/gL, as does entry via direct penetration (25). The envelope proteins gG, gE, gI, gJ, gM, UL45, and Us9 are dispensable for low pH entry (48, 49). Proteins that specifically function in low pH fusion and entry of HSV-1 have not been identified.
Low pH-independent entry pathways of herpesviruses
The focus of this article is the contribution of mildly acidic intracellular pH to herpesviral entry, but it is important to note that bona fide herpesvirus entry routes involve pH-independent mechanisms. These routes include endocytosis followed by pH-independent membrane fusion and fusion with the plasma membrane. When herpesvirus entry into a given cell type is only modestly inhibited by lysosomotropic agents, this may indicate that both pH-dependent and pH-independent pathways are being utilized. Also, the same cell type maintained in different laboratories can yield differential sensitivity to lysosomotropic agents (34, 36, 38, 50–52).
A low pH requirement has not been reported for Epstein Barr virus (EBV) entry into any cell type. EBV enters B cells by pH-independent endocytosis and epithelial cells by direct penetration (53, 54). Human cytomegalovirus enters fibroblasts and dendritic cells by a pH-independent mechanism (40, 55–58). Many studies using several cell types report HSV-1 entry that is resistant to inhibition by lysosomotropic agents (36, 38, 51, 52, 59, 60). Polyethylene glycol-induced fusion of HSV-1 with the plasma membrane of wild type CHO or B78 cells results in viral entry (44), suggesting that these cells are capable of mediating HSV-1 entry via a non-endocytic, pH-independent route.
Conformational changes in herpesviral fusion protein gB triggered by mildly acidic pH
Glycoprotein gB is conserved among all herpesviruses and is considered the primary fusion protein (13–15). By definition, the pre-fusion conformation of gB is present in infectious virions. Pre-fusion HSV-1 gB is altered in direct response to low pH (43, 61–63). I propose that this provides part of a molecular explanation for why herpesviruses require endosomal pH for entry. A mildly acidic pH of < 6.4 causes specific antigenic changes in virion gB. These include changes in Domains I and V, which constitute the functional region of gB containing the hydrophobic, bipartite fusion loops. Conformation is not globally altered by low pH as Domains II, III and IV are not affected (Fig. 1). Importantly, gB conformational change is also detected during viral entry by endocytosis, when the incoming virus arrives in an acidic compartment (43). Low pH-dependent antigenic changes in gB from murid herpesvirus 4 have also been detected during viral entry (64). Low pH-triggered changes in other herpesvirus gBs have not yet been reported. Mildly acidic pH causes a change in the oligomeric structure of HSV-1 gB, as demonstrated by several approaches. Following density centrifugation, gB from low pH-treated virions sediments at a lower density than gB from untreated virions. Virions previously treated with low pH and blotted to nitrocellulose have reduced reactivity with oligomer-specific monoclonal antibody DL16 (43). Analysis of gB by different iterations of mildly denaturing PAGE also detects an alteration in oligomer structure (43, 61–63, 65). The nature and significance of the change in gB quaternary structure remains to be determined.
Figure 1.
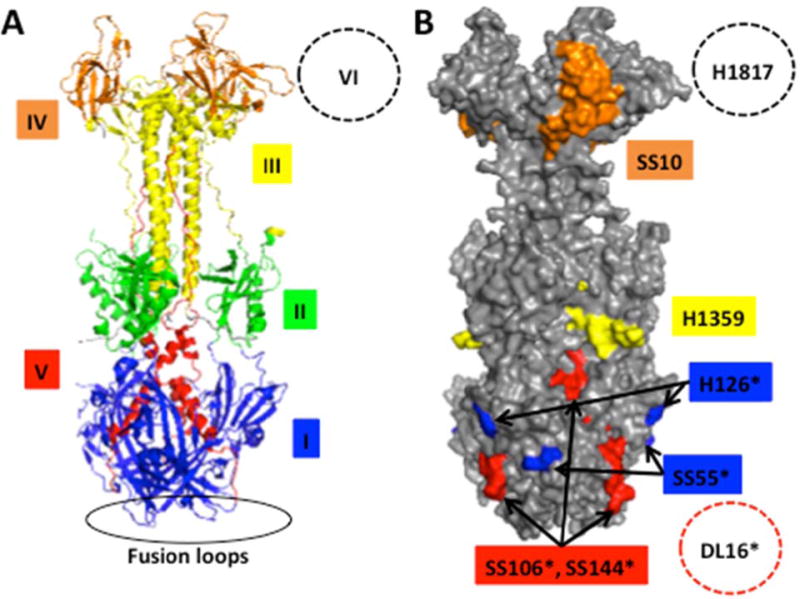
Effect of low pH on the antigenic structure of HSV-1 gB (A) gB ectodomain trimer representing a post-fusion conformation. (B) Location of monoclonal antibody binding sites. Monoclonal antibody-resistant mutations in Domain I, which contains bipartite hydrophobic fusion loops, map to amino acid residue 303 for H126 and residues 203, 335 and 199 for SS55 (108, 109). MAb H1359 to Domain III maps approximately to 487–505 (110). The MAb SS10 epitope in Domain IV maps to 640–670 based on binding to fragments and peptides (111). MAbs SS106 and SS144 to Domain V both bind to a 697–725 peptide (71). The oligomer-specific MAb DL16 binds to Domain V as determined by electron microscopy (109). The MAb H1817 epitope in Domain VI (not resolved in the structure) maps to 31–43 based on binding to fragments and peptides (111). MAbs that exhibit reduced reactivity with pre-fusion gB that has been pre-treated with pH <6.2 are marked with (*). The reactivity of unmarked MAbs is unchanged by low pH pre-treatment (43, 61, 63).
Low pH triggers gB to become more hydrophobic as measured by Triton X-114 partitioning (43, 63). This may suggest that membrane-interacting regions are revealed. Fusion-from-without (FFWO) is the rapid induction of cell fusion by the contact of virions with target cells. The combination of two amino acid mutations in gB, one in the ectodomain (V553A) and one in the cytoplasmic tail (A855V), confers FFWO activity to wild type HSV (66, 67). FFWO gB differs from wild type gB in that it has reduced reactivity with monoclonal antibodies DL16 and H126. Interestingly, low pH-treated wild type gB has reduced reactivity with these same antibodies (68), suggesting that a highly fusogenic form of gB resembles gB that has undergone low pH-triggered conformation changes. However, a more direct link between pH-triggered changes in gB and fusion activity has yet to be established. Mildly acidic pH has no detectable effect on gD antigenic structure, nor does it affect gD binding to its nectin-1 or HVEM receptors (63). Low pH affects the antigenic structure of gH/gL and some of the detected changes are irreversible (62). Whether these alterations influence viral entry is of importance and unknown. HSV-2 gB, which can substitute for HSV-1 gB in cell-cell fusion (69), also undergoes pH-triggered changes (43, 70).
Low pH alters the conformation of soluble forms of HSV gB
Mildly acidic pH alters the conformation of recombinant, purified forms of gB, indicating that low pH directly affects gB structure in absence of other virion components (43, 62, 63, 65). A membrane-truncated, ectodomain construct of gB (gB730), for which the x-ray structure is known and which currently represents the post-fusion conformation of gB (71), binds to liposomes at neutral pH via its exposed fusion loops (72). However, HSV-1 particles do not bind to liposomes at neutral pH likely because the pre-fusion form of gB present in virions has hidden fusion loops. A soluble form of gB containing the membrane-proximal region, missing from gB730, fails to bind to liposomes (73, 74). In the presence of the soluble gD-receptor HVEM and mildly acidic pH, HSV-1 associates with liposomes (75). Soluble nectin-1 receptor does not enable virus association with membranes under the same conditions for reasons that are not clear. Soluble gH/gL does not associate with liposomes at neutral pH. At low pH, it does so only in the presence of gB730 with functional fusion loops, suggesting a pH-induced association of gB and gH/gL (62). Fusion loop 2 in the gB730 x-ray structure at pH 5.5 is flipped outward relative to the neutral pH structure (65). As compared to full-length, pre-fusion gB present in the viral envelope, gB730 undergoes only limited conformational changes in response to mildly acidic pH, consistent with gB730 representing a post-fusion conformation. Another soluble form of gB undergoes conformational change(s) at pH 5.1 as measured by intrinsic fluorescence spectroscopy, a biophysical technique independent of antibody binding or detergent treatment of gB (63). In addition to virion and soluble gBs, gB from transfected cells and HSV-infected cells also undergo conformational changes in response to low pH (63, 70).
Virus fusion mechanisms are diverse, yet all involve large-scale conformational changes in fusion proteins (2). During fusion, there may be a large-scale rearrangement of gB folded domains such as proposed for another class III fusion protein, vesicular stomatitis virus G (76). However, the extent of gB conformational change(s) is an unresolved issue, as a pre-fusion structure is not yet available. The structure of a full-length, membrane-bound form of gB is distinct from the post-fusion conformation (77). Whether this structure resembles pre-fusion gB or an intermediate conformation remains to be determined. In addition to mildly acidic pH, the full range of conformational changes in gB during endocytic entry into epithelial cells may require gD, gH/gL and additional host factors, including a receptor that binds to gD.
If the detected conformational changes in gB indeed mediate membrane fusion during low pH entry, how might pH-independent entry proceed? One possibility is that surface receptor binding may functionally substitute for endosomal low pH in cases of entry via penetration at the plasma membrane. Neutral pH conformational changes in gB are expected to occur during plasma membrane fusion. They have not been identified but are predicted to be similar to those induced by pH. In the case of HSV-1, several cellular molecules bind to gB including paired immunoglobulin-like type 2 receptor alpha, non-muscle myosin IIA, and myelin-associated glycoprotein (78–80). It remains to be seen whether they trigger pH-independent conformational changes.
Reversibility of gB conformational changes
The pH-triggered conformational changes in HSV gB detected to date are reversible. These include changes in antigenic reactivity (43, 61, 63), oligomeric conformation (43, 61–63, 65), and intrinsic fluorescence (63). Reversible changes induced by pH have been described for the class III fusion proteins VSV G (76, 81) and baculovirus gp64 (82). Unlike class I and II fusion proteins, which undergo irreversible conformational changes, pre-fusion and post-fusion forms of class III proteins are proposed to exist in a pH equilibrium (83, 84). The equilibrium is shifted toward the post-fusion state at low pH. HSV-1 and pseudorabies virus travel through acidic compartments during egress (85, 86). Reversibility may allow fusion proteins to avoid nonspecific activation during assembly and egress through the low pH environment of the secretory pathway. While reversibility is a hallmark of class III proteins, its molecular determinants and its relationship to fusion activity are not clear. The energy released during a reversible conformational change may be limited relative to the irreversible changes of class I fusion proteins, suggesting important ramifications for fusion and entry, such as the minimum number important ramifications for fusion and entry, such as the minimum number of trimers required for fusion (87).
Inactivation of HSV-1 infectivity by low pH
Inactivation of virions by pretreatment with mildly acidic pH is a trait common to viruses that enter via an acid-activated mechanism. In the absence of a target membrane, low pH prematurely activates the fusion protein, so when the pH-treated virion is added to cells, it is incompetent for fusion and entry (88, 89). Viruses that enter exclusively via a pH-independent mechanism, such as Sendai virus (90) and HIV (91), are typically not inactivated by low pH pre-treatment.
The infectivity of isolated HSV particles exposed to low pH in vitro is rapidly and irreversibly reduced in a temperature-dependent manner (34). Inactivation begins at pH ~ 6.0. The part(s) of the virion that are inactivated is not known. The pH-dependent changes in gB conformation detected thus far are likely not responsible for inactivation since they are reversible. FFWO strains of HSV-1 with highly fusogenic forms of gB are sensitive to low pH inactivation similar to wild type strains (61). HSV-1 mutants individually deleted for envelope proteins gG, gE, gI, gJ, gM, UL45, and Us9 are acid-inactivated similar to wild type HSV (48, 49), suggesting that none of these proteins is responsible for low pH inactivation. The target of inactivation may be one or more components of the required entry machinery, gD, gH/gL, a region of gB, or one of the remaining envelope proteins yet to be analyzed. Infectivity of varicella-zoster virions is also diminished by pretreatment with mildly acidic pH (41). Identification of the virion target of herpesvirus inactivation by mildly acidic pH and the mechanism of inactivation may reveal key features of the low pH entry process.
Penetration of cell-bound herpesvirions is irreversibly blocked by exposure to a highly acidic glycine or sodium citrate buffer of pH 3.0 (92, 93). Inactivation of non-penetrated, non-internalized attached herpesvirions by pH 3.0 buffer is often employed as an experimental step in determination of the kinetics of viral uptake from the cell surface. It remains to be seen whether inactivation by pH 3.0 and the more physiologically relevant mildly acidic pH share a similar mechanism.
Summary and future efforts
It has become increasingly clear that herpesviruses utilize endosomal acidic pH entry pathways in a cell-specific manner. The precise activating role that pH plays is a key unanswered question. The full complement of herpesviral and host factors required for cell entry has remained elusive. Endosomal pH is likely one of several cues for herpesviral entry, including cellular receptors that bind either to the conserved herpesviral gB or gH or to subfamily-specific envelope proteins. Two or more of these cellular triggers may be needed to complete the entry process. The sequence of these virus-host interactions and where endosomal pH fits in is an outstanding question. If fusion can proceed in acid-dependent and independent manners, how similar is the execution of fusion? The acidic compartment that serves as the site of intracellular fusion following endocytic uptake also remains to be identified. Low pH-dependent conformational changes in the core fusion protein gB have been identified, but their specific role in the fusion mechanism is another critical unresolved issue.
Synopsis.
Herpesviridae comprise a large family of enveloped DNA viruses that utilize several cellular entry pathways in a cell type dependent manner. This review focuses primarily on our understanding of low pH entry mechanisms, with emphasis on the prototype alphaherpesvirus, herpes simplex virus 1 (HSV-1). In our model of infection, HSV-1 enters epithelial cells by a mildly acidic endosomal pathway, and HSV-1 entry into neurons, the site of latency, occurs by direct penetration at the cell surface. Low pH-triggered conformational changes in envelope glycoproteins, particularly the main fusion protein gB, are also described.
Acknowledgments
Work in the author’s laboratory is supported by Public Health Service grant AI119159 from the National Institute of Allergy and Infectious Diseases. Thank you to Darin Weed for constructing the figure and to Hector C. Aguilar and the members of my laboratory for critical reading of the manuscript.
Footnotes
The author declares no conflict of interest.
References
- 1.Cossart P, Helenius A. Endocytosis of viruses and bacteria. Cold Spring Harb Perspect Biol. 2014;6(8) doi: 10.1101/cshperspect.a016972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison SC. Viral membrane fusion. Nature structural & molecular biology. 2008;15(7):690–698. doi: 10.1038/nsmb.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roizman B, Knipe DM, Whitley RJ. Herpes simplex viruses. In: Knipe DM, Howley PM, editors. Fields Virology. 6th. Lipincott Williams & Wilkins; 2013. pp. 1823–1897. [Google Scholar]
- 4.Cotter K, Stransky L, McGuire C, Forgac M. Recent Insights into the Structure, Regulation, and Function of the V-ATPases. Trends Biochem Sci. 2015;40(10):611–622. doi: 10.1016/j.tibs.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of lysosomal function during dendritic cell maturation. Science. 2003;299(5611):1400–1403. doi: 10.1126/science.1080106. [DOI] [PubMed] [Google Scholar]
- 6.Breton S, Brown D. Regulation of luminal acidification by the V-ATPase. Physiology (Bethesda) 2013;28(5):318–329. doi: 10.1152/physiol.00007.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrow E, Nicola AV, Liu J. Multiscale perspectives of virus entry via endocytosis. Virol J. 2013;10:177. doi: 10.1186/1743-422X-10-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helenius A, Kartenbeck J, Simons K, Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980;84(2):404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Duve C. Lysosomes revisited. Eur J Biochem. 1983;137(3):391–397. doi: 10.1111/j.1432-1033.1983.tb07841.x. [DOI] [PubMed] [Google Scholar]
- 10.Werner G, Hagenmaier H, Drautz H, Baumgartner A, Zahner H. Metabolic products of microorganisms. 224. Bafilomycins, a new group of macrolide antibiotics Production, isolation, chemical structure and biological activity. J Antibiot (Tokyo) 1984;37(2):110–117. doi: 10.7164/antibiotics.37.110. [DOI] [PubMed] [Google Scholar]
- 11.Bowman EJ, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(21):7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Feng Y, Forgac M. Proton conduction and bafilomycin binding by the V0 domain of the coated vesicle V-ATPase. The Journal of biological chemistry. 1994;269(38):23518–23523. [PubMed] [Google Scholar]
- 13.Krummenacher C, Carfi A, Eisenberg RJ, Cohen GH. Entry of herpesviruses into cells: the enigma variations. Adv Exp Med Biol. 2013;790:178–195. doi: 10.1007/978-1-4614-7651-1_10. [DOI] [PubMed] [Google Scholar]
- 14.Campadelli-Fiume G, Menotti L, Avitabile E, Gianni T. Viral and cellular contributions to herpes simplex virus entry into the cell. Curr Opin Virol. 2012;2(1):28–36. doi: 10.1016/j.coviro.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Connolly SA, Jackson JO, Jardetzky TS, Longnecker R. Fusing structure and function: a structural view of the herpesvirus entry machinery. Nat Rev Microbiol. 2011;9(5):369–381. doi: 10.1038/nrmicro2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shukla D, Spear PG. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J Clin Invest. 2001;108(4):503–510. doi: 10.1172/JCI13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280(5369):1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 18.Cocchi F, Menotti L, Mirandola P, Lopez M, Campadelli-Fiume G. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J Virol. 1998;72(12):9992–10002. doi: 10.1128/jvi.72.12.9992-10002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi K, Nakanishi H, Miyahara M, Mandai K, Satoh K, Satoh A, Nishioka H, Aoki J, Nomoto A, Mizoguchi A, Takai Y. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with Afadin, a PDZ domain-containing protein. J Cell Biol. 1999;145(3):539–549. doi: 10.1083/jcb.145.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montgomery RI, Warner MS, Lum BJ, Spear PG. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87(3):427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 21.Cai WH, Gu B, Person S. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J Virol. 1988;62(8):2596–2604. doi: 10.1128/jvi.62.8.2596-2604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ligas MW, Johnson DC. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. Journal of Virology. 1988;62(5):1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forrester A, Farrell H, Wilkinson G, Kaye J, Davis-Poynter N, Minson T. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J Virol. 1992;66(1):341–348. doi: 10.1128/jvi.66.1.341-348.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roop C, Hutchinson L, Johnson DC. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J Virol. 1993;67(4):2285–2297. doi: 10.1128/jvi.67.4.2285-2297.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicola AV, Straus SE. Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J Virol. 2004;78(14):7508–7517. doi: 10.1128/JVI.78.14.7508-7517.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dingwell KS, Brunetti CR, Hendricks RL, Tang Q, Tang M, Rainbow AJ, Johnson DC. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J Virol. 1994;68(2):834–845. doi: 10.1128/jvi.68.2.834-845.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner A, Bruun B, Minson T, Browne H. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J Virol. 1998;72(1):873–875. doi: 10.1128/jvi.72.1.873-875.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan C, Rose HM, Mednis B. Electron microscopy of herpes simplex virus. I. Entry. J Virol. 1968;2(5):507–516. doi: 10.1128/jvi.2.5.507-516.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith JD, de Harven E. Herpes simplex virus and human cytomegalovirus replication in WI-38 cells. II. An ultrastructural study of viral penetration. J Virol. 1974;14(4):945–956. doi: 10.1128/jvi.14.4.945-956.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuller AO, Spear PG. Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc Natl Acad Sci U S A. 1987;84(15):5454–5458. doi: 10.1073/pnas.84.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koyama AH, Uchida T. The mode of entry of herpes simplex virus type 1 into Vero cells. Microbiol Immunol. 1987;31(2):123–130. doi: 10.1111/j.1348-0421.1987.tb03075.x. [DOI] [PubMed] [Google Scholar]
- 32.Wittels M, Spear PG. Penetration of cells by herpes simplex virus does not require a low pH-dependent endocytic pathway. Virus Res. 1991;18(2–3):271–290. doi: 10.1016/0168-1702(91)90024-p. [DOI] [PubMed] [Google Scholar]
- 33.Sodeik B, Ebersold MW, Helenius A. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J Cell Biol. 1997;136(5):1007–1021. doi: 10.1083/jcb.136.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicola AV, McEvoy AM, Straus SE. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J Virol. 2003;77(9):5324–5332. doi: 10.1128/JVI.77.9.5324-5332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lycke E, Hamark B, Johansson M, Krotochwil A, Lycke J, Svennerholm B. Herpes simplex virus infection of the human sensory neuron. An electron microscopy study. Arch Virol. 1988;101(1–2):87–104. doi: 10.1007/BF01314654. [DOI] [PubMed] [Google Scholar]
- 36.Nicola AV, Hou J, Major EO, Straus SE. Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytic pathway. J Virol. 2005;79(12):7609–7616. doi: 10.1128/JVI.79.12.7609-7616.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maurer UE, Sodeik B, Grunewald K. Native 3D intermediates of membrane fusion in herpes simplex virus 1 entry. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(30):10559–10564. doi: 10.1073/pnas.0801674105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahn E, Petermann P, Hsu MJ, Rixon FJ, Knebel-Morsdorf D. Entry pathways of herpes simplex virus type 1 into human keratinocytes are dynamin-and cholesterol-dependent. PLoS One. 2011;6(10):e25464. doi: 10.1371/journal.pone.0025464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akula SM, Naranatt PP, Walia NS, Wang FZ, Fegley B, Chandran B. Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) infection of human fibroblast cells occurs through endocytosis. J Virol. 2003;77(14):7978–7990. doi: 10.1128/JVI.77.14.7978-7990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryckman BJ, Jarvis MA, Drummond DD, Nelson JA, Johnson DC. Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. J Virol. 2006;80(2):710–722. doi: 10.1128/JVI.80.2.710-722.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finnen RL, Mizokami KR, Banfield BW, Cai GY, Simpson SA, Pizer LI, Levin MJ. Postentry events are responsible for restriction of productive varicella-zoster virus infection in Chinese hamster ovary cells. J Virol. 2006;80(21):10325–10334. doi: 10.1128/JVI.00939-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frampton AR, Jr, Stolz DB, Uchida H, Goins WF, Cohen JB, Glorioso JC. Equine herpesvirus 1 enters cells by two different pathways, and infection requires the activation of the cellular kinase ROCK1. J Virol. 2007;81(20):10879–10889. doi: 10.1128/JVI.00504-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dollery SJ, Delboy MG, Nicola AV. Low pH-induced conformational change in herpes simplex virus glycoprotein B. J Virol. 2010;84(8):3759–3766. doi: 10.1128/JVI.02573-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker EB, Pritchard SM, Cunha CW, Aguilar HC, Nicola AV. Polyethylene glycol-mediated fusion of herpes simplex type 1 virions with the plasma membrane of cells that support endocytic entry. Virol J. 2015 doi: 10.1186/s12985-015-0423-0. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Helenius A, Marsh M, White J. Inhibition of Semliki forest virus penetration by lysosomotropic weak bases. The Journal of general virology. 1982;58(Pt 1):47–61. doi: 10.1099/0022-1317-58-1-47. [DOI] [PubMed] [Google Scholar]
- 46.Marsh M, Wellsteed J, Kern H, Harms E, Helenius A. Monensin inhibits Semliki Forest virus penetration into culture cells. Proceedings of the National Academy of Sciences of the United States of America. 1982;79(17):5297–5301. doi: 10.1073/pnas.79.17.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marsh M, Bron R. SFV infection in CHO cells: cell-type specific restrictions to productive virus entry at the cell surface. J Cell Sci. 1997;110(Pt 1):95–103. doi: 10.1242/jcs.110.1.95. [DOI] [PubMed] [Google Scholar]
- 48.Dollery SJ, Lane KD, Delboy MG, Roller DG, Nicola AV. Role of the UL45 protein in herpes simplex virus entry via low pH-dependent endocytosis and its relationship to the conformation and function of glycoprotein B. Virus Res. 2010;149(1):115–118. doi: 10.1016/j.virusres.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Komala Sari T, Pritchard SM, Cunha CW, Wudiri GA, Laws EI, Aguilar HC, Taus NS, Nicola AV. Contributions of herpes simplex virus type 1 envelope proteins to entry by endocytosis. J Virol. 2013;87(24):13922–13926. doi: 10.1128/JVI.02500-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gianni T, Campadelli-Fiume G, Menotti L. Entry of herpes simplex virus mediated by chimeric forms of nectin1 retargeted to endosomes or to lipid rafts occurs through acidic endosomes. J Virol. 2004;78(22):12268–12276. doi: 10.1128/JVI.78.22.12268-12276.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chowdhury S, Chouljenko VN, Naderi M, Kousoulas KG. The amino terminus of herpes simplex virus 1 glycoprotein K is required for virion entry via the paired immunoglobulin-like type-2 receptor alpha. Journal of virology. 2013;87(6):3305–3313. doi: 10.1128/JVI.02982-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Devadas D, Koithan T, Diestel R, Prank U, Sodeik B, Dohner K. Herpes simplex virus internalization into epithelial cells requires Na+/H+ exchangers and p21-activated kinases but neither clathrin-nor caveolin-mediated endocytosis. Journal of Virology. 2014;88(22):13378–13395. doi: 10.1128/JVI.03631-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nemerow GR, Cooper NR. Early events in the infection of human B lymphocytes by Epstein-Barr virus: the internalization process. Virology. 1984;132(1):186–198. doi: 10.1016/0042-6822(84)90102-8. [DOI] [PubMed] [Google Scholar]
- 54.Miller N, Hutt-Fletcher LM. Epstein-Barr virus enters B cells and epithelial cells by different routes. J Virol. 1992;66(6):3409–3414. doi: 10.1128/jvi.66.6.3409-3414.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Compton T, Nepomuceno RR, Nowlin DM. Human cytomegalovirus penetrates host cells by pH-independent fusion at the cell surface. Virology. 1992;191(1):387–395. doi: 10.1016/0042-6822(92)90200-9. [DOI] [PubMed] [Google Scholar]
- 56.Vanarsdall AL, Wisner TW, Lei H, Kazlauskas A, Johnson DC. PDGF receptor-alpha does not promote HCMV entry into epithelial and endothelial cells but increased quantities stimulate entry by an abnormal pathway. PLoS Pathog. 2012;8(9):e1002905. doi: 10.1371/journal.ppat.1002905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haspot F, Lavault A, Sinzger C, Laib Sampaio K, Stierhof YD, Pilet P, Bressolette-Bodin C, Halary F. Human cytomegalovirus entry into dendritic cells occurs via a macropinocytosis-like pathway in a pH-independent and cholesterol-dependent manner. PLoS One. 2012;7(4):e34795. doi: 10.1371/journal.pone.0034795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hetzenecker S, Helenius A, Krzyzaniak MA. HCMV induces macropinocytosis for host cell entry in fibroblasts. Traffic. 2015 doi: 10.1111/tra.12355. [DOI] [PubMed] [Google Scholar]
- 59.Milne RS, Nicola AV, Whitbeck JC, Eisenberg RJ, Cohen GH. Glycoprotein D receptor-dependent, low-pH-independent endocytic entry of herpes simplex virus type 1. J Virol. 2005;79(11):6655–6663. doi: 10.1128/JVI.79.11.6655-6663.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gianni T, Cerretani A, Dubois R, Salvioli S, Blystone SS, Rey F, Campadelli-Fiume G. Herpes simplex virus glycoproteins H/L bind to cells independently of {alpha}V{beta}3 integrin and inhibit virus entry, and their constitutive expression restricts infection. J Virol. 2010;84(8):4013–4025. doi: 10.1128/JVI.02502-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siekavizza-Robles CR, Dollery SJ, Nicola AV. Reversible conformational change in herpes simplex virus glycoprotein B with fusion-from-without activity is triggered by mildly acidic pH. Virol J. 2010;7:352. doi: 10.1186/1743-422X-7-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cairns TM, Whitbeck JC, Lou H, Heldwein EE, Chowdary TK, Eisenberg RJ, Cohen GH. Capturing the herpes simplex virus core fusion complex (gB-gH/gL) in an acidic environment. J Virol. 2011;85(13):6175–6184. doi: 10.1128/JVI.00119-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dollery SJ, Wright CC, Johnson DC, Nicola AV. Low-pH-dependent changes in the conformation and oligomeric state of the prefusion form of herpes simplex virus glycoprotein B are separable from fusion activity. J Virol. 2011;85(19):9964–9973. doi: 10.1128/JVI.05291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gillet L, Colaco S, Stevenson PG. Glycoprotein B switches conformation during murid herpesvirus 4 entry. J Gen Virol. 2008;89(Pt 6):1352–1363. doi: 10.1099/vir.0.83519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stampfer SD, Lou H, Cohen GH, Eisenberg RJ, Heldwein EE. Structural basis of local, pH-dependent conformational changes in glycoprotein B from herpes simplex virus type 1. J Virol. 2010;84(24):12924–12933. doi: 10.1128/JVI.01750-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Falke D, Knoblich A, Muller S. Fusion from without induced by herpes simplex virus type 1. Intervirology. 1985;24(4):211–219. doi: 10.1159/000149645. [DOI] [PubMed] [Google Scholar]
- 67.Saharkhiz-Langroodi A, Holland TC. Identification of the fusion-from-without determinants of herpes simplex virus type 1 glycoprotein B. Virology. 1997;227(1):153–159. doi: 10.1006/viro.1996.8327. [DOI] [PubMed] [Google Scholar]
- 68.Roller DG, Dollery SJ, Doyle JL, Nicola AV. Structure-function analysis of herpes simplex virus glycoprotein B with fusion-from-without activity. Virology. 2008;382(2):207–216. doi: 10.1016/j.virol.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 69.Muggeridge MI. Characterization of cell-cell fusion mediated by herpes simplex virus 2 glycoproteins gB, gD, gH and gL in transfected cells. J Gen Virol. 2000;81(Pt 8):2017–2027. doi: 10.1099/0022-1317-81-8-2017. [DOI] [PubMed] [Google Scholar]
- 70.Muggeridge MI. Glycoprotein B of herpes simplex virus 2 has more than one intracellular conformation and is altered by low pH. J Virol. 2012;86(12):6444–6456. doi: 10.1128/JVI.06668-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heldwein EE, Lou H, Bender FC, Cohen GH, Eisenberg RJ, Harrison SC. Crystal structure of glycoprotein B from herpes simplex virus 1. Science. 2006;313(5784):217–220. doi: 10.1126/science.1126548. [DOI] [PubMed] [Google Scholar]
- 72.Hannah BP, Cairns TM, Bender FC, Whitbeck JC, Lou H, Eisenberg RJ, Cohen GH. Herpes simplex virus glycoprotein B associates with target membranes via its fusion loops. J Virol. 2009;83(13):6825–6836. doi: 10.1128/JVI.00301-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shelly SS, Cairns TM, Whitbeck JC, Lou H, Krummenacher C, Cohen GH, Eisenberg RJ. The membrane-proximal region (MPR) of herpes simplex virus gB regulates association of the fusion loops with lipid membranes. MBio. 2012;3(6) doi: 10.1128/mBio.00429-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maurer UE, Zeev-Ben-Mordehai T, Pandurangan AP, Cairns TM, Hannah BP, Whitbeck JC, Eisenberg RJ, Cohen GH, Topf M, Huiskonen JT, Grunewald K. The structure of herpesvirus fusion glycoprotein B-bilayer complex reveals the protein-membrane and lateral protein-protein interaction. Structure. 2013;21(8):1396–1405. doi: 10.1016/j.str.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whitbeck JC, Zuo Y, Milne RS, Cohen GH, Eisenberg RJ. Stable association of herpes simplex virus with target membranes is triggered by low pH in the presence of the gD receptor, HVEM. J Virol. 2006;80(8):3773–3780. doi: 10.1128/JVI.80.8.3773-3780.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roche S, Rey FA, Gaudin Y, Bressanelli S. Structure of the prefusion form of the vesicular stomatitis virus glycoprotein g. Science. 2007;315(5813):843–848. doi: 10.1126/science.1135710. [DOI] [PubMed] [Google Scholar]
- 77.Zeev-Ben-Mordehai T, Vasishtan D, Hernandez Duran A, Vollmer B, White P, Prasad Pandurangan A, Siebert CA, Topf M, Grunewald K. Two distinct trimeric conformations of natively membrane-anchored full-length herpes simplex virus 1 glycoprotein B. Proceedings of the National Academy of Sciences of the United States of America. 2016 doi: 10.1073/pnas.1523234113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Satoh T, Arii J, Suenaga T, Wang J, Kogure A, Uehori J, Arase N, Shiratori I, Tanaka S, Kawaguchi Y, Spear PG, Lanier LL, Arase H. PILRalpha is a herpes simplex virus-1 entry coreceptor that associates with glycoprotein B. Cell. 2008;132(6):935–944. doi: 10.1016/j.cell.2008.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arii J, Goto H, Suenaga T, Oyama M, Kozuka-Hata H, Imai T, Minowa A, Akashi H, Arase H, Kawaoka Y, Kawaguchi Y. Non-muscle myosin IIA is a functional entry receptor for herpes simplex virus-1. Nature. 2010;467(7317):859–862. doi: 10.1038/nature09420. [DOI] [PubMed] [Google Scholar]
- 80.Suenaga T, Satoh T, Somboonthum P, Kawaguchi Y, Mori Y, Arase H. Myelin-associated glycoprotein mediates membrane fusion and entry of neurotropic herpesviruses. Proc Natl Acad Sci U S A. 2010;107(2):866–871. doi: 10.1073/pnas.0913351107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Doms RW, Keller DS, Helenius A, Balch WE. Role for adenosine triphosphate in regulating the assembly and transport of vesicular stomatitis virus G protein trimers. J Cell Biol. 1987;105(5):1957–1969. doi: 10.1083/jcb.105.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou J, Blissard GW. Mapping the conformational epitope of a neutralizing antibody (AcV1) directed against the AcMNPV GP64 protein. Virology. 2006;352(2):427–437. doi: 10.1016/j.virol.2006.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gaudin Y, Tuffereau C, Segretain D, Knossow M, Flamand A. Reversible conformational changes and fusion activity of rabies virus glycoprotein. J Virol. 1991;65(9):4853–4859. doi: 10.1128/jvi.65.9.4853-4859.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roche S, Albertini AA, Lepault J, Bressanelli S, Gaudin Y. Structures of vesicular stomatitis virus glycoprotein: membrane fusion revisited. Cell Mol Life Sci. 2008;65(11):1716–1728. doi: 10.1007/s00018-008-7534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harley CA, Dasgupta A, Wilson DW. Characterization of herpes simplex virus-containing organelles by subcellular fractionation: role for organelle acidification in assembly of infectious particles. Journal of Virology. 2001;75(3):1236–1251. doi: 10.1128/JVI.75.3.1236-1251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hogue IB, Bosse JB, Hu JR, Thiberge SY, Enquist LW. Cellular mechanisms of alpha herpesvirus egress: live cell fluorescence microscopy of pseudorabies virus exocytosis. PLoS Pathog. 2014;10(12):e1004535. doi: 10.1371/journal.ppat.1004535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roche S, Gaudin Y. Characterization of the equilibrium between the native and fusion-inactive conformation of rabies virus glycoprotein indicates that the fusion complex is made of several trimers. Virology. 2002;297(1):128–135. doi: 10.1006/viro.2002.1429. [DOI] [PubMed] [Google Scholar]
- 88.Edwards J, Mann E, Brown DT. Conformational changes in Sindbis virus envelope proteins accompanying exposure to low pH. Journal of Virology. 1983;45(3):1090–1097. doi: 10.1128/jvi.45.3.1090-1097.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bron R, Wahlberg JM, Garoff H, Wilschut J. Membrane fusion of Semliki Forest virus in a model system: correlation between fusion kinetics and structural changes in the envelope glycoprotein. EMBO J. 1993;12(2):693–701. doi: 10.1002/j.1460-2075.1993.tb05703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chejanovsky N, Henis YI, Loyter A. Fusion of fluorescently labeled Sendai virus envelopes with living cultured cells as monitored by fluorescence dequenching. Exp Cell Res. 1986;164(2):353–365. doi: 10.1016/0014-4827(86)90034-0. [DOI] [PubMed] [Google Scholar]
- 91.McClure MO, Marsh M, Weiss RA. Human immunodeficiency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. Embo J. 1988;7(2):513–518. doi: 10.1002/j.1460-2075.1988.tb02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang AS, Wagner RR. Penetration of herpes simplex virus into human epidermoid cells. Proc Soc Exp Biol Med. 1964;116:863–869. doi: 10.3181/00379727-116-29392. [DOI] [PubMed] [Google Scholar]
- 93.Johnson DC, Spear PG. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982;43(3):1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clement C, Tiwari V, Scanlan PM, Valyi-Nagy T, Yue BY, Shukla D. A novel role for phagocytosis-like uptake in herpes simplex virus entry. J Cell Biol. 2006;174(7):1009–1021. doi: 10.1083/jcb.200509155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Delboy MG, Patterson JL, Hollander AM, Nicola AV. Nectin-2-mediated entry of a syncytial strain of herpes simplex virus via pH-independent fusion with the plasma membrane of Chinese hamster ovary cells. Virol J. 2006;3(1):105. doi: 10.1186/1743-422X-3-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tiwari V, Oh MJ, Kovacs M, Shukla SY, Valyi-Nagy T, Shukla D. Role for nectin-1 in herpes simplex virus 1 entry and spread in human retinal pigment epithelial cells. Febs J. 2008;275(21):5272–5285. doi: 10.1111/j.1742-4658.2008.06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shah A, Farooq AV, Tiwari V, Kim MJ, Shukla D. HSV-1 infection of human corneal epithelial cells: receptor-mediated entry and trends of re-infection. Mol Vis. 2010;16:2476–2486. [PMC free article] [PubMed] [Google Scholar]
- 98.Akhtar J, Tiwari V, Oh MJ, Kovacs M, Jani A, Kovacs SK, Valyi-Nagy T, Shukla D. HVEM and nectin-1 are the major mediators of herpes simplex virus 1 (HSV-1) entry into human conjunctival epithelium. Invest Ophthalmol Vis Sci. 2008;49(9):4026–4035. doi: 10.1167/iovs.08-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gianni T, Gatta V, Campadelli-Fiume G. {alpha}V{beta}3-integrin routes herpes simplex virus to an entry pathway dependent on cholesterol-rich lipid rafts and dynamin2. Proc Natl Acad Sci U S A. 2010;107(51):22260–22265. doi: 10.1073/pnas.1014923108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hasebe R, Sasaki M, Sawa H, Wada R, Umemura T, Kimura T. Infectious entry of equine herpesvirus-1 into host cells through different endocytic pathways. Virology. 2009;393(2):198–209. doi: 10.1016/j.virol.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang D, Yu QC, Schroer J, Murphy E, Shenk T. Human cytomegalovirus uses two distinct pathways to enter retinal pigmented epithelial cells. Proc Natl Acad Sci U S A. 2007;104(50):20037–20042. doi: 10.1073/pnas.0709704104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scrivano L, Esterlechner J, Muhlbach H, Ettischer N, Hagen C, Grunewald K, Mohr CA, Ruzsics Z, Koszinowski U, Adler B. The m74 gene product of murine cytomegalovirus (MCMV) is a functional homolog of human CMV gO and determines the entry pathway of MCMV. Journal of Virology. 2010;84(9):4469–4480. doi: 10.1128/JVI.02441-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Inoue N, Winter J, Lal RB, Offermann MK, Koyano S. Characterization of entry mechanisms of human herpesvirus 8 by using an Rta-dependent reporter cell line. Journal of Virology. 2003;77(14):8147–8152. doi: 10.1128/JVI.77.14.8147-8152.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Raghu H, Sharma-Walia N, Veettil MV, Sadagopan S, Chandran B. Kaposi’s sarcoma-associated herpesvirus utilizes an actin polymerization-dependent macropinocytic pathway to enter human dermal microvascular endothelial and human umbilical vein endothelial cells. Journal of Virology. 2009;83(10):4895–4911. doi: 10.1128/JVI.02498-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Greene W, Gao SJ. Actin dynamics regulate multiple endosomal steps during Kaposi’s sarcoma-associated herpesvirus entry and trafficking in endothelial cells. PLoS Pathog. 2009;5(7):e1000512. doi: 10.1371/journal.ppat.1000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kerur N, Veettil MV, Sharma-Walia N, Sadagopan S, Bottero V, Paul AG, Chandran B. Characterization of entry and infection of monocytic THP-1 cells by Kaposi’s sarcoma associated herpesvirus (KSHV): role of heparan sulfate, DC-SIGN, integrins and signaling. Virology. 2010;406(1):103–116. doi: 10.1016/j.virol.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang W, Zhou F, Greene W, Gao SJ. Rhesus rhadinovirus infection of rhesus fibroblasts occurs through clathrin-mediated endocytosis. Journal of Virology. 2010;84(22):11709–11717. doi: 10.1128/JVI.01429-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kousoulas KG, Huo B, Pereira L. Antibody-resistant mutations in cross-reactive and type-specific epitopes of herpes simplex virus 1 glycoprotein B map in separate domains. Virology. 1988;166(2):423–431. doi: 10.1016/0042-6822(88)90513-2. [DOI] [PubMed] [Google Scholar]
- 109.Cairns TM, Fontana J, Huang ZY, Whitbeck JC, Atanasiu D, Rao S, Shelly SS, Lou H, Ponce de Leon M, Steven AC, Eisenberg RJ, Cohen GH. Mechanism of neutralization of herpes simplex virus by antibodies directed at the fusion domain of glycoprotein B. Journal of Virology. 2014;88(5):2677–2689. doi: 10.1128/JVI.03200-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pereira L, Ali M, Kousoulas K, Huo B, Banks T. Domain structure of herpes simplex virus 1 glycoprotein B: neutralizing epitopes map in regions of continuous and discontinuous residues. Virology. 1989;172(1):11–24. doi: 10.1016/0042-6822(89)90102-5. [DOI] [PubMed] [Google Scholar]
- 111.Bender FC, Samanta M, Heldwein EE, de Leon MP, Bilman E, Lou H, Whitbeck JC, Eisenberg RJ, Cohen GH. Antigenic and mutational analyses of herpes simplex virus glycoprotein B reveal four functional regions. J Virol. 2007;81(8):3827–3841. doi: 10.1128/JVI.02710-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


