Abstract
The Médecins Sans Frontières (MSF) ethics review board (ERB) has been solicited in an unprecedented way to provide advice and review research protocols in an ‘emergency’ mode during the recent Ebola epidemic. Twenty-seven Ebola-related study protocols were reviewed between March 2014 and August 2015, ranging from epidemiological research, to behavioural research, infectivity studies and clinical trials with investigational products at (very) early development stages. This article examines the MSF ERB’s experience addressing issues related to both the process of review and substantive ethical issues in this context. These topics include lack of policies regarding blood sample collection and use, and engaging communities regarding their storage and future use; exclusion of pregnant women from clinical and vaccine trials; and the difficulty of implementing timely and high-quality qualitative/anthropological research to consider potential upfront harms. Having noticed different standards across ethics committees (ECs), we propose that when multiple ethics reviews of clinical and vaccine trials are carried out during a public health emergency they should be accompanied by transparent communication between the ECs involved. The MSF ERB experience should trigger a broader discussion on the ‘optimal’ ethics review in an emergency outbreak and what enduring structural changes are needed to improve the ethics review process.
Introduction
The size and scale of the 2014–2015 Ebola virus disease (EVD) epidemic in West Africa, due to the Guinean Zaire Strain, were unprecedented. Starting in March 2014, when the Ebola virus was identified as the causative agent of a haemorrhagic fever infection in Guinea (Baize et al., 2014; Dixon and Schafer, 2014) the epidemic eventually spread over several countries, Guinea, Liberia and Sierra Leona being the most affected. While Médecins Sans Frontières (MSF) has helped to control Ebola outbreaks in nine countries over the past 20 years, the recent epidemic that raged in West Africa proved uniquely catastrophic. MSF’s West Africa Ebola response started in March 2014 pushing it to the limits and beyond. MSF set up 15 Ebola management and transit1 centres in the three most affected countries (Marchbein, 2015). As of November 2015, a total of 10,363 Ebola-suspected patients had been admitted to MSF treatment centres, which corresponds to 36 per cent of all confirmed, probable and suspect cases reported by World Health Organization (WHO). In total 5226 confirmed Ebola cases were treated by MSF, a third of the confirmed cases reported by WHO (2015).
Although Ebola outbreaks have been occurring for almost 40 years (WHO, 1978) no specific vaccines or curative treatments for EVD had been developed up to 2014 (Goodman, 2014). Thus, throughout (most of) the West African epidemic, EVD treatment has primarily been supportive, including intravenous or oral rehydration, nutrition, pain killers, treatment of co-infections with antibacterial and antimalarial drugs and blood transfusion when appropriate (Feldmann and Geisbert, 2011; Chertow et al., 2014). Despite these interventions, mortality remained high (WHO Ebola Response Team, 2014).
The unprecedented scale of this Ebola epidemic, the lack of effective preventive or curative interventions, the fact that the outbreak initially seemed to be able to cross national borders and reach high-income countries, combined with mathematical models predicting continued explosive growth led to an equally unprecedented, although delayed and initially slow-paced response (Lancet editorial, 2014; Gulland, 2015) to develop and test new treatments and vaccines to prevent and control the further spread of the epidemic. WHO held a series of relevant meetings in August and September 2014. The Emergency Committee invoked the International Health Regulations to declare the EVD outbreak a public health emergency of international concern. Shortly thereafter, an ethics panel convened by WHO unanimously declared that it was ethically permissible to use unregistered interventions (including those not previously used or tested in humans) in the treatment of Ebola patients assuming that certain conditions were met. Subsequent meetings elaborated principles related to ethics and clinical trials (WHO, 2014a, b, c). Soon after the WHO panel, MSF convened an internal meeting where reluctance concerning clinical trials was voiced, since these require significant resources for complying with Good Clinical Practices and regulatory requirements, thus diverting attention and energy away from patients in very resource-limited settings. The appropriateness of ‘compassionate use’ in this context was also discussed.
Given that MSF was providing care to Ebola patients since the start of the epidemic, has considerable experience in managing Ebola treatment centres and was attending an important proportion of suspected and confirmed Ebola cases in the three most affected countries, the organization was immediately contacted by many commercial and non-commercial research groups interested in testing preventive and therapeutic tools to fight EVD. MSF itself as a medical humanitarian organization felt a moral duty to carry out research to improve its interventions in several aspects, despite some initial reluctance, expressed at the meeting noted above. In addition, the question of ‘compassionate use’, later reworded into ‘monitored emergency use’ of experimental drugs and vaccine2 appeared early on in the epidemic, and some humanitarian workers evacuated to their countries of origin received experimental interventions. The issue of ‘monitored emergency use’ was felt as very relevant at MSF. After initial general joint reflection among MSF medical directors and the MSF ethics review board (ERB), these programs were submitted for ethical review, aiming at ensuring the best possible protection of subjects and the best possible knowledge gain.
Since the beginning of 2014, the MSF ERB has been solicited in an exceptional way to provide advice and review research protocols in an ‘emergency’ mode. This article examines the MSF ERB experience in reviewing the research MSF has carried out, either as the coordinating or as a partner institution, during the West African Ebola epidemic. The functioning of the MSF ERB has been described in two previous articles and will not be further discussed here (Schopper et al., 2009, 2015). We will describe MSF ERB activity, address issues related to the review process itself and analyse substantive ethical issues raised by EVD research (proposed or conducted). Particular focus will be placed on how the ERB responded to a surge in protocols under tight timelines and the problems raised by simultaneous reviews by multiple committees. Key substantive ethical issues include collection, storage and future use of blood samples; exclusion of pregnant women; and poorly designed anthropological research. Some of these present such a high degree of complexity that they deserve further analysis elsewhere.
Thankfully, the devastating West African Ebola emergency appears to be at an end, leading to a time for reflection on necessary reforms before the next pandemic (Moon et al., 2015). We hope that the MSF ERB experience may trigger a broader discussion of ‘optimal’ procedures for ethics review in an emergency outbreak and enduring structural changes needed to improve ethics review processes in such complex, extraordinary, high-stake settings.
Procedural Issues Faced by the MSF ERB during the West African EVD Epidemic
Range of Protocols Reviewed and MSF ERB Workload
The MSF ERB reviewed 27 Ebola-related study protocols between March 2014 and August 2015 (Table 1). Research protocols reviewed covered a variety of different types of study, including epidemiological research, behavioural research, infectivity studies 3 and clinical trials with investigational products at (very) early development stages. Some other elements added to the complexity of the ethics review process are as follows: research was done during an epidemic, involving highly vulnerable populations faced with a deadly disease; research activities spanned over three low-income countries, with fragile health systems, poor infrastructure and little experience of medical research (and in particular for clinical trials); some research was carried out in collaboration with academic institutions, which required setting up new collaborative research agreements very quickly; and clinical trials with investigational products were sponsored by not-for-profit organizations other than MSF and/or by pharmaceutical companies. For drug and vaccine trials, the pharmaceutical companies played a key role even if they were not the legal sponsor of the trial, since they provided the investigational product. For example, in one trial which investigated the efficiency of a new drug, the legal sponsor (a University) could not oppose the company’s decision to withdraw the drug soon after inclusion of the first patients. The studies reviewed by the MSF ERB were all carried out inside MSF sites and with few exceptions directly involved patients or health staff at MSF Ebola Treatment Centres (ETCs).
Table 1.
Ebola-related study protocols reviewed by the MSF ERB between March 2014 and August 2015
| Type of research | Number of protocols | MSF’s relationship with the proposed research |
|---|---|---|
| Clinical trials testing treatments of unknown effectiveness (Brincidofivir/NCT02271347; Favipiravir/NCT02329054; Convalescent plasma/NCT02342171) | 3 | MSF not the legal sponsor; in partnership with academic institutions |
| Vaccine trial (Part A: ring vaccination; Part B: vaccination of front line workers)/PACTR201503001057193 | Two distinct protocols for two substudies (Parts A and B) | WHO was the sponsor, but MSF took responsibility for implementing Part B, as this was carried out on frontline workers employed and under the responsibility of MSF |
| Anthropological/qualitative studies (perceptions, knowledge, experience EVD) | 9 | Seven MSF sponsored, including one generic protocola Two developed and sponsored by academic institutions as companion research to clinical trials |
| Infectivity studies (one on patients; one on environmental samples) | 2 | MSF sponsored |
| Retrospective mortality studies | 2 | MSF sponsored |
| Diagnostic research (Cepheid Xpert diagnostic test) | 1 | MSF sponsored |
| ‘Compassionate use’/MEUURI (ZMapp. Mil77, Favipiravir, Convalescent plasma) | 5 | MSF sponsored |
| Pre- and post-exposure prophylaxis (VSV-ZEBO, ZMapp) | 3 | MSF sponsored |
| Total | 27 |
aGeneric protocol: A protocol submitted for ERB review and approval before the exact location of the disaster is known. This procedure was adopted by the MSF ERB to facilitate research in disaster situations. Once the disaster happens and location is known, the details can be filled in and subjected to expedited review to allow the protocol to be applied in a specific setting.
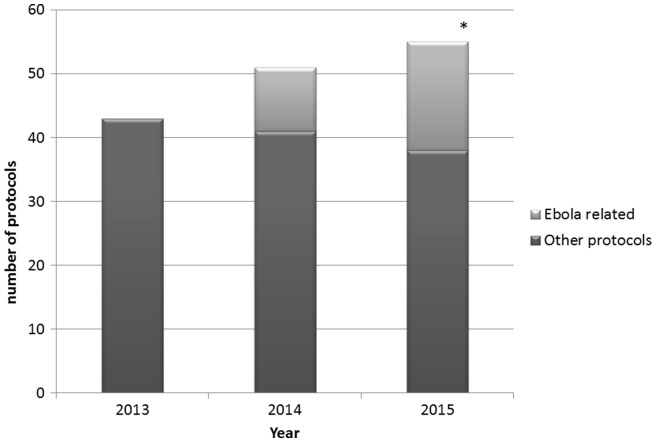
As shown in Figure 1 Ebola-related protocols added substantially to the MSF ERB’s workload particularly in 2015. In the first semester of 2015, 17 new protocols were submitted while the epidemic was declining.
Figure 1.
MSF ERB workload before and during the Ebola epidemic. *Includes only protocols submitted by August 2015.
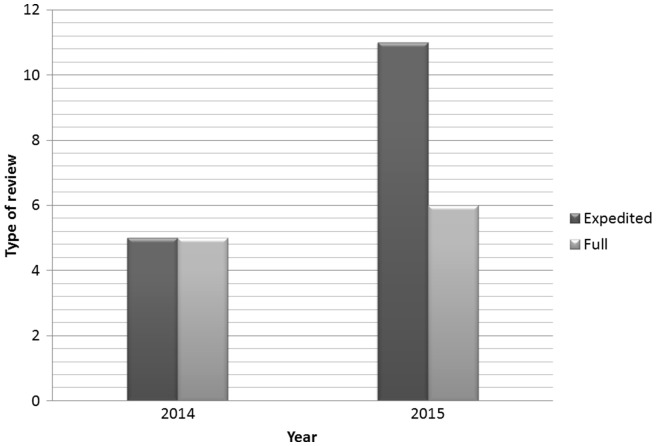
The workload did not only increase due to the numbers of protocols, but also because a higher proportion of the protocols than usual needed full review which implies involvement of all MSF ERB members (Schopper et al., 2009). Usually less than 10 per cent of protocols submitted to the MSF ERB are submitted to full review. As shown in Figure 2 in the case of Ebola-related research overall 40 per cent of the protocols required full review, and up to half of them in 2014 including most of the clinical trials. The combination of increased workload and unpredictability of the timing of new submissions created difficulties for individual members given other work and personal commitments.
Figure 2.
Type of review for Ebola protocols. *The vaccine trial is counted as two protocols: Parts A and B.
With the exception of four protocols, all studies were submitted for MSF ERB review after a 2-day meeting, held in mid-October 2014, and attended by all MSF ERB members, MSF medical directors and relevant researchers. The exchanges at that meeting provided mutual understanding of the upcoming ethical challenges and a consensus as to how they could be addressed. For example, detailed discussions were held about trial methodology, and a consensus developed to use historical controls rather than randomization between treatment options or placebos. This discussion was heavily influenced by the need to remain true to MSF’s humanitarian mission and an ethical concern to ensure that no potential participants were disadvantaged in terms of a chance to receive a potentially life-saving intervention in a situation with such high mortality rates. This very usefully informed the review process without affecting the independence of the MSF ERB judgements. It was essential in ensuring the rapid turnaround and a constructive debate before, during and after ethics review.
Timeliness and Consistency of MSF ERB Review
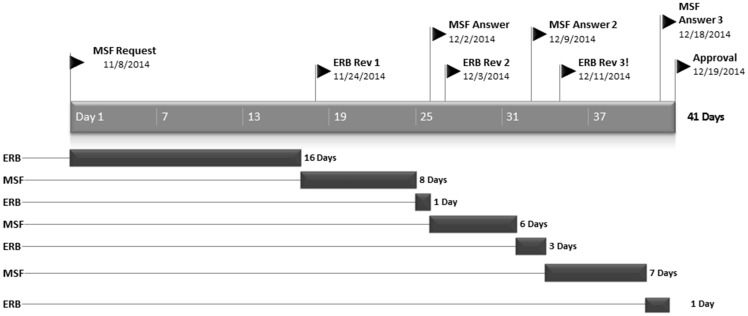
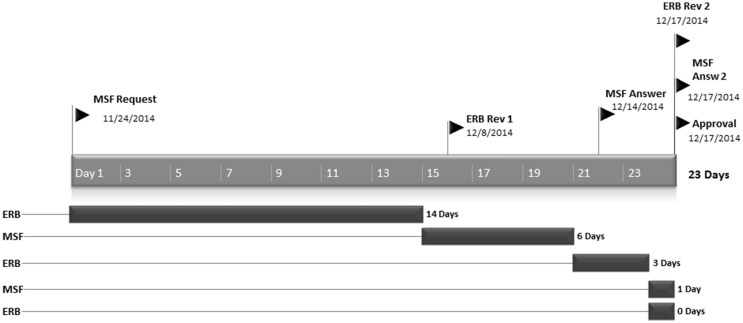
The MSF ERB responded promptly to the initial request as well as the responses of the investigators. On average it took 12.4 days to provide a review after the initial request. Thereafter, the MSF ERB responded within 1–4 days to the replies of the investigators. This was particularly remarkable for the clinical and vaccine trials meaning complex protocols with unknown risk of potential harm, thus needing full review and particularly stringent scrutiny. Figures 3 and 4 illustrate this with the timeline for two complex clinical protocols.
Figure 3.
Timeline of review process for RAPIDE Trial (Brincidofivir, Liberia).
Figure 4.
Timeline of review process for Jiki Trial (Favipiravir, Guinea).
The average time from request to approval of the five clinical research protocols by the MSF ERB was 35 days, ranging from 23 to 43 days. MSF ERB follow-up after initial approval of each protocol included further formal approval of one or several amendments, assessment of interim reports and assessing the potential impact of the research.
As the MSF ERB reviewed many Ebola-related protocols almost simultaneously, it was important for us to ensure similar standards, in particular across the clinical trials and the protocols for monitored emergency use of unregistered interventions (MEUURI), a term adopted by the WHO Ebola Ethics Working Group to replace the expression ‘compassionate use’. Major ethical issues, such as storage and future use of blood/plasma samples, post-trial access, consent of highly vulnerable patients, inclusion of pregnant women were identified across trials. Although the design was different, many ethical issues raised by the vaccine trial were comparable. Some of these will be briefly discussed later in this article.
How to Deal with Multiple Review Processes
Most Ebola research protocols reviewed by the MSF ERB during the West African EVD were also reviewed by other institutional review boards (IRBs) and research ethics committees (ECs) in the countries where studies were undertaken and at key research partner institutions. These included: the University of Liberia, Pacific Institute for Research and Evaluation, Institutional Research Board; Comité National d'Ethique pour la Recherche en Santé de Guinée; the WHO Ethical Review Committee; the ECs of the Oxford University and of the London School of Hygiene and Tropical Medicine in the UK; the IRB of the Institute of Tropical Medicine; the Ethics Evaluation Committee of INSERM (the French National Institute of Health and Medical Research); and the Ethics Committee of the Antwerp University Hospital in Belgium. Of the 27 protocols reviewed by the MSF ERB 11 were in addition reviewed by a national EC only, while 7 were reviewed by a national EC and one or several ECs/IRBs from other international institutions or academic centres. While it is the rule of the MSF ERB that all protocols must also be submitted to the relevant national EC, in eight instances this was not the case. As pre- and post-exposure prophylaxis could initially only be provided outside of the affected country, these protocols did not receive local approval, but underwent ethical scrutiny in the country where prophylactic treatment was going to be provided. Furthermore, two protocols were exempted from review; in two instances advice only was provided on MEUURI: one generic protocol was pre-approved and only later submitted to the national EC and one low-risk qualitative study was carried out in the UK.
Double ethics review, in the study country(ies) and in the country of the sponsor, has been recommended for ensuring protection of subjects and their communities in medical research sponsored, coordinated or funded by foreign organizations in low and middle income countries (Ravinetto et al., 2011). But it also raises important regulatory and feasibility issues, e.g. it is recommended but generally not mandatory—with the notable exception of a few African regulators (Ethiopia Ministry of Science and Technology, 2014; Uganda National Council for Science and Technology, 2014), and its usefulness may be limited if the different IRBs/ECs cannot have access to each other’s reviews (in practice, often only researchers have access to the different reviews). During the West African EVD epidemic, one study group positively evaluated the receipt of multiple ethics reviews, noting that the complementarity of the reviews raised the quality of the research and the protection of participants and community (De Crop et al., 2016) but they also argued that more should be done to harmonize the multiple review process in ‘urgency’ situations, by fostering direct dialogue among ECs. This is in line with the recommendation of the WHO’s 2009 report on Research Ethics in International Epidemic Response (‘it is crucial to streamline the ethics review process and to establish appropriate, flexible mechanisms and procedures for ethical oversight not limited to traditional REC systems’) (WHO, 2009), and with the more recent WHO Background Document on Potential Ebola Therapies and Vaccines (WHO, 2014b), which stated that ‘flexible approaches are required to harmonize various review processes, and ensure that the various ECs can review the projects simultaneously and share and discuss the review outcomes with each other’. Unfortunately, during this EVD epidemic no coordination mechanisms were set to implement such a ‘simultaneous ethical review’. This may be due to a mix of different reasons, e.g. there was no time to be spent in organizing meetings and teleconferences; there was no specific funding for supporting physical meetings (up to six bodies were involved in the review of a same protocol, and it is not clear who would be responsible to fund the meetings); and the availability of new technologies for audio- and videoconferences has been seriously underused. In some cases the MSF ERB (as well as, presumably, the other concerned IRB/ECs) was initially not even informed about which other ethical review boards or committees were going to assess a same protocol.
The lack of joint ethical review for Ebola clinical trials, or at least of proactive communication among ECs reviewing the same Ebola trial protocol(s), may have been a missed opportunity to streamline the different reviews into a comprehensive review (potentially, an advantage for the researchers) and to foster dialogue and mutual learning among the different ECs/IRBs (potentially, an advantage for the ECs/IRBs). Even if it ensured independence of judgment, the fact that the different ECs/IRBs were ‘blinded’ to each other’s reviews prevented the exchange of views, shared approaches to new dilemmas and agreement on common review policies (e.g. the MSF ERB possibility to pre-review generic protocols for research in emergency (Schopper et al., 2015) could be interesting also for other EC/IRBs). Also, the lack of mutual knowledge and communication may have potentially weakened the impact of the ethics review. The striking differences in issues raised by ECs in certain instances may have been difficult to reconcile for the researchers (De Crop M et al., 2016). Also, research groups might ‘shop around’ ECs, and use one favourable opinion from a less stringent body to influence other ECs to agree on it.
These difficulties, even if exacerbated by the context of a public health emergency, are not unique to Ebola research. While double or multiple reviews of international research protocols are common, we are not aware of any successful mechanisms for joint ethics review (Meslin et al., 2014; Tierney et al., 2013), with a single reported exception from the field of human African trypanosomiasis. A ‘pre-review’ process of the protocols for DNDi and partners’ pivotal Phase II/III fexinidazole study brought together in 2012 the Ethics unit of WHO and representatives of ECs from six African countries with the aim of reducing duplication of efforts (Coleman et al., 2015). This model of joint ‘pre-review’ of the ethical challenges foreseeable within a whole research plan, may be feasible for a public health emergency, if representatives of relevant organizations are pre-identified, available to meet on short notice, the resultant consensus review is agreed in advance to be acceptable to all and some organization takes on the role of coordination and engagement with the researchers.
Substantive Ethical Issues of Concern
The fact that research was done during an epidemic, involving highly vulnerable populations faced with a deadly disease and that research activities spanned over three low-income countries, with poor health infrastructure and governance—where trust in the healthcare system and/or international healthcare providers was revealed to already be lacking—implied in our view the need for especially careful and stringent ethics review. The question of the trial designs to be chosen that dominated the ethical debate in 2014 had been resolved upfront in discussions with MSF based on the WHO consultation (WHO, 2014c). Some of the issues the MSF ERB consistently raised in its reviews were how to obtain truly informed consent of patients facing a high chance of death in a high-safety environment, the high potential for therapeutic or philanthropic misconception4 as MSF was the only healthcare provider for Ebola patients in many instances (Ahmad and Mahmud, 2010) and community engagement. In addition, two questions were particularly difficult to address in a satisfactory manner: the storage of blood samples for future use and the exclusion of pregnant women in clinical trials.
Blood Samples: Collection, Storage, Future
Twelve of the 27 protocols reviewed involved the collection of blood samples as one of the inclusion criteria and as part of efficacy/safety assessment in the trial. The MSF ERB policy (Schopper et al., 2009) requires investigators to clearly indicate if blood samples will be destroyed after use in the study and if not that ECs and patients be informed about storage and potential future use of the samples. In case of potential future use, our policy requires that the patient be explicitly informed about the conditions for future use, such as not using the sample for any commercial purposes, and a statement that samples will only be used in any future research after ethics approval and that results will be made available to all those in need by using all possible ‘access mechanisms’. We also require that patients be given the option of declining future use. It became clear that the views on this matter were strikingly different between ECs. Given the low to non-existent capacity for safe storage and analysis of the samples in the three countries concerned, samples were exported to other countries. The MSF ERB insisted on proper Material Transfer Agreements as is standard international practice.5
In addition, we insisted on the urgent need to develop policies regarding sample collection and use and engage communities regarding their storage and future use. This does not only apply to blood collected during research with explicit consent, but more generally to biological specimens collected while providing clinical care over the duration of the outbreak. It is estimated that overall about 80,000 specimens now exist in the aftermath of the epidemic. Their precise location is unreported (Marchbein, 2015). There are plans to create a biobank to drive research and development for diagnostics, vaccines and therapeutics as well as for further understanding the molecular and virological characteristics of Ebola virus. The nature of this biobank, where it will be located, how it will be governed, who will have access, all remain unclear.
It is important to note that no explicit informed consent was received for the use of the clinically collected specimens for the purpose of future research. It might be argued that consent is no longer feasible and failure to use these specimens to advance scientific knowledge would be unethical. While it may be tenable to claim that the urgency of a response trumped the necessity for appropriate collection of samples and that consent was not feasible in the context of the outbreak, given well-documented concerns around biopiracy and exploitation in the context of colonial past, it is a moral failure not to have considered how this issue may be addressed in other ways. Given the special value commonly placed on blood in West African countries, adverse implications regarding local communities’ trust in healthcare systems and/or international healthcare providers should not be underestimated. There clearly needs to be an agreed upon process as to how the samples are stored and anonymized, how researchers access the samples and who has the authority to permit access and use. Proactive strategies to encourage scientific capacity in the affected nations could be an enduring positive legacy from the outbreak.
Excluding Pregnant Women
Pregnant women infected with Ebola were excluded from two of the clinical trial proposals and the vaccine trial reviewed by the MSF ERB despite evidence of higher than average fatality rates during pregnancy (Baggi et al., 2014) and extremely poor survival rates of the foetus. Among the clinical trials with therapeutic agents, only the convalescent plasma studies did not stipulate pregnancy as an exclusion criterion. In our view there was no strong justification for excluding pregnant women from the Ebola clinical trials. The reason usually cited for excluding pregnant women was possible embryotoxicity. Most made this prediction based on data from animal models. However, across Ebola outbreaks since 1995 there is a reported 100 per cent fatality rate for foetuses of infected women (Kitching et al., 2015). Only recently has one baby survived after delivery in an ETC,6 and she was given investigational treatments from birth. Her mother sadly died from the Ebola infection. In addition, data collected during an outbreak in 1996 in the Democratic Republic of the Congo indicate that pregnant women are more likely to have haemorrhagic complications associated with delivery or termination, including vaginal and uterine bleeding (Jamieson et al., 2014). Because of the context and known outcomes of Ebola on pregnancies, we found prioritizing concerns about potential foetal harm to be inappropriate and so focused on how to better evaluate the risks and potential benefits of trial participation by women during pregnancy. There was no evidence that the investigational agents would cause specific risks to the women’s health in pregnancy and the evidence indicates extremely low chance of in utero survival. As a result, we chose to explore how the women’s interests could be fairly respected, and whether the exclusion criteria were justifiable. Our conclusion, based on the information above, was that their exclusion promoted unfair access to any potential benefits of enrolment in these trials so we chose instead to emphasize inclusion of pregnant women with appropriate informed consent.
Special consideration of pregnant women is a universally accepted norm in research ethics guidance, but recent refinements in its application emphasize that exclusion should not be applied without strong justification (Baylis and Halperin, 2012; Foulkes et al., 2011; Lyerly et al., 2008). It is increasingly recognized that wholesale exclusion of pregnant women from clinical trials unjustly denies them access to the benefits of participating in research, and makes them more vulnerable to potentially unsafe practices such as informal off-label use of medications or unguided use in special access programs which normally do not have formal oversight. The CIOMS Guideline 15 and 197 reflect this discourse in bioethics which is increasingly advocating for researchers to provide justifications for exclusion rather than inclusion as has been the tradition so far. While we accept there is no individual right to access to research interventions, we argue there is a right not to be excluded from research for unreasonable factors. Thus we considered the following to help evaluate risks and benefits for pregnant women in clinical trials for Ebola, and would use these in other contexts as well. The primary question was: Is the exclusion of pregnant women justified in this study? This was examined on the following considerations: What are the clinical outcomes for pregnancies in this context? What is the average time from infection/diagnosis to mortality? Is it altered during pregnancy? Do the criteria for exclusion include a balance of risks and benefits for both the foetus and the mother? What phase is the study? Are there any existing safety data for the intervention? What other interventions are available? Whose interests should be considered in our determination? As new disease outbreaks emerge, such as Zika virus, it is less and less likely that excluding pregnant women from research will be feasible. With proper study design and proper research oversight risks in research can be mitigated or justified for any vulnerable population—including pregnant women.
In the interests of timely review, we did not refuse to approve the protocols that excluded pregnant women. We did however stress our concern and MSF responded where it could by implementing MEUURI programmes so pregnant women could obtain experimental treatments that were unavailable for them in trials. As it turned out, these were not drugs undergoing ‘First in Human’ trials, which it had been anticipated would be the only option, but new (‘off-label’) use of existing treatments already used for care of pregnant women with other infections or in the case of blood plasma transfusions which pregnant women are not usually excluded from (Kombe et al., 2016). We saw this as a tolerable compromise. We emphasized the importance of proper oversight and data collection on the treatment and outcomes for these patients, because even incremental knowledge of this sort can contribute to improving outcomes for pregnant women in future outbreaks. Interestingly, only one national EC and one further EC of an international organization took a similar stand on this issue.
Qualitative Research
In total, the MSF ERB received nine qualitative studies for review. The issues addressed in the protocols ranged from program assessment to knowledge, attitudes, perceptions and practices related to acceptability, suitability and barriers to Ebola trials and interventions.
EVD being a highly infectious disease with rapid progression and high fatality rate, it is critical to understand the knowledge, attitudes, beliefs and practices of the community of research to adapt the response. In West Africa, low levels of formal education, religious notoriety, deep-seated cultural beliefs and values, suspicion and mistrust of government and international agencies must be taken into consideration in control efforts and when implementing clinical and vaccine trials (Gidado et al., 2015; Abramowitz et al., 2015; Peters, 2014). For example, to limit further spread of the disease, MSF and other agencies initially isolated and quickly disposed of the bodies of those who died to avoid further infections. But this disappearance of friends and relatives when they were taken to a treatment unit raised suspicions and fears (Omidian and Monger, 2014).
In reviewing qualitative research protocols, the MSF ERB noted that implementing timely qualitative research, safeguarding ethical standards, seemed problematic because:
Lines between what constitutes qualitative data collection for programmatic purposes (often labelled rapid assessment) and actual research are blurred;
Qualitative studies are considered to be low risk because no physical interventions or biological materials are introduced or extracted from study participants, and thus less importance may be given to sound study design and ethical issues which can arise in the conduct of qualitative studies. Such an approach can underestimate the potential harms that can affect respondents and communities that take part in qualitative studies (Townsend et al., 2010);
Most ECs/IRBs are not equipped to examine such research protocols as they have limited experience reviewing them, little social science and anthropological expertise, and because of the ‘low risk’ perception they tend not to seek external advice (Molyneux et al., 2009).
Among the nine, seven qualitative studies were explicitly designed to improve the programmatic response, while two studies were meant to inform and accompany clinical trials. In some cases, the research questions and methodologies were not well laid out (which is not unexpected in this context) and may have had potential of generating unreliable and invalid results. This would not only be a waste of resources, but present the potential danger of eroding trust in science. In addition, qualitative studies may pose serious and often underestimated risks, such as confidentiality breaches during or after focus group discussions, social stigma and psychological harms (Oakes, 2002). The MSF ERB thus insisted that qualitative study designs require meticulous planning and intensive engagement with the community of research, and need to carefully weigh benefits and risks. Two studies were cancelled after our initial ethics review raised such concerns.
Researchers should not be left alone to address the challenges which appear in Ebola-like contexts. The challenge of time pressure in emergency settings should, in particular, be countered by funding agencies that should make resources available for sound qualitative research at the same time as for clinical trials; and by ECs, that may accept the development of generic qualitative protocols that could be quickly and immediately adapted and implemented in the case of an emergency outbreak.
Lessons Learned from the MSF ERB Experience
A paper published in 2009 discussed the ethical challenges of research on Ebola and Marburg haemorrhagic fevers (Calain et al., 2009). The lack of ethical oversight, challenges to obtaining voluntary informed consent, issues raised by collection of blood samples and future use without consent, the need to design generic research protocols and standards prior to emergencies and the establishment or strengthening of national and independent ethics research committees were highlighted. So what have we additionally learned from the West African EVD epidemic?
One of the remarkable features of this Ebola epidemic has certainly been the great effort to rapidly set up clinical and vaccine trials, including ethics assessments. The ethics panel convened by WHO recommended proceeding to do research on 11 August and the first patients were enrolled in late December 2014. This is a remarkably quick turnaround, given that funding mechanisms, research partnerships, protocols, drug procurement and clinical sites set-up, all started from scratch. Ethics review of the trials was all the more important as this was emergency research carried out in an extremely vulnerable population faced with a deadly disease. In our experience, it is possible to provide relevant and very timely ethical advice even for complex protocols. We strictly applied the usual ethical standards, as defined in the MSF ERB framework (Schopper et al., 2015), reinforcing them as needed. In our view ethics corner-cutting is neither justified nor necessary even in an emergency. This however implied a very considerable effort on the part of the MSF ERB which could not possibly have been sustained for an extended period of time. In the future the substantially increased workload of ECs should be taken into consideration in advanced planning of emergency research.
In our view the multiple ethics reviews of clinical and vaccine trials carried out during a public health emergency should be accompanied by transparent communication between ECs reviewing a given protocol. In some cases, the MSF ERB (as well as, presumably, the other concerned IRB/ECs) was initially not even informed about which other ethical bodies were going to assess each protocol. To be feasible and efficient, communication mechanisms should be planned well before the emergence of the next outbreak. Joint pre-review or review mechanisms would be beneficial, and they could become feasible via upfront planning and a better use of communication technologies for audio- and videoconferences, which have been seriously underused. In addition, a ‘generic pre-review meeting’ could be organized, where at the start of an outbreak representatives of the ECs/IRBs who will be likely to review the clinical trials protocols could come together to discuss general issues and approaches to foreseeable ethical dilemmas (along the lines of the joint meeting of the MSF REB and MSF Medical Directors). This raises the issue of funding such meetings. To ease communication between ethics bodies, WHO could possibly sponsor an EC register for such purposes akin to a clinical trial register.
As qualitative and anthropological research is essential to improve the response and to prepare and accompany clinical trials, more attention must be given to the soundness of research protocols and ethical oversight. Generic protocols should be prepared well in advance, so that they can be rapidly approved for a specific setting; unanticipated projects should be developed as carefully and fully as clinical trial protocols; risks should not be underestimated; qualitative researchers such as anthropologists and sociologists should be engaged early on in the development of clinical trials; and social science expertise should be included in all ECs.
The MSF ERB has consistently highlighted two major ethical issues. (i) Pregnant women should obtain access to experimental treatments either in clinical trials, or through MEUURI. (ii) The management of bio-samples is an integral element of protocol review (Schopper et al., 2009). In future outbreaks, the possibility for collecting and storing specimens should be anticipated and an explicit process should be developed to ensure the interests of patients and communities are protected, including proper informed consent and strategies for community engagement.
The big irony is that despite the rapidity with which all research was executed, the epidemic was very rapidly waning by the time the studies were initiated. Besides encouraging results from one vaccine trial, there are no significant therapeutic advances yet, although ZMapp may hold some promise (NIH, 2016). We thus know that the research effort will have to be faster and as intensive in the next epidemic. This is clearly highlighted in the recent paper on 10 essential reforms before the next pandemic (Moon et al. 2015), calling for appropriate conduct of research including improved ethical standards. However, the issue of ethical standards and oversight of Ebola protocols has been surprisingly underrepresented in the vast number of Ebola publications in 2014–2015. We hope that this article could lead to a discussion on the ‘optimal’ way for ethics review in an emergency outbreak and what enduring structural changes are needed to improve the ethics review process in response to emergencies such as infectious disease outbreaks.
Acknowledgments
The ERB members would like to thank the international medical director of MSF and past and current medical directors of the operational centres for their trust and the many open and very frank discussions. The authors are also very grateful to Patricia Chavez and Grace Ku who have provided invaluable support in the preparation of this manuscript.
Notes
1. Transit centres are short-stay centres for people to await blood test results. If the test comes back negative, they will be discharged. If positive, they will be transferred to an Ebola management centre.
2. The switch from the language of ‘compassionate use’ to the expression MEUURI, adopted by the WHO Ebola Ethics Working Group (see: http://www.who.int/csr/resources/publications/ebola/ethical-evd-therapeutics/en/, accessed: August 2016), was partly motivated by concern that (i) the language of ‘compassionate use’ seems to imply that it would be beneficial to receive the intervention in question (which may not be appropriate in the case of interventions which have not previously been tested in humans), and (ii) because ‘compassionate use’ is often technically defined as use of an (unregistered) intervention when use within a clinical trial is not possible (whereas a key question regarding the potential Ebola interventions in question was whether or not their use should actually be part of a clinical trial, which was a live possibility). The use of ‘monitored’ emphasises a key point of the WHO Ethics Panel, i.e. that we should aim to learn as much as possible from the use in question (i.e. by collecting data) whether a formal clinical trial is conducted (whereas ‘compassionate use’ does not imply/highlight a data collection imperative).
3. The infectivity studies examined the infection hazard for Ebola virus in body fluids of patients and in environmental samples.
4. Philanthropic misconception is comparable to therapeutic misconception. It is likely to exist among beneficiaries of humanitarian aid when these agencies sponsor or conduct research projects. The participants may believe that the aim of such researchers is primarily human welfare and the best interests of the individuals, instead of research.
5. This ensures that the person, whom biological samples belong to, sometimes referred to as the ‘owner’, sets the terms and conditions for the use those samples through informed consent. The institution/researcher that takes the samples then becomes the ‘custodian’ of the samples and agrees to their use as outlined by the consent. If the custodian has to send the samples to another party (if allowed by the consent) then it is custodian’s responsibility to make sure by having a material transfer agreement that use of these samples by the next party that will become the ‘possessor’ still remains confined to the boundaries of the consent under which it was taken.
6. The first newborn ever surviving Ebola was reported on 3 December 2015 by MSF https://msf.exposure.co/nubia [accessed: August 2016].
7. CIOMS Revision of the 2002 International Ethical Guidelines for Biomedical Research Involving Human Subjects draft 2015, available at: http://www.encepp.eu/documents/e-mailgroupsCIOMSdraft30sept2015_gsf.pdf [accessed: 07 October 2015].
References
- Abramowitz S. A., McLean K. E., McKune S. L., Bardosh K. L., Fallah M., Monger J., Tehounge K., Omidian P. (2015). Community-Centered Responses to Ebola in Urban Liberia: The View from Below. PLoS Neglected Tropical Diseases, 9, e0003706. doi:10.1371/journal.pntd.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A., Mahmud S. M. (2010). Philanthropic Misconception. Asian Bioethics Review, 2, 154–161. [Google Scholar]
- Baggi F. M., Taybee A., Kurth A., Van Herp M., Di Caro A., Wölfel R., Günther S., Decroo T., Declerck H., Jonckheere S. (2014). Management of Pregnant Women Infected with Ebola Virus in a Treatment Centre in Guinea. Euro Surveillance, 19, pii=20983.. [DOI] [PubMed] [Google Scholar]
- Baize S., Pannetier D., Oestereich L., Rieger T., Koivogui L., Magassouba N. F., Soropogui B., Sow M. S., Keïta S., De Clerck H., Tiffany A., Dominguez G., Loua M., Traoré A., Kolié M., Malano E. R., Heleze E., Bocquin A., Mély S., Raoul H., Caro V., Cadar D., Gabriel M., Pahlmann M., Tappe D., Schmidt-Chanasit J., Impouma B., Diallo A. K., Formenty P., Van Herp M., Günther S. (2014). Emergence of Zaire Ebola Virus Disease in Guinea. New England Journal of Medicine, 371, 1418–1425. [DOI] [PubMed] [Google Scholar]
- Baylis F., Halperin S. A. (2012). Research Involving Pregnant Women: Trials and Tribulations. Clinical Investigation, 2, 139–146. [Google Scholar]
- Calain P., Fiore N., Poncin M., Hurst S. (2009). Research Ethics and International Epidemic Response: The Case of Ebola and Marburg Hemorrhagic Fevers. Public Health Ethics, 2, 7–29. [Google Scholar]
- Chertow D. S., Kleine C., Edwards J. K., Scaini R., Giuliani R., Sprecher A. (2014). Ebola Virus Disease in West Africa—Clinical Manifestations and Management. New England Journal of Medicine, 371, 2054–2057. [DOI] [PubMed] [Google Scholar]
- Coleman C. H., Ardiot C., Blesson S., Bonnin Y., Bompart F., Colonna P., Dhai A., Ecuru J., Edielu A., Hervé C., Hirsch F., Kouyaté B., Mamzer-Bruneel M. -F., Maoundé D., Martinent E., Ntsiba H., Pelé G., Quéva G., Reinmund M. -C., Sarr S. C., Sepou A., Tarral A., Tetimian D., Valverde O., Van Nieuwenhove S., Strub-Wourgaft N. (2015). Improving the Quality of Host Country Ethical Oversight of International Research: The Use of a Collaborative ‘Pre-Review’ Mechanism for a Study of Fexinidazole for Human African Trypanosomiasis. Developing World Bioethics, 15, 241–247. [DOI] [PubMed] [Google Scholar]
- De Crop M., Delamou A., van Griensven J., Ravinetto R. (2016). The Multiple Ethical Review in North-South Collaborative Research: The Experience of the Ebola-Tx Trial in Guinea. IJME, 1, 76–82. [DOI] [PubMed] [Google Scholar]
- Dixon M., Schafer I. (2014). Ebola Viral Disease Outbreak–West Africa, 2014. Morbidity and Mortality Weekly Report, 63, 548–551. [PMC free article] [PubMed] [Google Scholar]
- Ethiopia Ministry of Science and Technology (2014). National Research Ethics Review Guideline, 5th edn, available from: http://www.most.gov.et/index.php/documents/legislations/most-guidelines [accessed 12 August 2016]
- Feldmann H., Geisbert T. W. (2011). Ebola Haemorrhagic Fever. Lancet, 377, 849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes M. A., Grady C., Spong C. Y., Bates A., Clayton J. A. (2011). Clinical Research Enrolling Pregnant Women: A Workshop Summary. Journal of Women's Health, 20, 1429–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidado S. O. A., Roberts A. A., Nguku P., Nwangwu I. G., Waziri N. E., Shuaib F., Oguntimehin O., Musa E., Nzuki C., Nasidi A., Adewuyi P., Daniel T., Olayinka A., Odubanjo O., Poggensee G. (2015). Public Knowledge, Perception and Source of Information on Ebola Virus Disease—Lagos, Nigeria; September, 2014. PLOS Currents Outbreaks, 7. doi: 10.1371/currents.outbreaks.0b805cac244d700a47d6a3713ef2d6db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J. L. (2014). Studying "Secret Serums"—Toward Safe, Effective Ebola Treatments. New England Journal of Medicine, 371, 1086–1089. [DOI] [PubMed] [Google Scholar]
- Gulland A. (2015). Institutional Failure Led to Ebola Outbreak "Spiralling Out of Control," Says MSF. BMJ, 350, h1619. doi: 10.1136/bmj.h1619. [DOI] [PubMed] [Google Scholar]
- Jamieson D. J., Uyeki T. M., Callaghan W. M., Meaney-Delman D., Rasmussen S. A. (2014). What Obstetrician-Gynecologists Should Know About Ebola: A Perspective From the Centers for Disease Control and Prevention. Obstetrics and Gynecology, 124, 1005–1010. [DOI] [PubMed] [Google Scholar]
- Kitching A., Walsh A., Morgan D. (2015). Ebola in Pregnancy: Risk and Clinical Outcomes. BJOG: An International Journal of Obstetrics and Gynaecology, 122, 287–287. [DOI] [PubMed] [Google Scholar]
- Kombe F., Folayan M. O., Ambe J., Igonoh A., Abayomi A. (2016). Taking the Bull by the Horns: Ethical Considerations in the Design and Implementation of an Ebola Virus Therapy Trial. Social Science and Medicine, 148, 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancet editorial (2014). Ebola: A Failure of International Collective Action. The Lancet, 384, 637.. [DOI] [PubMed] [Google Scholar]
- Lyerly A. D., Little M. O., Faden R. (2008). The Second Wave: Toward Responsible Inclusion of Pregnant Women in Research. International Journal of Feminist Approaches to Bioethics, 1, 5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchbein D. (2015). The Response to Ebola—Looking Back and Looking Ahead: The 2015 Lasker-Bloomberg Public Service Award. JAMA, 314, 1115–1116. [DOI] [PubMed] [Google Scholar]
- Meslin E., Ayuku D., Were E. (2014). "Because It Was Hard …"; Some Lessons Developing a Joint IRB Between Moi University (Kenya) and Indiana University (USA). American Journal of Bioethics: AJOB, 14, 17–19. [DOI] [PubMed] [Google Scholar]
- Molyneux C., Goudge J., Russell S., Chuma J., Gumede T., Gilson L. (2009). Conducting Health-Related Social Science Research in Low Income Settings: Ethical Dilemmas Faced in Kenya and South Africa. Journal of International Development, 21, 309–326. [Google Scholar]
- Moon S., Sridhar D., Pate M. A., Jha A. K., Clinton C., Delaunay S., Edwin V., Fallah M., Fidler D. P., Garrett L., Goosby E., Gostin L. O., Heymann D. L., Lee K., Leung G. M., Morrison J. S., Saavedra J., Tanner M., Leigh J. A., Hawkins B., Woskie L. R., Piot P. (2015). Will Ebola Change the Game? Ten Essential Reforms Before the Next Pandemic. The Report of the Harvard-LSHTM Independent Panel on the Global Response to Ebola. The Lancet, 386, 2204–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH. (2016). Experimental Ebola Drug ZMapp May Benefit Patients, but Insufficient Data to be Certain, Study Finds. In, News Releases, available from: https://www.niaid.nih.gov/news/newsreleases/2016/Pages/CROI-ZMapp.aspx [accessed 12 August 2016].
- Oakes J. M. (2002). Risks and Wrongs in Social Science Research: An Evaluator's Guide to the IRB. Evaluation Review, 26, 443–479. [DOI] [PubMed] [Google Scholar]
- Omidian P., Tehoungue K., Monger J. (2014). Medical Anthropology Study of the Ebola Virus Disease (EVD) Outbreak in Liberia/West Africa. Brazzaville: WHO Press. [Google Scholar]
- Peters M. M. (2014). Community Perceptions of Ebola Response Efforts in Liberia: Montserrado and Nimba Counties, Study Report for Oxfam, available from: http://www.ebola-anthropology.net/wp-content/uploads/2015/02/Oxfam-MMinorPeters-Liberia-Anthro-report_Dec2014.pdf [accessed 12 August 2016]
- Ravinetto R., Buve A., Halidou T., Lutumba P., Talisuna A. (2011). Double Ethical Review of North-south Collaborative Clinical Research: Hidden Paternalism or Real Partnership? Tropical Medicine and International Health, 16, 527–530. [DOI] [PubMed] [Google Scholar]
- WHO (1978). Ebola Virus Haemorrhagic Fever in Zaire, 1976. Report from an International Commission. Bulletin of the World Health Organization, 56, 271–293. [PMC free article] [PubMed] [Google Scholar]
- Schopper D., Dawson A., Upshur R., Ahmad A., Jesani A., Ravinetto R., Segelid M. J., Sheel S., Singh J. (2015). Innovations in Research Ethics Governance in Humanitarian Settings. BMC Medical Ethics, 16, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopper D., Upshur R., Francine M., Singh J., Sheel S., Ahmad A., van Dongen E. (2009). Research Ethics Review in Humanitarian Contexts: The Experience of the Independent Ethics Review Board of Médecins Sans Frontières. PLoS Medicine, 6, e1000115. doi: 10.1371/journal.pmed.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Ebola Response Team (2014). Ebola Virus Disease in West Africa—The First 9 Months of the Epidemic and Forward Projections. The New England Journal of Medicine, 2014 371, 1481–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uganda National Council for Science and Technology (2014). National Guidelines for Research Involving Humans as Research Participants, available from: http://www.uncst.go.ug/dmdocuments/Human%20Subjects%20Protection%20Guidelines%20July%202014.pdf [accessed 12 August 2016]
- Tierney W. M., Nyandiko W. N., Siika A. M., Wools-Kaloustian K., Sidle J. E., Kiplagat J., Bell A., Inui T. S. (2013). "These are Good Problems to Have…": Establishing a Collaborative Research Partnership in East Africa. Journal of General Internal Medicine, 28 (Suppl 3):625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A., Cox S. M., Li L. C. (2010). Qualitative Research Ethics: Enhancing Evidence-based Practice in Physical Therapy. Physical Therapy, 90, 615–628. [DOI] [PubMed] [Google Scholar]
- WHO (2009). Research Ethics in International Epidemic Response: WHO Technical Consultation 10-11 June 2009 Meeting Report. Geneva: WHO Press
- WHO (2014a). Ethical Considerations for Use of Unregistered Interventions for Ebola Viral Disease: Report of an Advisory Panel. Geneva: WHO Press. [Google Scholar]
- WHO (2014b). Potential Ebola Therapies and Vaccines. Geneva: WHO Press. [Google Scholar]
- WHO (2014c). Ethical Issues Related to Study Design for Trials on Therapeutics for Ebola Virus Disease, available from: http://apps.who.int/iris/bitstream/10665/137509/1/WHO_HIS_KER_GHE_14.2_eng.pdf?ua=1 [accessed 12 August 2016].
- WHO (2015). Ebola Situation Report 16 December 2015, available from: http://apps.who.int/ebola/current-situation/ebola-situation-report-16-december-2015 [accessed 12 August 2016]