Abstract
Background:
Oral pharmacotherapies to treat overactive bladder (OAB) are used less in men despite a similar prevalence of storage symptoms as women. The efficacy and safety of once-daily mirabegron 50 mg was evaluated in male OAB patients from five phase III studies that included placebo or antimuscarinic (tolterodine ER 4 mg or solifenacin 5 mg) as a comparator.
Methods:
Three pooled 12-week placebo-controlled studies (mirabegron 50 mg versus placebo) and one 12-week non-inferiority phase IIIb study (BEYOND; mirabegron 50 mg versus solifenacin 5 mg) were used for efficacy (daily micturition frequency, urgency and incontinence episodes) and safety analyses. An additional 52-week active-controlled phase III safety study (mirabegron 50 mg versus tolterodine ER 4 mg) was included in the safety analysis. Male patients aged ⩾18 years with OAB for ⩾3 months were included in the analyses. Patients may also have a history of lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH)/benign prostatic enlargement (BPE) or concomitant use of α1-blockers.
Results:
In the pooled studies, mirabegron 50 mg demonstrated superiority versus placebo (treatment difference: −0.37 [95% confidence interval (CI): −0.74, −0.01]) for reducing micturition frequency; improvements in urgency and incontinence were not significantly different between mirabegron 50 mg and placebo. In BEYOND, mirabegron 50 mg was comparable with solifenacin 5 mg for reducing micturition frequency, urgency, and incontinence episodes. Mirabegron was well tolerated at 12 and 52 weeks and overall treatment-emergent adverse events (AEs) were similar to those with placebo.
Conclusions:
In a male OAB population with or without LUTS associated with BPH/BPE, mirabegron 50 mg provided similar improvements in urgency, frequency, and incontinence as solifenacin 5 mg, and is a well-tolerated alternative to antimuscarinics. In the three pooled 12-week studies, significant differences were not seen for urgency and incontinence versus placebo, although mirabegron 50 mg did demonstrate significant improvements versus placebo for frequency.
Keywords: benign prostatic enlargement, benign prostatic hyperplasia, LUTS, male patients, mirabegron, overactive bladder
Introduction
Lower urinary tract symptoms (LUTS) can be classified into storage, voiding, and post-micturition symptoms. Most patients experience a diversity of LUTS, which often develop via similar pathophysiology. Nevertheless, male and female LUTS are often regarded as two distinct conditions, related to prostate pathology in men and bladder pathology in women.1 The prevalence of storage (51.3% and 59.2%), voiding (25.7% and 19.5%), and post-micturition LUTS (16.9% and 14.2%) are generally similar between men and women, respectively.2 This includes overactive bladder (OAB), defined as urinary urgency usually accompanied by frequency and nocturia, with or without urgency incontinence, in the absence of urinary tract infection or other obvious pathologies.3,4 OAB affects approximately 12% of men and women aged >40 years, and its prevalence increases with advancing age.2 OAB can impact significantly on quality of life (QoL) in men and women5,6 and is generally perceived as more bothersome than voiding symptoms;7 the latter is usually associated with benign prostatic obstruction (BPO) in men.
Despite the similar prevalence of OAB symptoms in men and women, there are important differences in predominating symptoms and their management. Men are more likely to experience urgency, frequency and nocturia accompanied by LUTS associated with voiding dysfunction,1,6,7 whereas women are twice as likely to experience incontinence (including stress and mixed incontinence).6,7
Oral antimuscarinics or β3-adrenoceptor agonists (mirabegron) are recommended as first-line pharmacotherapy for the treatment of OAB.8 Long-term persistence of treatment is often poor with antimuscarinics due to inadequate efficacy or anticholinergic adverse events (AEs), such as dry mouth or constipation,9,10 and in male patients there remains a perception of an increased risk of acute urinary retention, despite that risk being low.11 Mirabegron has demonstrated similar efficacy to antimuscarinics, without the bothersome AEs associated with antimuscarinics, in pivotal 12-week phase III studies and pooled data,12–16 including phase III studies in Japanese and Asian populations,17,18 and long-term tolerability in a 52-week phase III study.19 This improved tolerability profile is reflected by significantly higher 12-month adherence and persistence rates in patients taking mirabegron versus antimuscarinics.20 In a previous phase II study in males with LUTS/bladder outlet obstruction (BOO), mirabegron did not adversely affect voiding urodynamics [maximum urinary flow (Qmax), detrusor pressure at maximum urinary flow (Pdet.Qmax), or detrusor contractility] and was not associated with acute urinary retention after 12 weeks’ treatment.21 Additionally, mirabegron was efficacious for several OAB outcome variables.21 However, mirabegron is not recommended in patients with severe uncontrolled hypertension.22
In men with LUTS, the 2015 European Association of Urology guidelines recommend antimuscarinics or β3-adrenoceptor agonists to treat moderate-to-severe LUTS where bladder storage symptoms predominate, and a combination of α1-adrenoceptor antagonist (α1-blocker) and antimuscarinic to treat troublesome moderate-to-severe LUTS if symptom relief with either monotherapy is insufficient.23 Male patients often receive α1-blockers first to treat bladder storage symptoms (i.e. urgency) due to the perception that benign prostatic enlargement (BPE) is the underlying cause; however, storage LUTS remain bothersome in two-thirds of such men.24
Combining an antimuscarinic or mirabegron with an α1-blocker improves efficacy versus monotherapy in males with LUTS/BPE.25–27 However, the potential for anticholinergic AEs is higher with antimuscarinics, which may worsen treatment persistence.9 There have also been reports of increased post-void residual (PVR) volume in men with BPO treated with mirabegron or antimuscarinics combined with an α1-blocker; however, these were volumes considered clinically irrelevant (i.e. <50 ml).24–27
Despite the underrepresentation of male OAB patients in phase III trials (~20–25% of the study population), there is growing evidence about the efficacy and safety of mirabegron in men. The objective of this critical analysis of male data from five phase III studies was to evaluate the efficacy of mirabegron 50 mg once daily based on 12-week pooled data15 from three phase III studies (SCORPIO,12 ARIES,13 CAPRICORN14) and a phase IIIb non-inferiority study (BEYOND),16 and to evaluate 12-week safety from these studies and a 52-week phase III safety study (TAURUS).19
Methods
Study design and population
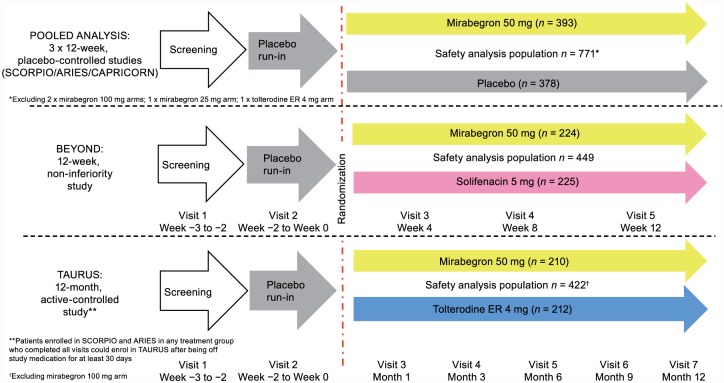
Efficacy and safety have been previously reported in the overall OAB population in the five phase III studies included in this analysis of male data.12–16,19 The five studies consisted of three 12-week placebo-controlled phase III studies [SCORPIO (ClinicalTrials.gov identifier: NCT00689104), ARIES (ClinicalTrials.gov identifier: NCT006- 62909), and CAPRICORN (ClinicalTrials.gov identifier: NCT00912964)], one 12-week non-inferiority phase IIIb study [BEYOND (Clinical Trials.gov identifier: NCT01638000)], and one 52-week phase III active-controlled safety study [TAURUS (ClinicalTrials.gov identifier: NCT0-0688688)] (Figure 1). Patients enrolled in SCORPIO and ARIES in any treatment group who completed all visits could enroll in TAURUS after discontinuing study medication for at least 30 days. Mirabegron 25 mg was only studied in one (CAPRICORN) of the five phase III studies. Due to the limited number of male patients administered once-daily 25 mg mirabegron [recommended starting dose in the United States (US) and several other countries], the 25 mg data were excluded in addition to those for the unlicensed 100 mg mirabegron dose (SCORPIO, ARIES, and TAURUS).
Figure 1.
Summary of the study designs for the phase III randomized controlled trials included in the analysis of male patients.
Men enrolled in these studies were aged ⩾18 years with OAB for ⩾3 months, and the studies could include men with a history of LUTS associated with BPH/BPE and/or concomitant use of α1-blockers (Tables 1 and 2). In BEYOND, the population consisted of patients dissatisfied (based on the Treatment Satisfaction Likert questionnaire) with the efficacy of their last antimuscarinic (excluding solifenacin). Following a 2-week, single-blind, placebo run-in to determine baseline symptoms and eligibility, patients were randomized if, during a 3-day micturition diary period, they recorded ⩾8 micturitions/24 h and ⩾3 urgency episodes, based on urgency grade 3 or 4 according to the Patient Perception of Intensity of Urgency Scale (PPIUS)28 with or without urgency incontinence. Patients with clinically significant BOO at risk of urinary retention were excluded from the pooled studies or TAURUS (at the discretion of the investigator), and excluded from BEYOND if PVR volume was >200 ml (Supplementary material S1: Key inclusion and exclusion criteria and Supplementary material S2: Randomization and blinding).
Table 1.
Summary of phase III studies investigating efficacy and safety of mirabegron in a male OAB population.
| Study |
|||||
|---|---|---|---|---|---|
| SCORPIO (046) | ARIES (047) | CAPRICORN (074) | TAURUS (049) | BEYOND | |
| Study duration | 12 weeks | 12 weeks | 12 weeks | 52 weeks | 12 weeks |
| Study design | Phase III, randomized, placebo-controlled, double-blind | Phase III, randomized, placebo-controlled, double-blind | Phase III, randomized, placebo-controlled, double-blind | Phase III, randomized, active-controlled, double-blind | Phase IIIb, non-inferiority, randomized, double-blind |
| Patient population | Adults with OAB ⩾ 3 months | Adults with OAB ⩾ 3 months | Adults with OAB ⩾ 3 months | Adults with OAB ⩾ 3 months | Adult OAB patients dissatisfied with their previous antimuscarinic due to lack of efficacy |
| Treatment arms/daily dose | Placebo; mirabegron 50 mg, mirabegron 100 mg,† tolterodine ER 4 mg‡ | Placebo, mirabegron 50 mg, mirabegron 100 mg† | Placebo, mirabegron 25 mg,* mirabegron 50 mg | Mirabegron 50 mg, mirabegron 100 mg,† tolterodine ER 4 mg | Mirabegron 50 mg, solifenacin 5 mg |
| Total proportion of males in the overall SAF | 549/1978 (27.8%) | 341/1328 (25.7%) | 408/1305 (31.3%) | 634/2444 (25.9%) | 449/1870 (24.0%) |
| Total proportion of males in the overall FAS | 534/1906 (28.0%) | 320/1270 (25.2%) | 394/1251 (31.5%) | 615/2382 (25.8%) | 443/1833 (24.2%) |
| Treatment arms included in efficacy analysis (FAS) | Placebo (n = 362), mirabegron 50 mg (n = 382) | Not applicable | Mirabegron 50 mg (n = 222), solifenacin 5 mg (n = 221) | ||
| Treatment arms included in SAF | Placebo (n = 378), mirabegron 50 mg (n = 393) | Mirabegron 50 mg (n = 210), tolterodine ER 4 mg (n = 212) | Mirabegron 50 mg (n = 224), solifenacin 5 mg (n = 225) | ||
| Efficacy data available by sex | Change from baseline to EoT: • Mean number of micturitions/24 h • Mean number of incontinence episodes/24 h • Mean number of urgency episodes (grade 3 or 4)/24 h |
No post hoc efficacy analysis in males was conducted as this study was designed primarily to assess 12-month safety | Change from baseline to EoT: • Mean number of micturitions/24 h • Mean number of incontinence episodes/24 h • Mean urgency episodes (grade 3 or 4)/24 h |
||
| Safety data available by sex | • Incidence of TEAEs • Change from baseline in vital signs (blood pressure and pulse rate) • AEs of special interest (antimuscarinic AEs, urinary retention) • Change from baseline in PVR |
• Incidence of TEAEs • Change from baseline in vital signs (blood pressure and pulse rate) • AEs of special interest (antimuscarinic AEs, urinary retention) |
• Incidence of TEAEs • AEs of special interest (antimuscarinic AEs, urinary retention) |
||
Mirabegron 25 mg results excluded as this dose was restricted to a single study (074); †Mirabegron 100 mg results not shown as this is not a licensed dose; ‡Tolterodine ER 4 mg active-control excluded from pooled analysis.
The efficacy and safety endpoints only reflect those included in this publication and do not represent all endpoints investigated in the total population in ARIES, SCORPIO, CAPRICORN, BEYOND, and TAURUS.
AE, adverse event; EoT, end of treatment; ER, extended release; FAS, full analysis set; OAB, overactive bladder; PVR, post-void residual; SAF, safety analysis set; TEAE, treatment-emergent adverse event.
Table 2.
Male patient demographics and baseline characteristics (FAS).
| Pooled studies (SCORPIO/ARIES/CAPRICORN) |
TAURUS |
BEYOND |
||||
|---|---|---|---|---|---|---|
| Placebo (n = 362) | Mirabegron 50 mg (n = 382) | Mirabegron 50 mg (n = 204) | Tolterodine ER 4 mg (n = 206) | Mirabegron 50 mg (n = 222) | Solifenacin 5 mg (n = 221) | |
| Sex, n (% of total study population) | ||||||
| Male/total | 362/1328 (27.3) | 382/1324 (28.9) | 204/789 (25.9) | 206/791 (26.0) | 222/921 (24.1) | 221/912 (24.2) |
| Age, years | ||||||
| Mean (SD) | 62.1 (12.8) | 62.8 (11.3) | 61.6 (11.5) | 61.7 (12.0) | 58.1 (15.1) | 59.5 (14.6) |
| Range | 23–86 | 21–85 | 24–87 | 24–83 | 22–87 | 19–87 |
| Race | ||||||
| White | 329 (90.9) | 356 (93.2) | 194 (95.1) | 197 (95.6) | 220 (99.1) | 217 (98.2) |
| Black/African American | 25 (6.9) | 16 (4.2) | 7 (3.4) | 5 (2.4) | 0 | 1 (0.5) |
| Asian | 4 (1.1) | 8 (2.1) | 3 (1.5) | 2 (1.0) | 1 (0.5%) | 3 (1.4) |
| Other | 4 (1.1) | 2 (0.5) | 0 | 2 (1.0) | 1 (0.5) | 0 |
| BMI, kg/m2 | ||||||
| Mean (SD) | 28.6 (5.5) | 28.2 (4.8) | 28.1 (4.4) | 28.4 (4.8) | 27.1 (4.0) | 27.6 (4.0) |
| Range | 15.9–56.6 | 19.4–45.5 | 19.4–51.9 | 19.4–45.1 | 20–41 | 20–44 |
| Type of OAB, n (%) | ||||||
| Urgency incontinence | 130 (35.9) | 141 (36.9) | 76 (37.3) | 67 (32.5) | 74 (33.3) | 78 (35.3) |
| Mixed | 16 (4.4) | 30 (7.9) | 6 (2.9) | 12 (5.8) | 19 (8.6) | 14 (6.3) |
| Frequency | 216 (59.7) | 211 (55.2) | 122 (59.8) | 127 (61.7) | 129 (58.1) | 129 (58.4) |
| Use of α1-blocker at baseline | 78 (21.5) | 82 (21.5) | 40 (19.6) | 43 (20.9) | 45 (20.3) | 50 (22.6) |
| History of BPH†, n (%) | 147 (40.6) | 142 (37.2) | 84 (41.2) | 78 (37.9) | 73 (32.9) | 80 (36.2) |
| Duration of OAB, months | ||||||
| Mean (SD) | 66.9 (78.6) | 73.4 (86.7) | 77.9 (94.6) | 62.7 (56.6) | 55.7 (75.4) | 51.7 (68.0) |
| Range | 3−688 | 3−688 | 3−534 | 5−427 | 3.2−568.0 | 3.0−568.0 |
| Previous OAB drug, n (%) | ||||||
| Yes | 133 (36.7) | 153 (40.1) | 91 (44.6) | 96 (46.6) | 222 (100.0) | 221 (100.0) |
| Reasons for previous OAB drug discontinuation,‡ n (%) | ||||||
| Insufficient effect | ||||||
| Yes | 95 (71.4) | 108 (70.6) | 62 (68.1) | 60 (62.5) | 222 (100.0) | 221 (100.0) |
| No | 38 (28.6) | 45 (29.4) | 29 (31.9) | 36 (37.5) | 0 | 0 |
| Poor tolerability | ||||||
| Yes | 30 (22.6) | 29 (19.0) | 14 (15.4) | 22 (22.9) | 34 (15.3) | 43 (19.5) |
| No | 103 (77.4) | 124 (81.0) | 77 (84.6) | 74 (77.1) | 188 (84.7) | 178 (80.5) |
| Mean number of incontinence episodes/24 h (FAS)* | ||||||
| Mean (SD) | 0.9 (2.09) | 1.0 (1.97) | 0.7 (1.74) | 0.7 (1.80) | ||
| Range | 0–26 | 0–15 | 0–11 | 0–17 | ||
| Mean number of incontinence episodes/24 h (FAS-I) | (n = 154) | (n = 168) | (n = 77) | (n = 76) | (n = 58) | (n = 59) |
| Mean (SD) | 2.1 (2.77) | 2.2 (2.45) | 1.9 (2.41) | 1.9 (2.55) | 2.13 (1.85) | 2.31 (2.16) |
| Range | 0−26 | 0−15 | 0–11 | 0–17 | 0.3–6.7 | 0.3–7.7 |
| Mean number of micturitions/24 h | ||||||
| Mean (SD) | 11.7 (3.4) | 12.0 (3.4) | 11.4 (2.8) | 11.4 (3.0) | 11.8 (3.1) | 11.6 (2.4) |
| Range | 4–40 | 7–33 | 6–25 | 7–25 | 8–27 | 8–22 |
| Mean number of urgency episodes/24 h | ||||||
| Mean (SD) | 5.0 (3.2) | 5.4 (3.9) | 5.2 (3.5) | 4.8 (3.6) | 7.6 (4.9) | 7.3 (4.4) |
| Range | 0–15 | 0–33 | 1–18 | 1–19 | 1–27 | 1–22 |
In BEYOND, summaries for incontinence episodes are only for patients with baseline value >0 (i.e. FAS-I).
History of BPH was defined using the following selected preferred terms based on MedDRA version 9.1 for the pooled studies and TAURUS and MedDRA version 12.1 for the BEYOND study for medical history: BPH, prostatic obstruction, prostatism, benign neoplasm of prostate, prostatic adenoma, transurethral prostatectomy, prostatomegaly, nocturia, terminal dribbling, urine flow decreased, strangury, and urinary hesitation.
Patients could choose more than one reason for discontinuation.
BMI, body mass index; BPH, benign prostatic hyperplasia; ER, extended release; FAS, full analysis set; FAS-I, full analysis set-incontinence; OAB, overactive bladder; SD, standard deviation.
Efficacy assessment
Efficacy was assessed according to sub-analyses of pooled data from SCORPIO, ARIES, and CAPRICORN (mirabegron 50 mg or placebo once daily), and data from BEYOND (mirabegron 50 mg or solifenacin 5 mg [active comparator] once daily) after 12 weeks’ treatment. Safety was the primary objective for the 52-week TAURUS study and was not designed or powered for efficacy comparisons so efficacy results are not presented. The pooled male sub-analysis for mean number of micturitions/24 h and mean number of incontinence episodes/24 h was prespecified prior to database lock and unblinding of treatment groups for the three studies included in the pooled analysis (i.e. prior to analyzing any data from any study by sex). The pooled male sub-analysis for mean number of urgency episodes/24 h was a post hoc analysis. In BEYOND, the analysis for micturition frequency was prespecified, and analysis on incontinence and urgency were post hoc.
Patients completed a 3-day micturition diary prior to clinic visits at baseline, 4, 8, and 12 weeks/end of treatment (EoT; last on-treatment assessment including patients who did not complete week 12 visit). The three key OAB symptoms were assessed based on the 24-h change from baseline to EoT: mean number of micturitions; mean number of urgency episodes (PPIUS grade 3 or 4); and mean number of incontinence episodes. Nocturia is often multifactorial and clinical trials do not differentiate between nocturnal polyuria and nocturia,29 hence its exclusion.
Safety assessment
Safety was assessed based on pooled data in males from SCORPIO, ARIES, and CAPRICORN (mirabegron 50 mg or placebo), post hoc analysis in BEYOND (mirabegron 50 mg or solifenacin 5 mg), and the 52-week TAURUS study [mirabegron 50 mg or tolterodine extended-release (ER) 4 mg (active control)].
Assessments included the incidence of treatment-emergent AEs (TEAEs), including those of special interest (e.g. antimuscarinic-related AEs and urinary retention). Vital signs were assessed using patient-recorded 5-day diaries (pooled analysis and TAURUS) and/or by the investigator on site (pooled analysis, TAURUS, and BEYOND). Patient-recorded vital signs in men are presented only for the pooled studies and TAURUS since this analysis was not conducted in BEYOND. Hypertension was reported as an AE according to three prespecified criteria in the pooled studies and TAURUS: systolic blood pressure (SBP) >140 mmHg and/or diastolic blood pressure (DBP) >90 mmHg at two consecutive visits post-baseline in normotensive patients at baseline; SBP increased by ⩾20 mmHg and/or DBP increased by ⩾10 mmHg at two consecutive visits post-baseline in hypertensive patients at baseline; treatment with an antihypertensive was initiated to treat hypertension or the dose of a prior antihypertensive was increased due to increased blood pressure. In contrast, hypertension as an AE was based on spontaneous reporting in BEYOND. Change in PVR volume from baseline to EoT was also assessed in men with or without a history of LUTS associated with benign prostatic hyperplasia (BPH)/BPE in the pooled analysis. PVR volume was not measured in TAURUS and only measured at screening in BEYOND.
Statistical analysis
Demographic and baseline characteristics were summarized by descriptive statistics. Micturition frequency and urgency were assessed in the full analysis set (FAS; patients receiving ⩾1 dose of double-blind treatment at baseline and ⩾1 post-baseline micturition measurement) and incontinence was assessed in the FAS-incontinence (FAS-I; FAS patients with ⩾1 incontinence episode at baseline).
In the pooled analysis, changes from baseline in daily micturitions, urgency episodes, and incontinence episodes for the comparison of mirabegron 50 mg versus placebo were analyzed using an analysis of covariance (ANCOVA) model including treatment, sex, study, and treatment-by-sex interaction as fixed factors, and baseline as a covariate.
In BEYOND, non-inferiority analyses of daily micturitions in the per protocol set were similar to the FAS;16 to be consistent with the pooled data, only outcomes in the FAS are reported. Change from baseline in daily micturitions, urgency episodes, and incontinence episodes was analyzed using an ANCOVA model with treatment, age (<65 years and ⩾65 years), number of prior antimuscarinics (1 antimuscarinic and ⩾2 antimuscarinics), and geographic region as fixed factors, and baseline as a covariate.
Least squares mean estimates and two-sided 95% confidence intervals (CIs) for mean changes from baseline were derived within treatment groups and between mirabegron 50 mg and placebo or solifenacin 5 mg.
Descriptive statistics were presented for the safety analysis set (SAF; randomized patients who received ⩾1 dose of double-blind treatment). Vital signs on the pooled studies were analyzed using the same ANCOVA model as the efficacy variables. Vital signs for TAURUS were assessed using an ANCOVA model with treatment group, previous study history, sex, geographical region, and treatment-by-sex interaction as fixed factors, and baseline as a covariate.
Results
Demographic and baseline characteristics
Overall, 1187 men were included in the FAS and 1642 men in the SAF (Table 1 and Figure 1). In the pooled 12-week studies and the 52-week TAURUS study, of the male patients who had prior OAB treatment (41%), approximately 70% had discontinued treatment because of insufficient efficacy; discontinuation of a previous antimuscarinic due to insufficient efficacy was a prerequisite for inclusion in BEYOND. Patient demographics and baseline characteristics were consistent across treatment groups and generally similar across studies, although patients in BEYOND had a higher average number of urgency episodes/24 h at baseline (>7 episodes) versus the pooled studies (~5 episodes) (Table 2). Approximately 37% of men had a history of LUTS associated with BPH/BPE and 22% were receiving an α1-blocker, indicating a substantial proportion had underlying LUTS/BPO. In the FAS-I, baseline incontinence (~2.0 episodes/24 h) was comparable across the pooled studies, TAURUS, and BEYOND.
Efficacy
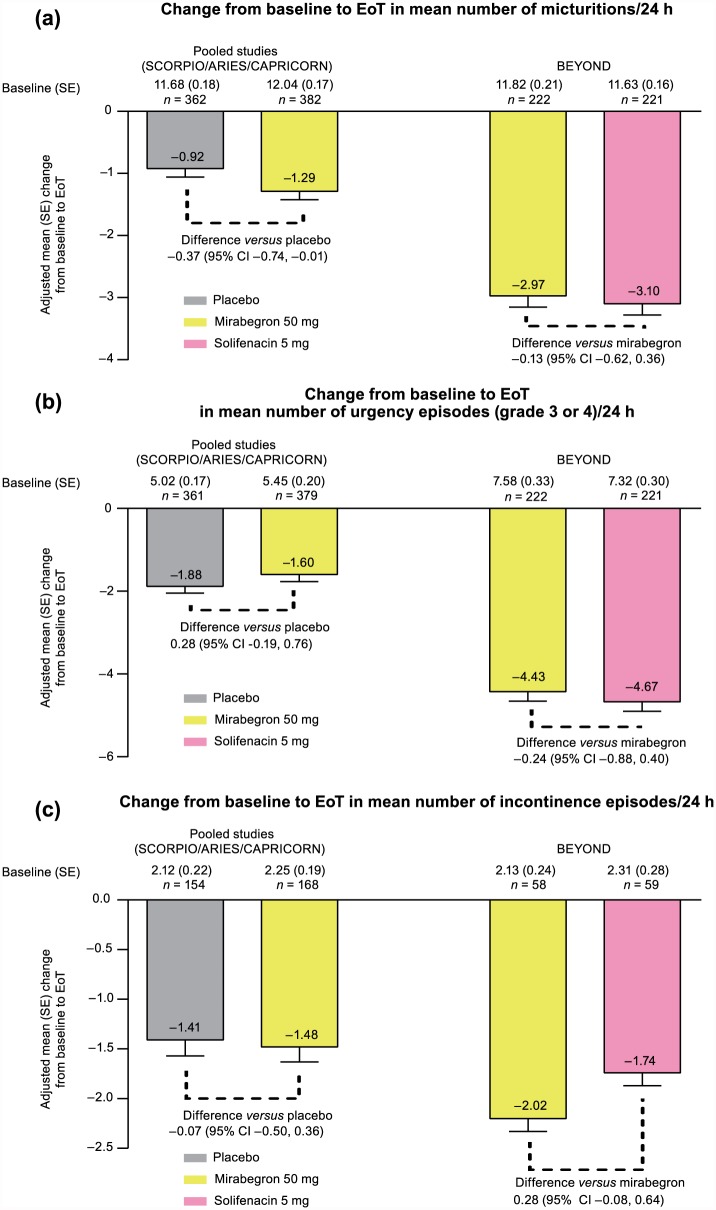
In the FAS, mirabegron 50 mg was associated with a statistically significantly greater reduction in daily micturitions versus placebo in the pooled analysis (Figure 2a). Adjusted mean (95% CI) change from baseline to EoT was −1.29 (−1.55, −1.04) and −0.92 (−1.18, −0.66) in the mirabegron 50 mg and placebo groups, respectively: significant treatment difference in favor of mirabegron versus placebo of −0.37 (95% CI: −0.74, −0.01). In BEYOND, an adjusted mean (95% CI) change from baseline to EoT of −2.97 (−3.32, −2.63) and −3.10 (−3.45, −2.76) for micturition frequency was observed with mirabegron 50 mg and solifenacin 5 mg, respectively: mean treatment difference versus mirabegron 50 mg of −0.13 (−0.62, 0.36), favoring solifenacin 5 mg, but was not statistically significant.
Figure 2.
Adjusted mean change from baseline to EoT in male patients for the key OAB efficacy parameters in the pooled phase III studies (SCORPIO, ARIES, CAPRICORN), and phase IIIb study (BEYOND): (a) mean number of micturitions/24 h (FAS), (b) mean number of urgency episodes (grade 3 or 4 of PPIUS)/24 h (FAS), and (c) mean number of incontinence episode/24 h (FAS-I).
CI, confidence interval; EoT, end of treatment; FAS, full analysis set; FAS-I, full analysis set-incontinence; OAB, overactive bladder; PPIUS, Patient Perception of Intensity of Urgency Scale; SE, standard error.
The mean number of urgency episodes (PPIUS grade 3 or 4)/24 h was improved with mirabegron in the pooled studies and BEYOND. However, there was a significant placebo effect in the pooled analysis, with an adjusted mean (95% CI) change from baseline to EoT of −1.60 (−1.93, −1.27) and −1.88 (−2.23, −1.54) with mirabegron 50 mg and placebo, respectively: mean treatment difference versus placebo of +0.28 (−0.19, 0.76). In BEYOND, the adjusted mean (95% CI) change from baseline to EoT was −4.43 (−4.88, −3.98) and −4.67 (−5.12, −4.22) with mirabegron 50 mg and solifenacin 5 mg, respectively: mean treatment difference versus mirabegron of −0.24 (−0.88, 0.40), favoring solifenacin 5 mg, but not statistically significant (Figure 2b).
In the FAS-I, improvements in daily incontinence episodes were similar with mirabegron 50 mg and placebo in the pooled analysis. Adjusted mean (95% CI) change from baseline to EoT in the mean number of incontinence episodes/24 h was −1.48 (−1.78, −1.18) and −1.41 (−1.72, −1.10) with mirabegron 50 mg and placebo, respectively: mean treatment difference versus placebo of −0.07 (−0.50, 0.36) favoring mirabegron 50 mg. In BEYOND, the adjusted mean (95% CI) change from baseline to EoT was −2.02 (−2.27, −1.77) and −1.74 (−1.99, −1.49) with mirabegron 50 mg and solifenacin 5 mg, respectively: mean treatment difference versus mirabegron 50 mg of +0.28 (–0.08, 0.64), which favored mirabegron, but was not statistically significant (Figure 2c).
Safety
After 12 weeks of treatment, the overall incidence of TEAEs was similar between mirabegron 50 mg (46.1%) and placebo (45.2%) in the pooled studies, and between mirabegron 50 mg (24.5%) and solifenacin 5 mg (26.2%) in BEYOND. After 52 weeks’ treatment, the incidence of TEAEs in TAURUS was higher than the other studies, likely due to additional observation time, but was similar between mirabegron 50 mg (60.0%) and tolterodine ER 4 mg (62.3%). In each case, the incidence of TEAEs in men was similar to the overall OAB population (Table 3). Common TEAEs (⩾2% in any group) in the pooled studies (mirabegron 50 mg versus placebo), BEYOND (mirabegron 50 mg versus solifenacin 5 mg), and TAURUS (mirabegron 50 mg versus tolterodine ER 4 mg), respectively, were nasopharyngitis (5.9% versus 2.1%; 2.2% versus 1.3%; and 3.3% versus 3.3%), headache (2.5% versus 1.3%; 3.1% versus 1.3%; and 2.4% versus 3.3%), constipation (2.0% versus 1.6%; 1.8% versus 2.7%; and 2.9% versus 1.9%), and dry mouth (1.5% versus 2.4%; 3.5% versus 6.7%; and 3.3% versus 8.0%) (Table 3). Arterial hypertension, reported according to three prespecified criteria, was the most frequently reported TEAE in the pooled studies (~10% with placebo and mirabegron 50 mg) and TAURUS (~12% with mirabegron 50 mg and tolterodine ER 4 mg). In BEYOND, hypertension according to spontaneous reporting as an AE was reported in 0.4% of men treated with mirabegron 50 mg or solifenacin 5 mg. Overall, three cases of acute urinary retention were reported, two in the pooled analysis [placebo n = 1 (0.3%) at day 83; mirabegron 50 mg n = 1 (0.3%) at day 47] and one in TAURUS [tolterodine ER 4 mg n = 1 (0.5%) at day 21].
Table 3.
Overall incidence of TEAEs, most common TEAEs (⩾2% in any treatment group), and TEAEs of special interest in male patients from the pooled phase III studies, 12-month study, and phase IIIb study (SAF).
| Pooled studies (12 weeks) (SCORPIO/ARIES/CAPRICORN) |
TAURUS (52 weeks) |
BEYOND (12 weeks) |
||||
|---|---|---|---|---|---|---|
| TEAE (preferred term), n (%) | Placebo (n = 378) | Mirabegron 50 mg (n = 393) | Mirabegron 50 mg (n = 210) | Tolterodine ER 4 mg (n = 212) | Mirabegron 50 mg (n = 224) | Solifenacin 5 mg (n = 225) |
| Overall TEAE in males | 171/378 (45.2) | 181/393 (46.1) | 126/210 (60.0) | 132/212 (62.3) | 55/224 (24.5) | 59/225 (26.2) |
| Overall TEAE in total population | 658/1380 (47.7) | 647/1375 (47.1) | 485/812 (59.7) | 508/812 (62.6) | 274/936 (29.3) | 282/934 (30.2) |
| Common TEAEs (⩾2% in any group) | ||||||
| Hypertension | 35 (9.3) | 43 (10.9) | 26 (12.4) | 25 (11.8) | – | – |
| Nasopharyngitis | 8 (2.1) | 23 (5.9) | 7 (3.3) | 7 (3.3) | 5 (2.2) | 3 (1.3) |
| Headache | 5 (1.3) | 10 (2.5) | 5 (2.4) | 7 (3.3) | 7 (3.1) | 3 (1.3) |
| Cough | – | – | 5 (2.4) | 1 (0.5) | – | – |
| Constipation | 6 (1.6) | 8 (2.0) | 6 (2.9) | 4 (1.9) | 4 (1.8) | 6 (2.7) |
| Diarrhea | – | – | 2 (1.0) | 5 (2.4) | – | – |
| Dry mouth | 9 (2.4) | 6 (1.5) | 7 (3.3) | 17 (8.0) | 8 (3.5) | 15 (6.7) |
| Urinary tract infection | – | – | 4 (1.9) | 5 (2.4) | – | – |
| Dizziness | – | – | 5 (2.4) | 7 (3.3) | – | – |
| Back pain | 8 (2.1) | 4 (1.0) | 9 (4.3) | 2 (0.9) | – | – |
| Tachycardia | – | – | 2 (1.0) | 8 (3.8) | – | – |
| Nausea | – | – | 1 (0.5) | 6 (2.8) | – | – |
| Fatigue | – | – | 2 (1.0) | 5 (2.4) | – | – |
| Influenza | – | – | 3 (1.4) | 6 (2.8) | – | – |
| Pain in extremity | – | – | 5 (2.4) | 1 (0.5) | – | – |
| Cystitis | – | – | 3 (1.4) | 5 (2.4) | – | – |
| TEAEs of special interest | ||||||
| Urinary retention | 1 (0.3) | 1 (0.3) | 0 | 3 (1.4) | 1 (0.1) | 0 |
| Acute urinary retention | 1 (0.3) | 1 (0.3) | 0 | 1 (0.5) | 0 | 0 |
| Blurred vision | 0 | 1 (0.3) | 3 (1.4) | 1 (0.5) | 2 (0.2) | 0 |
| Dyspepsia | 4 (1.1) | 2 (0.5) | 1 (0.5) | 4 (1.9) | 1 (0.1) | 1 (0.1) |
| Hypertension | 35 (9.3) | 43 (10.9) | 26 (12.4) | 25 (11.8) | 4 (0.4) | 4 (0.4) |
ER, extended release; SAF, safety analysis set; TEAE, treatment-emergent adverse events
In the pooled analysis at EoT, mean SBP and DBP increased by ~1 mmHg and 0.5 mmHg, respectively, and mean pulse rate increased by approximately 1 beat per minute (bpm) with mirabegron 50 mg versus placebo. In TAURUS, changes in SBP and DBP after 52 weeks were of a similar magnitude to the 12-week pooled studies, and the difference between mirabegron 50 mg and tolterodine ER 4 mg was also similar to the 12-week studies (Table 4).
Table 4.
Change from baseline to EoT in vital signs as measured by patient diary in the morning and afternoon (SAF).
| Vital signs | Pooled studies (SCORPIO/ARIES/CAPRICORN) |
TAURUS |
||
|---|---|---|---|---|
| Placebo | Mirabegron 50 mg | Mirabegron 50 mg | Tolterodine ER 4 mg | |
| SBP (a.m.), mmHg | n = 363 | n = 383 | n = 205 | n = 206 |
| Baseline mean (SE) | 132.2 (0.87) | 132.6 (0.81) | 133.3 (1.03) | 132.2 (1.02) |
| Adjusted change from baseline (SE) [95% CI] | 0.2 (0.47) | 1.7 (0.46) | 1.7 (0.66) [0.4, 3.0] | 0.9 (0.66) [−0.4, 2.2] |
| Mean difference versus placebo (SE) [95% CI] | – | 1.5 (0.65) [0.2, 2.8] | N/A | N/A |
| SBP (p.m.), mmHg | n = 361 | n = 383 | n = 204 | n = 206 |
| Baseline mean (SE) | 130.9 (0.79) | 131.5 (0.73) | 132.2 (0.90) | 131.3 (0.93) |
| Adjusted change from baseline (SE) [95% CI] | 1.4 (0.49) | 1.9 (0.48) | 1.9 (0.65) [0.6, 3.2] | 1.2 (0.65) [−0.1, 2.4] |
| Mean difference versus placebo (SE) [95% CI] | – | 0.5 (0.68) [−0.8, 1.9] | N/A | N/A |
| DBP (a.m.), mmHg | n = 363 | n = 383 | n = 205 | n = 206 |
| Baseline mean (SE) | 78.6 (0.50) | 79.3 (0.44) | 79.5 (0.61) | 79.0 (0.61) |
| Adjusted change from baseline (SE) [95% CI] | 0.0 (0.29) | 0.6 (0.29) | −0.2 (0.41) [−1.0, 0.6] | 0.3 (0.41) [−0.5, 1.1] |
| Mean difference versus placebo (SE) [95% CI] | – | 0.5 (0.41) [−0.3, 1.3] | N/A | N/A |
| DBP (p.m.), mmHg | n = 361 | n = 383 | n = 204 | n = 206 |
| Baseline mean (SE) | 76.5 (0.47) | 76.9 (0.46) | 77.4 (0.63) | 77.0 (0.60) |
| Adjusted change from baseline (SE) [95% CI] | 0.8 (0.32) | 1.0 (0.31) | 0.6 (0.42) [−0.2, 1.4] | 0.6 (0.42) [−0.2, 1.5] |
| Mean difference versus placebo (SE) [95% CI] | – | 0.2 (0.44) [−0.6, 1.1) | N/A | N/A |
| Pulse rate (a.m.), bpm | n = 363 | n = 383 | n = 205 | n = 206 |
| Baseline mean (SE) | 67.5 (0.56) | 67.3 (0.55) | 69.3 (0.79) | 66.5 (0.73) |
| Adjusted change from baseline (SE) [95% CI] | 0.3 (0.34) | 1.3 (0.33) | −0.6 (0.44) [−1.4, 0.3] | 0.4 (0.45) [−0.5, 1.3] |
| Mean difference versus placebo (SE) [95% CI] | – | 1.0 (0.47) [0.1, 1.9] | N/A | N/A |
| Pulse rate (p.m.), bpm | n = 361 | n = 383 | n = 204 | n = 206 |
| Baseline mean (SE) | 73.2 (0.60) | 72.9 (0.55) | 73.2 (0.73) | 71.4 (0.76) |
| Adjusted change from baseline (SE) [95% CI] | 0.2 (0.35) | 0.8 (0.34) | −1.1 (0.47) [−2.1, −0.2] | 0.8 (0.47) [−0.2, 1.7] |
| Mean difference versus placebo (SE) [95% CI] | – | 0.6 (0.49) [−0.3, 1.6] | N/A | N/A |
Adjusted change from baseline was generated from the ANCOVA model with treatment, sex, study, and treatment-by-sex as fixed factors, and baseline as a covariate. Difference of the adjusted means versus placebo was calculated by subtracting the adjusted mean of placebo from the adjusted mean of the treatment group.
ANCOVA, analysis of covariance; bpm, beats per minute; CI, confidence interval; DBP, diastolic blood pressure; ER, extended release; EoT, end of treatment; N/A, not applicable; SBP, systolic blood pressure; SAF, safety analysis set; SE, standard error.
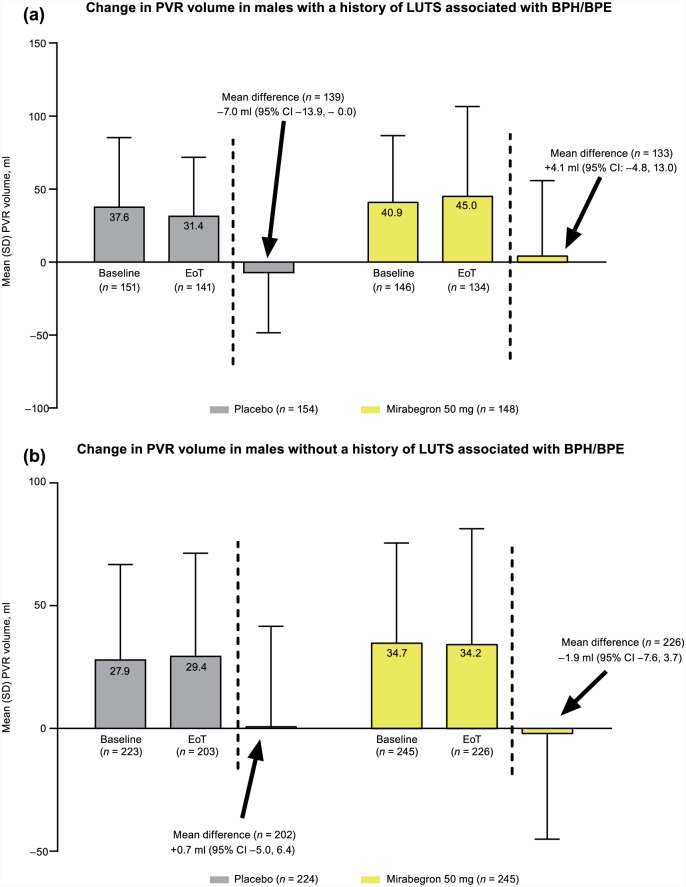
In the pooled analysis, there were no notable changes in PVR volume at EoT with mirabegron 50 mg or placebo in patients with or without history of LUTS associated with BPH/BPE (Figure 3). Overall, two patients experienced a post-baseline PVR >300 ml [placebo n = 1 (339 ml) and mirabegron 50 mg n = 1 (450 ml)], although baseline PVR volumes were already relatively high [172 ml (placebo) and 161 ml (mirabegron 50 mg)].
Figure 3.
Change from baseline to EoT in PVR volume (pooled 12-week studies only) (SAF): (a) males with a history of LUTS associated with BPH/BPE, and (b) males without a history of LUTS associated with BPH/BPE.
BPE, benign prostatic enlargement; BPH, benign prostatic hyperplasia; CI, confidence interval; EoT, end of treatment; LUTS, lower urinary tract symptoms; PVR, post-void residual; SAF, safety analysis set; SD, standard deviation.
Discussion
In this analysis of male OAB patients, including a significant proportion with a history of LUTS associated with BPH/BPE (~37%) or use of α1-blockers at baseline (~22%), mirabegron 50 mg demonstrated superiority versus placebo, and was comparable with solifenacin 5 mg in improving micturition frequency. In the pooled studies, despite a clear improvement in urgency with mirabegron 50 mg, the result did not differentiate from placebo possibly due to a high placebo response. Although incontinence is uncommon in men, it was improved with mirabegron 50 mg by a similar magnitude as solifenacin 5 mg, but mirabegron 50 mg did not differentiate from placebo in the pooled studies.
Although OAB affects men and women equally in terms of prevalence and impact on QoL, it is important to recognize sex differences in etiology, underlying pathology and symptom presentation. Men tend to report more LUTS with greater severity than the general OAB population, and are particularly bothered by urgency, frequency, and nocturia.7,30,31 The proportion of men with OAB in this analysis was relatively high (24–29%) compared with the Healthcore Integrated Research Database (17%),32 but was less than the EPIC study (35%).5 The FAS population was representative of male OAB patients in clinical practice with low baseline incontinence (~0.9 episodes/24 h).
The incidence of TEAEs in men treated with mirabegron 50 mg was similar to the overall population and placebo, suggesting there is no requirement for dose adjustment based on sex. The incidence of dry mouth was two-fold higher with solifenacin or tolterodine versus mirabegron, and was the most frequent TEAE reported in BEYOND. The higher incidence of TEAEs in TAURUS with mirabegron 50 mg and tolterodine ER 4 mg was expected given the longer duration of treatment. The lower incidence of TEAEs in BEYOND could be attributed to selection bias of patients previously treated with antimuscarinics and hence more likely to tolerate bothersome AEs. Arterial hypertension, the most frequent TEAE in the pooled studies and TAURUS, was reported at a similar rate with mirabegron, tolterodine, and placebo, and comparable with the overall population in these studies. Arterial hypertension was almost absent in BEYOND, which is probably explained by the different reporting criteria (prespecified versus spontaneous reporting) and frequency of assessment (on-site investigator versus 5-day diaries). The change in vital signs in men treated with mirabegron 50 mg was comparable with vital sign results from a recent systematic literature review of cardiovascular safety with mirabegron, which reported a mean increase in SBP/DBP and pulse rate of ⩽1 mmHg and 1 bpm, respectively, with mirabegron 50 mg versus placebo, and a rate of arterial hypertension of 8.7% and 8.5% with mirabegron 50 mg and placebo, respectively.33
These data offer an interesting insight in patients with a history of LUTS associated with BPH/BPE and OAB, and confirm the safety of mirabegron and solifenacin in this population. In the pooled studies, PVR volume, an important predictor of acute urinary retention, was comparable between mirabegron and placebo, and was unaffected by history of LUTS associated with BPH/BPE. Acute urinary retention was reported in only 3 cases (placebo n = 1; mirabegron 50 mg n = 1; tolterodine ER 4 mg n = 1).
Approximately half of men with symptomatic BPE also have bladder storage symptoms,24,34 and it is these storage symptoms, mainly urgency, frequency, and nocturia, which usually prompt them to seek advice. However, the underlying cause of storage LUTS may be unrelated to BPE. Furthermore, it is often difficult to differentiate symptoms of detrusor overactivity and BPO without a comprehensive urodynamic assessment.35 Consequently, storage LUTS are often treated with α1-blockers assuming underlying BPO without an additional medication to target residual storage symptoms. This results in the insufficient control of urgency and frequency in as many as two-thirds of males with LUTS.24
Mirabegron has previously been shown to be efficacious and well tolerated in a phase II study investigating urodynamics and safety in 200 men with LUTS/BOO.21 Micturition frequency, urgency, and incontinence episodes/24 h were reduced by a similar magnitude with mirabegron 50 mg versus placebo (−1.35, −1.60, and −0.89, respectively) as the pooled 12-week studies, and differences were statistically significant for urgency (p < 0.01) and frequency (p < 0.05). Furthermore, there were no detrimental effects on voiding phase urodynamics such as Qmax, Pdet.Qmax, or bladder contractility index. The overall incidence of TEAEs (mirabegron 40.0% versus placebo 43.1%) in men with LUTS/BOO was comparable with the pooled 12-week studies, and there were no reports of acute urinary retention.21 The rate of hypertension in males with LUTS/BOO was comparable between the mirabegron 50 mg (4.3%) and placebo (3.1%) groups.21
The absence of any detrimental effect on voiding phase parameters (Qmax and Pdet.Qmax) with mirabegron in males with LUTS/BPE is related to its mode of action. Stimulation of the β3-adrenoceptor mediates detrusor relaxation and increases bladder capacity during the storage phase, but there are no changes during the voiding phase, thus there are no effects on micturition pressure, flow rate, or residual volume. Concerns that antimuscarinics may impair voiding pressure during bladder emptying and thereby increase the risk of acute urinary retention, particularly in men with underlying BPO, appear to be unfounded based on two literature reviews of antimuscarinics in men with LUTS.11,36 After comparing acute urinary retention rates in the general male population and in men with LUTS (PVR ⩽ 200 ml), the risk with antimuscarinics with or without α1-blockers may be increased during short-term treatment but, if patients do not develop acute urinary retention within the first 3-4 months, their subsequent risk is lower than the untreated, symptomatic population.11 Furthermore, a smaller study suggested long-term antimuscarinic use (>1 year) was a risk factor for increasing PVR volume by >50 ml.37 Although significant increases in PVR volumes have been reported (>20 ml to <40 ml) in men with storage symptoms and BPE following combination therapy with antimuscarinic plus α1-blocker,26 or mirabegron plus α1-blocker,27 these were considered clinically irrelevant, as reflected by the single case of acute urinary retention in the latter study.27 These data support the safety for mirabegron and solifenacin in patients with LUTS associated with BPH and PVR < 200 ml.
The efficacy observed with mirabegron in this analysis, primarily deriving from US and European male data, is consistent with unpublished male data from a Japanese phase III study.17 In Japanese males with OAB, a similar benefit in reducing micturition frequency was observed with mirabegron 50 mg (−1.03) versus placebo (−0.74); a treatment difference of −0.30 (95% CI: −1.21, 0.61). However, relative improvements in urinary incontinence and urgency were not demonstrated. In a more diverse Asian population (Taiwan, Korea, China, and India),18 unpublished male data indicate that mirabegron 50 mg versus placebo improved micturition frequency (−2.25 versus −1.69) and incontinence (−1.30 versus −0.80); however, as observed with the US/European data and Japanese data, improvements in urgency did not differentiate from placebo. Interestingly, a clear treatment effect was observed for volume voided per micturition (the most objective bladder diary outcome) in favor of mirabegron (11.46 ml) versus placebo (−0.73 ml).
Study limitations
One of the main limitations is the small amount of pooled data available at the time of this male sub-analysis. The 12-week data were pooled as a requirement of the US and European regulatory submission process in 2012 and, therefore, only included North American and European studies. Studies conducted outside these regions with data available at the time of pooling (i.e. Japanese phase III data17) were not included. There are plans to integrate mirabegron phase II–IV data from all geographic regions, which will provide a larger sample size for men to be analyzed at the 12-week time point. When available, these results should add to the volume of available data on men with OAB. However, in the interim, the unpublished efficacy data in Japanese and Asian men are presented in the discussion to highlight potential regional differences.
The limited number of studies that shared the same design and population will have undoubtedly contributed to the observed heterogeneity with mirabegron. For instance, the inclusion of superiority versus non-inferiority study designs and studies that allowed only prior-treated patients versus those that also allowed naïve patients. Further factors known to affect homogeneity of OAB treatment effect estimates, which differed between the studies in this analysis, include small patient numbers, severity of incontinence and urgency at baseline, and placebo response rates. A high placebo response is not unusual in OAB trials and may be a consequence of counselling and lifestyle changes, self-reported diaries, subjective assessment of urgency, trials of shorter duration, and the concomitant use of α1-blockers. Markedly higher placebo responses were evident in male patients versus female patients for urgency (−1.88 versus −1.06) and incontinence (−1.41 versus −1.03), but not micturitions (−0.92 versus −1.31) in the pooled studies, which contributed in part to the lower treatment effect versus placebo observed in male patients for these two variables. In the overall populations in OAB registration trials, treatment differences between active drug and placebo are often limited due to the high placebo response inherent in OAB trials. The reduction in micturition frequency in this male OAB population is lower than seen in the overall OAB population, yet the results are statistically significant. The relatively small sample size in this analysis could have impacted the urgency and incontinence results, especially taking into consideration that a smaller proportion of male OAB patients have urinary incontinence compared with females. Ongoing mirabegron phase IV male OAB studies shall provide more conclusive evidence in the future. Although women are more likely to experience incontinence than men, a significant proportion of male patients experience some element of post-micturition dribble,38 which could be perceived by the patient as incontinence, and is less likely to be responsive to treatment. Therefore, one might expect to observe reductions in urgency incontinence of a similar magnitude in men versus women. Nevertheless, the limited response observed in men in this analysis is consistent with a recent literature review and meta-analysis of OAB medications, which found incontinence outcomes less favorable in men.39 The authors attributed this to discrepancies in anatomy and pathophysiology, particularly comorbid conditions such as BPH, which can precipitate episodes of post-micturition dribble and overflow incontinence.39 Furthermore, this analysis was not powered for the male population and lacked a common comparator across the studies, so efficacy and safety results are not interpretable for relative comparisons. The use of an active comparator (solifenacin) in BEYOND, in patients who were unresponsive to previous antimuscarinic therapy, could be seen to potentially bias the results in favor of mirabegron. However, this was not evident in the overall population or in male patients, which supports the original evidence-based premise that solifenacin is effective in both treatment-naïve OAB patients and those who failed previous antimuscarinic therapy, and supported the decision to use solifenacin as an active comparator for this non-inferiority study. The inclusion of a placebo group in BEYOND might have allowed a more meaningful assessment of the treatment effect observed with mirabegron and solifenacin.
The limitations of this analysis could be addressed in the future via a network meta-analysis with corresponding female data after accounting for differences between the male and female populations, which would allow direct and indirect treatment comparisons and estimation of relative efficacy (including patient-reported outcomes) and safety with mirabegron by sex; male and female data from the phase III Japanese and Asian studies17,18 could also be potentially included. Post hoc analyses could explore which male patient factors influence efficacy and safety of mirabegron (e.g. age, previous or concomitant medical therapy for LUTS/BPH, duration and severity of symptoms, previously treated versus treatment-naïve) and whether a similar response is observed in men treated with the 25-mg mirabegron dose. Future analyses could also benefit from the inclusion of efficacy and safety data from phase II studies and efficacy data in treatment-naïve patients from TAURUS.19
The two large randomized controlled trials [ClinicalTrials.gov identifiers: NCT02757768 and NCT02656173] designed to investigate the efficacy and safety of add-on mirabegron in men with OAB symptoms taking an α-blocker (tamsulosin hydrochloride) for LUTS due to BPH are currently recruiting participants. A small Japanese study has already reported significant improvements in OAB and good tolerability following add-on mirabegron to tamsulosin versus tamsulosin monotherapy in men with OAB and BPE.27
Conclusion
In male OAB patients with or without underlying BPE, mirabegron 50 mg improved urgency, frequency, and incontinence, as did solifenacin 5 mg in BEYOND. Although statistically significant differences were not seen for urgency and incontinence versus placebo in the pooled studies, mirabegron 50 mg did demonstrate statistically significant improvements in frequency versus placebo. Mirabegron 50 mg is a well-tolerated alternative to antimuscarinics, without the same potential concerns over voiding difficulty. No significant increase in PVR volume or in the incidence of acute urinary retention was observed with mirabegron. More robust randomized controlled studies are required to confirm the benefit of mirabegron in men, to explore why mirabegron is more effective in improving frequency than other OAB symptoms, and to investigate mirabegron monotherapy and add-on therapy to α1-blockers in men with and without underlying BPE/BPO, with the focus on improving storage symptoms of frequency, urgency, and nocturia. Post hoc analyses could also explore whether certain patient categories (e.g. age, severity of symptoms) are more predictive of treatment success.
Acknowledgments
The authors received medical writing assistance from Stuart Murray, MSc (Envision Scientific Solutions, Horsham, UK), for preparation of the initial and final drafts of the manuscript. The authors and study sponsor acknowledge the assistance of the study investigators.
Footnotes
Institutional Review Board approval of the protocol, and informed patient consent was obtained prior to each study commencing. Studies were conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki, Good Clinical Practice, and International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use guidelines and applicable laws and regulations.
Funding: All studies contributing to this analysis were funded by Astellas Pharma Europe BV, Astellas Pharma Europe Ltd, or Astellas Pharma Global Development (Chertsey, UK). Editorial assistance was funded by Astellas Pharma Global Development.
Conflict of interest statement: A.T. has received consultancy fees from Allergan (Dublin, Ireland), Astellas (Tokyo, Japan), AMS/Boston Scientific (Marlborough, USA), Bayer (Leverkusen, Germany), GSK (Middlesex, UK), and Shionogy (Florham Park, USA); investigator for GSK, Shionogy, Bayer, and Pierre Fabre (Paris, France).
J.E.B. received investigator and speaker fees for Astellas and course coordinator fees from Gebro Pharma (Fieberbrunn, Austria).
V.W.N. is an investigator for Allergan (Dublin, Ireland), Astellas, and Cook Myosite (Pittsburgh, USA).
S.H. has received consultancy fees from Astellas, Allergan, Pfizer (New York City, USA), Merus (Utrecht, The Netherlands), and Ferring (Saint-Prex, Switzerland); grants from Astellas and Allergan.
C.R.C. has received consultancy, research, and speaker fees from Allergan, Astellas, Medtronic, and Recordati (Milan, Italy); consultancy and speaker fees from Lilly (Indiana, USA); research and speaker fees from ONO (Osaka, Japan) and Pfizer; and speaker fees from Ranbaxy (Haryana, India).
M.B.B., E.S., and M.H. are employees of Astellas.
M.O. has received speaker and consultancy fees from Apogepha (Dresden, Germany), Astellas, Bayer, Duchesnay (Québec, Canada), Ferring, GlaxoSmithKline, Lilly, Pfizer, and Recordati; and grants from Astellas.
Contributor Information
Andrea Tubaro, Department of Urology, Sant’Andrea Hospital, ‘Sapienza’ University, Via di Grottarossa 1035–1039, 00189 Rome, Italy.
José E. Batista, Urodynamics Unit, URD/Hospital Quiron Teknon, Barcelona, Spain
Victor W. Nitti, Department of Urology, NYU Langone Medical Center, New York, NY, USA
Sender Herschorn, Department of Surgery/Urology, University of Toronto, Toronto, ON, Canada.
Christopher R. Chapple, Department of Urology, Royal Hallamshire Hospital, Sheffield, UK
Mary Beth Blauwet, Department of Biostatistics, Astellas, Northbrook, IL, USA.
Emad Siddiqui, Astellas Pharma Europe Ltd, Chertsey, Surrey.
Moses Huang, Astellas Pharma Europe Ltd, Chertsey, Surrey.
Matthias Oelke, Department of Urology, Academic Medical Hospital, University of Maastricht, The Netherlands.
References
- 1. Sexton CC, Coyne KS, Kopp ZS, et al. The overlap of storage, voiding and postmicturition symptoms and implications for treatment seeking in the USA, UK and Sweden: EpiLUTS. BJU Int 2009; 103(Suppl. 3): 12–23. [DOI] [PubMed] [Google Scholar]
- 2. Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol 2006; 50: 1306–1314. [DOI] [PubMed] [Google Scholar]
- 3. Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 2002; 21: 167–178. [DOI] [PubMed] [Google Scholar]
- 4. Drake MJ. Do we need a new definition of the overactive bladder syndrome? ICI-RS 2013. Neurourol Urodyn 2014; 33: 622–624. [DOI] [PubMed] [Google Scholar]
- 5. Coyne KS, Sexton CC, Irwin DE, et al. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU Int 2008; 101: 1388–1395. [DOI] [PubMed] [Google Scholar]
- 6. Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol 2003; 20: 327–336. [DOI] [PubMed] [Google Scholar]
- 7. Agarwal A, Eryuzlu LN, Cartwright R, et al. What is the most bothersome lower urinary tract symptom? Individual- and population-level perspectives for both men and women. Eur Urol 2014; 65: 1211–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gormley EA, Lightner DJ, Faraday M, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment. J Urol 2015; 193: 1572–1580. [DOI] [PubMed] [Google Scholar]
- 9. Benner JS, Nichol MB, Rovner ES, et al. Patient-reported reasons for discontinuing overactive bladder medication. BJU Int 2010; 105: 1276–1282. [DOI] [PubMed] [Google Scholar]
- 10. Wagg A, Compion G, Fahey A, et al. Persistence with prescribed antimuscarinic therapy for overactive bladder: a UK experience. BJU Int 2012; 110: 1767–1774. [DOI] [PubMed] [Google Scholar]
- 11. Oelke M, Speakman M, Desgrandchamps F, et al. Acute urinary retention rates in the general male population and in adult men with lower urinary tract symptoms participating in pharmacotherapy trials – a literature review. Urology 2015; 86: 654–665. [DOI] [PubMed] [Google Scholar]
- 12. Khullar V, Amarenco G, Angulo JC, et al. Efficacy and tolerability of mirabegron, a β(3)-adrenoceptor agonist, in patients with overactive bladder: results from a randomised European-Australian phase 3 trial. Eur Urol 2013; 63: 283–295. [DOI] [PubMed] [Google Scholar]
- 13. Nitti VW, Auerbach S, Martin N, et al. Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J Urol 2013; 189: 1388–1395. [DOI] [PubMed] [Google Scholar]
- 14. Herschorn S, Barkin J, Castro-Diaz D, et al. A phase III, randomized, double-blind, parallel-group, placebo-controlled, multicentre study to assess the efficacy and safety of the β3 adrenoceptor agonist, mirabegron, in patients with symptoms of overactive bladder. Urology 2013; 82: 313–320. [DOI] [PubMed] [Google Scholar]
- 15. Nitti V, Khullar V, van Kerrebroeck P, et al. Mirabegron for the treatment of overactive bladder: a prespecified pooled efficacy analysis and pooled safety analysis of three randomised, double-blind, placebo-controlled, phase III studies. Int J Clin Pract 2013; 67: 619–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Batista JE, Kölbl H, Herschorn S, et al. The efficacy and safety of mirabegron compared with solifenacin in overactive bladder patients dissatisfied with previous antimuscarinic treatment due to lack of efficacy: results of a non-inferiority, randomised, phase IIIb trial. Ther Adv Urol 2015; 7: 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamaguchi O, Marui E, Kakizaki H, et al. Phase III, randomised, double-blind, placebo-controlled study of the β3-adrenoceptor agonist mirabegron, 50 mg once daily, in Japanese patients with overactive bladder. BJU Int 2014; 113: 951–960. [DOI] [PubMed] [Google Scholar]
- 18. Kuo HC, Lee KS, Na Y, et al. Results of a randomized, double-blind, parallel-group, placebo- and active-controlled, multicenter study of mirabegron, a β3-adrenoceptor agonist, in patients with overactive bladder in Asia. Neurourol Urodyn 2015; 34: 685–692. [DOI] [PubMed] [Google Scholar]
- 19. Chapple CR, Kaplan SA, Mitcheson D, et al. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a β3-adrenoceptor agonist, in overactive bladder. Eur Urol 2013; 63: 296–305. [DOI] [PubMed] [Google Scholar]
- 20. Wagg A, Franks B, Ramos B, et al. Persistence and adherence with the new beta-3 receptor agonist, mirabegron, versus antimuscarinics in overactive bladder: early experience in Canada. Can Urol Assoc J 2015; 9: 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nitti VW, Rosenberg S, Mitcheson DH, et al. Urodynamics and safety of the β3-adrenoceptor agonist mirabegron in males with lower urinary tract symptoms and bladder outlet obstruction. J Urol 2013; 190: 1320–1327. [DOI] [PubMed] [Google Scholar]
- 22. Astellas Pharma US, Inc. Prescribing information for MYRBETRIQ® (mirabegron) extended-release tablets, for oral use, http://www.us.astellas.com/docs/Myrbetriq_WPI.pdf (2016, accessed 31 March 2017).
- 23. Gravas S, Bach T, Bachmann A, et al. European Association of Urology Guidelines on the management of non-neurogenic male lower urinary tract symptoms (LUTS) including benign prostatic obstruction, http://uroweb.org/wp-content/uploads/EAU-Guidelines-Non-Neurogenic-Male-LUTS-Guidelines-2015-v2.pdf (2015, accessed 31 March 2017). [DOI] [PubMed]
- 24. Lee JY, Kim HW, Lee SJ, et al. Comparison of doxazosin with or without tolterodine in men with symptomatic bladder outlet obstruction and an overactive bladder. BJU Int 2004; 94: 817–820. [DOI] [PubMed] [Google Scholar]
- 25. Masumori N, Tsukamoto T, Yanase M, et al. The add-on effect of solifenacin for patients with remaining overactive bladder after treatment with tamsulosin for lower urinary tract symptoms suggestive of benign prostatic obstruction. Adv Urol. Epub ahead of print 26 October 2010. DOI: 10.1155/2010/205251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee KS, Choo MS, Kim DY, et al. Combination treatment with propiverine hydrochloride plus doxazosin controlled release gastrointestinal therapeutic system formulation for overactive bladder and coexisting benign prostatic obstruction: a prospective, randomized, controlled multicenter study. J Urol 2005; 174: 1334–1338. [DOI] [PubMed] [Google Scholar]
- 27. Ichihara K, Masumori N, Fukuta F, et al. A randomized controlled study of the efficacy of tamsulosin monotherapy and its combination with mirabegron for overactive bladder induced by benign prostatic obstruction. J Urol 2015; 193: 921–926. [DOI] [PubMed] [Google Scholar]
- 28. Notte SM, Marshall TS, Lee M, et al. Content validity and test-retest reliability of Patient Perception of Intensity of Urgency Scale (PPIUS) for overactive bladder. BMC Urol 2012; 12: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oelke M, Adler E, Marschall-Kehrel D, et al. Nocturia – state of the art and critical analysis of current assessment and treatment strategies. World J Urol 2014; 32: 1109–1117. [DOI] [PubMed] [Google Scholar]
- 30. Irwin DE, Milsom I, Kopp Z, et al. Prevalence, severity, and symptom bother of lower urinary tract symptoms among men in the EPIC study: impact of overactive bladder. Eur Urol 2009; 56: 14–20. [DOI] [PubMed] [Google Scholar]
- 31. Oelke M, Wiese B, Berges R. Nocturia and its impact on health-related quality of life and health care seeking behaviour in German community-dwelling men aged 50 years or older. World J Urol 2014; 32: 1155–1162. [DOI] [PubMed] [Google Scholar]
- 32. Andersson KE, Sarawate C, Kahler KH, et al. Cardiovascular morbidity, heart rates and use of antimuscarinics in patients with overactive bladder. BJU Int 2010; 106: 268–274. [DOI] [PubMed] [Google Scholar]
- 33. Rosa GM, Ferrero S, Nitti VW, et al. Cardiovascular safety of β3-adrenoceptor agonists for the treatment of patients with overactive bladder syndrome. Eur Urol 2016; 69: 311–323. [DOI] [PubMed] [Google Scholar]
- 34. Knutson T, Edlund C, Fall M, et al. BPH with coexisting overactive bladder dysfunction–an everyday urological dilemma. Neurourol Urodyn 2001; 20: 237–247. [DOI] [PubMed] [Google Scholar]
- 35. Oelke M, Baard J, Wijkstra H, et al. Age and bladder outlet obstruction are independently associated with detrusor overactivity in patients with benign prostatic hyperplasia. Eur Urol 2008; 54: 419–426. [DOI] [PubMed] [Google Scholar]
- 36. Athanasopoulos A, Chapple C, Fowler C, et al. The role of antimuscarinics in the management of men with symptoms of overactive bladder associated with concomitant bladder outlet obstruction: an update. Eur Urol 2011; 60: 94–105. [DOI] [PubMed] [Google Scholar]
- 37. Sung Yong C, Hyun Dong S, In Rae C, et al. The risk factors increasing post-void residual urine volume after long term anticholinergics therapy over 1 year in patients with benign prostatic hyperplasia accompanied with overactive bladder. Eur Urol Suppl 2009; 8: 237. [Google Scholar]
- 38. Furuya S, Ogura H, Tanaka M, et al. Incidence of postmicturition dribble in adult males in their twenties through fifties (English abstract). Acta Urologica Japonica 1997; 43: 407–410. [PubMed] [Google Scholar]
- 39. Hartmann KE, McPheeters ML, Biller DH, et al. Treatment of overactive bladder in women. Evidence Report/Technology Assessment No.187 (Prepared by the Vanderbilt Evidence-based Practice Center under Contract No. 290–2007–10065-I). AHRQ Publication No. 09-E017. Rockville, MD: Agency for Healthcare Research and Quality, 2009. [Google Scholar]