Abstract
Background:
Autologous chondrocyte implantation (ACI) has been shown to provide adequate durability, pain relief, and improved long-term functional outcomes in the average patient, but proof of its efficacy in individuals with greater than average physical demands is scarce. Further knowledge is required to understand which patients may benefit from ACI and to identify which risk factors are associated with failure to return to the preinjury activity level.
Purpose:
To determine the occupational outcomes, rates of reoperation, and variables predictive of suboptimal outcomes after ACI.
Study Design:
Case series; Level of evidence, 4.
Methods:
All active-duty military servicemembers in the United States who underwent ACI of the knee between 2004 and 2014 were identified. Demographic information, injury characteristics, surgical variables, and clinical and surgical outcomes were extracted from the medical record. Univariate and multivariate analyses were used to determine significant independent predictors of clinical and surgical failures.
Results:
A total of 90 patients (91 knees) met the inclusion criteria. The cohort was predominantly male (86%), with a mean age of 34.5 ± 6.3 years (range, 20-50 years). The most common location of the articular cartilage lesion was the patellofemoral compartment (54 lesions, 59%), and the mean Outerbridge grade and size were 3.8 ± 0.4 and 4.00 ± 2.77 cm2 (range, 1.2-15.0 cm2), respectively. A total of 72 patients (79%) had at least 1 previous knee procedure. Nearly three-quarters of patients (71%) underwent concomitant procedures. At a mean follow-up of 59.9 ± 27.1 months (range, 24.0-140.1 months), 60% of our patients reported significant improvement in knee pain and did not require further surgical intervention. Multivariate analysis identified age <30 years as the only significant independent predictor of both clinical (P = .011) and overall failure (P = .014). Moderate-demand military occupational specialties (P = .036), exclusive involvement of the patellofemoral compartment (P = .045), and use of a periosteal patch (P = .0173) were additionally found to be independent predictors of surgical failure.
Conclusion:
Treatment of articular cartilage defects of the knee with ACI in physically active young individuals can return nearly two-thirds of individuals to daily activity with decreased pain and improved function. Risk factors for failure after ACI surgery were age younger than 30 years, lower demand occupation, exclusive involvement of the patellofemoral compartment, prior microfracture, and use of a periosteal patch.
Keywords: autologous chondrocyte implantation, ACI, athletic activity, knee, articular cartilage
Focal articular cartilage defects of the knee can cause significant pain and disability and can ultimately lead to degenerative arthritis if left untreated. While there are a number of treatment options, each must be tailored to the demographic and functional demands of the patient. Management may also differ significantly depending on lesion size, location, knee alignment, meniscal integrity, and ligament status. There are various operative options that can be divided into 3 broad categories: palliative (eg, chondroplasty), reparative (eg, microfracture and subchondral drilling), and restorative (eg, osteochondral autograft transplantation [OATS], autologous chondrocyte implantation [ACI], and osteochondral allograft transplantation [OCA]).12,13
ACI was first published as a restorative treatment option in 1994.2,8 Compared with alternative techniques, ACI offers the ability to re-create hyaline-like cartilage with reproducible longevity and wear characteristics.22 ACI has been shown to reduce pain in young active populations and improve knee function by returning athletes to their preinjury level of activity at rates between 73% and 84%.3,10,20
The active-duty military population is similarly subject to regular daily lower extremity demands. However, ACI has not been evaluated on a large scale in the military population. When deciding to intervene surgically, the military orthopaedic surgeon must consider the techniques that will allow patients to return to their preinjury level of function. Accordingly, the aim of the current study was to evaluate the short- to midterm surgical and functional outcomes of United States (US) military servicemembers after ACI of the knee. Additionally, we sought to determine the rates of reoperation and variables predictive of suboptimal outcomes.
Methods
We conducted a retrospective review of the Management Analysis and Reporting Tool (M2) database to identify all tri-service US active-duty military servicemembers who underwent ACI (Current Process Terminology [CPT] code 27412) between the years 2004 and 2014 in the Military Health System. The M2 is an established health care management database that can be used to perform clinical outcomes research related to a variety of knee6,24 injuries and other musculoskeletal conditions.12
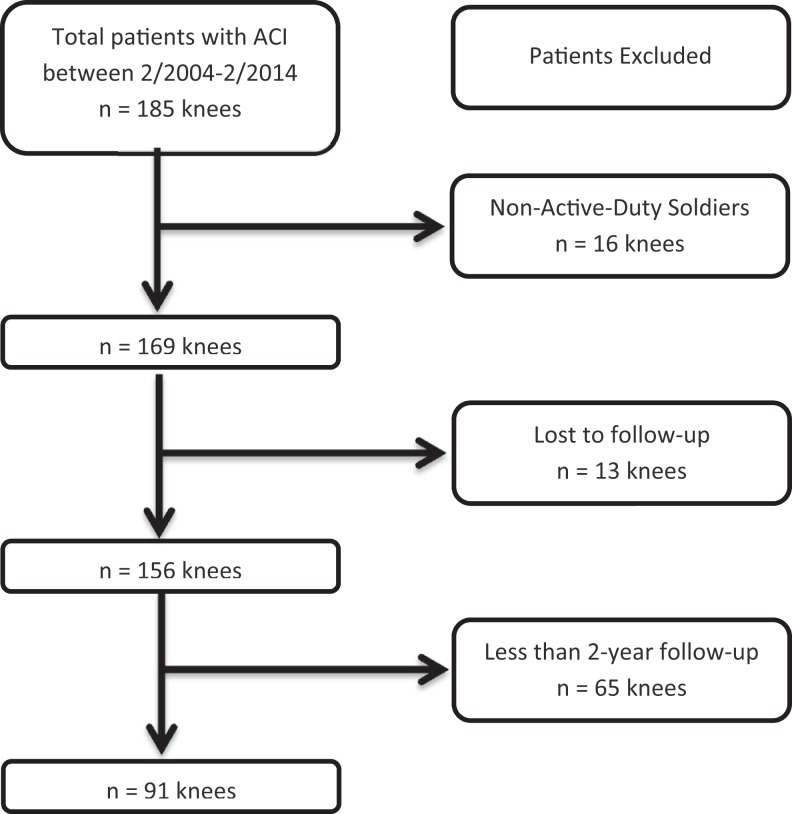
Inclusion criteria were patients with primary ACI for a high-grade, Outerbridge grade III or IV chondral defect of the knee, minimal 2-year clinical follow-up, and active-duty military status at the time of surgery. Exclusion criteria were applied to patients with nonmilitary or retired status at time of surgery, clinical follow-up of less than 2 years, hybrid chondral procedure other than ACI, previous ipsilateral ACI of the knee, and/or those with insufficient documentation (Figure 1).
Figure 1.
Primary autologous chondrocyte implantation (ACI) patient selection diagram.
Demographic data, including patient identifiers, age, sex, military rank, and branch of service, were initially extracted from the M2 database. These identifiers were cross-referenced with the military electronic medical record (Armed Forces Health Longitudinal Technology Application [AHLTA] and e-Profile [version 3.17; Medical Operational Data System]), and chart review was conducted to confirm the diagnosis and procedure. Further analysis yielded additional patient-based (laterality, race, body mass index [BMI], military rank, military occupational specialty [MOS], tobacco use), lesion-related (location, size, grade), and surgical variables (type of patch, prior and concomitant procedures [eg, tibial tubercle or proximal tibial osteotomy]).
Rank was stratified into junior enlisted (E1-5) versus senior enlisted (E6-9) and commissioned officers (O1-6). Junior enlisted soldiers are less able to modify their daily physical activities than their senior enlisted or officer counterparts. Junior enlisted rank has furthermore been correlated with lower socioeconomic and education status, as these servicemembers are typically younger, earn lower salaries, and more often have only secondary school education level at the time of enlistment.19 MOS designations were classified collectively as combat arms (CA), combat support (CS), or combat service support (CSS) based on inherent occupational demands. CA and CS occupational specialties are actively engaged in conflict and generally require greater functional demands than those of the more ancillary CSS MOS.
The primary outcomes of interest were (1) clinical failure, defined as inability to return to active military duty or medical separation from the military secondary to persistent disability attributable to the operative extremity and (2) surgical failure, constituting revision chondral procedure or conversion to arthroplasty due to persistent symptomatology after index ACI. Overall failure was defined as meeting the criteria for clinical and/or surgical failure. Complications and reoperations other than revision procedures were also recorded.
Statistical Analysis
Summary statistics including means and standard deviations were reported for continuous variables. Frequencies were calculated for categorical variables. Bivariate logistic regression analysis was performed to test the significance of demographic, injury-related, and surgical risk factors as predictors of clinical, surgical, and overall (either clinical or surgical, or both) failure. Multivariate regression analysis was subsequently performed for any risk factor with P < .05 on initial bivariate analysis. Significant independent predictors were defined as those with P < .05 and 95% confidence interval (CI) for the odds ratio (OR) exclusive of 1.0 on multivariable logistic regression analysis. ORs with corresponding 95% CIs and P values are reported for each variable. All statistical calculations were performed using SAS version 9.3 (SAS Institute Inc).
Results
Demographics
A total of 90 patients (91 knees) met the inclusion criteria (Table 1). The mean patient age was 34.5 ± 6.3 years (range, 20-50 years), and 86% were male. The mean BMI was 25.5 ± 3.6 kg/m2; 35% of patients had a BMI ≥30 kg/m2. More than one-third of patients (38%) reported regular use of tobacco products. MOS was divided nearly equally between CA/CS (54%) and CSS (46%).
TABLE 1.
Patient Demographics and Clinical Profilea
| Patients/knees, n | 90/91 |
| Follow-up, mo, mean (SD) | 59.9 (27.1) |
| Age, y, mean (SD) | 34.5 (6.3) |
| Sex, n (%) | |
| Men | 78 (86) |
| Women | 13 (14) |
| Body mass index (kg/m2), mean (SD) | 25.5 (3.6) |
| Service, n (%) | |
| Army | 81 (89) |
| Navy/Air Force/Marines/Coast Guard | 10 (11) |
| Rank, n (%) | |
| Junior enlisted | 12 (13) |
| Senior enlisted | 62 (68) |
| Officer | 17 (19) |
| Military occupational specialty, n (%) | |
| Combat arms | 25 (28) |
| Combat support | 24 (26) |
| Combat service support | 42 (46) |
| Tobacco, n (%) | |
| Yes | 35 (38) |
| No | 56 (62) |
aPercentages were calculated per total number of knees (N = 91).
Surgical Variables
With regard to lesion characteristics, nearly three-quarters (77%) were isolated, and most (59%) commonly involved the patellofemoral compartment (Table 2). The mean overall lesion Outerbridge grade and size were 3.8 ± 0.4 and 4.00 ± 2.77 cm2 (range, 1.2-15.0 cm2), respectively. The largest lesions were located in the patellofemoral compartment (5.61 ± 4.13 cm2). Most patients had undergone at least 1 previous knee procedure (79%), which was most often either chondroplasty (37%), meniscal debridement (21%), and/or microfracture (17%) (Table 3).
TABLE 2.
Articular Cartilage Injury Characteristicsa
| Outerbridge grade, mean (SD) | 3.8 (0.4) |
| Defects on index knee, n (%) | |
| Multiple (≥2 locations) | 21 (23) |
| Single | 70 (77) |
| Defects by location, n (%) | |
| Patella | 40 (44) |
| Trochlea | 35 (38) |
| Medial femoral condyle | 28 (31) |
| Lateral femoral condyle | 10 (11) |
| Medial tibial plateau | 1 (1) |
| Lateral tibial plateau | 1 (1) |
| Defects by knee compartment, n (%) | |
| Patellofemoral compartment | 54 (59) |
| Bipolar patellofemoral defectsb | 11 (12) |
| Tibiofemoral compartment | 27 (30) |
| Bipolar tibiofemoral compartmentc | 1 (1) |
| Total defect surface area, cm2, mean (SD) | 4.00 (2.77) |
| Total defect surface area by knee compartment, cm2, mean (SD) | |
| Patellofemoral | 5.61 (4.13) |
| Tibiofemoral | 5.36 (4.52) |
| Defect surface area, cm2, n (%) | |
| ≥6 | 24 (26) |
| 4-5.9 | 28 (31) |
| 2-3.9 | 25 (28) |
| <2 | 14 (15) |
aPercentages were calculated per total number of knees (N = 91).
bBipolar patellofemoral lesions represent lesions present on the articular surface of both the patella and the trochlea.
cBipolar tibiofemoral lesions represent lesions present on the articular surface of both the femoral condyle and the adjacent tibial plateau.
TABLE 3.
Prior and Concomitant Knee Proceduresa
| Procedure | n (%)b |
|---|---|
| Any knee surgery prior to ACI procedure | 72 (79) |
| Chondroplasty | 34 (37) |
| Meniscal debridement | 19 (21) |
| Microfracture | 15 (17) |
| Anterior cruciate ligament reconstruction | 12 (13) |
| Meniscal repair | 5 (6) |
| Lateral release | 5 (6) |
| High tibial osteotomy | 4 (4) |
| Osteochondral autograft transplantation | 2 (2) |
| Meniscal allograft transplantation | 1 (1) |
| Medial collateral ligament reconstruction | 1 (1) |
| Posterior cruciate ligament reconstruction | 1 (1) |
| Any concomitant surgery during ACI procedure | 65 (71) |
| Tibial tubercle osteotomy | 55 (60) |
| High tibial osteotomy | 6 (7) |
| Meniscal allograft transplantation | 4 (4) |
| Lateral release | 3 (3) |
| Microfracture | 1 (1) |
| Anterior cruciate ligament reconstruction | 1 (1) |
| Medial patellofemoral ligament reconstruction | 1 (1) |
aACI, autologous chondrocyte implantation.
bPercentages were calculated per total number of knees in each group.
A type I/III collagen xenograft bilayer membrane was used in the majority (69%) of cases, while the remainder used a periosteal patch harvested from the ipsilateral tibia (31%). Most patients (71%) underwent concomitant procedures, including tibial tubercle osteotomy (60%), high tibial osteotomy (7%), and meniscal allograft transplantation (4%) (Table 3). The indications for concomitant anteromedializing tibial tubercle osteotomy when performed included lateral subluxation of the patella, distal and lateral patellar chondral defects, and/or tibial tuberosity trochlear groove distance >15 mm. Any patient who underwent a high tibial osteotomy was found to have a concurrent varus mechanical alignment with a chondral defect located within the medial compartment of the knee.
Outcomes
At a mean follow-up of 59.9 ± 27.1 months (range, 24.0-140.1 months), 60% of patients had significant improvement of their knee pain and did not require any further surgical intervention. Furthermore, 82% of patients reported minimal to no pain at final follow-up. Nearly one-third (35%) of patients were considered clinical failures due to the inability to return to military duty or were medically separated due to persistent, rate-limiting symptoms in the operative extremity. At the time of final follow-up, 10 patients (11%) qualified as surgical failures. Of these, 6 (7%) patients required revision chondral procedure (3 microfracture, 3 OATS), and an additional 4 (4%) individuals underwent subsequent knee arthroplasty (3 total knee arthroplasty, 1 patellofemoral arthroplasty).
A total of 16 complications (18%) occurred in 16 patients and included graft hypertrophy (8%, n = 7) with first-generation technique, arthrofibrosis (6%, n = 5), superficial infection requiring irrigation and debridement (2%, n = 2), as well as proximal tibial fracture (1%, n = 1) and nonunion at the site of concomitant tibial tubercle osteotomy (1%, n = 1). Thirty patients (33%) underwent a total of 36 reoperations, composed mostly of hardware removal (12%, n = 11), second-look diagnostic arthroscopy (8%, n = 7), periosteal graft debridement (8%, n = 7), and manipulation under anesthesia (6%, n = 5) (Table 4). Of the 7 patients with second-look arthroscopy, indications were persistent pain despite normal examination and diagnostic findings (n = 2), arthrofibrosis (n = 2), mechanical symptoms (n = 2), and loose body formation (n = 1). However, none of these second-look arthroscopies documented any graft hypertrophy at the previous ACI location. Both cases of infection required a single repeat irrigation and debridement and were resolved with oral antibiotic use. One patient was noncompliant with premature weightbearing and activity outside of his brace, and he sustained a displaced proximal tibial fracture at 8 weeks postoperatively after combined patellar ACI and tibial tubercle osteotomy that required open reduction and internal fixation (ORIF) with a lateral proximal tibia plate. One additional patient had a persistent symptomatic nonunion that underwent curettage and revision ORIF with successful union.
TABLE 4.
Outcomes and Complications After ACIa
| Outcome/complication | n (%)b |
|---|---|
| Clinical outcomes | |
| Persistent pain | 16 (18) |
| Return to duty | 54 (59) |
| Deployment | 23 (25) |
| Clinical failure | |
| Medical separation | 32 (35) |
| Surgical failure | 10 (11) |
| Arthroplasty | 4 (4) |
| Chondral procedure | 6 (7) |
| Microfracture | 3 (3) |
| Osteochondral autograft transplant | 3 (3) |
| Other reoperation besides surgical failures | |
| Removal of hardware | 11 (12) |
| Manipulation under anesthesia | 5 (6) |
| Periosteal graft hypertrophy debridement | 7 (8) |
| Diagnostic arthroscopy | 7 (8) |
| Irrigation and debridement | 2 (2) |
| ORIF nonunion | 1 (1) |
| ORIF proximal tibia fracture | 1 (1) |
| Meniscal debridement | 1 (1) |
| Lateral release | 1 (1) |
| Complications | |
| Periosteal graft hypetrophy | 7 (8) |
| Periosteal patch | 6 (7) |
| Collagen xenograft bilayer membrane | 1 (1) |
| Arthrofibrosis | 5 (6) |
| Infection | 2 (2) |
| Osteotomy nonunion | 1 (1) |
| Proximal tibia fracture | 1 (1) |
aACI, autologous chondrocyte implantation; ORIF, open reduction internal fixation.
bPercentages were calculated per total number of knees (N = 91).
Risk Factors
Univariate logistic regression analysis yielded several risk factors for clinical, surgical, and overall failure (Table 5). Subsequent multivariate analysis identified age <30 years as the only significant independent predictor of both clinical (OR, 4.01; 95% CI, 1.37-11.73) and overall failure (OR, 3.84; 95% CI, 1.32-11.16) (Table 6). Moderate demand, CS MOS (OR, 19.59; 95% CI, 1.21-316.12), exclusive involvement of the patellofemoral compartment (OR, 6.98; 95% CI, 1.04-46.80), and use of a periosteal patch (OR, 10.75; 95% CI, 1.52-75.81) were additionally found to be independent predictors of surgical failure.
TABLE 5.
Univariate Logistic Regression Analyses of Clinical, Surgical, and Overall Failuresa
| Variable | Clinical Failure, OR (95% CI) | P Value | Surgical Failure, OR (95% CI) | P Value | Overall Failure, OR (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Ageb | 0.92 (0.86-0.99) | .033c | 0.99 (0.89-1.10) | .841 | 0.94 (0.88-1.01) | .087 |
| <30 y | 2.99 (1.14-7.82) | .026c | 2.03 (0.52-7.94) | .307 | 2.86 (1.10-7.48) | .031c |
| Female sex | 1.71 (0.52-5.62) | .374 | 0.64 (0.07-5.51) | .684 | 1.97 (0.60-6.44) | .261 |
| Tobacco use | 2.11 (0.87-5.09) | .098 | 1.08 (0.28-4.12) | .916 | 1.84 (0.78-4.36) | .167 |
| BMIb | 1.02 (0.90-1.14) | .810 | 0.97 (0.81-1.17) | .748 | 1.00 (0.89-1.13) | .972 |
| ≥30 kg/m2 | 1.17 (0.48-2.87) | .731 | 0.77 (0.19-3.20) | .718 | 1.07 (0.45-2.58) | .878 |
| Army branch of service | 5.58 (0.67-46.21) | .111 | 1.13 (0.13-9.94) | .916 | 6.85 (0.83-56.61) | .074 |
| Military occupational specialty | ||||||
| Combat arms | 0.85 (0.30-2.42) | .757 | 7.81 (0.82-74.34) | .074 | 1.08 (0.39-2.99) | .877 |
| Combat support | 1.08 (0.38-3.06) | .885 | 10.79 (1.18-98.80) | .035c | 1.16 (0.42-3.23) | .775 |
| Combat service support | — | — | — | — | — | — |
| Exclusive patellofemoral lesion(s) | 1.18 (0.45-3.09) | .741 | 4.86 (1.11-21.25) | .036c | 1.71 (0.67-4.38) | .263 |
| Multiple (≥2) lesions | 1.53 (0.57-4.16) | .402 | 0.82 (0.16-4.17) | .807 | 1.19 (0.44-3.21) | .725 |
| Total size, cm2 b | 1.00 (0.99-1.01) | .331 | 1.00 (0.99-1.01) | .338 | 1.00 (0.99-1.01) | .766 |
| ≥6 | 5.08 (0.93-27.75) | .061 | 0.55 (0.07-4.28) | .568 | 2.12 (0.52-8.67) | .298 |
| 4-5.9 | 4.50 (0.84-23.99) | .078 | 0.46 (0.06-3.68) | .465 | 1.88 (0.47-7.45) | .372 |
| 2-3.9 | 2.33 (0.41-13.20) | .338 | 1.14 (0.18-7.19) | .887 | 1.41 (0.34-5.81) | .638 |
| <2 | — | — | — | — | — | — |
| Any previous procedures | 2.39 (0.72-7.93) | .156 | 2.57 (0.31-21.66) | .385 | 3.00 (0.91-9.93) | .072 |
| Previous microfracture procedure | 0.62 (0.18-2.15) | .454 | 4.24 (1.03-17.48) | .046c | 1.02 (0.33-3.17) | .970 |
| Concomitant procedure | 0.82 (0.32-2.10) | .677 | 0.35 (0.09-1.33) | .123 | 0.55 (0.22-1.38) | .200 |
| Periosteal patch | 1.29 (0.52-3.25) | .584 | 6.67 (1.58-28.16) | .010c | 1.86 (0.76-4.60) | .177 |
aBMI, body mass index; OR, odds ratio.
bAge, BMI, and total size were analyzed as continuous variables.
cStatistically significant at P < .05.
TABLE 6.
Multivariate Analysis for Risk Factors for Clinical, Surgical, and Overall Failuresa
| Outcome | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Clinical failure | |||
| Age <30 ya | 4.01 | 1.37-11.73 | .011 |
| Surgical failure | |||
| Military occupational specialty | |||
| Combat arms | 10.58 | 0.73-153.58 | .084 |
| Combat support | 19.59 | 1.21-316.12 | .036 |
| Combat service support | — | — | — |
| Exclusive patellofemoral lesion(s) | 6.98 | 1.04-46.80 | .046 |
| Previous microfracture procedure | 2.06 | 0.33-12.80 | .437 |
| Periosteal patch | 10.75 | 1.52-75.81 | .017 |
| Overall failure | |||
| Age <30 yb | 3.84 | 1.32-11.16 | .014 |
aSelected multivariate regression analysis performed on variables identified to be P < .05 on univariate analysis.
bAge was analyzed as a continuous variable.
Discussion
Resolution of symptoms and return to activity after ACI for focal articular cartilage defects of the knee present a significant challenge. Our study presents a retrospective case series of physically active young servicemembers with symptomatic focal articular cartilage defects of the knee treated with ACI. Within this analysis, we demonstrate that age <30 years, combat support, exclusive involvement of the patellofemoral compartment, prior microfracture, and use of a periosteal patch are predictors of failure after ACI.
The military represents an ideal population for evaluating the outcomes of restorative chondral procedures of the lower extremities. On average, active-duty patients are younger, maintain regular lower extremity physical activity, and, ultimately, desire return to a high level of function. At a mean 59.9-month follow-up, the current study demonstrated that 60% of patients who had ACI for a symptomatic focal chondral defect of the knee were able to return to military duty and their occupational demands. This rate is slightly lower than previously reported success rates of 73% to 84%3,9,11,17,21,22,25 but may be commensurate with the intense physical profile of this demographic and the limited time frame allotted for rehabilitation within the military framework.
A systematic review by Campbell et al3 reported on cartilage repair in the athlete’s knee. The authors sought to determine which surgical techniques returned athletes to competition and correlated patient- and defect-specific factors that were associated with successful return to sport. The authors reported an 84% rate of returning athletes to sport participation after ACI for a focal symptomatic cartilage lesion. They concluded that younger patients with smaller chondral defects, shorter duration of symptoms, knee without history of microfracture, and those who participated in a dedicated rehabilitation protocol would have a better prognosis. Additionally, a retrospective case series assessing return to sports after ACI21 showed that 73.1% of patients were able to resume some form of sports activity. The authors of this study further analyzed the different sports activities that patients were involved in prior to and after ACI. Their findings revealed that after ACI, patients transitioned from high-impact, start-stop sports to less demanding, low-impact and endurance activities.
When critically evaluating our clinical failure rate of 35%, analogous findings from the civilian literature reveal an inability to return to previous sports activity in 16% to 27% of patients after ACI of the knee.3,11,21 Within our cohort, we attribute this mainly to the increased activity-related demands placed on our population and the larger overall size of articular lesions (5.06 ± 3.78 cm2) treated. Additionally, it is exceedingly difficult to expeditiously restore military patients to active duty status due to a number of unique considerations, including the inherent strenuous occupational demands, physically active lifestyle, limited timeline allotted for full recovery (ie, 6-12 months depending on service branch), and variable capacity for activity modifications or work restrictions. An additional challenge in the military framework is defining realistic expectations for not only the patient but also the patient’s superiors within the chain of command. A patient’s specific occupation or function (ie, MOS) is often one of the most critical variables in determining return to unrestricted duty. While protective physical limitations can be imposed by the treating orthopaedic surgeon, a medical separation may still be initiated for a given patient if the anticipated recovery time exceeds certain established thresholds, despite a favorable prognosis for return to unrestricted duty and continued clinical improvement at up to 24 months postoperatively.21 If patients within the military system were afforded greater latitudes for modified daily activity or occupational specialty postoperatively, we believe that this would translate to more favorable short- to midterm overall outcomes after ACI.
We also found that patients younger than 30 years were more prone to overall failure postoperatively. This is in stark contrast to previous studies that have largely found that younger patient age is associated with superior outcomes.3,17 Nawaz et al17 reported on the mid- to long-term results of skeletally mature individuals undergoing ACI, with 78% and 51% graft survival at 5 and 10 years, respectively, and younger patients serving as the ideal candidates for ACI due to limited degeneration of adjacent compartments. In the current population, these disparate findings may reflect disproportionately greater physical demands associated with junior military status as well as the importance of peak physical fitness in career progression. Furthermore, younger servicemembers often have broader occupational skill sets, lesser degrees of specialized training, and limited mission-critical leadership roles, making them easier to replace. While failure in the general population may be more attributable to age-related decline in cartilage quality and viability, younger military patient populations are more likely to fail due to more activity-related reasons and inability to modify their occupational activities.
In the current series, patellofemoral lesions accounted for 2 of 3 defects, and this subgroup was found to be at increased risk for surgical failure when compared with articular lesions exclusive to the tibiofemoral compartment. In addition, these defects were larger than lesions located elsewhere (5.61 cm2 vs 5.36 cm2). Traditionally, success after ACI for lesions within the patellofemoral joint has been much lower when compared with other locations within the knee (60%-80% vs 80%-95%, respectively).2,9,15,18,22,23 The general consensus is that the treatment options are less reliable for restoration of patellofemoral lesions. This is due to the unique articular topography and the biomechanical forces that create a formidable challenge to articular cartilage restoration,15 particularly in the presence of bipolar or so-called “kissing” lesions (n = 12) that are typically contraindicated for cell-based treatment. Although not demonstrated in our results, other studies have shown that correction of rotational malalignment and/or offloading of symptomatic bipolar defects with a tibial tubercle osteotomy can improve the outcomes after ACI in the patellofemoral joint by correcting the abnormal shear and compressive forces.5,7,23
Prior marrow stimulation techniques (microfracture, abrasion chondroplasty) were associated with unsatisfactory surgical outcomes on univariate analysis but failed to achieve statistical significance on multivariate testing. Current trends in the surgical management of articular cartilage defects show comparatively lower rates of cell-based chondral restoration and osteochondral reconstruction when compared with other palliative techniques, such as microfracture or limited chondroplasty, as the initial surgery of choice.4,13,16 Ostensibly, this practice of initially selecting a microfracture or limited chondroplasty first could complicate outcomes for revision cartilage surgery with subsequent ACI, in large due to reactive, osseous overgrowth with intralesional osteophyte formation and disruption of the normal subchondral plate architecture.14 Minas et al14 compared ACI after previous marrow stimulation techniques with individuals undergoing primary ACI for symptomatic chondral defects. Although objective functional knees scores were not obtained in the study, the authors demonstrated an increased failure rate of ACI after marrow stimulation (26%) versus a control group (8%). Zaslav et al,25 in a prospective multicenter study, further demonstrated that although there is an increased failure rate for patients who had ACI after failed microfracture, these individuals can still have clinically significant improvements in both pain and function. Similar functional outcome scores and failure rates were found in studies with mid- to long-term follow-up.15,20 Minas et al15 showed a 62% survival rate of ACI grafts at 15 years among patients with failed marrow stimulation surgery (microfracture, subchondral drilling, or abrasion chondroplasty), and patients with prior microfracture (44%) had significantly worse survivorship than those with primary ACI (79%) for chondral defects. Pestka et al,20 in a retrospective matched-pair study, analyzed ACI after failed microfracture treatment versus those who underwent ACI alone for articular cartilage defects of the knee. Their findings demonstrated significant differences between groups. The group with prior microfracture had lower survival rates (75% vs 96%, respectively), and lower Knee injury and Osteoarthritis Outcome Score (KOOS) pain and KOOS activities of daily living subscores.
Graft hypertrophy requiring debridement occurred in 7 (8%) knees from the entire cohort, including 1 patient with a second-generation technique. The association between graft hypertrophy and first-generation ACI technique is well established in the literature, and patients treated with a periosteal patch may require secondary debridement in up to 15% to 50% of cases.9,15,18 Additionally, periosteal patch coverage with ACI may also portend an increased risk of surgical or clinical failure. Subgroup analysis of those patients in the current cohort with the first-generation ACI group revealed that 6 patients (21%) required arthroscopic debridement, with 4 of these 6 meeting the criteria for overall failure (microfracture, n = 1; patellofemoral arthroplasty, n = 1; medical discharge, n = 2). We theorize that repeated insult to the hyaline-like cartilage regenerated at the graft site may disrupt the architectural reorganization and structural integrity, increasing the risk for abnormal loading patterns and subsequent failure.
A large percentage of our cohort (71%) had a concomitant surgical procedure at the time of ACI, which may serve as a source of confounding. In particular, it may be difficult to ascertain whether outcomes were attributable to adjunctive procedures, such as offloading osteotomy, or chondral restoration alone. As with any retrospective series from the military setting, certain inherent limitations must be acknowledged. Foremost, the definition of clinical failure may be overly stringent and lack external validity to other less-active or sedentary patient populations. Similarly, knee-related medical discharge may reflect inadequate rehabilitation or compliance with commonly accepted postoperative limitations typically observed at up to 12 to 18 months after ACI surgery,1 and this could artificially inflate rates of clinical failure at short-term follow-up. Additionally, there is the potential for nonresponder bias with patients who complete their military service obligation and exit the military or choose to follow up in the civilian health care network. Given its retrospective nature, the current study also lacks validated patient-reported outcomes and objective knee measures, which would increase the generalizability of our findings and allow for quantification of pain and functional outcomes.
Conclusion
This study evaluated the short- to midterm clinical and occupational outcomes of ACI in a physically active military population and demonstrated that nearly two-thirds of individuals can return to daily activity with improved pain and function after ACI surgery. Specific risk factors for failure after ACI surgery were age younger than 30 years, lower demand occupation, exclusive involvement of the patellofemoral compartment, prior microfracture, and use of a periosteal patch. Future research is needed to provide long-term outcomes; however, the present findings allow us to appropriately counsel the expected outcomes for a young active patient who would undergo ACI for a symptomatic chondral defect of the knee.
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.
Ethical approval for this study was obtained from the William Beaumont Army Medical Center Institutional Review Board–Package #14:09.
References
- 1. Brigham & Women’s Hospital. Standard of Care: Autologous Chondrocyte Implantation Physical Therapy management. Centers for Disease Control and Prevention http://www.brighamandwomens.org/Patients_Visitors/pcs/RehabilitationServices/Physical-Therapy-Standards-of-Care-and-Protocols/Knee%20-%20ACI.pdf. Updated December 2012. Accessed September 21, 2016.
- 2. Brittberg M, Lindahl Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. [DOI] [PubMed] [Google Scholar]
- 3. Campbell AB, Pineda M, Harris JD, Flanigan DC. Return to sport after cartilage repair in athletes’ knees: a systematic review. Arthroscopy. 2016;32:651–668. [DOI] [PubMed] [Google Scholar]
- 4. Engen CN, Ången CN, Engebretsen L. Incidence of knee cartilage surgery in Norway, 2008-2011. BMJ Open. 2015;5:e008423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farr J. Autologous chondrocyte implantation improves patellofemoral cartilage treatment options. Clin Orthop Relat Res. 2007;467:187–194. [PubMed] [Google Scholar]
- 6. Fisher TF, Waterman BR, Orr JD, Holland CA, Bader J, Belmont PJ., Jr Tibial tubercle osteotomy for patellar chondral pathology in an active United States military population. Am J Sports Med. 2016;32:2342–2349. [DOI] [PubMed] [Google Scholar]
- 7. Gillogly SD, Arnold RM. Autologous chondrocyte implantation and anteromedialization for isolated patellar articular cartilage lesions: 5- to 11-year follow-up. Am J Sports Med. 2014;42:912–919. [DOI] [PubMed] [Google Scholar]
- 8. Gikas PD, Morris T, Carrington R, Skinner J, Bentley G, Briggs T. A correlation between the timing of biopsy after autologous chondrocyte implantation and the histological appearance. J Bone Joint Surg Br. 2009;91-B:1172–1177. [DOI] [PubMed] [Google Scholar]
- 9. Gomoll AH, Gillogly SD, Cole BJ, et al. Autologous chondrocyte implantation in the patella: a multicenter experience. Am J Sports Med. 2014;42:1074–1081. [DOI] [PubMed] [Google Scholar]
- 10. Goyal D, Goyal A, Keyhani S, Lee EH, Hui JH. Evidence-based second- and third-generation autologous chondrocyte implantation over first generation: a systematic review of level I and II studies. Arthroscopy. 2013;29:1872–1878. [DOI] [PubMed] [Google Scholar]
- 11. Harris JD, Brophy RH, Siston RA, Flanigan DC. Treatment of chondral defects in the athlete’s knee. Arthroscopy. 2010;26:841–852. [DOI] [PubMed] [Google Scholar]
- 12. Kusnezov N, Dunn JC, DeLong JM, Waterman BR. Sternoclavicular reconstruction on the young active patient: risk factor analysis and clinical outcomes at short-term follow-up. J Orthop Trauma. 2016;30:e111–e117. [DOI] [PubMed] [Google Scholar]
- 13. McCormick F, Harris JD, Abrams GD, et al. Trends in the surgical treatment of articular cartilage lesions in the United States: an analysis of a large private-payer database over a period of 8 years. Arthroscopy. 2014;30:222–226. [DOI] [PubMed] [Google Scholar]
- 14. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37:902–908. [DOI] [PubMed] [Google Scholar]
- 15. Minas T, Voon Keudell A, Bryant T, Gomoll AH. The John Insall Award. A minimum 10-year outcomes study of autologous chondrocyte implantation. Clin Orthop Relat Res. 2014;472:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Montgomery SR, Foster BD, Ngo SS, et al. Trends in the surgical treatment of articular cartilage defects of the knee in the United States. Knee Surg Sports Traumatol Arthrosc. 2014;22:2070–2075. [DOI] [PubMed] [Google Scholar]
- 17. Nawaz SZ, Bentley G, Briggs TW, Carrington RW, Skinner JA, Dhinsa BS. Autologous chondrocyte implantation in the knee: mid-term to long-term results. J Bone Joint Surg Am. 2014;21:824–830. [DOI] [PubMed] [Google Scholar]
- 18. Neimeyer P, Pestka JM, Kreuz PC, et al. Characteristic complications after autologous chondrocyte implantation for cartilage defects of the knee joint. Am J Sports Med. 2008;36:2091–2099. [DOI] [PubMed] [Google Scholar]
- 19. Orr JD, Dawson LK, Garcia E, Kirk KL. Incidence of osteochondral lesions of the talus in the United States military. Foot Ankle Int. 2011;32:948–954. [DOI] [PubMed] [Google Scholar]
- 20. Pestka JM, Bode G, Salzmann G, Südkamp NP, Niemeyer P. Clinical outcomes of autologous chondrocyte implantation for failed microfracture treatment of full-thickness cartilage defects of the knee joint. Am J Sports Med. 2012;40:325–331. [DOI] [PubMed] [Google Scholar]
- 21. Pestka JM, Feucht MJ, Porichis S, Bode G, Südkamp NP, Niemeyer P. Return to sport activity and work after autologous chondrocyte implantation of the knee. Am J Sports Med. 2015;44:370–377. [DOI] [PubMed] [Google Scholar]
- 22. Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow up. Am J Sports Med. 2010;38:1117–1124. [DOI] [PubMed] [Google Scholar]
- 23. Trinh TQ, Harris JD, Siston RA, Flanigan DC. Improved outcomes with combined autologous chondrocyte implantation and petellofemoral osteotomy versus isolated autologous chondrocyte implantation. Arthroscopy. 2013;29:566–574. [DOI] [PubMed] [Google Scholar]
- 24. Waterman BR, Hoffman JD, Laughlin MD, et al. Success of high tibial osteotomy in the United states Military. Orthop J Sports Med. 2015;3:2325967115574670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zaslav K, Cole B, Brewster R, et al. A prospective study of autologous chondrocyte implantation in patients with failed prior treatment for articular cartilage of the knee: results of the Study of the Treatment of Articular Repair (STAR) clinical trial. Am J Sports Med. 2009;37:42–55. [DOI] [PubMed] [Google Scholar]