Introduction
Mansonella perstans is a human filarial parasite endemic in many countries of sub-Saharan Africa, as well as in parts of South and Central America. M. perstans is spread by biting midges of the genus Culicoides. The adult parasites are thought to live in serous body cavities, and the female parasites release microfilariae into the blood [1, 2]. The clinical manifestations of M. perstans infection are poorly defined, but possible symptoms are swellings in extremities or face, itching, rash, exhaustion, and pain from serous cavities [1–4]. Few recent reports exist that describe the symptoms or treatment of travelers and migrants with M. perstans infection [5–8]. With increasing migration and travel, an increase in masonelliasis cases diagnosed in nonendemic areas may, however, be expected. M. perstans has been regarded as one of the most difficult filarial infections to treat. Antihelminthig drugs that have been tried against the infection include diethylcarbamazine (DEC), the benzimidazoles (e.g., albendazole and mebendazole), and ivermectin [1, 9, 10]. After the discovery of the symbiotic bacteria Wolbachia in M. perstans strains from certain endemic regions, doxycycline has, however, emerged as a promising treatment alternative [11, 12]. Here, the symptoms and treatment of 2 imported cases of M. perstans infection are described.
Presentation of cases
Case 1
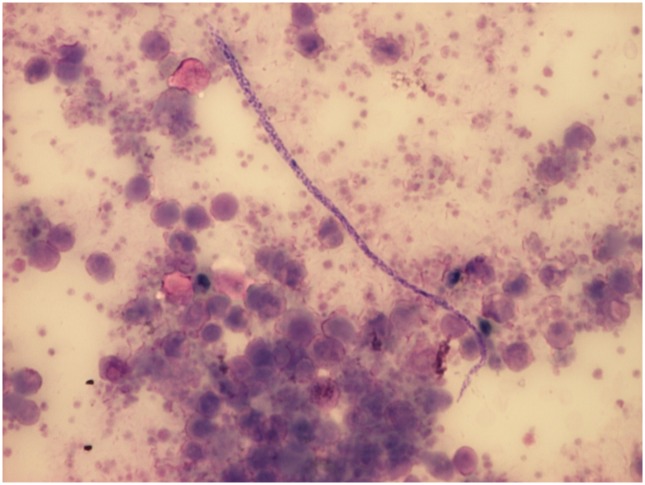
A 57-year-old woman complained of 4–6 weeks of fatigue, feeling feverish, and extensive night sweats and 3 weeks of coughing and dyspnea. She had returned from Mozambique 2 weeks earlier, where she had been living for the past 5 years. She was of Swedish origin, previously healthy, took no medications, and had no known allergies. The white blood cell count was 37.3 x 109/L, with eosinophils 24.8 x 109/L. Serum immunoglobulin E (IgE) was 220 kE/L. Antineutrophil cytoplasmic antibodies were not present. Computed tomography of the thorax and abdomen visualized bilateral peribronchial infiltrates. Bone marrow biopsy revealed a cell-rich marrow with high-grade eosinophilia, without suspicion of malignancy. Multiple stool and urine analyses for ova and parasites, including cultures for Strongyloides, did not identify any pathogens. Peripheral blood microscopy (membrane filtration technique) revealed M. perstans 1 microfilaria/ml (Fig 1). Filaria serology (in-house ELISA based on cross-reaction of antibodies directed against common Acanthocheilonema vitae antigens) was strongly positive (209 arbitrary units, cutoff 17), while for Strongyloides, optical density (OD) of 1.5 units (cutoff 0.5) was a suspected cross-reaction. Serological analyses for Fasciola, Schistosoma, Toxocara, and Trichinella were all negative.
Fig 1. Mansonella perstans microfilaria as seen in microscopy of peripheral blood sediment after Knott’s concentration (Giemsa stain, 200X).
Microfilariae were unsheathed and measured approximately 200 x 4 μm, the nuclei extending to the tip of the characteristically blunt tail. (Source: Silvia Botero).
Prednisolone 30 mg/day was initiated and tapered over 7 weeks. Concurrently, doxycycline 200 mg/day was prescribed for 6 weeks. Initially, the symptoms improved and the eosinophil count normalized, but shortly after having finished the doxycycline and prednisolone treatments, the patient again complained of increasing fatigue. Blood test revealed that the eosinophils had risen to 7.9 x 109/L, and repeat blood microscopy showed M. perstans 3 microfilariae/ml. Prednisolone again had good symptomatic effects (tapered over 12 weeks). Albendazole 1,000 mg/day (13 mg/kg/day in 2 doses) was prescribed for 4 weeks, combined with ivermectin 15 mg and a second dose 4 months later. Microscopy for microfilariae 8 months after the second ivermectin dose was negative. Nine months later (2 years after the first contact), the patient was still feeling well and had a negative microscopy. The filaria serology titer had decreased to 42 units, with normalized serum IgE (63 kE/L) and eosinophil count (0.4 x 109/L).
Case 2
A previously healthy 40-year-old male presented with fever, fatigue, loose stools, and mild pruritus 1 month after immigrating from the Democratic Republic of Congo (DRC). Plasmodium vivax malaria was diagnosed and chloroquine phosphate prescribed. As an incidental finding during malaria microscopy, microfilariae were noticed. The eosinophil count was 0.5x109/L. Most of the symptoms improved after malaria treatment, but he continued complaining of fatigue and itching. Further analysis revealed M. perstans 83 microfilariae/ml and Loa loa 670 microfilariae/ml peripheral blood (membrane filtration technique). Filaria ELISA was positive, 1.19 OD (cutoff 0.5). Stool and urine analyses for ova and parasites were negative. Doxycycline 200 mg/day for 6 weeks and albendazole 400 mg/day (6 mg/kg/day) for 3 weeks were initiated, combined with ivermectin 12 mg after 4 weeks and a second dose after 3 months (considered safe to use, since L. loa microfilaremia level was not high)[13]. Blood microscopy 10 weeks after the doxycycline treatment was still positive for L. loa (130 microfilariae/ml) but negative for M. perstans. A 3-week albendazole treatment was repeated, with 2 doses ivermectin (diethylcarbamazine, usually regarded as the drug of choice for loiasis, is not easily available in Sweden). Microscopy 3 months later (10 months after the first contact) showed decreased L. loa microfilaremia (<1 microfilariae/ml) and remained negative for M. perstans. Sixteen months after the first contact, the patient was still feeling well and had negative microscopy for microfilariae.
Discussion
Here, we have described 2 cases of M. perstans infection imported to Europe and successfully treated with combination treatments including doxycycline, albendazole, and ivermectin. In both cases, microfilariae were identified by blood microscopy, and filaria serology was positive. In the first patient, the amount of microfilariae in blood was very low, but it nevertheless caused a severe hypersensitivity reaction. Although uncommon, high-grade eosinophilia has previously been reported in association with M. perstans infection in travelers [5–7]. In the second patient, the only symptoms were mild itching and fatigue. Hence, both patients had unspecific symptoms with various possible differential diagnoses. This highlights the importance of awareness of M. perstans infection in travelers and migrants coming from endemic areas.
It is not known how commonly M. perstans strains in Mozambique and DRC harbor Wolbachia. If our patients’ strains harbored Wolbachia, doxycycline treatment alone would possibly have been sufficient. Doxycycline is thought to have an effect on adult parasites and on female parasite fertility and embryogenesis rather than having direct effect on microfilariae [11]. Therefore, disappearance of M. perstans microfilariae from the blood should not be expected directly following doxycycline therapy (because of the life span of the microfilariae). However, albendazole (possible effect on adult parasites) and ivermectin (possible effect on microfilariae) were added in the first case because of the severity of disease and persisting hypereosinophilia and in the second case because of the concomitant L. loa infection. The cases nevertheless illustrate that it is possible to clear M. perstans microfilaremia with antihelminthic therapy containing doxycycline.
In conclusion, hypersensitivity reaction, possibly severe, with eosinophilia may be a manifestation of M. perstans infection in travelers and migrants coming from endemic areas. Treatment regimens including doxycycline may be effective in clearing M. perstans microfilariae.
Key learning points
M. perstans infection commonly presents with unspecific symptoms.
M. perstans infection may present as hypersensitivity reaction, possibly severe, with eosinophilia in travelers and migrants coming from endemic areas.
Antihelminthic treatment regimens including doxycycline may be effective in clearing M. perstans microfilariae.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Simonsen PE, Onapa AW, Asio SM. Mansonella perstans filariasis in Africa. Acta Trop. 2011;120:S109–20. 10.1016/j.actatropica.2010.01.014 [DOI] [PubMed] [Google Scholar]
- 2.Klion AD, Nutman TB. Loiasis and Mansonella infections In: Guerrant DL, Walker DH, Weller PF, eds. Tropical infectious diseases: principles, pathogens, and practice. 3rd ed Philadelphia: Elsevier Saunders; 2011:735–40. [Google Scholar]
- 3.Bregani ER, Balzarini L, Mbaïdoum N, Rovellini A. Prevalence of filariasis in symptomatic patients in Moyen Chari district, south of Chad. Trop Doct. 2007;37:175–7. 10.1258/004947507781524629 [DOI] [PubMed] [Google Scholar]
- 4.Cobo F, Cabezas-Fernández MT, Salas-Coronas J, Cabeza-Barrera MI, Vázquez-Villegas J, Soriano-Pérez MJ. Filariasis in sub-Saharan immigrants attended in a health area of southern Spain: clinical and epidemiological findings. J Immigr Minor Health. 2015;17:306–9. 10.1007/s10903-013-9880-y [DOI] [PubMed] [Google Scholar]
- 5.Fux CA, Chappuis B, Holzer B, Aebi C, Bordmann G, Marti H, et al. Mansonella perstans causing symptomatic hypereosinophilia in a missionary family. Travel Med Infect Dis. 2006;4:275–80. 10.1016/j.tmaid.2005.07.001 [DOI] [PubMed] [Google Scholar]
- 6.Lipani F, Caramello P, Biglino A, Sacchi C. Albendazole for the treatment of Mansonella perstans filariasis. Trans R Soc Trop Med Hyg. 1997;91:221 [DOI] [PubMed] [Google Scholar]
- 7.Pavlovic M, Berdat P, Holzer B, Aebi C, Carrel T, Pfammatter JP. Severe mitral valve involvement in a child with hypereosinophilia secondary to parasitic infection. J Heart Valve Dis. 2003;12:649–51. [PubMed] [Google Scholar]
- 8.Van den Enden E, Van Gompel A, Van der Stuyft P, Vervoort T, Van den Ende J. Treatment failure of a single high dose of ivermectin for Mansonella perstans filariasis. Trans R Soc Trop Med Hyg. 1993;87:90 [DOI] [PubMed] [Google Scholar]
- 9.Bregani ER, Rovellini A, Mbaïdoum N, Magnini MG. Comparison of different anthelminthic drug regimens against Mansonella perstans filariasis. Trans R Soc Trop Med Hyg. 2006;100:458–63. 10.1016/j.trstmh.2005.07.009 [DOI] [PubMed] [Google Scholar]
- 10.Asio SM, Simonsen PE, Onapa AW. Mansonella perstans: safety and efficacy of ivermectin alone, albendazole alone and the two drugs in combination. Ann Trop Med Parasitol. 2009;103:31–7. 10.1179/136485909X384929 [DOI] [PubMed] [Google Scholar]
- 11.Coulibaly YI, Dembele B, Diallo AA, Lipner EM, Doumbia SS, Coulibaly SY, et al. A randomized trial of doxycycline for Mansonella perstans infection. N Engl J Med. 2009;361:1448–58. 10.1056/NEJMoa0900863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gehringer C, Kreidenweiss A, Flamen A, Antony JS, Grobusch MP, Bélard S. Molecular evidence of Wolbachia endosymbiosis in Mansonella perstans in Gabon, Central Africa. J Infect Dis. 2014;210:1633–8. 10.1093/infdis/jiu320 [DOI] [PubMed] [Google Scholar]
- 13.Gardon J, Gardon-Wendel N, Demanga-Ngangue, Kamgno J, Chippaux JP, Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet. 1997;350:18–22. 10.1016/S0140-6736(96)11094-1 [DOI] [PubMed] [Google Scholar]