Abstract
Background
To evaluate biological strategies that enhance tendon–bone healing in humans, it is imperative that suitable animal models accurately reproduce the pathological changes observed in the clinical setting following a tear. The purpose of the present study was to investigate rotator cuff degeneration in a rat, as well as assess the development of osteopenia at the enthesis following tendon detachment.
Methods
Eighteen female Wistar rats underwent unilateral detachment of the supraspinatus tendon. Specimens were retrieved at 4 weeks (n = 6), 6 weeks (n = 6) and 9 weeks (n = 6) postoperatively for histological analysis and peripheral quantitative computer tomography.
Results
Three weeks following tendon detachment, there was a significant increase in the modified Movin score, characterized by a loss of muscle mass, fatty infiltration, an increase in musculotendinous cellularity, loss of normal collagen fibre structure/arrangement, rounded tenocyte nuclei and an increase in the number of vascular bundles. This was accompanied by a reduction in bone mineral density at the tendon insertion site. After 3 weeks however, these changes were less prominent.
Conclusions
The rotator cuff tendon-muscle-bone unit in a rat model 3 weeks after detachment of supraspinatus represents a valid model for investigating rotator cuff degeneration.
Keywords: animal model, rotator cuff, tendon–bone healing, tendon degeneration
Introduction
Rotator cuff tendon degeneration is common and can result in the development of tears in susceptible tendons, associated with degenerative changes in the relevant rotator cuff muscles and in the humeral enthesis.1 Macroscopic structural changes include rotator cuff tendon thinning and retraction, muscle atrophy and fatty infiltration and compensatory hypertrophic of the intra-articular biceps tendon. Ultrastructural changes include alteration of tendon cellularity, degradation of tendon matrix quality, diminution of perfusion, microcalcification, amyloid deposition and synovial proliferation.1,2
Degeneration can be initiated by a number of factors that are either intrinsic or extrinsic to the cuff itself. Accumulation of degenerative microtrauma has been proposed as the most important intrinsic factor and encompasses age-related degeneration compounded by repetitive microtrauma, eventually resulting in the development of partial, and subsequently full-thickness tears.1 Extrinsic causes comprise environmental and anatomical influences. The former includes increasing age, shoulder overuse, smoking and any medical condition such as diabetes mellitus that disturbs healing by microvascular impairment.1 Abnormal acromial morphology has been postulated as the principal anatomical variant initiating the degenerative process.1 Progressive change in the topography and shape of the undersurface of the acromion and ‘spur’ formation at its antero-inferior border with thickening of the coracoacromial ligament (the coraco-acromial arch) lead to stenosis of the subacromial space and supraspinatus ‘outlet’ deforming the supraspinatus muscle and tendon passing under the coracoacromial arch, causing inflammation, physical damage to the muscle and musculotendinous junction, as well as the clinical presentation of ‘impingement syndrome’.
Poor healing and recurrent tears frequently occur following repair of a degenerative rotator cuff and are associated with a poor functional outcome.3 To select appropriate tendon graft materials and to determine the effect of biological augmentation on healing, it is useful to examine such strategies in a degenerative tendon model that replicates what is observed in the clinical setting. Several animal models of tendon degeneration have been developed: the rat shoulder is the most popular.4,5 The advantages of using the rat model as a surrogate for investigation of human rotator cuff function include the presence of an arch-like structure that encloses supraspinatus (similar to the coracoacromial arch) and the high functional loads generated in the tendon. Primate models have greater anatomical similarities to humans but, as a result of their expense and restricted use, they are an impractical alternative.6 Supraspinatus detachment has been shown, using a rat model, to lead to degenerative changes comparable to those seen in the clinical setting: tendon degeneration, inflammation and muscle atrophy combined with a persisting defect. These were most apparent after an interval of 3 weeks, with longer time points being associated with complete closure of the defect.5
The purpose of the present study was to investigate rotator cuff degeneration and assess the development of osteopenia at the bony insertion of supraspinatus following tendon detachment. Osteopenia of the humeral head occurs following a rotator cuff tear in humans and compromises fixation techniques where tendon is reattached to bone.7 It is therefore important to describe the osteopenia that develops in models of tendon degeneration following a chronic tear. The hypothesis was that detachment of supraspinatus from the humerus would result in tendon degeneration and osteopenia of the greater tuberosity in a rat model.
Materials and methods
Study design
All animal work was conducted in accordance with the UK Home Office Animals (Scientific Procedures) Act 1986. Eighteen randomly allocated (using simple randomization) female Wistar rats that had not previously been subject to any experimentation underwent unilateral detachment of the supraspinatus tendon. All procedures were carried out by one surgeon over several days. Using a power calculation and previously published data, an n of 6 has been shown to provide a power of 0.8, which provides significance at p = 0.05.1 Animals were allowed to freely mobilize immediately postoperatively (with cage mates and a constant supply of food and water) and specimens were retrieved after euthanasia at 3 weeks (n = 6), 6 weeks (n = 6) and 9 weeks (n = 6) postoperatively for histological analysis and peripheral quantitative computer tomography (pQCT).
Surgical technique
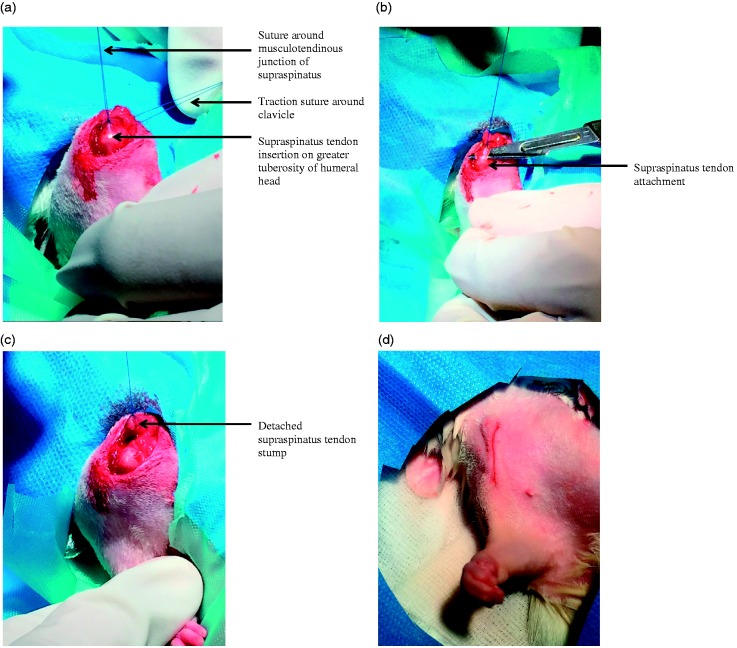
A chronic, degenerative full thickness rotator cuff tear model was developed from one that has been previously used to examine tendon degenerative changes.2 Anaesthesia was induced and maintained using 2% isoflurane mixed with pure oxygen via a facemask: this was undertaken by a veterinary anaesthetist experienced with the technique. Continuous monitoring of vital signs (heart rat, respiratory rate and temperature) was undertaken throughout surgery, which was performed in a dedicated operating theatre throughout the day. The right shoulder was used for tendon detachment in all cases and the contralateral left shoulder served as a control. A 1.5-cm skin incision was made directly over the anterolateral border of the acromion. The deltoid was detached from the anterior, lateral, and posterior margins of the acromion and split caudally for 0.5 cm. The acromioclavicular joint was divided and a traction suture was placed around the clavicle to facilitate visualization of supraspinatus (Fig. 1a). The bony end of the supraspinatus tendon was marked at its musculotendinous junction with a 5-0 Prolene suture (Ethicon; Johnson & Johnson Medical Ltd, Wokingham, UK) to assess retraction during tissue harvest. Under tension of the suture, the tendon was detached using sharp dissection from the greater tuberosity of the humeral head and allowed to retract medially (Fig. 1b, c). The deltoid muscle and fascia were closed with absorbable 5-0 Vicryl suture (Ethicon; Johnson & Johnson Medical Ltd). Skin closure was achieved using absorbable 5’0 Monocryl suture (Ethicon) and the animals were permitted unrestricted cage activity (Fig. 1d). Postoperative pain was assessed daily and analgesia (intramuscular buprenorphine 0.6 mg) was given every 12 hours for 3 days.
Figure 1.
Right shoulder joint. (a) Supraspinatus tendon insertion on the greater tuberosity. (b) Supraspinatus tendon detachment from greater tuberosity. (c) Supraspinatus tendon stump following detachment. (d) Surgical wound following closure.
Macroscopic assessment
Animals were euthanized at 3 weeks (n = 6), 6 weeks (n = 6) and 9 weeks (n = 6). Supraspinatus tendon–bone defects were visually assessed and classified as: persistent, partial and completely closed.
pQCT
After sacrifice, pQCT scanning was performed to measure bone mineral density at the humeral head. Using an XCT 2000 Bone Scanner (Stratec Medizintechnik GmbH, Pforzheim, Germany) with version 6.20 of the appropriate software, 1-mm CT slices were taken through the humeral head and supraspinatus musculotendinous unit.
Histological assessment
At euthanasia, the right shoulder was dissected and a specimen comprising the humerus with its attached supraspinatus musculotendinous unit was removed. The contralateral left shoulder served as a control (n = 6). Each sample was fixed in 10% formal saline and underwent decalcification in ethylenediaminetetraacetic acid. Decalcification was checked by radiography at weekly intervals. Following decalcification, the specimens were dehydrated in ascending graded alcohol dehydration, followed by defatting in chloroform, and embedding in paraffin wax. Multiple 4-µm sections were cut in the coronal plane through the humerus, enthesis, supraspinatus musculotendinous unit and any scar tissue that filled the gap between tendon and bone. Sections were stained with hematoxylin and eosin.
A double-blind evaluation of all sections was performed using an Olympus BH-2 light microscope (Olympus, Glasgow, UK). Using a semi-quantitative scoring system (0 = none, 1 = mild and 2 = severe), four high-powered fields were examined in each muscle to determine the extent of fatty infiltration, cellularity and inflammation.5
Tendon degeneration was assessed according to a modified Movin scale 10 and included the variables: (1) fibre structure, (2) fibre arrangement, (3) rounding of the nuclei, (4) regional variations in cellularity, (5) increased vascularity and (6) hyalinization. A four-point scoring system was used: 0 = normal appearance, 1 = slightly abnormal appearance, 2 = a moderately abnormal appearance and 3 = a markedly abnormal appearance.11 Based on this, the total score for any given slide could range from 0 (normal tendon) to 18 (the greatest level of degeneration).
Statistical analysis
Nonparametric statistical methods were used for all analyses because of the non-normality of the data in the groups being compared. Numerical data were input into SPSS, version 23 (SPSS Inc., IBM Corp., Armonk, NY, USA). The data are presented as median values (with 95% confidence intervals) unless otherwise stated. Mann–Whitney U-tests were used to compare between data sets for each group. p < 0.05 was considered statistically significant.
Results
All animals survived the duration of the study and none had postoperative infection. Limping was noted for all animals for the first 3 days to 5 days postoperatively, althoguh a normal gait pattern returned afterwards.
Macroscopic findings
Scar tissue was noted in all animals. Based on the position of the suture marker, the supraspinatus tendon had retracted approximately 5 mm in all cases. The muscle belly of supraspinatus was atrophic and was pale in appearance (Fig. 2). Some degree of tendon–bone defect closure occurred in all animals at all time points. At 3 weeks, partial defect closure was evident in all cases. At 6 weeks, two animals had partial closure of the defect and four animals had complete closure. All animals in the 9-week group had complete closure of the tendon–bone defect (Fig. 2).
Figure 2.
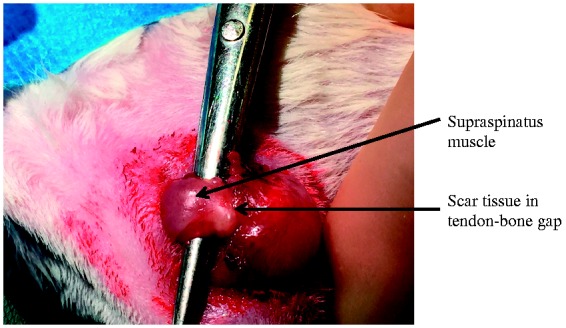
Scar tissue in supraspinatus tendon–bone gap at 9 weeks following tendon detachment.
pQCT scans
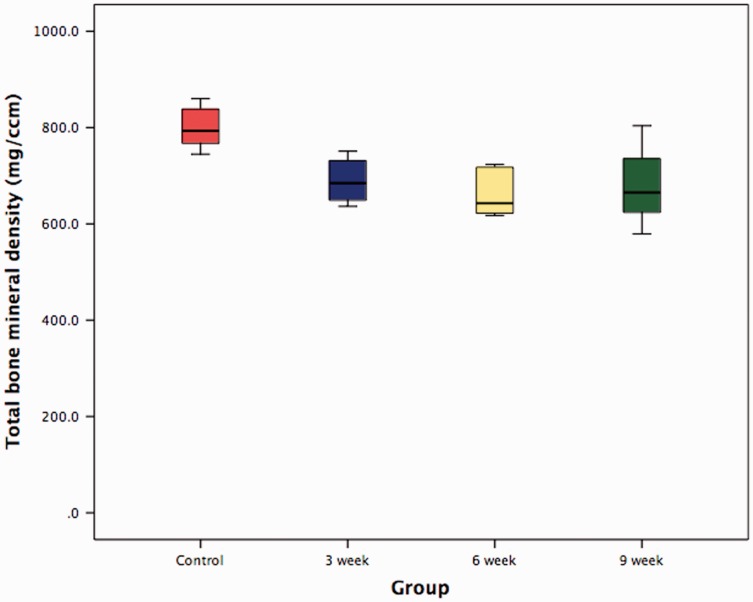
The contralateral shoulder in which the supraspinatus had not been detached represented control specimens. Median total bone mineral density significantly decreased 3 weeks (p = 0.006), 6 weeks (p = 0.004) and 9 weeks (p = 0.025) following tendon detachment (Fig. 3 and Table 1). No significant change in bone mineral density occurred between 3 weeks, 6 weeks and 9 weeks (Table 2).
Figure 3.
Box and whiskers plot showing total bone mineral density at the supraspinatus tendon–bone insertion 3 weeks, 6 weeks and 9 weeks following tendon detachment.
Table 1.
Median total bone mineral density at the supraspinatus tendon–bone insertion 3 weeks, 6 weeks and 9 weeks following tendon detachment.
| Control (non-operated shoulder) group (n = 6) | 3-week group (n = 6) | 6-week group (n = 6) | 9-week group (n = 6) | |
|---|---|---|---|---|
| Median total bone mineral density (mg/cm2) | 793.25 (95% CI 754.24 to 844.70) | 684.70 (95% CI 639.21 to 739.82) | 642.85 (CI 610.74 to 711.33) | 665.20 (CI 594.01 to 763.62) |
CI, confidence interval.
Table 2.
Statistical significance (p-values) between total bone mineral density at the supraspinatus tendon–bone insertion 3 weeks, 6 weeks and 9 weeks following tendon detachment.
| Control (non-operated shoulder) group (n = 6) | 3-week group (n = 6) | 6-week group (n = 6) | 9-week group (n = 6) | |
|---|---|---|---|---|
| Control (non-operated shoulder) group (n = 6) | – | 0.006 | 0.004 | 0.025 |
| 3-week group (n = 6) | 0.006 | – | 0.200 | 0.749 |
| 6-week group (n = 6) | 0.004 | 0.200 | – | 0.631 |
| 9-week group (n = 6) | 0.025 | 0.749 | 0.631 | – |
Histological findings
Muscle evaluation
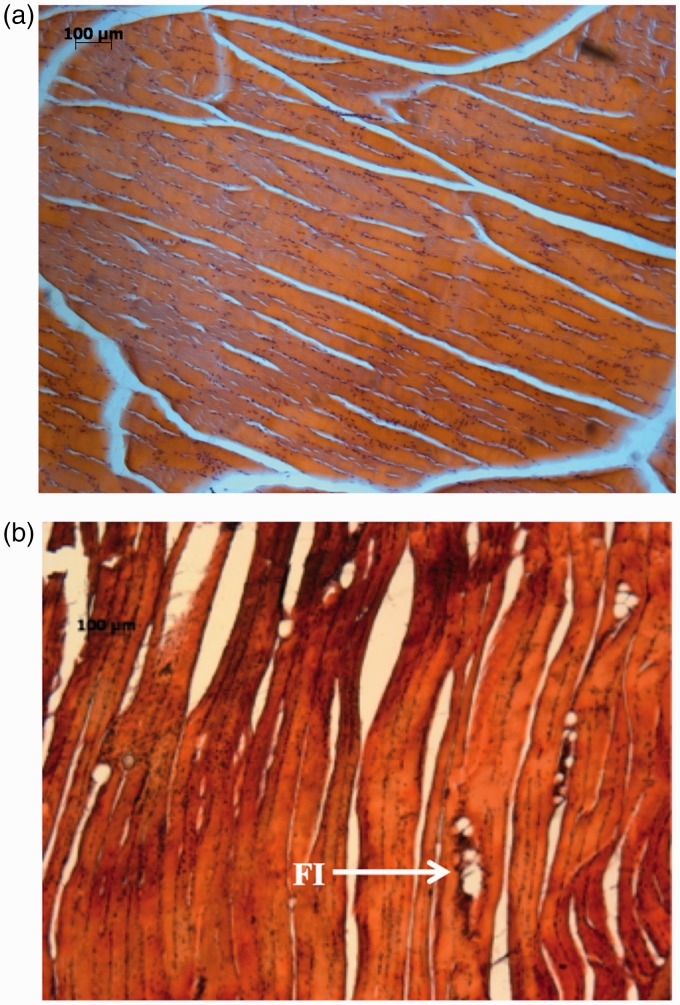
A loss of muscle mass was observed at all time points, and was accompanied by degenerative changes (characterized by increased amounts of fibrotic tissue) that were most prominent 3 weeks after detachment and less evident by 9 weeks. No inflammatory changes were present in any of the animals. All groups demonstrated a degree of fatty infiltration, which peaked at 3 weeks (Fig. 4 and Table 3). Compared to controls (where there was no fatty infiltration present) fatty infiltration significantly increased (p = 0.002) at 3 weeks but reduced at 6 weeks (p = 0.140) and 9 weeks (p = 0.138).
Figure 4.
Photomicrograph showing fatty infiltration within the supraspinatus muscle belly. (a) Control: no fatty infiltration. (b) Three-week specimen: fatty infiltration (FI).
Table 3.
Muscle and tendon histological outcome scores 3 weeks, 6 weeks and 9 weeks following tendon detachment.
| Control (non-operated shoulder) group (n = 6) | 3-week group (n = 6) | 6-week group (n = 6) | 9-week group (n = 6) | |
|---|---|---|---|---|
| Muscle: fatty infiltration | 0 (95% CI 0 to 0) | 0.5 (95% CI 0.40 to 0.94) | 0 (95% CI -0.19 to 0.69) | 0 (95% CI -0.10 to 0.44) |
| Muscle: cellularity | 0 (95% CI 0 to 0) | 2 (95% CI 2 to 2) | 1 (95% CI 0.91 to 1.69) | 1.5 (95% CI 1.02 to 1.81) |
| Modified Movin score | 0 (95% CI -0.27 to 0.60) | 8.75 (95% CI 7.08 to 11.26) | 7.75 (95% CI 6.45 to 9.38) | 8 (95% CI 7.53 to 9.87) |
| Tendon: fibre structure | 0 (95% CI 0 to 0) | 2 (95% CI 1.56 to 2.10) | 1.75 (95% CI 1.40 to 2.26) | 2.5 (95% CI 2.40 to 2.94) |
| Tendon: fibre arrangement | 0 (95% CI 0 to 0) | 2 (95% CI 1.52 to 2.31) | 1.5 (95% CI 1.20 to 1.63) | 1.5 (95% CI 1.02 to 1.81) |
| Tendon: Tenocyte nuclei | 0 (95% CI – 0.13 to 0.30) | 2.50 (95% CI 2.06 to 2.60) | 1.75 (95% CI 1.40 to 2.26) | 2 (95% CI 1.56 to 2.10) |
| Tendon: Cellularity | 0 (95% CI -0.13 to 0.30) | 1.25 (95% CI 0.84 to 2.16) | 1.75 (95% CI 1.40 to 2.26) | 1.75 (95% CI 1.46 to 2.03) |
| Tendon: Vascularity | 0 (95% CI 0 to 0) | 1.5 (95% CI 0.68 to 2.49) | 1 (95% CI 0.53 to 1.47) | 0.5 (95% CI 0.67 to 1.10) |
CI, confidence interval.
Cellularity significantly increased at 3 weeks (p = 0.001), at 6 weeks (p = 0.002) and at 9 weeks (p = 0.002), compared to controls (Table 3). Furthermore, cellularity was significantly greater in the 3-week group than in the 6-week and 9-week groups (p = 0.006 and 0.007 respectively).
Tendon evaluation
Modified Movin score
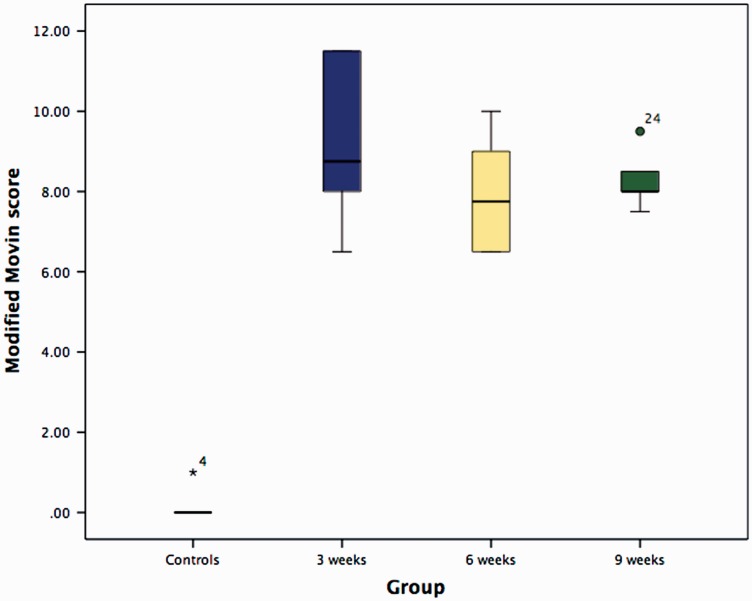
The modified Movin score was significantly higher (indicating degeneration) in the three experimental groups compared to the controls (p = 0.003: 3 weeks, 6 weeks and 9 weeks after supraspinatus tendon detachment) (Fig. 5 and Table 3). There were no significant inter-group differences (Table 4).
Figure 5.
Box and whiskers plot illustrating the modified Movin scores 3 weeks, 6 weeks and 9 weeks following tendon detachment.
Table 4.
Statistical significance (p-values) between modified Movin scores 3 weeks, 6 weeks and 9 weeks following tendon detachment.
| Control (non-operated shoulder) group (n = 6) | 3-week group (n = 6) | 6-week group (n = 6) | 9-week group (n = 6) | |
|---|---|---|---|---|
| Control (non-operated shoulder) group (n = 6) | – | 0.003 | 0.003 | 0.003 |
| 3-week group (n = 6) | 0.003 | – | 0.256 | 0.326 |
| 6-week group (n = 6) | 0.003 | 0.256 | – | 0.513 |
| 9-week group (n = 6) | 0.003 | 0.326 | 0.513 | – |
Fibre structure
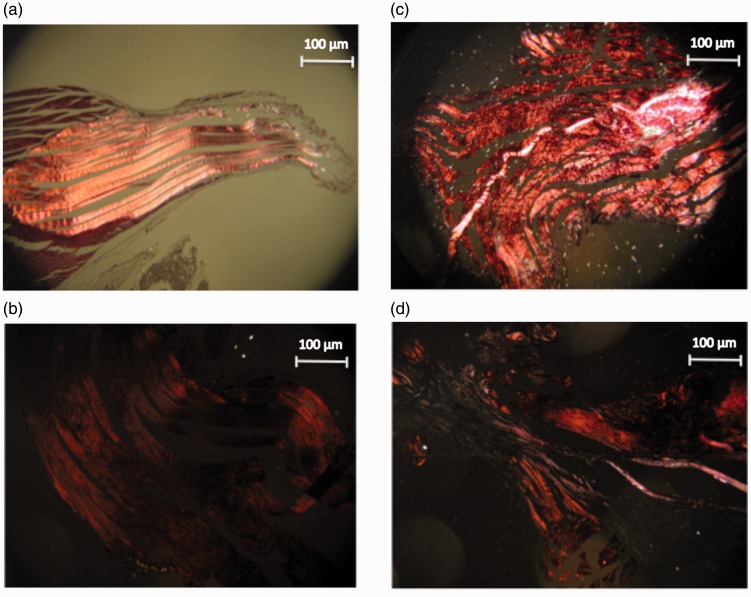
In control specimens, collagen fibres were close together and arranged in parallel. Abnormal specimens lost this uniform structure (increased waviness and distance between fibres) to differing degrees (Fig. 6 and Table 3). Fibre structure was significantly more abnormal in the 9-week group compared to the 3-week (p = 0.003) and 6-week groups (p = 0.007).
Figure 6.
Photomicrograph (under polarized light) showing collagen fibre structure. (a) Controls. (b) Three-week group. (c) Six-week group. (d) Nine-week group.
Fibre arrangement
In control specimens, the fibres were arranged in parallel. Abnormal specimens lost this arrangement to differing degrees (Fig. 6 and Table 3). Fibre arrangement was significantly more abnormal in the 3-week group compared to the controls (p = 0.002), the 6-week group (p = 0.001) and the 9-week group (p = 0.002).
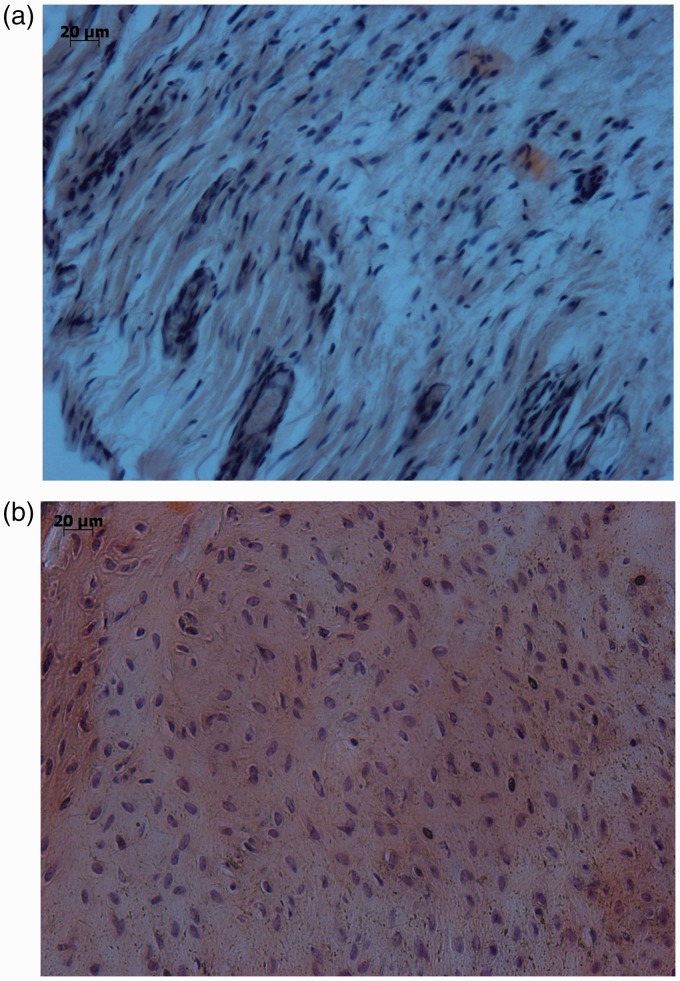
Tenocyte nuclei
Tenocyte nuclei were flattened and spindle-shaped in control specimens, but following tendon detachment became more rounded (Fig. 7 and Table 3). Tenocyte nuclei were significantly more abnormal than controls following tendon detachment (p = 0.002 at 3 weeks, p = 0.003 at 6 weeks and p = 0.002 at 9 weeks), with the 3-week group demonstrating more abnormal rounded nuclei than the 6-week and 9-week groups.
Figure 7.
Photomicrograph illustrating flattened and spindle-shaped nuclei in controls (a) and rounded nuclei 3 weeks after tendon detachment (b).
Cellularity
Specimens were evaluated for an increase in cellularity. There was a significant increase in cellularity following tendon detachment (p = 0.003 at 3 weeks, p = 0.003 at 6 weeks and p = 0.002 at 9 weeks); however, there were no significant differences between experimental groups (Tables 3 and 5).
Table 5.
Statistical significance (p-values) between cellularity 3 weeks, 6 weeks and 9 weeks following tendon detachment.
| Control (non-operated shoulder) group (n = 6) | 3-week group (n = 6) | 6-week group (n = 6) | 9-week group (n = 6) | |
|---|---|---|---|---|
| Control (non-operated shoulder) group (n = 6) | – | 0.003 | 0.003 | 0.002 |
| 3-week group (n = 6) | 0.003 | – | 0.246 | 0.315 |
| 6-week group (n = 6) | 0.003 | 0246 | – | 0.789 |
| 9-week group (n = 6) | 0.002 | 0.315 | 0.789 | – |
Vascularity
Vascular bundles ran with collagen fibres and increased in number with tendon degeneration.11 The number of vascular bundles significantly increased at 3 weeks (p = 0.002), 6 weeks (p = 0.002) and 9 weeks (p = 0.006) following supraspinatus detachment (Table 3). A significant reduction in vascularity was noted between 3 weeks and 9 weeks (p = 0.030).
Hyalinization
Hyalinization was not observed in any of the specimens.
Discussion
The present study describes a rat model for the investigation of chronic rotator cuff tears. Following detachment of supraspinatus, there was a significant rise in the modified Movin score characterized by a loss of muscle mass, fatty infiltration, an increase in musculotendinous cellularity, loss of normal collagen fibre structure/arrangement, rounded tenocyte nuclei and an increase in the number of vascular bundles. These results, in conjunction with those from the pQCT evaluation, support our hypothesis that tendon detachment induces supraspinatus musculotendinous degeneration and a reduction in bone mineral density at the enthesis. These changes occurred acutely, after a duration of 3 weeks. However, after this time, defect closure occurs, with complete closure of the defect seen at 9 weeks, and there appears to be no further degradation of the tendon or muscle. By contrast to previous reports, fatty infiltration was present in muscle specimens at 3 weeks but was no longer evident during the latter stages of the study.3,4 These transient changes in fatty infiltration suggest that, with time, there is gradual reconstitution of the tendon–bone interface with fibrous tissue that permits the transfer of load and subsequent remodelling of this neo-enthesis into a tendon-like structure.4,5
Chronic rotator cuff tears are characterized by retraction, muscle atrophy, reduced/increased cellularity, reduced/increased vascularity, fatty infiltration, calcification and degeneration of the muscle.6,7 In humans, fatty infiltration into the rotator cuff is irreversible and represents an important predisposing factor to repair failure and poor functional outcomes.8 Current rodent models have been unable to establish a significant amount of fat accumulation following tendon detachment, making it difficult to specifically examine hypotheses related to it.3,4 In the present study, there was a significant amount of fatty accumulation into the muscle belly of supraspinatus compared to controls, peaking at 3 weeks following tendon detachment and subsiding thereafter. This novel finding may be associated with fundamental interspecies differences between the Wistar rats used in this study and the Sprague–Dawley rats used in others.3,4 Lipoprotein lipase catalyzes the hydrolysis of triglycerides and is highly expressed in skeletal tissues. It is regulated differently between Wistar and Sprague–Dawley rats and may account for the lack of fat accumulation in otherwise degenerative muscle tissue in some studies.9
Rotator cuff tears can cause osteopenia at the enthesis as a result of a loss of physical stimuli10,11 During surgery, suture anchors are inserted into the greater tuberosity and therefore any reduction in bone mineral density may cause loosening or pullout before adequate tendon–bone healing can occur.12 Accordingly, this has been recognized as an independent risk factor predictive of healing, with a higher bone mineral density resulting in better outcomes.13,14 The majority of studies ascribe this alteration in bone mineral density to attritional changes secondary to tendon damage, although it is plausible that they may precede the tear and be causative in nature.15 To examine biological strategies that specifically address bone quality, relevant animal models are required. Although the anatomical similarities between the rat and human rotator cuff have been extensively described, to date, there are no studies evaluating the onset of osteopenia in the rat. In the present study, supraspinatus detachment caused a reduction in bone mineral density at 3 weeks, 6 weeks and 9 weeks, with no significant change between successive time-points. During a chronic rotator cuff tear, the forces borne by the greater tuberosity reduce and therefore cause an imbalance in bone turnover, favouring bone resorption over bone formation: a principle governed by Wolff’s law.13
The limitations of the present study include those associated with using the contralateral shoulder as a control given that its mechanical and histological properties may have altered during the few days when the animals were limping and therefore placing more weight through the non-operated limb. Additional time points (2 weeks and 12 weeks) would have been beneficial to evaluate the progression and further resolution of degenerative musculotendinous changes and alterations in bone mineral density.
In conclusion, the present study has shown that, 3 weeks following detachment, the supraspinatus musculotendinous unit in a rat undergoes degeneration, and the greater tuberosity exhibits a reduction in bone mineral density. These changes are similar to those that occur in the clinical setting following a chronic rotator cuff tear, with the difference that scar tissue bridges the defect in a rat, whereas, in a human, the tendon–bone gap is largely maintained. These findings suggest that the detached rat supraspinatus tendon, after 3 weeks, could represent a suitable model for investigating biological strategies targeted towards improving tendon–bone healing in chronic rotator cuff tears.
Acknowledgements
We thank The Royal College of Surgeons of England (RCS ART Research Fellowship).
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The paper has not been presented at any society or meeting.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by The Royal College of Surgeons of England (RCS ART Research Fellowship).
Ethical review and patient consent
All animal work was conducted in accordance with the UK Home Office Animals (Scientific Procedures) Act 1986.
References
- 1.Sundar S, Pendegrass CJ, Blunn GW. Tendon bone healing can be enhanced by demineralized bone matrix: a functional and histological study. J Biomed Mater Res Part B Appl Biomater 2009; 88: 115–122. [DOI] [PubMed] [Google Scholar]
- 2.Gulotta LV, Kovacevic D, Ehteshami JR, Dagher E, Packer JD, Rodeo SA. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med 2009; 37: 2126–2133. [DOI] [PubMed] [Google Scholar]
- 3.Buchmann S, Walz L, Sandmann GH, et al. Rotator cuff changes in a full thickness tear rat model: verification of the optimal time interval until reconstruction for comparison to the healing process of chronic lesions in humans. Arch Orthop Trauma Surg 2011; 131: 429–435. [DOI] [PubMed] [Google Scholar]
- 4.Barton ER, Gimbel JA, Williams GR, Soslowsky LJ. Rat supraspinatus muscle atrophy after tendon detachment. J Orthop Res 2005; 23: 259–265. [DOI] [PubMed] [Google Scholar]
- 5.Gimbel JA, Van Kleunen JP, Mehta S, Perry SM, Williams GR, Soslowsky LJ. Supraspinatus tendon organizational and mechanical properties in a chronic rotator cuff tear animal model. J Biomech 2004; 37: 739–749. [DOI] [PubMed] [Google Scholar]
- 6.Loew M, Magosch P, Lichtenberg S, Habermeyer P, Porschke F. How to discriminate between acute traumatic and chronic degenerative rotator cuff lesions: an analysis of specific criteria on radiography and magnetic resonance imaging. J Shoulder Elbow Surg 2015; 24: 1685–1693. [DOI] [PubMed] [Google Scholar]
- 7.Nho SJ, Yadav H, Shindle MK, Macgillivray JD. Rotator cuff degeneration: etiology and pathogenesis. Am Sports Med 2008; 36: 987–993. [DOI] [PubMed] [Google Scholar]
- 8.Deniz G, Kose O, Tugay A, Guler F, Turan A. Fatty degeneration and atrophy of the rotator cuff muscles after arthroscopic repair: does it improve, halt or deteriorate? Arch Orthop Trauma Surg 2014; 134: 985–990. [DOI] [PubMed] [Google Scholar]
- 9.Galan X, Llobera M, Ramirez I. Lipoprotein lipase in developing rat tissues: differences between Wistar and Sprague–Dawley rats. Biol Neonate 1993; 64: 295–303. [DOI] [PubMed] [Google Scholar]
- 10.Oh JH, Song BW, Kim SH, et al. The measurement of bone mineral density of bilateral proximal humeri using DXA in patients with unilateral rotator cuff tear. Osteoporos Int 2014; 25: 2639–48. [DOI] [PubMed] [Google Scholar]
- 11.Meyer DC, Fucentese SF, Koller B, Gerber C. Association of osteopenia of the humeral head with full-thickness rotator cuff tears. J Shoulder Elbow Surg 2004; 13: 333–337. [DOI] [PubMed] [Google Scholar]
- 12.Tingart MJ, Apreleva M, Zurakowski D, Warner JJ. Pullout strength of suture anchors used in rotator cuff repair. J Bone Joint Surg Am 2003; 85a: 2190–2198. [DOI] [PubMed] [Google Scholar]
- 13.Cadet ER, Hsu JW, Levine WN, Bigliani LU, Ahmad CS. The relationship between greater tuberosity osteopenia and the chronicity of rotator cuff tears. J Shoulder Elbow Surg 2008; 17: 73–77. [DOI] [PubMed] [Google Scholar]
- 14.Chung SW, Oh JH, Gong HS, Kim JY, Kim SH. Factors affecting rotator cuff healing after arthroscopic repair: osteoporosis as one of the independent risk factors. Am J Sports Med 2011; 39: 2099–2107. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Giambini H, Ben-Abraham E, An KN, Nassr A, Zhao C. Effect of bone mineral density on rotator cuff tear: an osteoporotic rabbit model. PloS ONE 2015; 10: e0139384–e0139384. [DOI] [PMC free article] [PubMed] [Google Scholar]