Abstract
Postprostatectomy vesicourethral anastomotic stenosis (VUAS) remains a challenging problem for both patient and urologist. Improved surgical techniques and perioperative identification and treatment of risk factors has led to a decline over the last several decades. High-level evidence to guide management is lacking, primarily relying on small retrospective studies and expert opinion. Endourologic therapies, including dilation and transurethral incision or resection with or without adjunct injection of scar modulators is considered first-line management. Recalcitrant VUAS requires surgical reconstruction of the vesicourethral anastomosis, and in poor surgical candidates, a chronic indwelling catheter or urinary diversion may be the only option. This review provides an update in the diagnosis and management of postprostatectomy VUAS.
Keywords: bladder neck contracture, prostate cancer, radical prostatectomy, urethral stricture, urinary incontinence, vesicourethral anastomotic stenosis
Introduction
Per the National Center for Health Statistics Hospital Discharge Survey, 138,000 prostatectomies were performed in 2010.1 Despite the decline in radical prostatectomy (RP) volume following the 2012 United States Preventive Services Task Force recommendation, the median radical prostatectomy volume per urologist per year remains six.2 A known complication from radical prostatectomy includes a fibrotic narrowing at the vesicourethral anastomotic site, or vesicourethral anastomotic stenosis (VUAS). Previously, this was termed bladder neck contracture, however stenosis is the more pathologically descriptive and accurate term and is now used in place of contracture.3 While the historically reported incidence of VUAS after RP was as high as 30%, most contemporary series demonstrate a much more modest rate of less than 5%.4–7
No consensus exists for the management of VUAS following prostatectomy. Various endoscopic therapies have been described for first-line management and those patients failing this approach often require an individualized complex reconstructive procedure. In this review, we aim to improve the understanding of how to effectively diagnose and manage VUAS.
Epidemiology
With refinement in surgical technique, the incidence of VUAS has continued to decrease over the last several decades. What was once thought to be as high as 30%, VUAS following RP carried an 8.4% (277/3310) risk in a 2007 review of CaPSURE data.5 While RP has the highest rate of VUAS among the various treatments for prostate cancer, the incidence may vary depending on the surgical approach. Radical retropubic prostatectomy causes VUAS in 0.5–30% of patients.8–16 Although long-term data are limited for VUAS following radical perineal prostatectomy, Gillitzer et al. reported an incidence of 3.8% (33/863) with a median follow up of 52 months.16 Robotic-assisted laparoscopic prostatectomy has been shown to cause VUAS in <1.4% of cases.17–23 Although not the primary focus of this review, the rate of VUAS following open prostatectomy for benign prostatic hyperplasia (BPH) ranges from 3.3% to 5.3%, based on contemporary series.24–26
Risk factors
A combination of preoperative patient characteristics and operative risk factors have been suggested to contribute to higher rates of VUAS. Higher-grade prostate cancer has consistently yielded increased rates of VUAS.27–29 This may be related to the burden of disease, or more likely surgical technique adjustments undertaken during the prostatectomy. In their large single-surgeon experience, Erickson and colleagues performed a multivariate risk factor analysis on 4132 men who underwent radical retropubic prostatectomy from 1993 to 2007 and found that non-nerve sparing technique was a risk factor for the development of a VUAS.29 It may be that these men with more advanced disease were not offered a nerve-sparing procedure.
Men with more advance disease are also more likely to undergo adjuvant or salvage radiation therapy, which can result in an obliterative endarteritis. This severe proliferating inflammation of the arterial intima can result in vessel occlusion, tissue necrosis, and fibrosis. Sowerby and colleagues reported a VUAS rate following adjuvant or salvage radiotherapy of 2.6%.30 Although they demonstrated no difference in rate when delivered in an adjuvant or salvage setting, delaying the administration of radiation after RP has been shown by others to decrease the rate of VUAS.31,32
Several patient factors have been associated with VUAS in different surgical series. Commonly cited comorbid conditions associated with higher likelihood of anastomotic stenosis are coronary artery disease, hypertension, diabetes mellitus, cigarette smoking, obesity, prior bladder outlet reduction surgery, and older age.13,19,27,28,33–35 Similar to the changes induced with radiation therapy, many of these conditions negatively affect vascular health, thereby increasing the risk of ischemia or poor tissue healing.
Other than attempting a nerve-sparing procedure, several other operative factors have been associated with VUAS. Anastomotic urine leak, intravesical foreign body, increased operative time and increased estimated blood loss have been cited by multiple studies as risk factors for VUAS.6,7,13,19,27,28,33–39 Given that most of these factors are caused by or increase the risk of poor anastomotic mucosal apposition, it is intuitive to assume this as an important risk factor for VUAS formation.29 To address this concern, Srougi et al. described a technique in which the bladder neck mucosa was everted to improve apposition and reduce VUAS, but an improvement in rates of VUAS with this technique was not noted.35 Similarly, the type of suture used for the anastomosis and duration of postoperative catheterization have not been shown to improve rates of VUAS.28 Hu and colleagues analyzed Medicare claims data collected from 1997 to 1998 and found that surgeon volume was highly predictive of VUAS. Low-volume surgeons (<40/year) had an incidence of VUAS of 27.7% compared with 22% for high-volume surgeons (>40/year).27 Although speculative, increased surgical experience may reduce the likelihood of a poor-quality anastomosis and reduce the risk for anastomotic distraction and urine leak.
The decrease seen in the incidence of VUAS over the last two decades can be explained through a combination of improved patient selection, improved surgical technique and technological advances. The advent of the DaVinci Robotic System and development of modern techniques for robot-assisted laparoscopic radical prostatectomy has propelled this surgical approach to the forefront. Three-dimensional visualization, operative field magnification and improved laparoscopic suturing with wristed instruments have created an environment for surgeons to perfect the vesicourethral anastomosis. Sandhu and colleagues nicely highlighted this in their 2011 analysis of over 4500 radical prostatectomies performed at Memorial Sloan-Kettering.40 They found an overall VUAS rate of 4% (198 patients) presenting an average of 3.5 months following prostatectomy. On multivariate analysis, open procedures were found to have a 10-times increased risk for VUAS compared with those performed minimally invasively. Other risk factors were those previously mentioned, including advanced age, higher body mass index, urinoma and hematoma.
Evaluation
Presentation
Symptomatic anastomotic stenosis generally presents within 6 months of prostatectomy and rarely after 24 months.5 Voiding complaints are primarily obstructive in nature and include weak stream, straining to void, hesitancy, and incomplete bladder emptying. A stenosis may also be discovered at the time of catheter placement for another procedure. In addition to obstructive symptoms, men who have received radiation therapy often complain of dysuria and urinary urgency or frequency. In some cases, recurrent urinary tract infections or urinary retention may be the first sign of VUAS.
Diagnosis
The evaluation of VUAS begins with a thorough history and physical examination. The history should elicit prior VUAS treatments, history of radiation therapy and presence of urinary incontinence. Patients with a VUAS may have minimal incontinence on presentation secondary to the stenotic outlet; however, during evaluation, all patients should be counseled about the possibility of VUAS treatment causing worsening of urinary incontinence. Laboratory evaluation consists of urinalysis to rule out hematuria or urinary tract infection and a serum prostate-specific antigen (PSA) to evaluate for recurrent or persistent prostate cancer.
In patients with a concerning history for VUAS, simple uroflowmetry and measurement of postvoid residual can be helpful to establish a baseline level of obstruction. Office cystoscopy is a reasonable initial diagnostic test because it allows direct visualization of the tissue and easily confirms the presence of an anastomotic stenosis. Depending on the severity of the stenosis, cystoscopic examination may also allow you to evaluate the bladder for other pathology. Retrograde urethrogram and voiding cystourethrogram are often not necessary as part of the initial evaluation unless complicating factors are present. However, these tests are essential in cases of recurrent stenosis that are being considered for reconstruction. Urodynamics are optional on initial evaluation, but if performed, should focus on determining bladder compliance and characterizing any incontinence. Lastly, imaging with ultrasound, computed tomography and magnetic resonance imaging has a limited role but may be necessary in specific cases.
Management
The goals of VUAS treatment include preventing urinary retention, allowing physiologic voiding and maintaining continence. Depending on patient desires, treatment options can include conservative management or endoscopic, open or minimally invasive surgical procedures. Poor surgical candidates or those refusing surgical intervention may be offered a chronic urethral or suprapubic catheter, clean intermittent catheterization or self-urethral dilation.
Rather than an indwelling catheter, we have found that patient-performed daily intermittent balloon dilation with a urethral balloon catheter (Cook® Medical) has been surprisingly effective. Patients pass the catheter all the way into the bladder to ensure proper positioning. The dilating balloon is then inflated with air and left inflated for 5–10 min before removal. If cystoscopy at 3 months demonstrates a patent bladder neck, then the daily regimen can be reduced over the next 6–9 months down to once per week or less. Although not curative, this may be an acceptable treatment for a subset of men with VUAS.
Endourologic treatment
Endoscopic management is first-line treatment for most men with anastomotic stenosis following prostatectomy.41 There are a variety of endoscopic options available, including dilation, internal urethrotomy, or transurethral resection. The choice of technique is largely surgeon preference, as none has been shown definitively to improve outcomes over another (Table 1). Direct-vision internal urethrotomy can be accomplished with a cold or hot knife, as well as a holmium laser. For recalcitrant stenosis failing prior management, repeat endoscopic management with injection of Mitomycin C (MMC) or steroids has been suggested to improve outcomes. Contrary to the treatment of bulbar urethral strictures, VUAS should be treated with multiple repeat endoscopic interventions prior to determining this less invasive treatment a failure. Although success with repeat endoscopic procedures for bulbar strictures approaches zero, most studies reporting outcomes following endoscopic management of VUAS find increasing overall success rates with repeat endoscopic attempts.41
Table 1.
Endoscopic management of vesicourethral anastomotic stenosis.
| Endoscopic modality | Study (reference no.) | n | Follow up (months) | Success after one treatment (%) | Success after multiple treatments (%) |
|---|---|---|---|---|---|
| Dilation | |||||
| 8 | 26 | 12 | NR | 92.30 | |
| 42 | 27 | 31.5 | 59 | NR | |
| 43 | 80 | NR | 45.5 | 100 | |
| 45 | 40 | NR | 93 | 100 | |
| 13 | 48 | NR | NR | 100 | |
| 28 | 38 | NR | 58 | NR | |
| 54 | 14 | 62.5 | 80 | 92.9 | |
| Incision | |||||
| 47 | 142 | 9.7 | 44.2 | 91 | |
| 49 | 50 | 12.9 | 72.9 | 86 | |
| 50 | 63 | 11 | 73 | NR | |
| 51 | 43 | 48 | 74 | 100 | |
| 53 | 24 | 24 | 83 | 96 | |
| 54 | 8 | 49.3 | 50 | 87.5 | |
| 56 | 18 | 12 | 72 | 94 | |
| 57 | 66 | 9.2 | 58 | 75 | |
| 58 | 40 | 20.5 | 75 | 87.5 | |
| TUR | |||||
| 61 | 10 | 18 | 100 | 100 | |
| 54 | 7 | 47.1 | 100 | 100 | |
| 9 | 24 | NR | 100 | 100 | |
| 63 | 28 | 24 | 82 | 89.2 |
NR, not recorded; TUR, transurethral resection.
Dilation
Although the success of urethral dilation for VUAS following RP has varied, there are some series with long-term favorable outcomes. In an office-based setting, Park et al. reported a 92.3% success rate using a Nottingham catheter passed over a guide cystoscopically.8 They recommended a 3-month self-catheterization protocol for all patients. A total of 7 of 26 men who were successfully managed with urethral dilation underwent more than one dilation. The mean time between dilation intervals was 2.75 months, and success was defined as having no evidence of recurrence 6 months after dilation. Success has also been achieved using male urethral sounds.42,43 In 2007, an S-shaped coaxial dilator was developed to follow the normal urethral contour.44 Most cases require multiple dilations and a period of self-dilation as single dilations have less favorable outcomes.13,28,34,42
Ramchandani and colleagues described a transurethral balloon dilation technique with regional anesthesia. A total of 16 of 27 anastomoses (59%) remained patent at a mean follow-up time of 31.5 months. Of note, the success of the modality did not appear to be related to the size of the balloon used, given the varied success and failure with 8 mm, 10 mm and 12 mm balloons. Furthermore, others have also used nephrostomy-tract dilation balloons for treatment of VUAS.45 At a mean follow-up time of 24 months, Ishii et al. reported a 100% success rate after 1–2 dilations, using a high-pressure balloon catheter that consists of a 6-French (Fr) open lumen, blunt-tip catheter, and 6 cm long balloon that inflates fully to 30 Fr at a maximum inflation pressure of 30 ATM.46
Holmium laser incisions
Results following endoscopic incision of a VUAS with holmium laser has been described in several series. LaBossiere and colleagues published the largest series to date comparing endoscopic modalities used to treat VUAS in 142 patients.47 In the holmium laser group, the fibrotic tissue was incised using a holmium laser at the 3 and 9 o’clock positions and an 18–20 Fr urethral catheter was left for 3–5 days. Using this technique, they report a significantly higher success rate with first (69%) or second treatments (58%) compared with a third (38%) or more (32%) treatments for a cumulative success rate of 91%, as defined by ability to pass a 16 Fr cystoscope. The mean time to recurrence was 6 months with knife or laser treatment, and patients underwent an average of 2.1 treatments to achieve success. Of the failures, 69% underwent open reconstruction. Albeit not a randomized comparison of treatment modalities, patients undergoing holmium laser incision had significantly higher success rates (69%) compared with other endoscopic management (0–39%) after a single treatment. Other risk factors for treatment failure included smoking and number of prior treatment attempts. Although incisions of the VUAS with a holmium can have good results, Raber et al. reports no difference in outcomes when comparing holmium laser versus cold knife incision.48
Cold- or hot-knife incisions
Stenosis incision with either a cold or hot knife has also been used to successfully treat VUAS with comparable outcomes to holmium laser incision in many series. Ramirez and colleagues performed deep lateral transurethral incisions for recurrent VUAS in 50 patients.49 Patients underwent balloon dilation to open the stenosis followed by incisions into the perivesical fat using a Collings knife on cutting current at the 3 and 9 o’clock positions. They reported being able to successfully pass a flexible cystoscope into the bladder in 72% of patients at a mean 13 months of follow up. Of the failures, seven (50%) underwent repeat transurethral incisions, five (35%) underwent suprapubic tube placement and two (15%) underwent open urethral reconstruction. Of note, 80% of the patients suffered from stress urinary incontinence and 65% of these patients underwent surgical reconstruction following endoscopic treatment.
In Brede and associate’s 15-year experience at the Cleveland Clinic, 63 patients were treated with three separate radial incisions down to the perivesical fat using a cold knife.50 A total of 46 (73%) patients were successfully treated with one or two endoscopic procedures and remained stenosis free after 11 months’ follow up. Giannarini and colleagues also reported excellent outcomes with cold-knife incision.51 Of the 43 patients in their series, they reported a 100% success rate with only 11 (26%) requiring more than one procedure. Interestingly, they report that de novo urinary incontinence was not encountered following stricture treatment, and of the 21 originally incontinent men, 11 became continent, 8 improved and 2 remained unchanged.51 This is a profound finding, given that most authors note de novo or worsening incontinence following VUAS treatment. This is an area that requires further investigation.
Incision/dilation + steroids
While patient outcomes are favorable in the majority of men following endoscopic treatment of VUAS using cold/hot knife or laser incision, more recently, efforts to improve outcomes have looked at injection of medication directly into the fibrosis following endoscopic treatment. Specifically, injection of steroids at the incision sites is thought to potentially prevent recurrent scar formation by enhancing endogenous collagenase and thus reduce contracture.52
In 2008, Eltahawy et al. described a series of 24 patients with bladder neck contractures who underwent deep holmium laser incisions followed by injection of triamcinolone at the incision sites.53 A mean follow up of 24 months revealed an 83% success rate with 70% requiring one treatment and the remainder requiring a second incision and injection at 6 weeks. Kravchick and colleagues also reported similar findings with an overall success rate of 93% with VUAS dilation followed by TRUS-guided injection of a long-acting steroid.54
Incision + Mitomycin C
MMC has a long history of use in various fields due to its antiproliferative properties that have been shown to inhibit fibroblasts and collagen deposition, thereby reducing subsequent scar formation.55 Its use in the treatment of VUAS was first described by Vanni et al. in 2011.56 The TURNS Study Group subsequently reported on 66 patients with recalcitrant VUAS. They found a 58% success rate after single treatment at an average 9.2 months of follow up. After completing a second treatment, the success rate increased to 75%. Despite its success, there was a 7% complication rate, most notably, osteitis pubis and rectourethral fistula and three patients were scheduled to undergo cystectomy at the time of publication.57
Not all series report such morbid complications using MMC in this setting. In a multi-institutional retrospective review over a 7-year period, durable results with acceptable complication rates were obtained with injection of MMC for refractory VUAS.58 At 20.5 months’ follow up, 75% had stable success after one incision and 87.5% after two incisions.58 No significant complications were noted and the 10% with refractory disease underwent open reconstruction.58
An important distinction between these two MMC series is the technique for incision/injection and concentration of medication used. In the TURNS Study Group series, a variety of concentrations of MMC were allowed and neither the injection nor incision technique was standardized. Nagpal and colleagues’ follow-on study to the original MMC description by Vanni et al. used a standard MMC dose (0.3–0.4 mg/ml) and shallow, rather than deep incisions into the fibrosis.58 Although speculative, this difference may account for at least some of the variability in complications noted between these two sets of patients. In addition to steroids and MMC, researchers note anecdotal improvement in stricture-free rates with injection of onabotulinum A.59,60
Transurethral resection
Another option for first-line treatment, as stated in the 2017 American Urologic Association Guidelines on Male Urethral Stricture, is transurethral resection of VUAS.41 Unlike transurethral incision, transurethral resection of the stenotic anastomosis involves endoscopic removal of the scar tissue. Lagerveld et al. reports 100% success rate with transurethral resection using a holmium: YAG laser.61 Using a 365-mum fiber at a setting of 2 J and frequency of 10–20 Hz, they created a deep incision of the scar at the 6 o’clock position, followed by a vaporizing resection of the remaining scar tissue between 3 and 9 o’clock. The mean follow up was 18 months and they experience no de novo incontinence. Although Kravchick and colleagues also reported a success rate of 100%, among the endoscopic therapies used for treatment, transurethral resection had the highest incontinence rate (57.1%).54 In 2007, Bach et al. reported a success rate of 86% using a 70 W 2-micron continuous wave laser that utilizes thulium as the active ion.62 Laser incisions were made at the 5 and 7 o’clock position and the remaining tissue between was vaporized. Plasma-button vaporization has also been used for resection of vesicourethral anastomotic stenoses.63
Open reconstruction
Several endoscopic attempts at treating VUAS should be undertaken prior to consideration for urethral reconstruction. In general, for men who fail endoscopic management and are considering open reconstruction, temporary suprapubic tube (SPT) drainage is performed to allow for urethral rest and time for surgical planning. A mature SPT tract is also helpful during reconstruction as it allows antegrade access to the anastomotic site to ensure a properly positioned revision anastomosis. Although most reconstructions can be completed with a progressive perineal approach, pure abdominal or combined abdominoperineal approaches have been described (Table 2). In men with an end-stage bladder, urinary diversion may also be an acceptable option.
Table 2.
Surgical management of vesicourethral anastomotic stenosis.
| Modality | Study (reference number) | n | Follow up (months) | Success (%) |
|---|---|---|---|---|
| Transperineal | ||||
| 65 | 15 | 20.5 | 93.3 | |
| 66 | 6 | 38 | 100 | |
| 68 | 23 | NR | 91.3 | |
| Abdominal | ||||
| 71 | 20 | 59.2 | 60 | |
| Abdominoperineal | ||||
| 67 | 6 | 24.2 | 83.3 | |
| Minimally invasive | ||||
| 72 | 4 | 4.2–17.5 | 100 |
NR, not recorded.
Transperineal technique
Most urethral reconstructions for a recalcitrant VUAS can be completed via a progressive perineal approach using a technique popularized for the treatment of pelvic fracture urethral injuries. This approach has many advantages, including virgin tissue dissection, proximal and distal mobilization/scar revision and anastomosis under direct vision. Additionally, it does not require entry into the peritoneum, which may have adhesions and adds additional patient morbidity.
To complete the procedure, the patient is placed in the lithotomy position, prepped and draped. A perineal incision (midline or lambda) is made followed by exposure and mobilization of the corpus spongiosum. The spongiosum may be freed from the underlying corpora as far distal as the penoscrotal junction and it is often helpful to do so. Buck’s fascia is excised from the spongiosum for improved elasticity of the tissue and ease of anastomosis. The bulb of the urethra is then dissected proximally, taking down the perineal body and dissecting proximally until the membranous urethra is reached (Figure 1).
Figure 1.
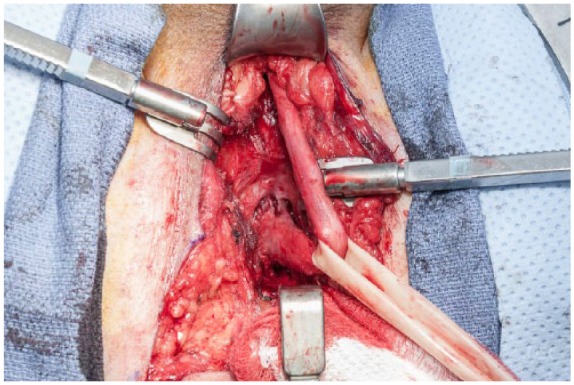
Complete urethral mobilization.
After complete urethral mobilization, the spongiosum is divided proximally and spatulated. A sound is then passed antegrade through the cystotomy tract and into the site of the anastomosis. This allows palpation of the anastomosis through the perineal incision. As the anastomotic site is frequently located quite proximal and superior within the incision, midline division of the corporal bodies is nearly universally required. This exposes the dorsal venous complex, which should be ligated (Figure 2). Occasionally an inferior pubectomy may be required if further exposure is necessary.
Figure 2.
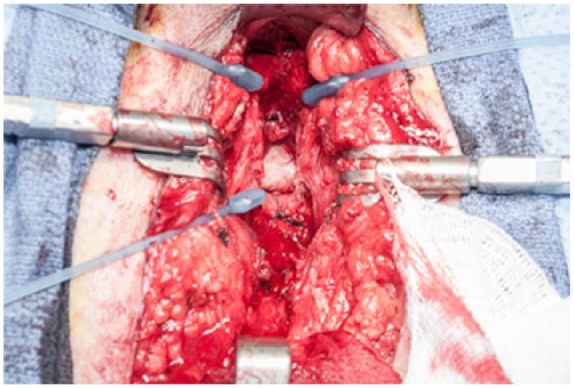
Urethra trascected, corpora split and dorsal venous complex (DVC) ligated, exposing the inferior pubis.
Once adequate exposure is attained and the proximal stenosis located, wide incision/excision of scar tissue is performed until healthy-appearing bladder is exposed for the revision anastomosis. The anastomosis should be completed with interrupted absorbable suture and is accomplished most easily by preplacing each of the proximal sutures before completing the anastomosis. After completing the anastomosis, the urethra often lies nicely in the groove created by dividing the corporal bodies allowing a tension-free anastomosis (Figure 3).
Figure 3.
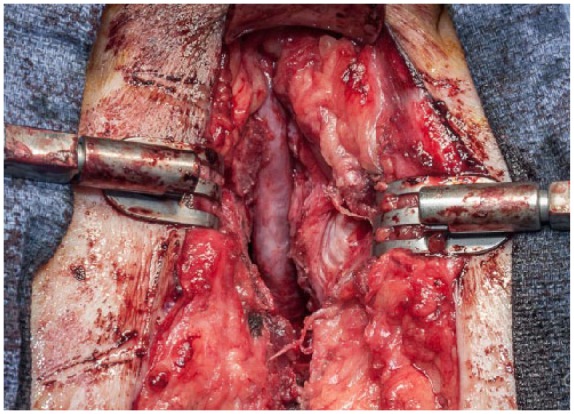
Completed anastomosis with spongiosum lying in groove between corporal bodies.
Urethral reconstruction outcomes
There is a paucity of published results for open reconstruction for VUAS failing endoscopic management. Surgical approaches for open reconstruction of the vesicourethral anastomosis include pure abdominal, perineal or abdominoperineal. The decision on which approach is utilized is generally surgeon preference, however a perineal approach can successfully treat most patients except for those with very extensive stenosis or other complicating factors.64 Reiss et al. reported on 15 patients who had three prior failed endoscopic treatments and then underwent open transperineal reanastomosis.65 Despite a mean stenosis length of 3.1 cm and follow up of 20.5 months, they found a 93.3% success rate. Although 87% reported high or very high satisfaction, 93.3% were incontinent and all complained of erectile dysfunction.65 Simonato et al. corroborates this relationship of incontinence following open transperineal VUAS reconstruction and notes that it can be successfully managed with artificial urinary sphincter (AUS) insertion.66 Some surgeons have assumed incontinence will occur in these patients and therefore place an AUS at the time of open reconstruction.67 Mundy et al. also used a transperineal approach and successfully treated 21/23 patients with recalcitrant VUAS.68 Eight patients required revision and 75% of these patients, as well as the two treatment failures, had received salvage radiotherapy which highlights the adverse effects of irradiation on the anastomosis. Prior radiotherapy was also reported as a risk factor for urethral reconstruction failure by Elliot and colleagues.69
Nikolavsky et al. retrospectively analyzed twelve cases of VUAS performed by a single surgeon over an 8-year period.70 Surgical approaches were either abdominal (7), perineal (3) or abdominoperineal (2). They reported a 92% success and 75% incontinence rate. Using a retropubic approach, Pfalzgraf and associate’s 2011 article detailed an initial success rate of 60%.71 Of the eight recurrences seven were successfully treated endoscopically, resulting in an overall 95% success rate. They only noted a de novo incontinence rate of 31%. Lastly, an abdominoperineal repair with simultaneous artificial urethral sphincter placement was found by Theodoros and colleagues to have an 83% success rate at 2 years.67
Very little has been published on laparoscopic or robotic-assisted treatment of complex recalcitrant VUAS. Hohenhorst and colleagues presented a poster on robot-assisted laparoscopic YV-plasty in a series of four patients who had three previously unsuccessful endoscopic treatments.72 At short-interval follow up (range 4.2–17.5 months), success was noted in all patients. No intraoperative or major postoperative complications occurred.
Concurrent procedures
A well known sequela of VUAS treatment is worsening or de novo incontinence. These procedures can damage the external urinary sphincter or unveil an already incompetent sphincter once patency is achieved. Repeat procedures and more invasive techniques may result in higher incontinence rates.73 Deep incision, transurethral resection and open repair of VUAS appear to consistently have the highest rates of incontinence. Therefore, it is important to counsel patients about the possibility that the cost of maintaining an open VUA may be incontinence.
The dilemma lies in whether to perform a concurrent anti-incontinence procedure or wait until the VUA has stabilized to place a sling or AUS. In their 2004 study, Anger et al. performed simultaneous deep hot-knife incision at the 3 and 9 o’clock positions and sphincter implantation in 33 men.74 All patients were continent and no patients required reincision of the VUA at an average follow up of 26.6 months. Concomitant AUS insertion with open repair of recalcitrant VUAS has also been reported.67 Although this strategy has demonstrated success in a handful of studies, most authors report deferring simultaneous AUS insertion due to the risk of VUAS recurrence and difficulty of treating VUAS with the cuff of the AUS in place. The exact timing of AUS insertion varies throughout the literature, however, most report waiting 6 months to ensure long-term stability of the VUA.
Conclusion
Modern VUAS rates range from 1% to 3% following radical prostatectomy. The basic work up includes a focused history and physical, cystoscopy, and urinalysis with urine culture. The treatment approach should be progressive in nature, starting with multiple endoscope procedures, direct-vision internal urethrotomy +/– injection of scar modulators, urethral dilation or transurethral resection, followed by reconstruction or urinary diversion.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Disclaimer: The views expressed in this article are those of the author(s) and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States Government.
Contributor Information
Nicholas R Rocco, Naval Medical Center San Diego, San Diego, CA, USA.
Jack M Zuckerman, Naval Medical Center San Diego, 34800 Bob Wilson Drive, San Diego, CA 92134, USA.
References
- 1. CDC. National Hospital Discharge Survey: 2010 table, Procedures by selected patient characteristics – Number by procedure category and age. 2016. [Google Scholar]
- 2. Halpern JA, Shoag JE, Artis AS, et al. National trends in prostate biopsy and radical prostatectomy volumes following the United States preventative services task force guidelines against prostate-specific antigen screening. JAMA Surg 2016; 152: 192–198. [DOI] [PubMed] [Google Scholar]
- 3. Latini JM, McAninch JW, Brandes SB, et al. SIU/ICUD Consultation on urethral strictures: Epidemiology, etiology, anatomy, and nomenclature of urethral stenoses, strictures, and pelvic fracture urethral disruption injuries. Urology 2014; 83: S1–S7. [DOI] [PubMed] [Google Scholar]
- 4. Erickson BA, McAninch JW, Eisenberg ML, et al. Management for prostate cancer treatment related posterior urethral and bladder neck stenosis with stents. J Urol 2011; 185: 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elliott SP, Meng MV, Elkin EP, et al. Incidence of urethral stricture after primary treatment for prostate cancer: data From CaPSURE. J Urol 2007; 178: 529–534; discussion 34. [DOI] [PubMed] [Google Scholar]
- 6. Parihar JS, Ha YS, Kim IY. Bladder neck contracture-incidence and management following contemporary robot assisted radical prostatectomy technique. Prostate Int 2014; 2: 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cho HJ, Jung TY, Kim DY, et al. Prevalence and risk factors of bladder neck contracture after radical prostatectomy. Korean J Urol 2013; 54: 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park R, Martin S, Goldberg JD, et al. Anastomotic strictures following radical prostatectomy: insights into incidence, effectiveness of intervention, effect on continence, and factors predisposing to occurrence. Urology 2001; 57: 742–746. [DOI] [PubMed] [Google Scholar]
- 9. Popken G, Sommerkamp H, Schultze-Seemann W, et al. Anastomotic stricture after radical prostatectomy. Incidence, findings and treatment. Eur Urol 1998; 33: 382–386. [DOI] [PubMed] [Google Scholar]
- 10. Tomschi W, Suster G, Höltl W. Bladder neck strictures after radical retropubic prostatectomy: still an unsolved problem. Br J Urol 1998; 81: 823–826. [DOI] [PubMed] [Google Scholar]
- 11. Dalkin BL. Endoscopic evaluation and treatment of anastomotic strictures after radical retropubic prostatectomy. J Urol 1996; 155: 206–208. [PubMed] [Google Scholar]
- 12. Garg T, See WA. Bladder neck contracture after radical retropubic prostatectomy using an intussuscepted vesico-urethral anastomosis: incidence with long-term follow-up. BJU Int 2009; 104: 925–928. [DOI] [PubMed] [Google Scholar]
- 13. Besarani D, Amoroso P, Kirby R. Bladder neck contracture after radical retropubic prostatectomy. BJU Int 2004; 94: 1245–1247. [DOI] [PubMed] [Google Scholar]
- 14. Catalona WJ, Carvalhal GF, Mager DE, et al. Potency, continence and complication rates in 1,870 consecutive radical retropubic prostatectomies. J Urol 1999; 162: 433–438. [PubMed] [Google Scholar]
- 15. Hisasue S, Takahashi A, Kato R, et al. Early and late complications of radical retropubic prostatectomy: experience in a single institution. Jpn J Clin Oncol 2004; 34: 274–279. [DOI] [PubMed] [Google Scholar]
- 16. Gillitzer R, Thomas C, Wiesner C, et al. Single center comparison of anastomotic strictures after radical perineal and radical retropubic prostatectomy. Urology 2010; 76: 417–422. [DOI] [PubMed] [Google Scholar]
- 17. Hu JC, Nelson RA, Wilson TG, et al. Perioperative complications of laparoscopic and robotic assisted laparoscopic radical prostatectomy. J Urol 2006; 175: 541–546; discussion 6. [DOI] [PubMed] [Google Scholar]
- 18. Fischer B, Engel N, Fehr JL, et al. Complications of robotic assisted radical prostatectomy. World J Urol 2008; 26: 595–602. [DOI] [PubMed] [Google Scholar]
- 19. Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA 2009; 302: 1557–1564. [DOI] [PubMed] [Google Scholar]
- 20. Carlsson S, Nilsson AE, Schumacher MC, et al. Surgery-related complications in 1253 robot-assisted and 485 open retropubic radical prostatectomies at the Karolinska University Hospital, Sweden. Urology 2010; 75: 1092–1097. [DOI] [PubMed] [Google Scholar]
- 21. Ahlering TE, Patel V, Lee DI, et al. Multiinstitutional review of complications after robotic-assisted laparoscopic prostatectomy (RLP). J Endourol 2006; 20: VP8–VP11. [Google Scholar]
- 22. Patel VR, Thaly R, Shah K. Robotic radical prostatectomy: outcomes of 500 cases. BJU Int 2007; 99: 1109–1112. [DOI] [PubMed] [Google Scholar]
- 23. Badani KK, Kaul S, Menon M. Evolution of robotic radical prostatectomy: assessment after 2766 procedures. Cancer 2007; 110: 1951–1958. [DOI] [PubMed] [Google Scholar]
- 24. Varkarakis I, Kyriakakis Z, Delis A, et al. Long-term results of open transvesical prostatectomy from a contemporary series of patients. Urology 2004; 64: 306–310. [DOI] [PubMed] [Google Scholar]
- 25. Helfand B, Mouli S, Dedhia R, et al. Management of lower urinary tract symptoms secondary to benign prostatic hyperplasia with open prostatectomy: results of a contemporary series. J Urol 2006; 176: 2557–2561; discussion 61. [DOI] [PubMed] [Google Scholar]
- 26. Serretta V, Morgia G, Fondacaro L, et al. Open prostatectomy for benign prostatic enlargement in southern Europe in the late 1990s: a contemporary series of 1800 interventions. Urology 2002; 60: 623–627. [DOI] [PubMed] [Google Scholar]
- 27. Hu JC, Gold KF, Pashos CL, et al. Role of surgeon volume in radical prostatectomy outcomes. J Clin Oncol 2003; 21: 401–405. [DOI] [PubMed] [Google Scholar]
- 28. Borboroglu PG, Sands JP, Roberts JL, et al. Risk factors for vesicourethral anastomotic stricture after radical prostatectomy. Urology 2000; 56: 96–100. [DOI] [PubMed] [Google Scholar]
- 29. Erickson BA, Meeks JJ, Roehl KA, et al. Bladder neck contracture after retropubic radical prostatectomy: incidence and risk factors from a large single-surgeon experience. BJU Int 2009; 104: 1615–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sowerby RJ, Gani J, Yim H, et al. Long-term complications in men who have early or late radiotherapy after radical prostatectomy. Can Urol Assoc J 2014; 8: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Merrick GS, Butler WM, Wallner KE, et al. Risk factors for the development of prostate brachytherapy related urethral strictures. J Urol 2006; 175: 1376–1380; discussion 81. [DOI] [PubMed] [Google Scholar]
- 32. Kowalczyk KJ, Gu X, Nguyen PL, et al. Optimal timing of early versus delayed adjuvant radiotherapy following radical prostatectomy for locally advanced prostate cancer. Urol Oncol 2014; 32: 303–308. [DOI] [PubMed] [Google Scholar]
- 33. Herschorn S, Elliott S, Coburn M, et al. SIU/ICUD Consultation on Urethral Strictures: Posterior urethral stenosis after treatment of prostate cancer. Urology 2014; 83: S59–S70. [DOI] [PubMed] [Google Scholar]
- 34. Surya BV, Provet J, Johanson KE, et al. Anastomotic strictures following radical prostatectomy: risk factors and management. J Urol 1990; 143: 755–758. [DOI] [PubMed] [Google Scholar]
- 35. Srougi M, Paranhos M, Leite KM, et al. The influence of bladder neck mucosal eversion and early urinary extravasation on patient outcome after radical retropubic prostatectomy: a prospective controlled trial. BJU Int 2005; 95: 757–760. [DOI] [PubMed] [Google Scholar]
- 36. Cormio L, Massenio P, Lucarelli G, et al. Hem-o-lok clip: a neglected cause of severe bladder neck contracture and consequent urinary incontinence after robot-assisted laparoscopic radical prostatectomy. BMC Urol 2014; 14: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ganpule A, Dhawan DR, Desai MR. Hem-o-Lokclip eroding into the urethra following laparoscopic radical prostatectomy: a case report and review of literature. Indian J Urol 2010; 26: 580–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shin YS, Doo AR, Cha JS, et al. Floating Hem-o-Lok clips in the bladder without stone formation after robot-assisted laparoscopic radical prostatectomy. Korean J Urol 2012; 53: 60–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yi JS, Kwak C, Kim HH, et al. Surgical clip-related complications after radical prostatectomy. Korean J Urol 2010; 51: 683–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sandhu JS, Gotto GT, Herran LA, et al. Age, obesity, medical comorbidities and surgical technique are predictive of symptomatic anastomotic strictures after contemporary radical prostatectomy. J Urol 2011; 185: 2148–2152. [DOI] [PubMed] [Google Scholar]
- 41. Wessells H, Angermeier KW, Elliott S, et al. Male Urethral Stricture: American Urological Association Guideline. J Urol 2017; 197: 182–190. [DOI] [PubMed] [Google Scholar]
- 42. Ramchandani P, Banner MP, Berlin JW, et al. Vesicourethral anastomotic strictures after radical prostatectomy: efficacy of transurethral balloon dilation. Radiology 1994; 193: 345–349. [DOI] [PubMed] [Google Scholar]
- 43. Geary ES, Dendinger TE, Freiha FS, et al. Incontinence and vesical neck strictures following radical retropubic prostatectomy. Urology 1995; 45: 1000–1006. [DOI] [PubMed] [Google Scholar]
- 44. Herschorn S, Carrington E. S-shaped coaxial dilators for male urethral strictures. Urology 2007; 69: 1199–1201. [DOI] [PubMed] [Google Scholar]
- 45. Zhang CY, Zhu Y, Li K, et al. Outcome of nephrostomy balloon dilation for vesicourethral anastomotic strictures following radical prostatectomy: a retrospective study. Asian J Androl 2014; 16: 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ishii G, Naruoka T, Kasai K, et al. High pressure balloon dilation for vesicourethral anastomotic strictures after radical prostatectomy. BMC Urol 2015; 15: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. LaBossiere JR, Cheung D, Rourke K. Endoscopic treatment of vesicourethral stenosis after radical prostatectomy: outcomes and predictors of success. J Urol 2016; 195:1495–1500. [DOI] [PubMed] [Google Scholar]
- 48. Raber M, Abdollah F, Matloob R, et al. Comparison of holmium laser, cold knife and diatermic incision for the endoscopic treatment of anastomotic stricture after radical retropubic prostatectomy. European Urology Supplements 2007; 6: 95. [Google Scholar]
- 49. Ramirez D, Zhao LC, Bagrodia A, et al. Deep lateral transurethral incisions for recurrent bladder neck contracture: promising 5-year experience using a standardized approach. Urology 2013; 82: 1430–1435. [DOI] [PubMed] [Google Scholar]
- 50. Brede C, Angermeier K, Wood H. Continence outcomes after treatment of recalcitrant postprostatectomy bladder neck contracture and review of the literature. Urology 2014; 83: 648–652. [DOI] [PubMed] [Google Scholar]
- 51. Giannarini G, Manassero F, Mogorovich A, et al. Cold-knife incision of anastomotic strictures after radical retropubic prostatectomy with bladder neck preservation: efficacy and impact on urinary continence status. Eur Urol 2008; 54: 647–656. [DOI] [PubMed] [Google Scholar]
- 52. Farah RN, DiLoreto RR, Cerny JC. Transurethral resection combined with steroid injection in treatment of recurrent vesical neck contractures. Urology 1979; 13: 395–397. [DOI] [PubMed] [Google Scholar]
- 53. Eltahawy E, Gur U, Virasoro R, et al. Management of recurrent anastomotic stenosis following radical prostatectomy using holmium laser and steroid injection. BJU Int 2008; 102: 796–798. [DOI] [PubMed] [Google Scholar]
- 54. Kravchick S, Lobik L, Peled R, et al. Transrectal ultrasonography-guided injection of long-acting steroids in the treatment of recurrent/resistant anastomotic stenosis after radical prostatectomy. J Endourol 2013; 27: 875–879. [DOI] [PubMed] [Google Scholar]
- 55. Ayyildiz A, Nuhoglu B, Gülerkaya B, et al. Effect of intraurethral Mitomycin-C on healing and fibrosis in rats with experimentally induced urethral stricture. Int J Urol 2004; 11:1122–1126. [DOI] [PubMed] [Google Scholar]
- 56. Vanni AJ, Zinman LN, Buckley JC. Radial urethrotomy and intralesional mitomycin C for the management of recurrent bladder neck contractures. J Urol 2011; 186: 156–160. [DOI] [PubMed] [Google Scholar]
- 57. Redshaw JD, Broghammer JA, Smith TG, et al. Intralesional injection of mitomycin C at transurethral incision of bladder neck contracture may offer limited benefit: TURNS study group. J Urol 2015; 193: 587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nagpal K, Zinman LN, Lebeis C, et al. Durable results of mitomycin C injection with internal urethrotomy for refractory bladder neck contractures: multi-institutional experience. Urology Practice 2015; 2: 250–255. [DOI] [PubMed] [Google Scholar]
- 59. Khera M, Boone TB, Smith CP. Botulinum toxin type A: a novel approach to the treatment of recurrent urethral strictures. J Urol 2004; 172: 574–575. [DOI] [PubMed] [Google Scholar]
- 60. Chen ML, Correa AF, Santucci RA. Urethral strictures and stenoses caused by prostate therapy. Rev Urol 2016; 18: 90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lagerveld BW, Laguna MP, Debruyne FM, et al. Holmium: YAG laser for treatment of strictures of vesicourethral anastomosis after radical prostatectomy. J Endourol 2005; 19: 497–501. [DOI] [PubMed] [Google Scholar]
- 62. Bach T, Herrmann TR, Cellarius C, et al. Bladder neck incision using a 70 W 2 micron continuous wave laser (RevoLix). World J Urol 2007; 25: 263–267. [DOI] [PubMed] [Google Scholar]
- 63. Öztürk H. Treatment of recurrent vesicourethral anastomotic stricture after radical prostatectomy using plasma-button vaporization. Scand J Urol 2015; 49: 371–376. [DOI] [PubMed] [Google Scholar]
- 64. Kovell RC, Terlecki RP. Management strategies for post-prostatectomy bladder neck contractures. Curr Urol Rep 2015; 16: 65. [DOI] [PubMed] [Google Scholar]
- 65. Reiss CP, Pfalzgraf D, Kluth LA, et al. Transperineal reanastomosis for the treatment for highly recurrent anastomotic strictures as a last option before urinary diversion. World J Urol 2014; 32: 1185–1190. [DOI] [PubMed] [Google Scholar]
- 66. Simonato A, Gregori A, Lissiani A, et al. Two-stage transperineal management of posterior urethral strictures or bladder neck contractures associated with urinary incontinence after prostate surgery and endoscopic treatment failures. Eur Urol 2007; 52: 1499–1504. [DOI] [PubMed] [Google Scholar]
- 67. Theodoros C, Katsifotis C, Stournaras P, et al. Abdomino-perineal repair of recurrent and complex bladder neck-prostatic urethra contractures. Eur Urol 2000; 38: 734–740; discusssion 40–41. [DOI] [PubMed] [Google Scholar]
- 68. Mundy AR, Andrich DE. Posterior urethral complications of the treatment of prostate cancer. BJU Int 2012; 110: 304–325. [DOI] [PubMed] [Google Scholar]
- 69. Elliott SP, McAninch JW, Chi T, et al. Management of severe urethral complications of prostate cancer therapy. J Urol 2006; 176: 2508–2513. [DOI] [PubMed] [Google Scholar]
- 70. Nikolavsky D, Blakely SA, Hadley DA, et al. Open reconstruction of recurrent vesicourethral anastomotic stricture after radical prostatectomy. Int Urol Nephrol 2014; 46: 2147–2152. [DOI] [PubMed] [Google Scholar]
- 71. Pfalzgraf D, Beuke M, Isbarn H, et al. Open retropubic reanastomosis for highly recurrent and complex bladder neck stenosis. J Urol 2011; 186: 1944–1947. [DOI] [PubMed] [Google Scholar]
- 72. Hohenhorst J, Musch M, Pailliart A, et al. Robot-assisted laparoscopic YV-plasty in patients with refractory bladder neck contracture. The Journal of Urology 2016; 193: e845. [DOI] [PubMed] [Google Scholar]
- 73. Song J, Eswara J, Brandes SB. Postprostatectomy anastomosis stenosis: a systematic review. Urology 2015; 86: 211–218. [DOI] [PubMed] [Google Scholar]
- 74. Anger JT, Raj GV, Delvecchio FC, Webster GD. Anastomotic contracture and incontinence after radical prostatectomy: a graded approach to management. J Urol 2005; 173: 1143–1146. [DOI] [PubMed] [Google Scholar]


