Abstract
Several types of associative learning are dependent upon the presence or absence of food, and are crucial for the survival of most animals. Target of rapamycin (TOR), a kinase which exists as a component of two complexes, TOR complex 1 (TORC1) and TOR complex 2 (TORC2), is known to act as a nutrient sensor in numerous organisms. However, the in vivo roles of TOR signaling in the nervous system remain largely unclear, partly because its multifunctionality and requirement for survival make it difficult to investigate. Here, using pharmacological inhibitors and genetic analyses, we show that TORC1 and TORC2 contribute to associative learning between salt and food availability in the nematode Caenorhabditis elegans in a process called taste associative learning. Worms migrate to salt concentrations experienced previously during feeding, but they avoid salt concentrations experienced under starvation conditions. Administration of the TOR inhibitor rapamycin causes a behavioral defect after starvation conditioning. Worms lacking either RICT-1 or SINH-1, two TORC2 components, show defects in migration to high salt levels after learning under both fed and starved conditions. We also analyzed the behavioral phenotypes of mutants of the putative TORC1 substrate RSKS-1 (the C. elegans homolog of the mammalian S6 kinase S6K) and the putative TORC2 substrates SGK-1 and PKC-2 (homologs of the serum and glucocorticoid-induced kinase 1, SGK1, and protein kinase C-α, PKC-α, respectively) and found that neuronal RSKS-1 and PKC-2, as well as intestinal SGK-1, are involved in taste associative learning. Our findings shed light on the functions of TOR signaling in behavioral plasticity and provide insight into the mechanisms by which information sensed in the intestine affects the nervous system to modulate food-searching behaviors.
Introduction
Target of rapamycin (TOR) is a serine/threonine kinase conserved from yeast to humans that performs many diverse functions by forming two structurally distinct protein complexes: TOR complex 1 (TORC1) and TOR complex 2 (TORC2) [1–3]. The use of rapamycin—a potent TORC1 inhibitor—has enabled considerable advancements in understanding the functions of the TORC1 signaling pathway, and TORC1 is now widely recognized for its role in monitoring nutrient information and regulating gene expression and metabolism, thereby controlling cell growth [4]. By contrast, assessing TORC2 function has been challenging due to a lack of specific inhibitors.
TORC2 contains four core components that are all highly conserved across species: rapamycin-insensitive companion of TOR (Rictor), stress-activated protein kinase-interacting protein (Sin1), lethal with SEC13 protein 8 (LST8), and TOR. Previous studies have identified certain AGC-subfamily kinases as putative TORC2 substrates, including Akt, protein kinase C-α (PKC-α), and serum and glucocorticoid-induced kinase 1 (SGK1), and TORC2 has been shown to control the actin cytoskeleton as well as gene transcription through phosphorylation of its substrates [5,6]. Furthermore, TORC2 has also been reported to regulate neuronal morphology, long-term memory formation, and chronic opiate-induced changes in neuronal excitation [7,8]. However, evaluation of the neuronal functions of TORC2 in vivo remains challenging because loss of TORC2 is lethal in many species. The nematode Caenorhabditis elegans is notable in this regard in that viable mutants of rict-1, the sole ortholog of Rictor, have been reported [9,10].
C. elegans has been widely used as a model organism in neuroscience research because it demonstrates various types of complex behaviors despite its morphological simplicity. Previously, we demonstrated that worms can exhibit a form of associative learning that depends on food availability and external salt concentration [11,12]: when worms were cultivated on a medium containing NaCl and bacterial food, they learned to migrate to the NaCl concentration at which they had been previously grown. Conversely, after exposure to NaCl in the absence of food, the worms learned to avoid the previously experienced NaCl concentration (S1 Fig). We call this salt and feeding state dependent behavioral plasticity “taste associative learning.” We have reported that certain evolutionarily conserved signaling pathways, including the insulin/PI3-kinase (PI3K) and the Gq/nPKC pathways, are required for taste associative learning [12,13]. Mutants of the insulin/PI3K pathway show defects in avoidance of NaCl concentrations after conditioning in the absence of food, although they show normal attraction to NaCl concentrations experienced during feeding [11]. After starvation at a high salt concentration, these mutants migrated towards high salt concentrations instead of low salt concentrations, in a pattern similar to worms that were previously fed at a high salt concentration. A mirror-image behavior, i.e. migration to low salt, was observed after starvation at a low salt concentration. These observations suggest that the insulin/PI3K pathway is required for associative learning between NaCl concentration and starvation, and/or learning-dependent switching from attraction to avoidance of a previously experienced salt concentration. On the other hand, increased activity of the Gq/PKC pathway promotes high salt migration irrespective of conditioning [11]. In particular, after either feeding at a low salt concentration or starvation at a high salt concentration, mutants with increased activity of the pathway migrated to high salt concentrations instead of low. These observations suggested that this pathway plays instructive roles in promoting high-salt migration, acting either at the memory acquisition step or during the chemotaxis behavior. So far it is not known at exactly which step of the learning and behavioral execution processes that these pathways actually regulate taste associative learning.
In this study, we aimed to identify new players in taste associative learning. By performing experiments in which we used pharmacological inhibitors and genetic mutants, we determined that disruption of the C. elegans TORC1 (CeTORC1) and TORC2 (CeTORC2) pathways resulted in abnormal behaviors in taste associative learning. We showed that RSKS-1, the homologue of S6 kinase (S6K), which acts downstream of TORC1 [4], functions in neurons. Moreover, we found that the putative CeTORC2 substrates PKC-2 and SGK-1 control taste associative learning by acting in distinct locations, in neurons and the intestine, respectively, and further that SGK-1 functions downstream of TORC2. Our results raise the possibility that the chemical information sensed in the intestine affects the nervous systems to modulate this food-related behavior in C. elegans.
Results
Loss of CeTORC1 signaling disrupts normal taste associative learning
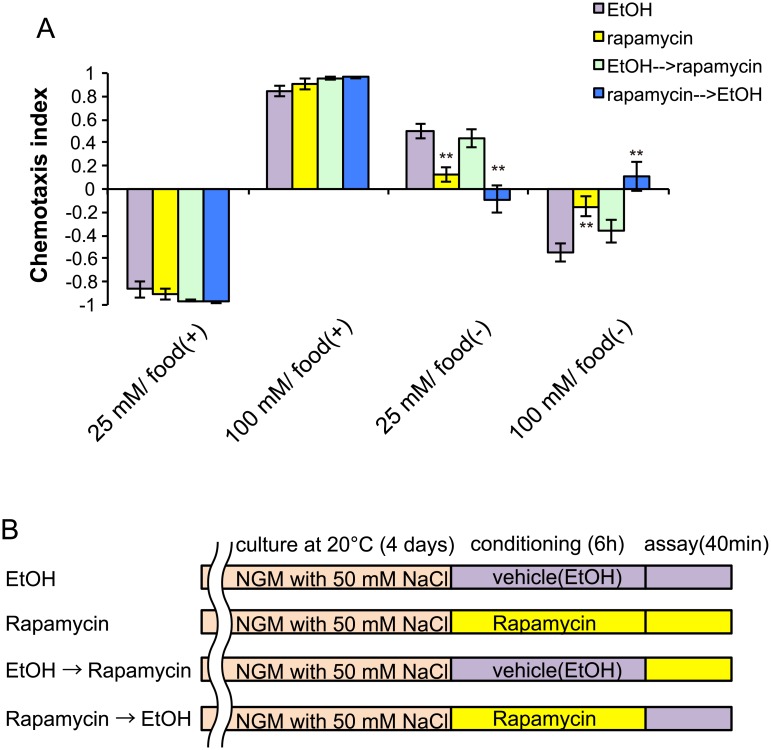
To examine the role of TOR signaling in taste associative learning, we inhibited TOR activity by using rapamycin. Rapamycin has been used as a TOR inhibitor in several organisms, and it is also effective in C. elegans at high concentrations (100 μM) [14]. We administered 100 μM rapamycin or vehicle to worms during both salt conditioning and the salt-chemotaxis test, and found that the treatment weakened the avoidance of the salt concentrations at which worms were starved: rapamycin-treated animals showed decreased migration to high or low salt levels after exposure to 25 or 100 mM NaCl in the absence of food, respectively, while chemotaxis after feeding was not affected (Fig 1A); these findings imply that post-developmental inhibition of TOR signaling may result in defective taste associative learning during starved conditions. We treated worms with rapamycin during either salt conditioning or salt-chemotaxis testing (Fig 1B). Rapamycin administration during chemotaxis testing was not sufficient to disrupt taste associative learning, whereas rapamycin exposure during salt conditioning disrupted normal chemotaxis behavior (Fig 1A).
Fig 1. TOR-specific inhibitor rapamycin disrupts normal taste associative learning.
Chemotaxis indices of wild-type worms after conditioning on low (25 mM) or high (100 mM) salt-containing agar plates with (+) or without (–) food. (B) Schematic representation of the timing of rapamycin treatment. Rapamycin or vehicle was administered to worms after 4 days of cultivation. Error bars, s.e.m.; **p < 0.01 (EtOH vs. each condition, one-way ANOVA with Dunnett’s post hoc test, N ≥ 4).
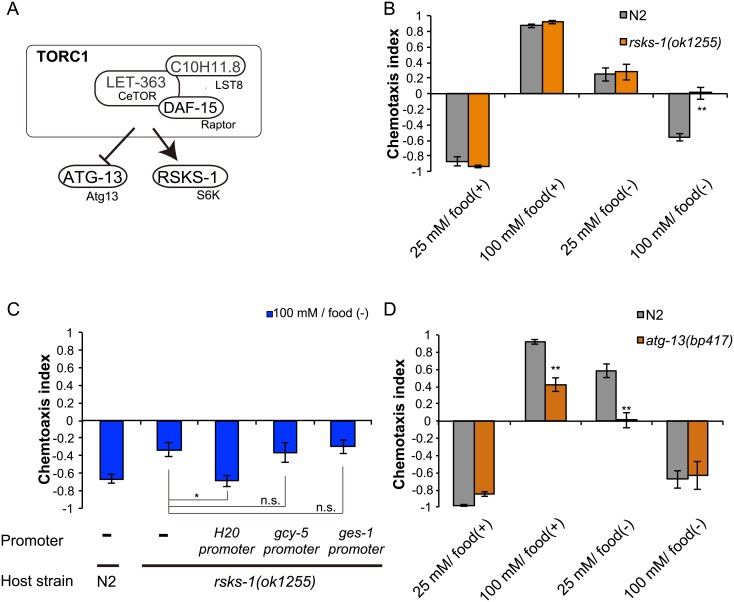
In mammalian cells, S6K, eIF4E-binding protein 1 (4E-BP1), and autophagy related gene 13 (Atg13) are major substrates of TORC1 (Fig 2A) [4]. However, null mutants of CeTORC1 core components, let-363/TOR, daf-15/Raptor, and C10H11.8/LST8, are all lethal, and no structural 4E-BP1 homolog has been found in the C. elegans genome [15]. Therefore, in our assay we tested mutants of the putative CeTORC1 targets rsks-1, the S6K homolog, and atg-13, the Atg13 homolog. Similar to rapamycin-treated animals, rsks-1 mutants failed to avoid the high salt concentration experienced during starvation: they did not migrate to low salt concentrations after exposure to 100 mM NaCl in the absence of food (high-salt/food(–) conditioning) (Fig 2B, S2 Fig). We investigated the sites of rsks-1 function by performing tissue-specific rescue experiments, and found that neuronal but not intestinal expression of rsks-1 cDNA rescued the chemotaxis abnormality (Fig 2C). However, rsks-1 cDNA expression in only the salt-sensing neuron ASER, which senses chloride ions and is considered to play a primary role in taste associative learning [11,13,16,17], failed to rescue the rsks-1 defects in taste associative learning. These results suggest that rsks-1 acts in neurons other than ASER to promote migration to low salt levels after high-salt/starvation conditioning. We also note that atg-13 mutants migrated to lower salt levels than wild-type animals after high-salt/food(+) and low-salt/food(–) conditioning (Fig 2D). Atg13 is known to be directly phosphorylated and inhibited by TORC1 in mammals [18–20]. Thus, TORC1 might negatively regulate ATG-13 function in salt chemotaxis in C. elegans (see also Discussion).
Fig 2. TORC1 signaling regulates migration to low salt concentrations through S6K.
(A) A schematic diagram of putative TORC1 signaling in C. elegans. (B) Salt chemotaxis of wild-type N2 worms and rsks-1(ok1255) mutants after salt conditioning. (C) Expression of rsks-1 cDNA under the pan-neuronal H20 promoter, but not ASER-specific gcy-5 promoter or intestinal ges-1 promoter, rescued the chemotaxis abnormality of rsks-1(ok1255) mutants. Salt chemotaxis was tested after high-salt/food(–) conditioning. (D) Salt chemotaxis of wild-type N2 animals and atg-13(bp417) mutants after salt conditioning. *p < 0.05, **p < 0.01, n.s. = not significant (one-way ANOVA with Dunnett’s post hoc test, N ≥ 9).
CeTORC2-pathway mutants show marked defects in associative learning
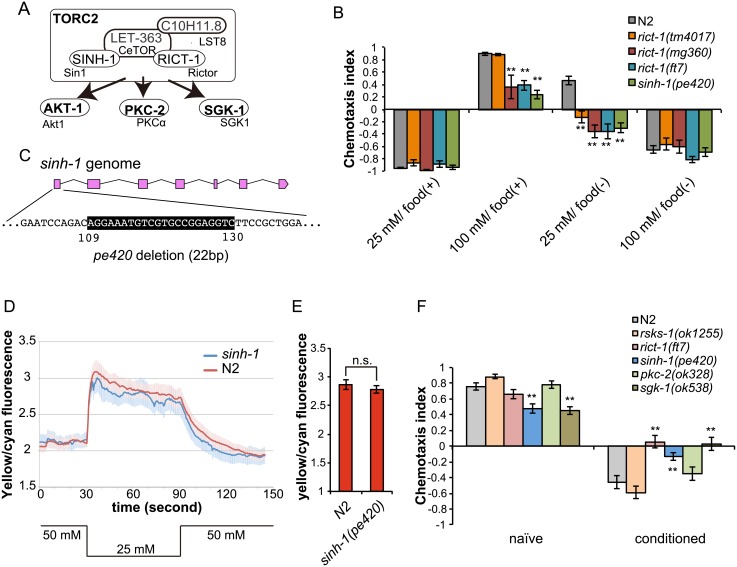
We note that the phenotype of the rsks-1 mutant mimics only a part of the rapamycin treated worms’ behavior. Because long-term administration of rapamycin has been reported to be capable of inhibiting TORC2 signaling [14,21], we suspected that in addition to CeTORC1, CeTORC2 might play a role in taste associative learning. As expected, mutants of rict-1, which encodes the homolog of the essential TORC2 component Rictor, also showed marked defects in taste associative learning (Fig 3A and 3B, S2 Fig). We tested three previously obtained alleles of rict-1 and found that the mutant worms migrated to low salt concentrations even after exposure to 25 mM NaCl in the absence of food (low-salt/food(–) conditioning). Previously, the rict-1(ft7) nonsense allele and the rict-1(mg360) missense allele were reported to cause severe defects in growth and body size and were considered to be loss of function alleles [9,10]. In addition to the chemotaxis defects observed after starvation conditioning, worms harboring these rict-1 alleles showed decreased migration to high salt concentration after exposure to 100 mM NaCl in the presence of food (high-salt/food(+) conditioning). The rict-1(tm4017) mutant showed a defect only after low-salt/food(–) conditioning (Fig 3B). The allele rict-1(tm4017) contains a deletion in an intronic region of rict-1, and worms carrying this allele showed no obvious defect in growth and body size, which implies that this is a reduction of function allele (S3 Fig). Thus, the differences in the behavioral phenotype might be due to a residual function of rict-1(tm4017). We also tested a mutant of sinh-1, which is the homolog of Sin1, another TORC2-specific component [22–24]. Because no sinh-1 mutant has been reported to date in C. elegans, we created a putative null sinh-1(pe420) mutant by using the CRISPR-Cas9 system (Fig 3C). The sinh-1(pe420) mutant worms showed an increase in body fat (S4 Fig), a phenotype similar to that of rict-1(ft7) mutants [9]. The results of behavioral assays further showed that sinh-1 mutants, like the rict-1 mutants, migrated to lower salt levels than wild-type worms after both high-salt/food(+) and low-salt/food(–) conditioning (Fig 3B, S2 Fig), which agrees with the previous report that Rictor and Sin1 function together in TORC2 [22–24]. Taken together, these data show that TORC2 disruption causes migration to lower salt levels than normal as a result of impairment of either the associative learning or execution of memory-dependent chemotaxis after high-salt/food(+) and low-salt/food(–) conditioning.
Fig 3. TORC2 components regulate migration to high salt concentrations.
(A) A schematic model of putative TORC2 signaling in C. elegans. (B) Salt chemotaxis of wild-type N2 worms and mutants of TORC2 components. TORC2-signaling mutants migrated to lower salt levels than did wild-type worms. (C) Genomic structure of sinh-1. Pink boxes indicate exons. The location of the pe420 deletion is shown below the sinh-1 locus. (D) Calcium responses of ASER of wild-type animals and sinh-1 mutants to an NaCl down-step from 50 to 25 mM and back to 50 mM (traces represent the means, the shadings the s.e.m.) after 5-h low-salt/food(–) conditioning. (E) Bar graphs showing averaged yellow/cyan fluorescence ratios during 10 s after downstep stimulation. Error bars, s.e.m.; n.s. = not significant (Student’s t test, N ≥ 9). (F) Benzaldehyde chemotaxis of wild-type N2 and mutant worms after benzaldehyde conditioning or in the naive state. rict-1(ft7), sinh-1(pe420), and sgk-1(ok538) mutants, but not pkc-2(ok328) and rsks-1(ok1255) mutants, showed defects in food-odor associative learning. Error bars, s.e.m.; **p < 0.01, n.s. = not significant (wild-type vs. each mutant, one-way ANOVA with Dunnett’s post hoc test, N ≥ 9).
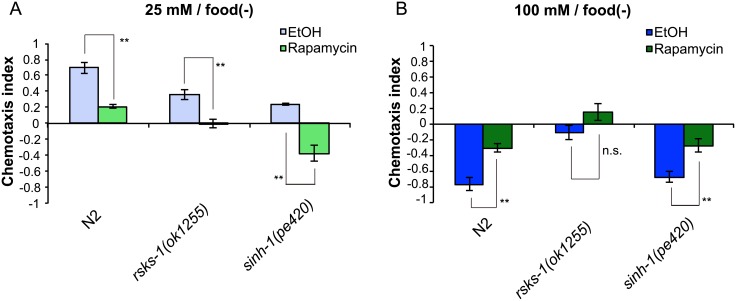
To assess the mode of action of rapamycin on taste associative learning, we treated rsks-1 and sinh-1 mutants with rapamycin. Rapamycin administration enhanced the sinh-1 phenotype after low-salt/food(–) conditioning (Fig 4A and 4B), which suggests that rapamycin inhibits migration to high salt levels through an unknown target other than TORC2. On the other hand, Rapamycin treatment did not cause statistically significant enhancement of the rsks-1 phenotype after high-salt/food(–) conditioning (Fig 4A and 4B). These data are consistent with the hypothesis that RSKS-1 promotes migration to low salt levels after high-salt/food(-) conditioning, which is inhibited by rapamycin.
Fig 4. Effects of rapamycin on taste associative learning of CeTOR signaling mutants.
(A and B) Salt chemotaxis of rapamycin-treated worms after starvation conditioning on low (A) or high (B) salt-containing agar plates; 100 μM rapamycin was used. Error bars, s.e.m.; *p < 0.05, **p < 0.01, n.s. = not significant (EtOH vs. rapamycin, Student’s t test, N ≥ 6).
Because mutants of TORC2 components showed decreased migration to high salt concentrations, we suspected that these mutants have defects in salt sensation. We monitored the response of the ASER sensory neuron to salt stimuli by using the calcium indicator Yellow Cameleon. Because ASER responds to a stepwise decrease in NaCl concentrations [25], we examined ASER responses to a NaCl down-step from 50 to 25 mM in wild-type and sinh-1 mutant worms after low-salt/food(–) conditioning, following which sinh-1 mutants showed a defect in salt chemotaxis. We observed no statistically significant difference between wild-type and sinh-1 mutant worms in ASER responses (Fig 3D and 3E), which suggests that animals lacking functional TORC2 can sense salt normally by ASER and that TORC2 might regulate sensory processing in ASER after salt sensation, or in the downstream neural circuit.
Our aforementioned results demonstrated that disruption of CeTOR and its putative substrates affect taste associative learning. To determine whether CeTOR signaling also regulates other types of learning, we analyzed the phenotypes of mutants of the CeTOR pathways in another associative learning paradigm, food-odor associative learning, in which the naive attractive response to the odorant benzaldehyde switches to avoidance after odor exposure in the absence of food [26]. Our analysis revealed that food-odor associative learning was defective in mutants of two TORC2 components, rict-1 and sinh-1, but not in a rsks-1 mutant (Fig 3F). We also investigated the rapamycin effect on food-odor associative learning and found that rapamycin treatment did not affect this type of learning (S5 Fig). The fact that mutants of TORC2 components showed reduced benzaldehyde avoidance after benzaldehyde exposure with starvation raises the possibility that CeTORC2 plays a general role in multiple types of behavioral plasticity. The sinh-1 mutant showed diminished migration toward benzaldehyde even under naive conditions, which further suggests that TORC2 signaling might be involved in the sensation and/or processing of odor information. By contrast, the phenotype of the rict-1(ft7) mutant under naive conditions was not statistically different from that of wild-type worms, suggesting that rict-1(ft7), but not sinh-1(pe420), spares the TORC2 function required for odor chemotaxis.
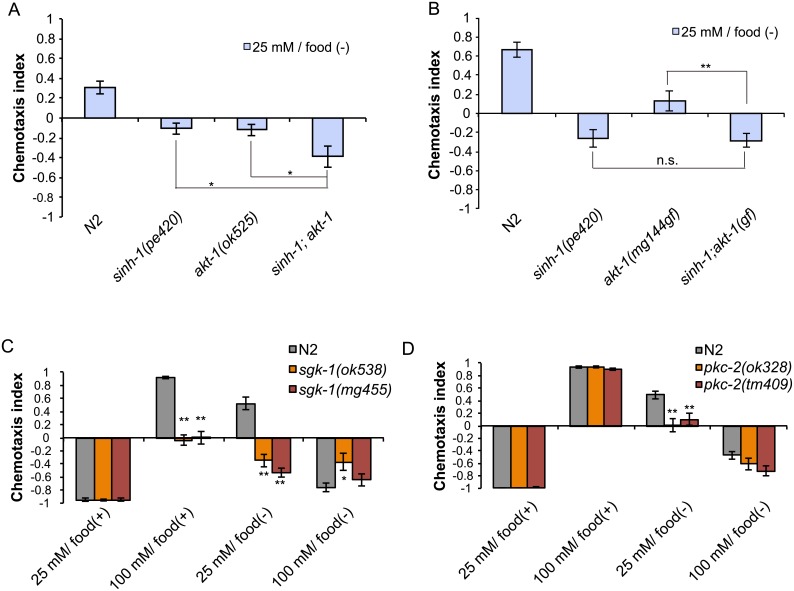
CeTORC2 functions in parallel with akt-1 in taste associative learning
TORC2 directly phosphorylates several members of the AGC kinase family, such as Akt1, SGK1, and PKC-α (Fig 3A) [4–6]. We have previously reported that akt-1 mutants show defects in taste associative learning [11,12,17], and thus we hypothesized AKT-1 may be a CeTORC2 substrate in taste associative learning. We have reported that akt-1(ok525) mutants fail to avoid salt concentrations that were associated with starvation [11]. We hypothesized that if AKT-1 acts independently of CeTORC2, the CeTORC2 mutation would further enhance the learning defect of the akt-1 mutant. Our analysis revealed that sinh-1(pe420);akt-1(ok525) double mutants migrated to lower salt levels than did sinh-1(pe420) or akt-1(ok525) single mutants after low-salt/food(-) conditioning (Fig 5A), which suggests that these mutations exert additive effects. We also tested the interaction between a gain-of-function mutation of akt-1, akt-1(mg144gf)[27], and sinh-1(pe420), and found that the akt-1(mg144gf) mutation did not suppress the sinh-1 phenotype (Fig 5B). These results argue against the possibility that AKT-1 functions as a CeTORC2 substrate in taste associative learning.
Fig 5. Putative TORC2 substrates akt-1, pkc-2, and sgk-1 are involved in taste associative learning.
(A) sinh-1 and akt-1 mutations have additive effects on salt chemotaxis after low-salt/food(–) conditioning. (B) akt-1(mg144gf) does not suppress the chemotaxis defect of sinh-1(pe420) after low-salt/food(–) conditioning. (C and D) Salt chemotaxis of the mutants of the putative TORC2 substrates sgk-1 (C) and pkc-2 (D). sgk-1 mutants showed abnormal migration to low salt levels after 25 mM salt/food(–) and 100 mM salt/food(+) conditioning (C), whereas pkc-2 mutants showed migration to low salt levels only after 25 mM salt/food(–) conditioning (D). Error bars, s.e.m.; *p < 0.05, **p < 0.01 (wild-type vs. each mutant, one-way ANOVA with Dunnett’s post hoc test, N ≥ 6).
Sgk and cPKC mutants show defects in taste associative learning
SGK1 was reported to be a substrate of TORC2 and to control the activities of ion channels and transporters by phosphorylating them [28]. Moreover, the SGK1 homolog in C. elegans, sgk-1, was reported to function downstream of TORC2 and play a role in controlling fat storage and aging [9,10,29]. Therefore, we examined the involvement of this candidate CeTORC2 substrate in taste associative learning. As observed in the case of TORC2 mutants, two sgk-1 mutants migrated to lower salt concentrations than wild-type worms did after low-salt/food(–) and high-salt/food(+) conditioning (Fig 5C).
pkc-2, the only gene encoding conventional PKC (cPKC) in the C. elegans genome, has been reported to function downstream of CeTORC2 [30]. We found that two pkc-2 mutants also migrated to lower salt levels than did wild-type worms, but the phenotype of these mutants after low-salt/food(–) conditioning was weaker than those of other CeTORC2-pathway mutants (Fig 5D). Unlike most of the TORC2 mutants tested in this study, the pkc-2 mutants showed no chemotaxis defect after high-salt/food(+) conditioning. Thus, PKC-2 appears to be involved in taste associative learning, but its contribution is less than that of other TORC2-pathway mutants.
We also tested whether these putative TORC2 substrates are involved in food-odor associative learning. Similar to the rict-1(ft7) mutant, the sgk-1(ok538) mutant showed weaker attraction to benzaldehyde under naive conditions and weaker avoidance after a 1-h exposure to benzaldehyde than did wild-type worms (Fig 3F). By contrast, both naive and conditioned pkc-2(ok328) mutants showed no marked defect in migration to benzaldehyde (Fig 3F). Thus, in contrast to its regulation of taste associative learning, pkc-2 does not appear to contribute to associative learning between odor and starvation.
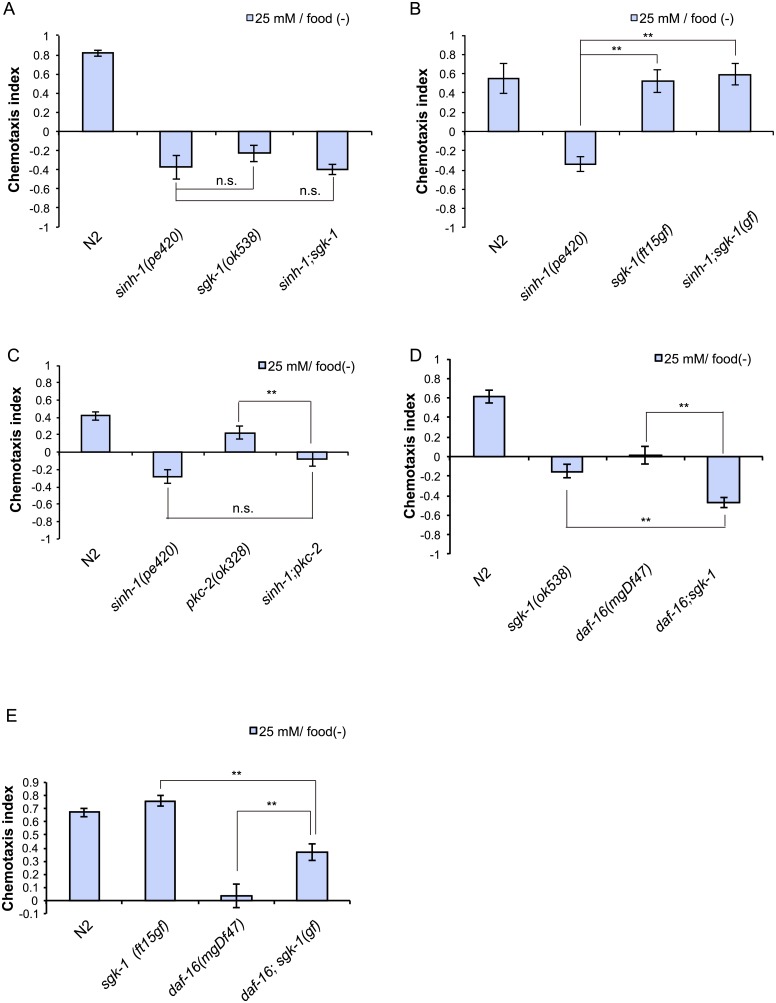
sgk-1 and possibly pkc-2 function downstream of CeTORC2 to control taste associative learning
To test whether sgk-1 and pkc-2 act downstream of CeTORC2, we conducted genetic-interaction analyses. The deletion mutation allele sgk-1(ok538) did not enhance the chemotaxis defect of sinh-1(pe420) (Fig 6A), which suggests that sinh-1 and sgk-1 function in the same pathway to control taste associative learning. We next examined the TORC2-sgk-1 interaction by using a putative gain of function allele of sgk-1, sgk-1(ft15gf), which was isolated through a genetic screen for suppressor mutations that block the high-fat-storage phenotype of a rict-1 loss of function mutant [9,31]. The sgk-1(ft15gf) mutant showed largely normal salt chemotaxis (S6 Fig), and the mutation drastically suppressed the abnormal chemotaxis of the sinh-1(pe420) mutants (Fig 6B). These results indicate that sgk-1 regulates taste associative learning downstream of CeTORC2. The pkc-2(ok328) mutation did not further enhance the abnormal migration to low salt concentrations of the sinh-1(pe420) mutant (Fig 6C), which is consistent with the notion that pkc-2 functions in the same pathway as sinh-1.
Fig 6. TORC2 and PKC-2 function in the same pathway and SGK-1 acts downstream of TORC2.
(A) sinh-1(pe420) and sgk-1(ok538) double mutants do not show an additive effect on chemotaxis after low-salt/food(–) conditioning when compared to single mutants. (B) sgk-1(ft15gf) suppresses the sinh-1(pe420) chemotaxis defect after low-salt/food(–) conditioning. (C) sinh-1(pe420) and pkc-2(ok328) mutations do not produce an additive effect on salt chemotaxis after low-salt/food(–) conditioning. (D) daf-16(mgDf47) and sgk-1(ok538) have an additive effect on chemotaxis after low-salt/food(–) conditioning. (E) sgk-1(ft15gf) suppresses the chemotaxis defect of daf-16(mgDf47) after low-salt/food(–) conditioning. Error bars, s.e.m.; **p < 0.01, n.s. = not significant (one-way ANOVA with Dunnett’s post hoc test, N ≥ 6).
We also investigated the interaction between sgk-1 and pkc-2. The pkc-2(ok328) sgk-1(ok538) double mutant migrated to lower salt levels than the sgk-1 or pkc-2 single mutants did after low-salt/food(−) conditioning (S7 Fig), which suggests that these two genes function in parallel. Collectively, the results of the genetic-interaction analyses support the idea that sgk-1 and pkc-2 act in parallel downstream of TORC2. However, we cannot exclude the possibility that pkc-2 functions independently of TORC2 signaling.
sgk-1 functions in parallel with daf-16 in taste associative learning
Mammalian SGK has been reported to directly phosphorylate a forkhead box O (FOXO) transcription factor and inhibit its activation [32]. In C. elegans, SGK-1 positively regulates DAF-16, the sole FOXO homolog [33], and thereby controls longevity [31,34]. Therefore, we investigated the genetic interaction between daf-16 and sgk-1. After low-salt/food(–) conditioning, the double mutant daf-16(mgDf47);sgk-1(ok538) showed a more severe phenotype than the single mutants did (Fig 6D), which indicates that DAF-16 and SGK-1 exert additive effects. Conversely, the gain of function mutation sgk-1(ft15gf) partially suppressed the phenotype of the daf-16 null mutant daf-16(mgDf47) (Fig 6E). If daf-16 functioned as the sole downstream effector of sgk-1, sgk-1(gf) would not be expected to suppress the chemotaxis defect of the daf-16 mutant. Collectively, these results suggest that daf-16 and sgk-1 act in parallel in taste associative learning.
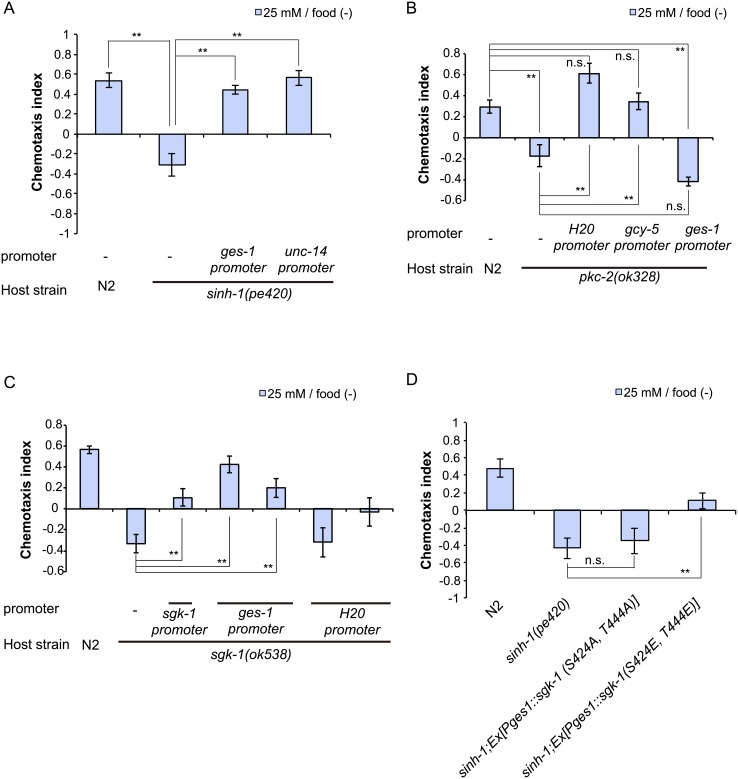
pkc-2 functions in neurons and sgk-1 functions in the intestine to regulate taste associative learning
Our results thus far demonstrated that the mutants of the CeTORC2 components and the sgk-1 and pkc-2 mutants showed abnormal taste associative learning, particularly after starvation conditioning. To identify the sites of action of these genes, we performed tissue-specific rescue experiments.
First, we investigated the site of function of sinh-1, the CeTORC2 component. Interestingly, intestinal expression of sinh-1 cDNA was sufficient for rescuing the sinh-1 chemotaxis defect (Fig 7A), which suggested that intestinal CeTORC2 functions contribute to taste associative learning. Furthermore, neuronal expression of the cDNA under a pan-neuronal promoter, the unc-14 promoter, also rescued the defect of the sinh-1 mutant (Fig 7A). Therefore, sinh-1 might act not only in the intestine, but also in neurons. However, we observed that in addition to driving neuronal expression, the unc-14 promoter drove weak expression in the intestine. Therefore, we cannot exclude the possibility that this weak expression in the intestine was sufficient for the rescue of the sinh-1 phenotype observed with the use of the unc-14 promoter.
Fig 7. TORC2/PKC-2 pathway functions in neurons, whereas TORC2/SGK-1 pathway functions in the intestine.
(A) Neuronal (unc-14 promoter) and intestinal (ges-1 promoter) expression of sinh-1 cDNA rescued the chemotaxis defects of the sinh-1(pe420) mutant after low-salt/food(–) conditioning. (B) Pan-neuronal expression (H20 promoter) and ASER-specific expression (gcy-5 promoter), but not intestine-specific expression (ges-1 promoter), of the pkc-2 cDNA rescued the chemotaxis abnormality of the pkc-2(ok328) mutant after low-salt/food(–) conditioning. (C) Intestine-specific expression (sgk-1B promoter or ges-1 promoter), but not pan-neuronal expression (H20 promoter), of sgk-1 isoform B was sufficient for rescuing the chemotaxis defect of the sgk-1(ok538) mutant after low-salt/food(–) conditioning. (D) Intestine-specific expression of the active form of sgk-1 suppressed the chemotaxis defect of the sinh-1(pe420) mutant after low-salt/food(–) conditioning. Error bars, s.e.m.; *p < 0.05, **p < 0.01, n.s. = not significant (Dunnett’s test, N ≥ 9).
We next investigated the sites of action of pkc-2 and sgk-1, the putative downstream components of TORC2. Previous works have shown that pkc-2 is expressed in multiple neurons, muscle cells, the gonads, and the intestine [35,36], and we found that expression of the pkc-2 cDNA in neurons or the ASER neuron, but not in the intestine, rescued the chemotaxis defect of pkc-2 mutants (Fig 7B); this result suggests that pkc-2 functions in ASER to regulate taste associative learning.
The translational reporter construct of sgk-1 was reported to be expressed mainly in several neurons and the intestine [37]. Therefore, we expressed the sgk-1 cDNA in neurons or the intestine of sgk-1(ok538) mutants. Intestine-specific sgk-1 expression under the ges-1 promoter rescued the chemotaxis defect of sgk-1(ok538) (Fig 7C). Moreover, sgk-1 expression driven by the pan-neuronal H20 promoter exerted a weak, but non-significant rescue trend on the sgk-1 defect. These results suggested the importance of intestinal CeTORC2/SGK-1 signaling in associative learning. We performed additional rescue experiments by using the sgk-1A and sgk-1B promoters, both of which drove expression only in the intestine (S8A–S8D Fig). The differences in the expression patterns observed here and in a previous study [37] that reported neuronal expression of sgk-1 might be explained by the use of not only the upstream region of sgk-1, but also exonic and intronic regions in the previous study. The sgk-1 expression driven by these promoters rescued the sgk-1 defects (Fig 7C, S8E Fig), which further supports the notion that intestinal SGK-1 functions in learning.
Lastly, to ascertain whether SGK-1 phosphorylation is involved in learning, we constructed mutant sgk-1 cDNAs that included unphosphorylatable and phosphomimetic substitutions. mTORC2 phosphorylates the hydrophobic motif (HM) and the turn motif (TM) of AGC kinases such as Akt1, PKC, and SGK1 [5,38]. We introduced substitution mutations at S424 in the TM and T444 in the HM of SGK-1, the residues equivalent to S379 and S424 in mouse SGK1, respectively [9]. Whereas expression of the cDNA encoding the unphosphorylatable sgk-1 (S424A, T444A) in the sinh-1 mutant produced no effect (Fig 7D), intestine-specific expression of the phosphomimetic sgk-1 cDNA (S424E, T444E) partially suppressed the abnormal chemotaxis of sinh-1 mutants (Fig 7D). These results are consistent with the possibility that direct phosphorylation of SGK-1 by CeTORC2 in the intestine is critical for taste associative learning.
Discussion
Since the discovery of C. elegans Raptor daf-15 [39] and C. elegans Rictor rict-1 [9,10], the functions of these molecules in development, metabolism, and longevity have been extensively studied [9,10,14,31,40]. By contrast, the roles of CeTOR signaling in neural functions have remained poorly investigated. In this study, we characterized for the first time the functions of CeTOR signaling in associative learning. Our results indicate that the functions of both CeTORC1 and CeTORC2 in neuronal or non-neuronal cells contribute to behavioral changes induced by salt and food availability. Although mammalian TOR signaling is recognized to be involved in neural functions such as long-term memory formation and neuronal excitation [7,8,41,42], the mechanistic details of mTOR control of neuronal activity remain unclear. Given that TOR signaling is highly conserved across species, elucidation of the role of CeTOR signaling in the regulation of neuronal functions will contribute toward the understanding of TOR signaling in other species.
The results of our genetic analyses suggest that CeTORC1 and CeTORC2 control learning-dependent behaviors in C. elegans. We evaluated the behaviors of the CeTOR signaling mutants using chemotaxis assays after four kinds of salt conditioning: attraction to high and low salt concentrations associated with food and avoidance of high and low salt concentrations associated with starvation (S1 Table). We demonstrated that neuronal RSKS-1, the putative CeTORC1 substrate, contributes to avoidance of high salt concentrations after high-salt/food(–) conditioning (Fig 2B, S1 Table). Previous studies have reported that mTORC1/S6K signaling plays critical roles in protein synthesis in neurons, where it contributes to synaptic plasticity and is essential for neuronal function [43–45]. Thus, in C. elegans, CeTORC1 might also control synaptic functions by regulating protein synthesis. On the other hand, mutants of CeTORC2-defining components RICT-1 and SINH-1, and its putative substrate SGK-1, showed decreased migration to high salt levels and/or increased migration to low salt levels after high-salt/food(+) and low-salt/food(–) conditioning (Fig 3C–3E, S1 Table), indicating that CeTORC2/SGK-1 signaling contributes to migration towards high salt concentrations. These defects were rescued by the intestinal expression of each of the responsible genes (Fig 7A, 7C and 7D). Our results obtained using the sgk-1(gf) mutant and the intestinal expression of phosphomimetic SGK-1 suggested that SGK-1 functions downstream of TORC2 in taste associative learning. Thus, CeTORC1 and CeTORC2 signaling pathways contribute to generating appropriate behavioral responses after learning by acting in different tissues, the nervous system and the intestine, respectively(S9 Fig).
Besides the above-mentioned mechanism, TORC1 and TORC2 signaling components appear to have additional functions in taste associative learning. Among the TORC2 signaling mutants tested in this study, only the sgk-1(ok538) mutant showed a defect in avoidance of high salt after high-salt/food(−) conditioning. Because SGK-1 is also regulated by insulin-like signaling, [37] and insulin-like pathway mutants showed strong defects in avoidance of high salt after high-salt/food(−) conditioning [11], SGK-1 might also promote migration to low salt after starvation conditioning through insulin-like signaling. Furthermore, there might also be some interactions between TORC1 and TORC2 pathways in neurons and the intestine. PKC-2 controls low salt avoidance possibly in the CeTORC2 pathway after low-salt/food(−) conditioning in neurons (Fig 7B, S1 Table), where PKC-2 might interact with the CeTORC1 signaling pathway. Although we have not determined the site of action of ATG-13 and its possible interaction with CeTORC1 in taste associative learning, the similar learning phenotypes of the atg-13 and CeTORC2 mutants (S1 Table) imply that ATG-13 and CeTORC2/SGK-1 pathways might control the same process in the intestine. These possibilities should be further investigated in future studies.
The analysis performed using the sinh-1;pkc-2 double mutant suggested that TORC2 and PKC-2 act in the same genetic pathway. In food-odor associative learning, the sgk-1 mutant showed a phenotype similar to those of TORC2-component mutants, whereas the pkc-2 mutant showed no notable defect (Fig 3F). Recently, PKC-α signaling was shown to control long-term memory formation through neuronal actin organization in mice and Drosophila melanogaster [7]. Intriguingly, the neuronal function of ADD-1, a C. elegans homolog of α-adducin, which promotes assembly of the spectrin-actin cytoskeleton, is required for several forms of associative learning [46]. Thus, in C. elegans, the neuronal PKC-2 pathway might function by regulating the actin cytoskeleton as in mouse and Drosophila neurons. It will be of interest to investigate how SGK-1 and PKC-2 regulate intestinal and neuronal functions, respectively, and clarify the roles of SGK-1 and PKC-2, including their target molecules, in TORC2 signaling in associative learning.
How could TORC2/SGK-1 signaling acting in the intestine control the neuronal functions that underlie chemotaxis behavior? Given that the intestine is a major organ for nutrient uptake and the TOR pathway is recognized as a nutrition sensor, it’s possible that TORC2/SGK-1 signaling functions as a mediator of nutrient signals. If this is the case, loss of sgk-1 and TORC2 components would result in defective chemotaxis after fed conditioning. Interestingly, mutants of sgk-1 and TORC2 components showed defects in migration to high, but not low, salt levels after fed conditioning. Furthermore, these mutants also showed defective migration to high salt levels after starvation conditioning. These observations imply that TORC2/SGK-1 signaling transmits salt-related information to promote migration to high salt levels after learning. One possibility is that intestinal TORC2/SGK-1 signaling acts in salt sensation. Previous studies have shown that non-neural organs, including the intestine, could also function as sensors for environmental conditions such as temperature [34,47]. In taste associative learning, the intestine might act as a chemical sensor of salt concentration and transmit information onto neurons through, for example, secretion of a hormone-like substance, to promote migration to high salt concentrations. Another possibility is that the sgk-1 phenotype is caused by changes in lipid metabolism. The results of previous studies and those obtained here using Oil-Red-O staining suggest that mutants of rict-1, sinh-1, and sgk-1 accumulate more lipid in the intestine than do wild-type animals [9,10] (S4 Fig). Moreover, we previously reported that mutants of a PTEN homolog, daf-18, in which PI3-kinase signaling is activated, and a novel protein kinase C-ε/η ortholog, pkc-1, in which the Gq/DAG/PKC pathway is inactivated, showed defective migration to high salt levels after starvation or fed conditioning similar to the sgk-1 and the TORC2-component mutants [11,12]. Both of these signaling pathways use lipids, such as phosphatidylinositols and diacylglycerol, as second messengers [11–13]. Dysfunction of intestinal SGK-1 might affect lipid metabolism not only in the intestine but also in neurons, and lead to altered actions of the PI3-kinase pathway or the Gq/DAG/PKC pathway, both of which are required for normal salt chemotaxis.
Rapamycin, which is used as an immunosuppressive drug, has drawn considerable attention because of its ability to increase longevity and its usefulness in the treatment of various diseases [48,49]. In relation to neural functions, chronic oral administration of rapamycin has been shown to exert beneficial effects on learning and memory [50–53]. However, certain studies have reported that direct rapamycin administration into the central nervous system impairs long-term potentiation in the hippocampus or learning ability [41,43,44,50,54–56]. In this study, we investigated the rapamycin effect only during conditioning and chemotaxis tests. This is considered an acute rapamycin effect rather than a chronic effect. This result agrees with the findings of previous studies showing that acute local administration of rapamycin impairs learning and memory formation [41,43,44,50,54–56]. Although rapamycin was previously reported to be capable of crossing the blood-brain barrier [57], the general whole-body effects of rapamycin and the local effect of rapamycin on the brain should be distinguished because the concentration and localization of rapamycin are considered to differ following peroral versus regional administration. Further investigation is required for a complete understanding of the beneficial and deleterious effects of rapamycin as a therapeutic compound.
The result obtained with rapamycin treatment of the sinh-1 mutants implies that an unknown rapamycin target other than TORC2 is involved in the learning paradigm studied here. Because the rapamycin concentration used here was high, unexpected side effects which have not been reported in other animals may have been observed. Exploiting the forward genetic screening possible in C. elegans might facilitate the identification of previously unrecognized rapamycin targets.
In this study, we characterized for the first time the functions of CeTORC2 signaling in learning. The simple nervous system of C. elegans and the viability of CeTORC2-component mutants will help us elucidate the functions of TORC2 in the nervous system. Therefore, future analysis of CeTORC2 using our experimental model may help clarify previously unknown conserved functions of TORC2 in learning.
Materials and methods
Strains and culture
Animals were grown at 20°C on Escherichia coli strain NA22 as a food source under standard conditions [58]. N2 Bristol was used as the wild-type strain. The C. elegans mutant and transgenic strains used in this study were as follows: Ex[Pgcy-5::YC2.60;Plin-44::mCherry], Ex[PH20::RSKS-1(F5A-T404E -R3A);Pmyo3::venus], Ex[Psgk-1A::venus], Ex[Psgk-1B::venus], daf-16(mgDf47)I, daf-16(mgDf47)I;sgk-1(ok538)X, daf-16(mgDf47)I;sgk-1(ft15)X, rict-1(ft7)II, rict-1(tm4017)II, rict-1(mg360)II, sinh-1(pe420)II, sinh-1(pe420)II;akt-1(ok525)V, sinh-1(pe420)II;akt-1(mg144)V, sinh-1(pe420)II;pkc-2(ok328)X, sinh-1(pe420)II;sgk-1(ok538)X, sinh-1(pe420)II;sgk-1(ft15)X, sinh-1(mg360);Ex[Pges-1::sgk-1(S424A,T444A);Pmyo-3::venus], sinh-1(mg360);Ex[Pges-1::sgk-1(S424E,T444E);Pmyo-3::venus], sinh-1(mg360);Ex[PH20::pkc-2CAT;Pmyo-3::venus], sinh-1(pe420)II;Ex[Pges-1::sinh-1;Pmyo-3::venus], sinh-1(pe420)II;Ex[Punc-14::sinh-1;Pmyo-3::venus], sinh-1(pe420)II;Ex[Pgcy-5::YC2.60;Plin-44::mCherry], rsks-1(ok1255)III, rsks-1(ok1255)III;Ex[PH20::rsks-1;Pmyo-3::venus], rsks-1(ok1255)III;Ex[Pgcy-5::rsks-1;Pmyo-3::venus], rsks-1(ok1255)III;Ex[Pges-1::rsks-1;Pmyo-3::venus]atg-13(bp414)III, atg-13(bp417)IV;bpIs267[PY37A1B.5::sqst-1::gfp+unc-76]V, akt-1(ok525)V, akt-1(mg144)V, pkc-2(ok328)X, pkc-2(tm409)X, pkc-2(ok328)X;Ex[PH20::pkc-2;Pmyo3::venus], pkc-2(ok328)X;Ex[Pgcy-5::pkc-2;Pmyo-3::venus], pkc-2(ok328)X;Ex[Pges-1::pkc-2cDNA;Pmyo-3::venus], pkc-2(ok328) sgk-1(ok538)X, sgk-1(mg455)X, sgk-1(ft15)X, sgk-1(ok538)X, sgk-1(ok538)X;Ex[Psgk-1A::sgk-1A;Pmyo-3::venus;], sgk-1(ok538)X;Ex[Psgk-1B::sgk-1B;Pmyo-3::venus;], sgk-1(ok538)X;Ex[Pges-1::sgk-1B;Pmyo-3::venus], sgk-1(ok538)X;Ex[PH20::sgk-1B;Pmyo-3::venus]
Statistical analyses
All data, except for the lifespan assay, were analyzed by Student’s t test or one-way ANOVA with Dunnett’s post-hoc test using Graph-pad Prism 5.0 statistical software (Graph-pad Software, San Diego, CA).
Behavioral assays
The salt-chemotaxis learning assays were performed as described previously [11]. Briefly, adult animals grown on standard nematode growth medium (NGM) were transferred for conditioning to NGM plates that contained NaCl at low (25 mM) or high (100 mM) concentration together with or without food. After 5–6 h, animals were washed out from the plates with chemotaxis buffer (50 mM NaCl, 25 mM potassium phosphate (pH 6.0), 1 mM CaCl2, 1 mM MgSO4), placed at the center of test plates featuring a salt gradient, and allowed to run for 40 min. The chemotaxis index was calculated as (A—B)/(N—C), where A is the number of animals within 2 cm of the highest point of the salt gradient, B is the number of animals within 2 cm of the lowest point of the salt gradient, N is the total number of animals on a test plate, and C is the number of animals that remained in the central region (S1B Fig).
The food-odor associative learning assays were performed using benzaldehyde, as described with modifications [59]. To test the odor chemotaxis of naive animals, well-fed worms were washed thrice with chemotaxis buffer (5 mM potassium phosphate (pH 6.0), 1 mM CaCl2, 1 mM MgSO4), placed at the center of test plates featuring a benzaldehyde gradient, and allowed to run for 30 min. To prepare the test plates with the benzaldehyde gradient, 1 μL of benzaldehyde (1:100 dilution) was spotted on each of two points at one end of the plates, 1 μL of ethanol was spotted on each of two points at the other end of the plates, and 1 μL of 0.5 M NaN3 was spotted on two points at both ends of the plates to anesthetize worms. For odor adaptation, animals were soaked in 500 μL of benzaldehyde (1:105 dilution) for 1 h, and after washing thrice with chemotaxis buffer, were placed at the center of the test plates and allowed to run for 30 min. Chemotaxis index for the olfactory-plasticity assays was calculated as (A—B)/(A + B), where A is the number of animals on the odorant-spotted side of the plate and B is the number of animals on the opposite side (S1C Fig).
In each behavioral experiment, we simultaneously performed behavioral assays with indicated conditions and worm strains and repeated the assays at least four times.
Plasmid constructions and germ-line transformation
All plasmid constructs were generated using the GATEWAY system (Invitrogen). To construct entry vectors harboring the promoter sequences of sgk-1, 2.0 kb upstream of the first exon of sgk-1 isoform A and 2.5 kb upstream of the first exon of sgk-1 isoform B were amplified from C. elegans genomic DNA, and then inserted into an entry vector, pENTR1A. The rsks-1, pkc-2, sgk-1, and sinh-1 cDNAs were cloned from C. elegans total cDNA, and inserted into a destination vector, pPD-DEST, to generate pDEST-rsks-1, pDEST-pkc-2, pDEST-sgk-1, and pDEST-sinh-1, respectively. To construct pDEST-rict-1, rict-1 cDNA (a kind gift from S. Takagi) was inserted into the destination vector pPD-DEST.
Germ-line transformation was performed using standard microinjection techniques, as described previously [60]. In rescue experiments, pG-myo-3p::Venus was used as a co-injection marker. The injection mixtures contained 10 ng/μL of each plasmid DNA.
Generation of sinh-1(pe420) mutant strain
The pe420 allele of sinh-1 was generated using the CRISPR-Cas9 system, as described previously [61]. The expression constructs of sgRNAs containing the target sequences 5'-GACAGGAAATGTCGTGCCGG-3', 5'-GACATTTCCTGTCTGGATTC-3', and 5'-GGCTTCTCCTGAATCCAGAC-3' were prepared using a PCR-fusion technique and injected at 10 ng/μL each together with 50 ng/μL pDD162-Peft-3::Cas9. After injection, F1 animals were screened by means of single-worm PCR performed using the primers 5'-ACGTAATTCGACATGAGCTTC-3' and 5'-ATATCATCAAATCCCGCATCC-3'.
Rapamycin administration
Rapamycin (LC laboratories) was dissolved in 10% Tween-20/90% ethanol, and 20 h before assays, rapamycin was added to conditioning plates or test plates at a final concentration of 100 μM. Control plates were prepared to contain the solvents at the same concentration as that in the rapamycin-containing plates.
Ca2+ imaging
We used animals expressing YC2.60 in the ASER neuron to visualize calcium ion dynamics in response to NaCl stimuli. Imaging experiments were performed as previously reported [11] with modification. In this study, animals were conditioned on normal NGM plates without glycerol.
Body fat assessment
Oil-Red-O staining was conducted as described previously [62]; images of stained animals were acquired using a Zeiss Axiovert S100 microscope and a CCD camera (Keyence VB-7010). To quantify Oil-Red-O staining, we manually determined the region of interest, which included the most of the anterior body region extending from the posterior edge of the pharynx to the vulva (S4B Fig) in each animal, and then measured red, green, and blue (RGB) intensities by using Image J software. Body fat levels were calculated as ratios of red to green intensities after subtraction of intensities measured in background regions.
Fluorescence microscopy
Well-fed adult worms were mounted on a 5% agar pad with 10 mM NaN3 and observed under a Leica confocal microscope (TCS-SP5).
Supporting information
(A) Wild-type C. elegans migrated to the NaCl concentration at which they were previously cultivated with food (orange bars). By contrast, the worms avoided the salt level at which they previously experienced starvation (blue bars). High and low chemotaxis indices indicate that the worms migrated to high and low salt levels, respectively. (B) Schematic diagram of a salt-chemotaxis test plate. The chemotaxis index was calculated as (N[A]–N[B])/(N[all]–N[C]), where N[A] is the number of animals within a 2 radius cm of the highest point in the salt gradient, N[B] is the number of animals within a 2 cm radius of the lowest point in the salt gradient, N[all] is the total number of animals on the test plate, and N[C] is the number of animals that remained in the central region. (C) Schematic diagram of a benzaldehyde-chemotaxis test plate. The chemotaxis index was calculated as (N[A]–N[B])/(N[A] + N[B]), where N[A] is the number of animals on the odorant-spotted side of the plate and N[B] is the number of animals on the opposite side.
(TIF)
A replicate experiment of those shown in Figs 2B and 3B. Taste associative learning of N2, rsks-1, sinh-1, and rict-1 mutants were assayed simultaneously. Error bars, s.e.m.; **p < 0.01 (Dunnett’s test, N ≥ 9).
(TIF)
Genomic structure of rict-1 (top), pkc-2 (middle), and sgk-1 (bottom). Black boxes indicate exons, red boxes indicate deletion mutations, and arrowheads indicate point mutations.
(TIF)
(A) Examples of Oil-Red-O-stained animals. Scale bars: 100 μm. (B) To quantify Oil-Red-O staining, we determined the R/G ratios of the region of interest, which included most of the anterior portion of the body: from the posterior edge of the pharynx to the vulva in each animal (indicated with a purple box). The details are listed in Materials and Methods. (C) Quantification of Oil-Red-O staining in wild-type, rict-1(ft7), and sinh-1(pe420) animals. As previously reported based on experiments conducted using Nile-Red staining [9], our Oil-Red-O staining results demonstrated a body fat increase in rict-1(ft7) animals. Similar to the rict-1(ft7) animals, sinh-1(pe420) animals also showed the increased body fat phenotype, which is in agreement with previous reports that Sin1 is an essential component of TORC2 in several species [22–24]. Data were normalized against the averaged value determined for wild-type. Error bars, s.e.m.; **p < 0.01 (wild-type vs. each mutant, Dunnett’s test, N = 40).
(TIF)
Rapamycin administration didn’t affect food-odor associative learning. This is consistent with the phenotype of rsks-1(ok1255) mutants, which showed no defect in food-odor associative learning. The learning assay was performed using benzaldehyde as an odorant as described in Fig 4F. Error bars, s.e.m.; n.s. = not significant (Student’s t test, N ≥ 8).
(TIF)
Chemotaxis of the sgk-1(gf) mutant after salt conditioning is shown. Error bars, s.e.m.; *p < 0.05, n.s. = not significant (Student’s t test, N ≥ 6).
(TIF)
sgk-1;pkc-2 double mutants showed enhanced migration to low salt levels as compared with each single mutant. Error bars, s.e.m.; **p < 0.01 (Dunnett’s test, N ≥ 9).
(TIF)
(A) Venus expression driven by the sgk-1 isoform A promoter (2.0 kb upstream of the first exon of sgk-1 isoform A). The sgk-1 isoform A promoter drives strong expression in the intestine, but not in neurons. Left, bright field; center, Venus; right, merged. Scale bar: 100 μm. (B) No signal was detected in the head ganglia. Scale bar: 30 μm. (C) Venus expression driven by the sgk-1 isoform B promoter (2.5 kb upstream of the first exon of sgk-1 isoform B). The sgk-1 isoform B promoter drives expression in the intestine. Scale bar: 100 μm. (D) No fluorescence was detected in the head ganglia. Scale bar: 30 μm. (E) Intestine-specific expression (sgk-1A promoter) of sgk-1 isoform A was sufficient for rescuing the chemotaxis defect of the sgk-1(ok538) mutant after low-salt/food(–) conditioning. Error bars, s.e.m.; **p < 0.01 (Dunnett’s test, N ≥ 9).
(TIF)
Intestinal TORC2/SGK-1 signaling and neuronal RSKS-1 and PKC-2 contribute to taste associative learning: TORC2/SGK-1 promotes migration to high salt levels through SGK-1 phosphorylation in the intestine after fed and starvation conditioning.
(TIF)
(TIF)
Acknowledgments
We thank the Caenorhabditis Genetics Center, Dr. Shohei Mitani and the National BioResource Project, Dr. Alexander Soukas, Dr. Ralf Baumeister, Dr. Shin Takagi, and Dr. Hong Zhang for providing strains; Dr. Sreekanth Chalasani and Dr. Noelle L’Etoile for helpful discussions; and the members of the Iino laboratory for helpful discussions, expert technical assistance, and critical reading of this manuscript. The primary data of this study are archived in the Iino laboratory in the Department of Biological Sciences, University of Tokyo, and are available upon request.
Data Availability
Data are available on figshare with the DOI 10.6084/m9.figshare.5001359.
Funding Statement
Y.I. was supported by a Grant-in-Aid for Scientific Research (B) 26291069, for Scientific Research on Innovative Areas 25115010, and for Challenging Exploratory Research 15K14310. M.T. was supported by a Grant-in-Aid for Japan Society for the Promotion of Science Young Scientists (B) 25870172. N.S. was supported by a Grant-in-Aid for Japan Society for the Promotion of Science Fellows 10978. The URL of Japan Society for the Promotion of Science; https://www.jsps.go.jp/english/index.html. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124: 471–484. 10.1016/j.cell.2006.01.016 [DOI] [PubMed] [Google Scholar]
- 2.Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10: 457–468. 10.1016/S1097-2765(02)00636-6 [DOI] [PubMed] [Google Scholar]
- 3.Jacinto E, Loewith R, Schmidt A, Lin S, Rüegg MA, Hall A, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6: 1122–1128. 10.1038/ncb1183 [DOI] [PubMed] [Google Scholar]
- 4.Laplante M, Sabatini DM. MTOR signaling in growth control and disease. Cell. Elsevier; 2012;149: 274–293. 10.1016/j.cell.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cybulski N, Hall MN. TOR complex 2: a signaling pathway of its own. Trends Biochem Sci. 2009;34: 620–627. 10.1016/j.tibs.2009.09.004 [DOI] [PubMed] [Google Scholar]
- 6.Oh WJ, Jacinto E. mTOR complex 2 signaling and functions. Cell Cycle. 2011;10: 2305–2316. 10.4161/cc.10.14.16586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang W, Zhu PJ, Zhang S, Zhou H, Stoica L, Galiano M, et al. mTORC2 controls actin polymerization required for consolidation of long-term memory. Nat Neurosci. 2013;16: 441–448. 10.1038/nn.3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazei-Robison MS, Koo JW, Friedman AK, Lansink CS, Robison AJ, Vinish M, et al. Role for mTOR signaling and neuronal activity in morphine-induced adaptations in ventral tegmental area dopamine neurons. Neuron. Elsevier Inc.; 2011;72: 977–990. 10.1016/j.neuron.2011.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones KT, Greer ER, Pearce D, Ashrafi K. Rictor/torc2 regulates Caenorhabditis elegans fat storage, body size, and development through sgk-1. PLoS Biol. 2009;7: 0604–0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soukas A a, Kane E a, Carr CE, Melo J a, Ruvkun G. Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans. Genes Dev. 2009;23: 496–511. 10.1101/gad.1775409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunitomo H, Sato H, Iwata R, Satoh Y, Ohno H, Yamada K, et al. Concentration memory-dependent synaptic plasticity of a taste circuit regulates salt concentration chemotaxis in Caenorhabditis elegans. Nat Commun. Nature Publishing Group; 2013;4: 2210 10.1038/ncomms3210 [DOI] [PubMed] [Google Scholar]
- 12.Tomioka M, Adachi T, Suzuki H, Kunitomo H, Schafer WR, Iino Y. The Insulin/PI 3-Kinase Pathway Regulates Salt Chemotaxis Learning in Caenorhabditis elegans. Neuron. 2006;51: 613–625. 10.1016/j.neuron.2006.07.024 [DOI] [PubMed] [Google Scholar]
- 13.Adachi T, Kunitomo H, Tomioka M, Ohno H, Okochi Y, Mori I, et al. Reversal of salt preference is directed by the insulin/PI3K and G q/PKC signaling in Caenorhabditis elegans. Genetics. 2010;186: 1309–1319. 10.1534/genetics.110.119768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robida-Stubbs S, Glover-Cutter K, Lamming DW, Mizunuma M, Narasimhan SD, Neumann-Haefelin E, et al. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. Elsevier Inc.; 2012;15: 713–724. 10.1016/j.cmet.2012.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Syntichaki P, Troulinaki K, Tavernarakis N. eIF4E function in somatic cells modulates ageing in Caenorhabditis elegans. Nature. 2007;445: 922–926. 10.1038/nature05603 [DOI] [PubMed] [Google Scholar]
- 16.Bargmann CI, Horvitz HR. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron. 1991;7: 729–742. 10.1016/0896-6273(91)90276-6 [DOI] [PubMed] [Google Scholar]
- 17.Ohno H, Kato S, Naito Y, Kunitomo H, Tomioka M, Iino Y. Role of synaptic phosphatidylinositol 3-kinase in a behavioral learning response in C. elegans. Science (80-). 2014;345: 313–317. 10.1126/science.1250709 [DOI] [PubMed] [Google Scholar]
- 18.Ganley IG, Lam DH, Wang J, Ding X, Chen S, Jiang X. ULK1·ATG13·FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem. 2009;284: 12297–12305. 10.1074/jbc.M900573200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, et al. Nutrient-dependent mTORC1 Association with the ULK1-Atg13-FIP200 Complex Required for Autophagy. Mol Biol Cell. 2009;20: 1981–1991. 10.1091/mbc.E08-12-1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung CH, Jun CB, Ro S-H, Kim Y-M, Otto NM, Cao J, et al. ULK-Atg13-FIP200 Complexes Mediate mTOR Signaling to the Autophagy Machinery. Mol Biol Cell. 2009;20: 1992–2003. 10.1091/mbc.E08-12-1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. Prolonged Rapamycin Treatment Inhibits mTORC2 Assembly and Akt/PKB. Mol Cell. 2006;22: 159–168. 10.1016/j.molcel.2006.03.029 [DOI] [PubMed] [Google Scholar]
- 22.Frias MA, Thoreen CC, Jaffe JD, Schroder W, Sculley T, Carr SA, et al. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16: 1865–70. 10.1016/j.cub.2006.08.001 [DOI] [PubMed] [Google Scholar]
- 23.Yang Q, Inoki K, Ikenoue T, Guan KL. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006;20: 2820–2832. 10.1101/gad.1461206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, et al. SIN1/MIP1 Maintains rictor-mTOR Complex Integrity and Regulates Akt Phosphorylation and Substrate Specificity. Cell. 2006;127: 125–137. 10.1016/j.cell.2006.08.033 [DOI] [PubMed] [Google Scholar]
- 25.Suzuki H, Thiele TR, Faumont S, Ezcurra M, Lockery SR, Schafer WR. Functional asymmetry in Caenorhabditis elegans taste neurons and its computational role in chemotaxis. Nature. 2008;454: 114–117. 10.1038/nature06927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nuttley WM, Atkinson-Leadbeater KP, Van Der Kooy D. Serotonin mediates food-odor associative learning in the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2002;99: 12449–54. 10.1073/pnas.192101699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from age-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 1998;12: 2488–2498. 10.1101/gad.12.16.2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang F, Strutz-Seebohm N, Seebohm G, Lang UE. Significance of SGK1 in the regulation of neuronal function. J Physiol. 2010;588: 3349–3354. 10.1113/jphysiol.2010.190926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizunuma M, Neumann-Haefelin E, Moroz N, Li Y, Blackwell TK. mTORC2-SGK-1 acts in two environmentally responsive pathways with opposing effects on longevity. Aging Cell. 2014;13: 869–878. 10.1111/acel.12248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nukazuka A, Tamaki S, Matsumoto K, Oda Y, Fujisawa H, Takagi S. A shift of the TOR adaptor from Rictor towards Raptor by semaphorin in C. elegans. Nat Commun. 2011;2: 484 10.1038/ncomms1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen ATY, Guo C, Dumas KJ, Ashrafi K, Hu PJ. Effects of Caenorhabditis elegans sgk-1 mutations on lifespan, stress resistance, and DAF-16/FoxO regulation. Aging Cell. 2013;12: 932–940. 10.1111/acel.12120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol Cell Biol. 2001;21: 952–65. 10.1128/MCB.21.3.952-965.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum H a, et al. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389: 994–999. 10.1038/40194 [DOI] [PubMed] [Google Scholar]
- 34.Xiao R, Zhang B, Dong Y, Gong J, Xu T, Liu J, et al. A genetic program promotes C. elegans longevity at cold temperatures via a thermosensitive TRP channel. Cell. Elsevier Inc.; 2013;152: 806–817. 10.1016/j.cell.2013.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Islas-Trejo A, Land M, Tcherepanova I, Freedman JH, Rubin CS. Structure and Expression of the Caenorhabditis elegans Protein Kinase C2 Gene ORIGINS AND REGULATED EXPRESSION OF A FAMILY OF Ca2+-ACTIVATED PROTEIN KINASE C ISOFORMS. J Biol Chem. 1997;272: 6629–6640. 10.1074/jbc.272.10.6629 [DOI] [PubMed] [Google Scholar]
- 36.Tabuse Y. Protein kinase C isotypes in C. elegans. J Biochem. 2002;132: 519–522. [DOI] [PubMed] [Google Scholar]
- 37.Hertweck M, Göbel C, Baumeister R. C. elegans SGK-1 is the critical component in the Akt/PKB kinase complex to control stress response and life span. Dev Cell. 2004;6: 577–588. 10.1016/S1534-5807(04)00095-4 [DOI] [PubMed] [Google Scholar]
- 38.García-Martínez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem J. 2008;416: 375–385. 10.1042/BJ20081668 [DOI] [PubMed] [Google Scholar]
- 39.Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131: 3897–3906. 10.1242/dev.01255 [DOI] [PubMed] [Google Scholar]
- 40.Zhu H, Shen H, Sewell AK, Kniazeva M, Han M. A novel sphingolipid-TORC1 pathway critically promotes postembryonic development in Caenorhabditis elegans. Elife. 2013;2013: 1–19. 10.7554/eLife.00429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, et al. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell. 1999;99: 221–237. 10.1016/S0092-8674(00)81653-0 [DOI] [PubMed] [Google Scholar]
- 42.Graber TE, McCamphill PK, Sossin WS. A recollection of mTOR signaling in learning and memory. Learn Mem. 2013;20: 518–30. 10.1101/lm.027664.112 [DOI] [PubMed] [Google Scholar]
- 43.Cammalleri M, Lutjens R, Berton F, King AR, Simpson C, Francesconi W, et al. Time-restricted role for dendritic activation of the mTOR-p70S6K pathway in the induction of late-phase long-term potentiation in the CA1. Proc Natl Acad Sci. 2003;100: 14368–14373. 10.1073/pnas.2336098100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang SJ, Reis G, Kang H, Gingras A-C, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99: 467–472. 10.1073/pnas.012605299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsokas P, Grace EA, Chan P, Ma T, Sealfon SC, Iyengar R, et al. Local protein synthesis mediates a rapid increase in dendritic elongation factor 1A after induction of late long-term potentiation. J Neurosci. 2005;25: 5833–43. 10.1523/JNEUROSCI.0599-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vukojevic V, Gschwind L, Vogler C, Demougin P, de Quervain DJ-F, Papassotiropoulos A, et al. A role for α-adducin (ADD-1) in nematode and human memory. EMBO J. Nature Publishing Group; 2012;31: 1453–1466. 10.1038/emboj.2012.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sugi T, Nishida Y, Mori I. Regulation of behavioral plasticity by systemic temperature signaling in Caenorhabditis elegans. Nat Neurosci. Nature Publishing Group; 2011;14: 984–992. 10.1038/nn.2854 [DOI] [PubMed] [Google Scholar]
- 48.Law BK. Rapamycin: An anti-cancer immunosuppressant? Crit Rev Oncol Hematol. 2005;56: 47–60. 10.1016/j.critrevonc.2004.09.009 [DOI] [PubMed] [Google Scholar]
- 49.Cai Z, Yan L-J. Rapamycin, Autophagy, and Alzheimer’s Disease. J Biochem Pharmacol Res. 2013;1: 84–90. Secreted [PMC free article] [PubMed] [Google Scholar]
- 50.Talboom JS, Velazquez R, Oddo S. The mammalian target of rapamycin at the crossroad between cognitive aging and Alzheimer’s disease. npj Aging Mech Dis. Nature Publishing Group; 2015;1: 15008 10.1038/npjamd.2015.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Halloran J, Hussong SA, Burbank R, Podlutskaya N, Fischer KE, Sloane LB, et al. Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience. 2012;223: 102–113. 10.1016/j.neuroscience.2012.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin A-L, Jahrling JB, Zhang W, DeRosa N, Bakshi V, Romero P, et al. Rapamycin rescues vascular, metabolic and learning deficits in apolipoprotein E4 transgenic mice with pre-symptomatic Alzheimer’s disease. J Cereb Blood Flow Metab. 2015; 10.1177/0271678X15621575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Majumder S, Caccamo A, Medina DX, Benavides AD, Javors MA, Kraig E, et al. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1β and enhancing NMDA signaling. Aging Cell. 2012;11: 326–335. 10.1111/j.1474-9726.2011.00791.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jobim PFC, Pedroso TR, Christoff RR, Werenicz A, Maurmann N, Reolon GK, et al. Inhibition of mTOR by rapamycin in the amygdala or hippocampus impairs formation and reconsolidation of inhibitory avoidance memory. Neurobiol Learn Mem. Elsevier Inc.; 2012;97: 105–112. 10.1016/j.nlm.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 55.Qi S, Mizuno M, Yonezawa K, Nawa H, Takei N. Activation of mammalian target of rapamycin signaling in spatial learning. Neurosci Res. 2010;68: 88–93. 10.1016/j.neures.2010.06.008 [DOI] [PubMed] [Google Scholar]
- 56.Tischmeyer W, Schicknick H, Kraus M, Seidenbecher CI, Staak S, Scheich H, et al. Rapamycin-sensitive signalling in long-term consolidation of auditory cortex-dependent memory. Eur J Neurosci. 2003;18: 942–950. 10.1046/j.1460-9568.2003.02820.x [DOI] [PubMed] [Google Scholar]
- 57.Cloughesy TF, Yoshimoto K, Nghiemphu P, Brown K, Dang J, Zhu S, et al. Antitumor activity of rapamycin in a phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5: 0139–0151. 10.1371/journal.pmed.0050008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamada K, Hirotsu T, Matsuki M, Butcher RA, Tomioka M, Ishihara T, et al. Olfactory Plasticity Is Regulated by Pheromonal Signaling in Caenorhabditis elegans. Science (80-). 2010;329: 1647–1650. 10.1126/science.1192020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10: 3959–70. 10.1016/0168-9525(92)90342-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dickinson DJ, Ward JD, Reiner DJ, Goldstein B. Engineering the Caenorhabditis elegans genome using Cas9-triggered homologous recombination. Nat Methods. 2013;10: 1028–1034. 10.1038/nmeth.2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Rourke EJ, Soukas AA, Carr CE, Ruvkun G. C. elegans Major Fats Are Stored in Vesicles Distinct from Lysosome-Related Organelles. Cell Metab. Elsevier Ltd; 2009;10: 430–435. 10.1016/j.cmet.2009.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Wild-type C. elegans migrated to the NaCl concentration at which they were previously cultivated with food (orange bars). By contrast, the worms avoided the salt level at which they previously experienced starvation (blue bars). High and low chemotaxis indices indicate that the worms migrated to high and low salt levels, respectively. (B) Schematic diagram of a salt-chemotaxis test plate. The chemotaxis index was calculated as (N[A]–N[B])/(N[all]–N[C]), where N[A] is the number of animals within a 2 radius cm of the highest point in the salt gradient, N[B] is the number of animals within a 2 cm radius of the lowest point in the salt gradient, N[all] is the total number of animals on the test plate, and N[C] is the number of animals that remained in the central region. (C) Schematic diagram of a benzaldehyde-chemotaxis test plate. The chemotaxis index was calculated as (N[A]–N[B])/(N[A] + N[B]), where N[A] is the number of animals on the odorant-spotted side of the plate and N[B] is the number of animals on the opposite side.
(TIF)
A replicate experiment of those shown in Figs 2B and 3B. Taste associative learning of N2, rsks-1, sinh-1, and rict-1 mutants were assayed simultaneously. Error bars, s.e.m.; **p < 0.01 (Dunnett’s test, N ≥ 9).
(TIF)
Genomic structure of rict-1 (top), pkc-2 (middle), and sgk-1 (bottom). Black boxes indicate exons, red boxes indicate deletion mutations, and arrowheads indicate point mutations.
(TIF)
(A) Examples of Oil-Red-O-stained animals. Scale bars: 100 μm. (B) To quantify Oil-Red-O staining, we determined the R/G ratios of the region of interest, which included most of the anterior portion of the body: from the posterior edge of the pharynx to the vulva in each animal (indicated with a purple box). The details are listed in Materials and Methods. (C) Quantification of Oil-Red-O staining in wild-type, rict-1(ft7), and sinh-1(pe420) animals. As previously reported based on experiments conducted using Nile-Red staining [9], our Oil-Red-O staining results demonstrated a body fat increase in rict-1(ft7) animals. Similar to the rict-1(ft7) animals, sinh-1(pe420) animals also showed the increased body fat phenotype, which is in agreement with previous reports that Sin1 is an essential component of TORC2 in several species [22–24]. Data were normalized against the averaged value determined for wild-type. Error bars, s.e.m.; **p < 0.01 (wild-type vs. each mutant, Dunnett’s test, N = 40).
(TIF)
Rapamycin administration didn’t affect food-odor associative learning. This is consistent with the phenotype of rsks-1(ok1255) mutants, which showed no defect in food-odor associative learning. The learning assay was performed using benzaldehyde as an odorant as described in Fig 4F. Error bars, s.e.m.; n.s. = not significant (Student’s t test, N ≥ 8).
(TIF)
Chemotaxis of the sgk-1(gf) mutant after salt conditioning is shown. Error bars, s.e.m.; *p < 0.05, n.s. = not significant (Student’s t test, N ≥ 6).
(TIF)
sgk-1;pkc-2 double mutants showed enhanced migration to low salt levels as compared with each single mutant. Error bars, s.e.m.; **p < 0.01 (Dunnett’s test, N ≥ 9).
(TIF)
(A) Venus expression driven by the sgk-1 isoform A promoter (2.0 kb upstream of the first exon of sgk-1 isoform A). The sgk-1 isoform A promoter drives strong expression in the intestine, but not in neurons. Left, bright field; center, Venus; right, merged. Scale bar: 100 μm. (B) No signal was detected in the head ganglia. Scale bar: 30 μm. (C) Venus expression driven by the sgk-1 isoform B promoter (2.5 kb upstream of the first exon of sgk-1 isoform B). The sgk-1 isoform B promoter drives expression in the intestine. Scale bar: 100 μm. (D) No fluorescence was detected in the head ganglia. Scale bar: 30 μm. (E) Intestine-specific expression (sgk-1A promoter) of sgk-1 isoform A was sufficient for rescuing the chemotaxis defect of the sgk-1(ok538) mutant after low-salt/food(–) conditioning. Error bars, s.e.m.; **p < 0.01 (Dunnett’s test, N ≥ 9).
(TIF)
Intestinal TORC2/SGK-1 signaling and neuronal RSKS-1 and PKC-2 contribute to taste associative learning: TORC2/SGK-1 promotes migration to high salt levels through SGK-1 phosphorylation in the intestine after fed and starvation conditioning.
(TIF)
(TIF)
Data Availability Statement
Data are available on figshare with the DOI 10.6084/m9.figshare.5001359.