Abstract
The mechanistic evidence to support the cardioprotective effects of polyunsaturated fatty acids (PUFA) are controversial. The aim was to test cardioprotective mechanisms induced by PUFA supplementation against cardiac ischemia-reperfusion (IR) injury. Ten-week-old male Wistar rats (225 ± 14 g, n = 14) were divided in two groups: rats without supplementation (n = 7) and a PUFA group, supplemented by PUFA (0.6 g/kg/day; DHA:EPA = 3:1) for eight weeks (n = 7). Hearts were perfused with Krebs–Henseleit buffer for 20 min (control conditions); others were subjected to control conditions, 30 min of global ischemia and 120 min of reperfusion (IR group). Infarct size (IS) and left ventricular developed pressure (LVDP) were measured at 120 min of reperfusion. Oxidative stress biomarkers (TBARS, total carbonyls), antioxidant status (CAT, catalase; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase activity and GSH/GSSG ratio), myeloperoxidase activity, ATP levels and nuclear transcription factor erythroid 2-related factor 2 (Nrf2) and nuclear factor kappaB (NF-κB) were determined in both experimental conditions. At the end of reperfusion, hearts supplemented with PUFA showed lower IS and a higher LVDP compared with the nonsupplemented rats. Hearts in the group supplemented with PUFA showed lower levels of oxidative stress markers and higher antioxidant activity, decreased MPO activity and NF-κB and Nrf2 activation compared with the nonsupplemented group. Cardioprotective effects of PUFA are exerted through induction of anti-inflammatory and antioxidant mechanism at tissue level.
Keywords: Polyunsaturated fatty acids, infarct size, ventricular function, oxidative stress, NF-κB and Nrf2
Introduction
Dietary omega 3 fatty acids can improve clinical outcomes in patients with heart disease and associated cardiovascular mortality.1,2 The cardioprotective effects of these compounds include improvement in vascular relaxation, inhibition of platelet aggregation and modulation of anti-inflammatory responses, among others.3,4 Indeed, omega 3 fatty acids have been shown to improve cardiac hemodynamic factors such as left ventricular function, heart rate and blood pressure.5,6 The specific supplementation with polyunsaturated fatty acids (PUFA) such as docosahexanoic acid [DHA (22:6n3)] and eicosapentaenoic acid [EPA (20:5n3)] has been used experimentally and clinically for the prevention of ischemia-reperfusion (IR) injury to the heart. The molecular mechanism of these beneficial effects has been attributed to antioxidant and anti-inflammatory properties of PUFA, such as the reduction of the arachidonic acid-derived prostaglandins and reinforcement of the antioxidant defense system.7,8 Some studies show that supplementation with a diet rich in n-3 fatty acids seems to be better in terms of myocardial resistance than a diet rich in saturated fatty acids (but poor in both n-3 PUFA and n-6 PUFA), because smaller myocardial infarct sizes (IS) were observed.9,10
The American Heart Association (AHA) published a Science Advisory “Omega-6 (n-6) Fatty Acids and Risk for Cardiovascular Disease” early in 2009.11 The main conclusion of the advisory was that “the AHA supports an n-6 PUFA intake of at least 5–10% of energy in the context of other AHA lifestyle and dietary recommendations.” This conclusion was based on aggregate data from randomized trials, case–control and cohort studies and long-term animal feeding experiments, indicating that the consumption of at least 5–10% of energy from n-6 PUFAs reduces the risk of cardiac health diseases (CHD) relative to lower intakes.
The possible adverse effects of n-6 PUFA are based on these compounds taking the position of arachidonic acid metabolites as potent inflammatory and aggregate molecules. Thus, an increase in consumption of n-6 PUFA would have adverse health effects. The AHA Science Advisory, however, states, “at present there is little direct evidence that supports a net proinflammatory, proatherogenic effect of linoleic acid in humans.”11 Another concern pertains to competition between linoleic acid and alpha-linolenic acid (ALA) for the desaturase and elongase enzymes involved in conversion to longer chain PUFAs. Indeed, recent research shows that decreasing dietary linoleic acid while maintaining constant ALA can slightly increase EPA, but it also decreases DHA to the same extent, resulting in no change in the sum of the two.12
An inverse association between LA and CHD has also been seen in most previous studies of LA biomarkers and CHD events. In a meta-analysis of 22 studies, including case–control and prospective cohort data, blood/tissue ALA concentrations were inversely associated with nonfatal CHD end points.13 Even in a population with high PUFA intake (mean intake, 10.1% of energy) and very high ALA adipose tissue content (25.6% of adipose tissue composition), a nonsignificant inverse association was found between adipose LA and acute myocardial infarction after controlling for other n-6 PUFA.14
Recently, pretreatment with PUFA effectively protected against intestinal and lung injury induced by intestinal IR, suppressing inflammation, inhibiting lung apoptosis, and improving the lung endothelial barrier in rats.15 In cardiac IR injury, the cardioprotective effects of PUFA are associated with signaling pathways involving the activation of phospholipases, the synthesis of eicosanoids, and the regulation of receptor-associated enzymes and protein kinases. However, the redox signaling pathways, which involve NF-κB and Nrf2 activation, as well as their implication on mitochondrial function during an IR cycle have not been well addressed, and controversial results have been found in various models, including in human endotoxemia and isolated macrophages.16,17
The overall aims of this work were to associate the changes in the cardiac redox state and lipid composition induced by PUFA with functional and structural improvement and the likely molecular pathways.
In this work, the use of an isolated heart model made it possible to investigate the myocardium response independently from other organs.
Materials and methods
Animals and diet administration
This study conformed to the Guide for the Care and Use of Laboratory Animals, published by the U.S. National Institutes of Health (NIH, Publication No. 85-23, revised in 1996) and was approved by the Institutional Ethics Review Committee at the Universidad de Chile (Study number CBA# 0627 FMUCH).
Triphenyltetrazolium chloride (TTC), glutathione (GSH) and all other chemicals were obtained from Sigma-Aldrich, USA. The NF-κB probe was obtained from Grupo Bios SA., Chile. DNA polymerase was obtained from Invitrogen Corp., CA, USA.
All experiments were conducted using 10-week-old male Wistar rats (225 ± 14 g). All animals received an experimental solid diet (15 g of standard pellet meals) and water ad libitum per day. The composition of the experimental diet is shown in Table 1. Daily fluid intake was measured with graduated Richter tubes. Food intake was also estimated by gravimetry. All rats were housed under conditions of constant temperature, humidity and 12 h light/dark cycle. The animals were divided in two groups: control rats, which not received PUFA, only experimental diet (n = 7); and PUFA group (n = 7), supplemented by PUFA (0.6 g/kg/day; DHA: EPA = 3:1) on a daily doses via gavage for eight weeks.
Table 1.
Composition of experimental diet (g/kg diet)
| Casein | 200 |
|---|---|
| DL-methionine | 3 |
| Corn starch | 297 |
| Sucrose | 225 |
| Canola oil | 100 |
| Potato starch | 25 |
| Water-soluble vitaminsa | 30 |
| Fat-soluble vitaminsb | 20 |
| Mineral mixturec | 50 |
| Fibre | 50 |
| Energy (MJ/kg) | 16.3II |
Modified: Araya et al. (2001).
The water-soluble vitamin composition was (g/kg diet) choline chloride 0.945, p-aminobenzoic acid 0.473, inositol 0.094, niacin 0.047, calcium pantothenate 0.024, riboflavin 0.024, thiamine hydrochloride 0.019, pyridoxine hydrochloride 0.005, folic acid 0.005, biotin and cyanocobalamin 0.0005.
The fat-soluble vitamins composition was (per kg diet) DL-α-tocopherol acetate 66 mg, all-trans-retinyl acetate 2.5 mg (7000 IU), menadione 60 µg and cholecalciferol 7.5 µg.
The mineral composition was (per kg diet) CaCO3 15.75, CaH2PO4·2H2O 3.35 g, K2HPO4 16.40 g, NaCl 8.52 g, MgSO4.7H2O 5.18 g, iron citrate 0.51 g, MnSO4 0.25 g, CuSO4.5 H2O 25.3 mg, ZnCl2 5.0 mg, KI 1.2 mg, sodium selenite 5.0 mg and NaF 5.0 mg.
II 3900 kcal/kg.
Following the supplementation period, the rats were anesthetized with pentobarbital (50 mg·kg−1 IP) for surgical intervention. Once confirm deep anesthesia, a sternotomy was performed, heparin 100 U·kg−1 intravenous (IV) was administered.
Ex vivo measurements
Langendorff perfusion
The heart was rapidly excised, mounted in a temperature regulated heart chamber and perfused retrograde via the ascending aorta using a peristaltic infusion pump (Gilson Minipuls3, France) at a constant flow of 10–14 ml/min to generate an initial mean coronary (aortic) perfusion pressure of 60–70 mmHg with physiological modified Krebs Henseleit Buffer solution containing (in mM) NaCl (128.3), KCl (4.7), CaCl2 (1.35), NaHCO3 (20.2), NaH2PO4 (0.4), MgSO4 (1.1), glucose (11.1), and pH 7.4 at 37℃ when equilibrated with a mixture of 95% O2/5% CO2. Then, a latex balloon inserted in the left ventricle through the mitral valve was connected to a pressure transducer (Bridge Amp ML221 AD Instruments, Australia) and filled with normal saline to produce a left ventricle end-diastolic pressure (LVEDP) of 5–10 mmHg. The volume of the balloon was maintained constant throughout the experiment. The right atrium was excised to eliminate the contribution of the primary intrinsic pacemaker. The stimulator generated a pacing stimulus of 1 ms of duration with an intensity of twice of the threshold current. The pacing was used to maintain a standard contractile response (300 ± 50 bpm).
The occurrence of ventricular premature beats (VBs), ventricular tachycardia (VT) and ventricular fibrillation (VF) during reperfusion period were monitored for 30 min. The arrhythmia severity index was calculated in order to quantify the arrhythmias.18
Ischemia/reperfusion induction
The hearts were perfused with Krebs–Henseleit buffer for a 20-min stabilization period (control conditions), hearts with a LVDP less than 60 mmHg were excluded from the study. The remaining hearts were subject to 30 min of no flow global ischemia and 120 min reperfusion. Perfusate and bath temperatures were maintained at 37℃ by a thermostatically controlled water circulator (B. Braun Thermomix 1420, Germany).
Myocardial injury
After the 120-min reperfusion, the hearts were weighed to estimate the degree of ventricular hypertrophy. After this procedure, the hearts were perfused with 15 ml 2,3,5-triphenyltetrazolium chloride 1% in phosphate buffer adjusted to pH 7.4, for 15 min at 37℃. After overnight storage in 10% formaldehyde, five to six slices uniform 2-mm covered by a glass. Then, a digital photography of the mounted tissue was taken. For each slice, measuring the size was performed by planimetry using the Image J program (NIH, USA). The IS was expressed as a percentage of the total ventricular volume.19
Measurement of left ventricular function
The left ventricle systolic pressure (LVSP), LVEDP and coronary perfusion pressure were measured and continuously recorded throughout the entire experiment on a personal computer using PowerLab (ML866 ADInstruments, Australia). Left ventricular developed pressure (LVDP) was calculated as follows: LVDP = LVSP−LVEDP (mm Hg).
Biochemical measurements
Antioxidant enzyme activity
Heart lysates were homogenized in 0.25 mol·L−1 sucrose for the determination of Cu-Zn SOD (superoxide dismutase) activity, or in 1.15% KCl 10 mmol−1·L−1 Tris/HCl buffer (pH 7.4) for CAT (catalase) and GSH-Px (glutathione peroxidase) activity. The Cu-Zn SOD assay is based on the SOD-mediated increase in the rate of autooxidation of catechols in an aqueous alkaline solution in order to yield a chromophore with a maximum absorbance at 525 nm.20 One Cu-Zn SOD unit is defined as the activity that doubles the auto-oxidation background, and the results are expressed as units (U)/mg of protein. CAT activity was assayed by the kinetic of breakdown of peroxide of hydrogen (H2O2) at 240 nm by an aliquot of the 2400 g supernatant and are expressed as the first-order reaction rate constant (k)/mg of protein.21 Soluble GSH-Px activity was measured spectrophotometrically in the cytosolic fraction (100,000 g supernatant) by the reduction of glutathione disulfide coupled to NADPH oxidation by glutathione reductase.22 One GSH-Px unit is defined as the activity that oxidizes 1 µmol of NADPH/min and is expressed as units (U)/mg of protein.
Oxidative stress markers
The intracellular redox status in atrial tissue was assessed by a fluorometric method in order to measure the oxidized glutathione (GSSG) and reduced glutathione (GSH).23 The inter- and intra-assay CVs for GSH and GSSG were 3.1% and 4.2%; and 2.7% and 3.5%, respectively. The GSH/GSSG ratio was then calculated. Lipid peroxidation was assessed by the thiobarbituric acid reaction at pH 3.5, followed by solvent extraction with a mixture of n-butanol/pyridine (15:1, v/v).24 Tetramethoxypropane was used as the external standard, and the levels of lipid peroxides were detected spectrophotometrically at 532 nm and were expressed as mmol TBARS/mg protein. The inter-assay and intra-assay CVs for TBARS were 10.5% and 4.8%, respectively. The total protein carbonyl in heart tissue was assessed by a method that quantified the reaction between 2,4-dinitrophenylhydrazine and carbonyls groups. The amount of protein hydrozone produced was quantified spectrophotometrically at an absorbance between 360 and 385 nm.25 The carbonyl content was expressed in nmol/mg of protein
Assessment of myeloperoxidase activity
Neutrophil infiltration was assessed through the determination of MPO activity. Cardiac tissue was homogenized in 50 mM PBS, pH 7.4, and centrifuged at 14,000 g for 10 min at 4℃. The pellet was homogenized again in 50 mM PBS, pH 6.0, containing 0.5% hexadecyltrimethylammonium bromide (HETAB) and 10 mM EDTA. This homogenate was subjected to one cycle of freezing–thawing and a brief period of sonication. An aliquot of homogenate was added to a solution containing 80 mM PBS, pH 5.4, 0.5% HETAB, and 1.6 mM 3,30,5,50-tetramethylbenzidine (TMB), and the reaction was started by the addition of 0.3 mM H2O2. Optical density was read at 655 nm. One unit of MPO activity was defined as the amount of enzyme that produced a change in absorbance of 1.0 unit/min at 37℃.26
ATP quantification
Intracellular ATP levels were quantified by luminescence using a CellTiter-Glo kit from Promega (Madison, WI, USA). Luminescence was measured using a Multi-Mode Microplate Reader (Synergy HT, BioTek, USA). Results are expressed as relative luminescence units (RLU) per milligram of protein.
Electromobility shift assay
Nuclear protein extracts from heart tissue samples were prepared according to Deryckere and Ganon.27 The samples were subjected to EMSA for the assessment of NF-κB DNA binding using the NF-κB probe 5′-GATCTCAGAGGGGACTTTCCGAG-3′ (GrupoBios SA, Chile) labelled with α-32P-dCTP using the Klenow DNA Polymerase Fragment I (Invitrogen Corp., Carlsbad, CA, USA). The specificity of the reaction was determined by a competition assay using 100-fold molar excess of unlabelled DNA probe. The subunit composition of DNA-binding protein was confirmed by supershift assay using specific antibodies from rat and rabbit IgG raised against NF-κB p50 and p65 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Samples were loaded on nondenaturing 6% polyacrylamide gels and run until the free probe reached the end of the gel; NF-κB bands were detected by autoradiography and quantified by densitometry using IMAGEJ (NIH Image, http://www.scioncorp.com, Scion Corporation, USA).
Transcription factor binding assay
The Nrf2 activation was assessed by other technique (ELISA, enzyme-linked immunosorbent assay) replaces the cumbersome radioactive electrophoretic mobility assay (EMSA; Cayman KIT # 600590). A specific double stranded DNA (ds DNA) sequence containing the Nrf2 response element is immobilized onto the wells of a 96-well plate. Nrf2 contained in a nuclear extract (NE) binds specifically to the Nrf2 response element. Nrf2 is detected by addition of a specific primary antibody directed against Nrf2. A secondary antibody conjugated to HRP is added to provide a sensitive colorimetric readout at 450 nm. The results were expressed as nuclear/cytosolic Nrf2 content.
Fatty acid analyses
Total lipids extracted were fractionated with a solvent system of chloroform ± methanol ±water (1:0.8:0.8, by vol.).28 Afterwards, the lipid spots on the chromatograms were scraped, methylated and the fatty acid composition was analyzed using a GC model Clarus 500 (Perkin Elmer, USA), equipped with capillary column SPTM 2380 (length 60 m, 0.25 mm × 0.2 µm film thickness, Supelco, USA). The oven temperature was programmed from 150 to 230℃, at 6℃/min, with a final hold, to separate the fatty acids from 14:0 to 22:6 n-3. For the identification and determination of the fatty acid composition, the standard retention times of fatty acid methyl esters were used as a reference (F.A.M.E. Mix C4-C24, Sigma, USA). The fatty acid methyl esters were identified by comparison with authentic standards retention times and peak areas were automatically computed as a percentage by a TotalChrom navigator version 6.3 (Perkin Elmer, USA).
Statistical analysis
A sample size calculation was performed on the basis of the IS reduction. The assumptions used for this purpose included an expected 30% of reduction in IS at the end of reperfusion in the supplemented rats with PUFA, compared with rats that did not receive any supplementation. The sample size calculation was based on the differences in the mean value between two groups with equal sample size, prespecified 5% alpha error and 80% power. This calculation was based on previous determination in our laboratory and publication (30.6% of IS reduction).8
The resulting sample size was 7.3 rats in each treatment group (Stata 10.0 for Windows). The infarct areas at the end of reperfusion were analyzed using unpaired t-test. The comparisons between two or more groups were analyzed using two-way analysis of variance. A Tukey post-hoc test was used to assess the differences in biomarkers of oxidative stress among groups. A value of P < 0.05 was considered for significant statistical difference. All data are expressed as mean ± SD.
Results
Effects of PUFA supplementation of cardiac mass
Feeding Wistar rats with an experimental diet (Table 1) for eight weeks resulted in similar and energy consumption in both groups. With respect to cardiac/body weight ratio, both groups did not show significant differences.
Regarding to lipid cardiac composition, the hearts of PUFA group showed a 65.1 and 66.8% higher levels of EPA and DHA fatty acids, respectively (P < 0.01), than the control group. Conversely, the rats supplemented with PUFA and subjected to IR, showed in cardiac samples, 41.3% lower levels of arachidonic acid (20:4 n-3), with respect to levels measured in control samples.
Effects of PUFA supplementation on myocardial IR injury and ventricular function
The heart weight-to-body weight ratio showed no significant differences between control and PUFA group, compared to basal values (baseline, control vs. PUFA: 0.29 ± 0.08 vs. 0.31 ± 0.07; after treatment: 0.37 ± 0.08 vs. 0.35 ± 0.11, respectively)
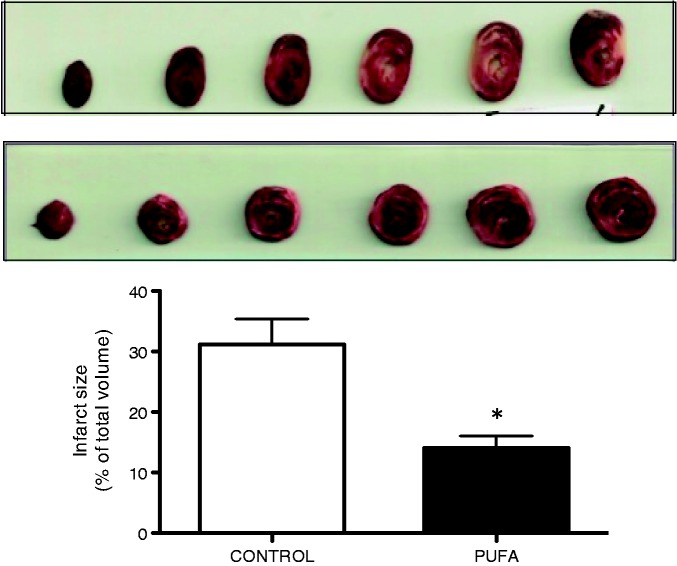
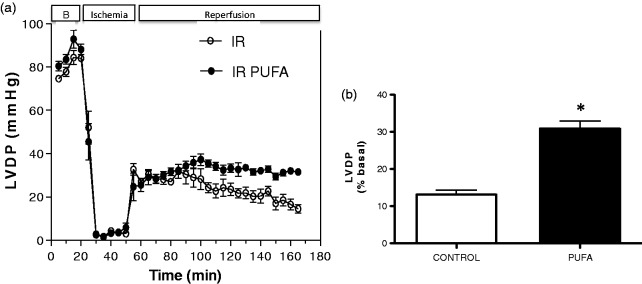
Indeed, PUFA supplementation is associated with a 54.8% lower IS with respect to hearts of rats without PUFA supplementation (P < 0.01; Figure 1). Regarding LVDP, the pre-ischemic values were similar for both groups, but this parameter was 17.8% lower at the end of reperfusion in the control groups (P < 0.05). These differences were calculated with respect to the highest value of LVDP developed during the baseline time (Figure 2).
Figure 1.
Effects of PUFA supplementation on myocardial IR injury. Infarct size was determined in rats trough ex vivo assessed in rats supplemented with PUFA by eight weeks. Values are expressed as mean ± SD of total ventricular volume (n = 7). Significant differences: *P < 0.05 vs. control. (A color version of this figure is available in the online journal.)
Figure 2.
Measurement of LV developed pressure (LVDP). (a) Through the study; (b) at the end of reperfusion, in rats supplemented with PUFA and Control. Values are expressed as mean ± SD (n = 7). Significant differences: *P < 0.05 vs. control
In the control group, the incidence of ventricular premature beats and ventricular tachycardia was 45%, whereas ventricular fibrillation occurred in 10.7% hearts. PUFA significantly reduced the incidence of ventricular arrhythmias incidences (number of hearts affected/number of hearts per group: VB 6/7, VT 2/7 and VF 1/7) with the arrhythmia severity index 3.6 (n = 5, P < 0.05).
Effect of PUFA supplementation on antioxidant enzyme activity and oxidative markers
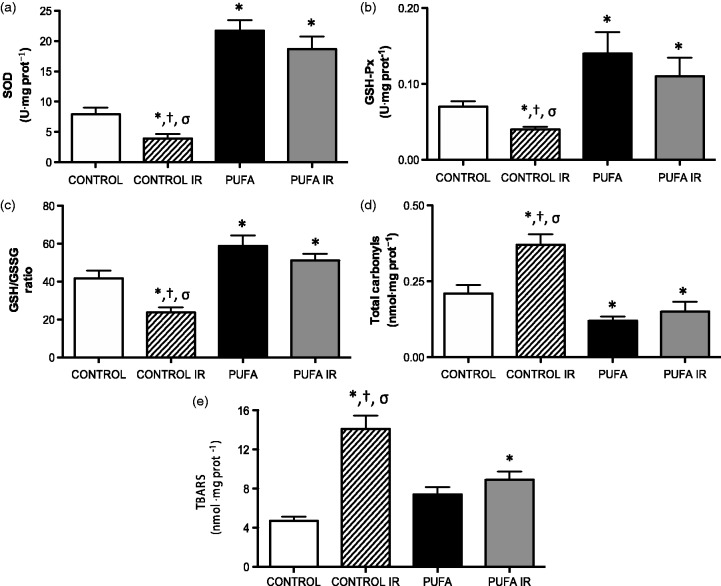
Cardiac antioxidant activity enzyme and GSH/GSSG ratio were significantly lower in samples obtained following IR cycle, compared to hearts from baseline control conditions (SOD, 7.90 ± 3.23 vs. 3.90 ± 2.1 U·mg protein−1; GSH-Px, 0,07 ± 0.02 vs. 0.04 ± 0.01 U·mg protein−1; GSH/GSSG ratio, 41.8 ± 11.3 vs. 23.8 ± 7.9, respectively; P < 0.05). PUFA administration is associated with a major recovery of these parameters (SOD, 21.7 ± 4.9 U·mg protein−1; GSH-Px, 0.14 ± 0.08 U·mg protein−1; GSH/GSSG ratio, 58.9 ± 15.4; P < 0.05). Indeed, samples of heart tissue obtained in PUFA group at the end of reperfusion time (PUFA IR) show no significant differences in antioxidant status parameters with respect the PUFA group in control conditions (Figure 3(a) to (c)).
Figure 3.
Oxidative stress related-parameters. (a) SOD, (b) GSH-Px, (c) GSH/GSSG ratio, (d) Total carbonyls and (e) lipid peroxidation TBARS in rats supplemented with PUFA and Control, at basal condition and at the end of reperfusion (120 min). Values are expressed as mean ± SD (n = 7).*P < 0.01 vs. control; †P < 0.01 vs. PUFA; σP < 0.01 vs. PUFA IR
The oxidative stress biomarkers, total carbonyls and TBARS shown higher levels in cardiac samples after IR (carbonyls, 0.21 ± 0.08 vs. 0.37 ± 0.12 nmol·mg protein−1; TBARS, 4.7 ± 1.2 vs. 14.1 ± 4.9 nmol·mg protein−1), compared with levels in control conditions (P < 0.05). The PUFA administration attenuate these changes, both control condition and following IR (PUFA: carbonyl, 0.12 ± 0.05; TBARS, 7.4 ±2.11 nmol·mg protein−1; P < 0.05; Figure 3(d) and (e)).
Effect of PUFA supplementation on Myeloperoxidase activity and ATP levels
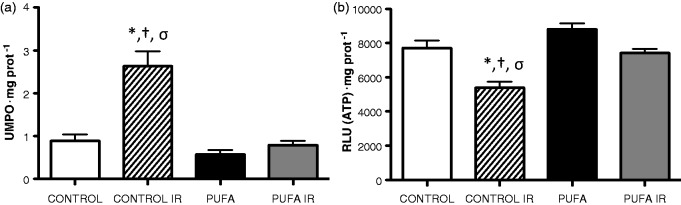
The hearts subjected to ex vivo IR showed a 195% higher MPO activity compared with baseline control levels (0.89 ± 0.41 vs. 2.63 ± 0.98 UMPO·mg protein−1; P < 0.01). PUFA administration totally prevented the increase in MPO activity in control conditions and induced by IR (PUFA, 0.58 ± 0.31 and PUFA IR, 0.74 ± 0.28 UMPO·mg protein−1; Figure 4(a)).
Figure 4.
Myeloperoxidase activity and ATP levels. (a) Neutrophil infiltration assessed by MPO activity and (b) ATP levels in rats supplemented with PUFA and Control group, at basal condition and at the end of reperfusion (120 min; IR). Values are expressed as mean ± SD (n = 7).*P < 0.01 vs. control; †P < 0.01 vs. PUFA; σP < 0.01 vs PUFA IR
Since any alteration in the electron transport chain energy affects the cell energy, we studied the intracellular ATP levels in heart samples. Following IR heart samples showed a drop in cellular ATP levels with respect to baseline values (7711 ±1235 vs. 5295 ± 1024, RLU ATP·mg protein−1; P < 0.05). These changes were attenuated by PUFA administration at control and IR conditions (PUFA, 8809 ± 977 and PUFA IR, 7015 ± 658; Figure 4(b)).
PUFA administration modulates antioxidant and pro-inflammatory signaling pathways
Nuclear factor kappaB activation
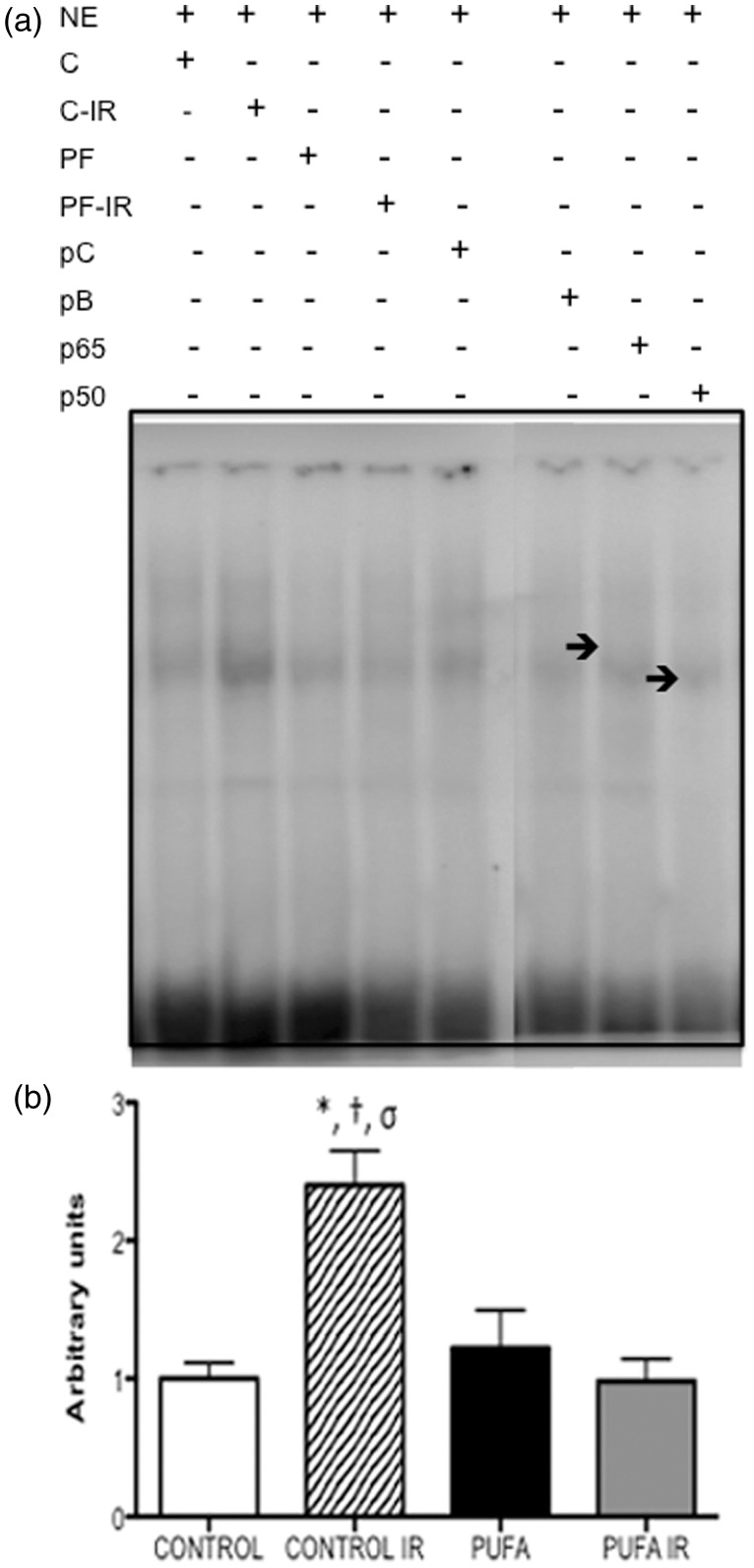
Heart tissue DNA binding of NF-κB in experimental conditions is shown in Figure 5. Cardiac tissue NF-κB DNA binding in rats subjected to IR was higher 2.4-fold compared with samples in control conditions (P < 0.05). PUFA administration attenuates these changes to similar levels to control conditions and PUFA baseline. Suppression of the EMSA NF-κB in control bands by 100-molar excess of the respective unlabelled DNA probes confirmed the specificity of the determinations. PUFA baseline samples in supershift analysis show both components of NF-κB rel p50 and p65 that are implicated in DNA binding and pro-inflammatory gene regulation.
Figure 5.
Heart nuclear factor-kappaB (NF-κB) DNA binding on electromobility shift assay. (a) NF-kB DNA-binding activity and subunit composition by EMSA and gel supershifts in nuclear extracts (NE) from control (C) and hearts supplemented by PUFA (PF) at baseline and at the end of reperfusion (C-IR; PF-IR). Arrows p50 and p50 indicate two different subunit-specific antibodies used in gel supershifts. Control group in competition experiments without [positive control (pC)] and with 100-fold molar excess of the unlabelled DNA probe (pB). (b) Bar graphs corresponding to densitometric quantification of relative NF-κB DNA binding represent means ± SD (n = 7) *P < 0.01 vs. control; †P < 0.01 vs. PUFA; σP < 0.01 vs. PUFA IR
Nuclear transcription factor erythroid 2-related factor 2
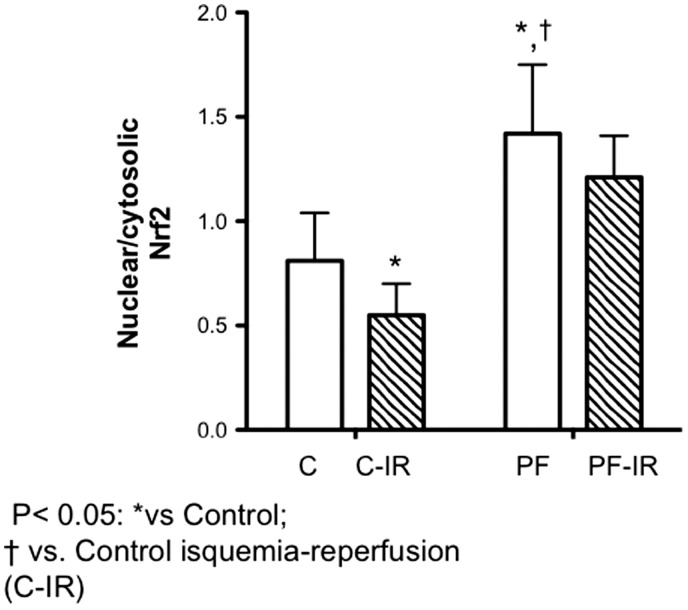
Following IR significant drop was observed in nuclear/cytosolic cardiac Nrf2 content. Following PUFA administration, cardiac nuclear content were 65.4% and 42.1% higher than hearts without supplementation in control and at the end of reperfusion time (P < 0.05). No significant differences were shown in hearts of rats supplemented with PUFA at the end of reperfusion respect to control conditions.
Discussion
In this experimental work, we show that PUFA administration attenuates the cardiac structural and functional damage induced by IR through the induction of the antioxidant and anti-inflammatory mechanism. These changes could be associated with the incorporation of these fatty acids into the cardiac membrane after PUFA supplementation in an ex vivo IR model.
Cardioprotective properties of PUFA are explained by pleiotropic effects including a reduction of plasma triglycerides levels lowers resting heart rate, and blood pressure and may also improve myocardial filling and vascular function.29 Additionally, PUFA can modulate several molecular pathways, such as the alteration of physical and chemical properties of cell membranes due to direct interaction with membrane channels and proteins, the regulation of gene expression via nuclear receptors and transcription factors, and the conversion of n-3 PUFA to bioactive metabolites.30 In conditions of high PUFA intake, the changes in eicosanoid profiles to inflammation-resolving lipid mediators and the suppression of acute phase reactants during IR have been well characterized.31 Eicosanoids produced from DHA and EPA are generally less inflammatory than their AA-derived eicosanoid counterparts. PUFA can reduce the production of AA-derived eicosanoids by competing with AA for incorporation into the cell membrane through the release of free AA by PLA2 or by inhibiting the enzyme COX-2 and 5-LOX.32 This inhibition may shift the production of inflammatory eicosanoids derived from n-6 PUFA to n-3 PUFA. In cardiac tissue, these anti-inflammatory effects are expressed through the reduction in neutrophil infiltration, the inhibition of NF-κB activation, the reduction of COX-2 expression and prostanoid synthesis.33,34 However, despite these findings only a few studies have estimated the association between the PUFA effects in inflammatory pathways with improvement in cardiac IR injury.
Reduction in IS in the group supplemented with PUFA (Figure 1) agrees with a previous study by Zeghichi-Hamri et al.,10 in which rats were also supplemented with a diet rich in n-3 PUFA and low in n-6 PUFA for eight weeks. In their work, the effects of supplementation did not show any differences in ventricular function as compared with the other fatty acids groups. This result is explained by the myocardial hibernation that may have masked the better recovery of post-ischemic function. In our work, the ischemic time was the same (30 min); however, we found significant recovery of LVDP at the end of reperfusion (Figure 2). Moreover, clinical studies have demonstrated that patients supplemented for three to six months with PUFA showed a correlation between the EPA+DHA content in their red blood cell membranes and the fatty acid content in their myocardial tissue.32 From this work, “the omega-3 index” is defined as the EPA+DHA percentage related to the total fatty acids in the red blood cell membranes. Lower omega-3 index values have been associated with epidemiological variables and risk factors of sudden death and major cardiovascular events35; therefore, the omega 3 index seems to be a good prognostic tool for evaluating the efficacy of PUFA supplementation. In Table 2, the results show a higher proportion of EPA (20:5 n-3) and DHA (22:6 n-3) in the cardiac membrane and lower amounts of AA (20:4 n-6). These findings may explain the anti-inflammatory portion of the cardioprotective effects.
Table 2.
Cardiac/body weight, energy consumption and cardiac lipid composition at final of IR protocol
| Parameter | Control (n = 7) | PUFA (n = 7) |
|---|---|---|
| Cardiac/body weight (mg/g) | 0.29 ± 0.08 | 0.32 ± 0.07 |
| Energy consumption (kcal 100 g BW/day) | 20.5 ± 2.2 | 22.8 ± 3.8 |
| Cardiac PUFA composition (percentage of total) | ||
| Polyunsaturated | ||
| 20:4(n-6) | 20.3 ± 2.7 | 11.5 ± 0.9* |
| 20:5(n-3) | 0.45 ± 0.2 | 1.29 ± 0.6* |
| 22:6(n-3) | 2.26 ± 2.1 | 6.61 ± 1.5* |
Values are expressed as mean ± SD.
P < 0.05 when compared with control.
PUFA, polyunsaturated fatty acids; BW, body weight.
The antioxidant effects of PUFA are mainly related to its incorporation into the cell membrane and the modulation of the antioxidant signaling pathways. The improvement of the antioxidant defenses and the attenuation of oxidative damage have been described in plasma and in cardiac tissue.36,37 Chronic fish oil supplementation increased the activity of SOD and decreased TBARS levels in plasma in a rat model. Additionally, the antioxidant GSH content and the activities of CAT, SOD and GSH-Px increased in rat hearts supplemented with higher doses of PUFA and subjected to IR injury.8,37 These data are consistent with other studies and other animal models that show not only the effects on the markers of antioxidant status but also increase the expression of antioxidant enzymes38,39 In our results, the antioxidant effects of PUFA are demonstrated by an increase in antioxidant enzyme activities (Figure 3(a) and (b)) and the GSH/GSSG ratio (Figure 3(c)) in the PUFA and the PUFA IR groups. The attenuation of oxidative damage was demonstrated through lower levels of total carbonyls (Figure 3(d)) and TBARS (Figure 3(e)) in the cardiac tissue.
Molecular mechanism of the effects of PUFA: role of NF-KappaB and Nrf2 in IR injury
The nuclear factor kappaB is the super family of transcription factors that have been implicated in the regulation of immune cell maturation, cell survival and inflammation in many cell types including cardiac myocytes.40 This factor is a redox-sensitive transcription factor existing in most cell types and has the common p50/65 heterodimer pattern. Generally, inactive NF-κB dimers bind with the inhibitor of NF-κB proteins (IkBs) and remain in the cytosol. Many stimuli, such as reactive oxygen species (ROS) and inflammatory factors, may lead to the activation of NF-κB. Activated NF-κB then translocates into the nucleus where it activates the corresponding target genes, many of which regulate apoptosis, inflammation and autophagy.41 In the context of IR injury, the activation of NF-κB without prior adaptation appears to be detrimental because the inhibition of NF-κB activation reduces IS and heart function.42,43 However, the role of NF-κB in myocardial ischemia and reperfusion might be dual, having both a cardioprotective role in ischemic preconditioning and a detrimental role during sustained IR.44 Indeed, the activity of p65, a subunit of NF-κB, has increased activity during reperfusion presumably due to the overwhelming release of ROS during reperfusion.45 It has been reported in hearts subjected to in vivo infarction that NFκB-activation is biphasic, peaking after 15 min and then again after 3 h of reperfusion, which may possibly correspond to primary activation by reactive oxygen intermediates and secondary activation by pro-inflammatory cytokines related to myocardial remodeling.46 A lethal role of NF-κB during reperfusion is suggested indirectly by functional studies of the inhibition of target genes and studies that use strategies that protect the heart against IR.47,48 Additionally, in isolated hearts, the NF-κB-activation started after the initiation of ischemia and increased following the reperfusion time.49 In a recent experiment, the use of NF-κB inhibitor resulted in a significant reduction in myocardial infarct volume, a preservation of left ventricular function, and a change associated with the inhibition of the NF-κB activation of the p65 subunit.50 In our results, the IR cycle induced a greater activation of NF-κB with respect to baseline samples assessed by the DNA union in the EMSA assay. PUFA administration attenuates these changes likely due to anti-inflammatory effects as previously described (Figure 5). The probable inhibition of this factor induced by PUFA supplementation has been shown in in vivo models and in some clinical trials of IR injury.17 More recently, diets high in DHA counteracted the reduced SOD and SIRT1 activity in rats after brain trauma.51 SIRT1 is associated directly with the p65 subunit and deacetylates lys310 residue, which is a site critical for NF-κB transcriptional activity. Moreover, SIRT1 deacetylates and suppresses the transcriptional activity of AP-1, which leads to downregulation of cyclooxygenase-2 gene expression.52 SIRT1 knockout or knockdown leads to an increase in NF-κB activation and pro-inflammatory cytokine release. In contrast, SIRT1 activation (e.g. SRT1720 and resveratrol) inhibits NF-κB-mediated inflammatory mediators, which suggests SIRT1 is involved in the regulation of inflammation.53 In this context, supplementary results (Figure S1) show that PUFA induces higher expression of SIRT-1 and attenuates the drop in IR cycle-associated levels.
Experimental studies have shown that the acute activation of Nrf2 prior to cardiac ischemia reperfusion reduced IS in vivo and improved recovery time in Langendorff-perfused mouse hearts.54,55 To investigate whether the IR-induced Nrf2 protein translation occurs in vivo and is related to the antioxidant response, we measured the Nrf2 protein levels in the myocardium at baseline conditions and following 120 min of reperfusion, which is known to induce oxidative stress.8 After 30 min of ischemia and 120 min of reperfusion, both cytosolic and nuclear levels of this factor showed no change (Figure 6). Moreover, the evidence suggests that severe ischemia and reperfusion stress, such as 30′min I/60′R and 60′I/90′R, did not cause Nrf2 protein elevation.56 This finding is very important, as it indicates that for Nrf2 to initiate antioxidant defenses against reperfusion injury, the length of the prior ischemic phase may be a critical factor. Early rescue from ischemia may attenuate Nrf2 response to oxidative stress upon reperfusion, which reduces the protection from reperfusion injury.57 With respect to PUFA administration, the observed effect in the Nrf2 content indicates that, following IR, the factor translocates to the nucleus, which would directly activate the factor and lead to the induction of an antioxidant response. This finding is complementary to the higher activity of the antioxidant enzymes and the GSH/GSSG ratio in rat hearts supplemented with PUFA (Figure 3). Moreover, there was no significant change in these markers at the end of reperfusion. These changes agree with the time-dependent effects of PUFA, antioxidant response system in the heart,58 and the attenuation of cardiac ischemic remodeling.
Figure 6.
Nuclear/cytosolic Nrf2 content. Nrf2 activation in cardiac tissue from rats without supplementation (n = 7), and hearts of rats supplemented with PUFA (PF; n = 7): C, baseline; CT-IR, cardiac samples at the end of reperfusion. Results are expressed as mean ± SD. Significant differences: *P < 0.05 vs. control, †P < 0.05 vs. IR conditions
Conclusions
This work shows that the cardioprotective effects of PUFA supplementation could be potentially determined by a DHA:EPA ratio and the probable incorporation in cardiac membrane. The molecular mechanisms would be based on the higher proportion of DHA incorporation and the induction of the antioxidant and anti-inflammatory response. The effects on the redox state allow the heart to improve its resistance to functional and structural damage caused following the IR event. Therefore, novel experimental evidence is needed to design effective pharmacological studies in animal models of IR and preclinical trials.
Acknowledgments
Drs Ramón Rodrigo and Gina Sánchez for facilitating the use of their laboratory equipment. Also, the technical assistance of Mr. Diego Soto. We thank the Fondo Nacional de Investigación Científica y Tecnológica and Comisión Nacional de Investigación Científica y Tecnológica (Fondecyt) into Research Grant (1161731; 11130232; RLC; CC-P.).
Authors' contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript. RLC, C-CP and JGF conducted the experiments, NS and JQ evaluated diet composition, and RLC, C-CP, MQ, PA, VM and JGF wrote the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Masson S, Marchioli R, Mozaffarian D, Bernasconi R, Milani V, Dragani L, Tacconi M, Marfisi RM, Borgese L, Cirrincione V, Febo O, Nicolis E, Maggioni AP, Tognoni G, Tavazzi L, Latini R. Plasma n-3 polyunsaturated fatty acids in chronic heart failure in the GISSI Heart Failure Trial: relation with fish intake, circulating biomarkers, and mortality. Am Heart J 2013; 165: 208–15.e4. [DOI] [PubMed] [Google Scholar]
- 2.Macchia A, Romero M, D’Ettorre A, Tognoni G, Mariani J. Exploratory analysis on the use of statins with or without n-3 PUFA and major events in patients discharged for acute myocardial infarction: an observational retrospective study. PLoS One 2013; 8: e62772–e62772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frenoux JM, Prost ED, Belleville JL, Prost JL. A polyunsaturated fatty acid diet lowers blood pressure and improves antioxidant status in spontaneously hypertensive rats. J Nutr 2001; 131: 39–45. [DOI] [PubMed] [Google Scholar]
- 4.Gortan Cappellari G, Losurdo P, Mazzucco S, Panizon E, Jevnicar M, Macaluso L, Fabris B, Barazzoni R, Biolo G, Carretta R, Zanetti M. Treatment with n-3 polyunsaturated fatty acids reverses endothelial dysfunction and oxidative stress in experimental menopause. J Nutr Biochem 2013; 24: 371–9. [DOI] [PubMed] [Google Scholar]
- 5.Mozaffarian D. Fish, n-3 fatty acids, and cardiovascular haemodynamics. J Cardiovasc Med (Hagerstown) 2007; 8: S23–6. [DOI] [PubMed] [Google Scholar]
- 6.McLennan PL, Abeywardena MY, Dallimore JA, Raederstorff D. Dietary fish oil preserves cardiac function in the hypertrophied rat heart. Br J Nutr 2012; 108: 645–54. [DOI] [PubMed] [Google Scholar]
- 7.Brahmbhatt V, Oliveira M, Briand M, Perrisseau G, Bastic Schmid V, Destaillats F, Pace-Asciak C, Benyacoub J, Bosco N. Protective effects of dietary EPA and DHA on ischemia-reperfusion-induced intestinal stress. J Nutr Biochem 2013; 24: 104–11. [DOI] [PubMed] [Google Scholar]
- 8.Castillo RL, Arias C, Farías JG. Omega 3 chronic supplementation attenuates myocardial ischaemia-reperfusion injury through reinforcement of antioxidant defense system in rats. Cell Biochem Funct 2014; 32: 274–81. [DOI] [PubMed] [Google Scholar]
- 9.Ogita H, Node K, Asanuma H, Sanada S, Takashima S, Minamino T, Soma M, Kim J, Hori M, Kitakaze M. Eicosapentaenoic acid reduces myocardial injury induced by ischemia and reperfusion in rabbit hearts. J Cardiovasc Pharmacol 2003; 41: 964–9. [DOI] [PubMed] [Google Scholar]
- 10.Zeghichi-Hamri S, de Lorgeril M, Salen P, Chibane M, de Leiris J, Boucher F, Laporte F. Protective effect of dietary n-3 polyunsaturated fatty acids on myocardial resistance to ischemia-reperfusion injury in rats. Nutr Res 2010; 30: 849–57. [DOI] [PubMed] [Google Scholar]
- 11.Harris WS, Mozaffarian D, Rimm EB, Kris-Etherton P, Rudel LL, Appel LJ, Engler MM, Engler MB, Sacks F. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Committee. Circulation 2009; 119: 902–7. [DOI] [PubMed] [Google Scholar]
- 12.Liou YA, King DJ, Zibrik D, Innis SM. Decreasing linoleic acid with constant alpha-linolenic acid in dietary fats increases (n-3) eicosapentaenoic acid in plasma phospholipids in healthy men. J Nutr 2007; 137: 945–52. [DOI] [PubMed] [Google Scholar]
- 13.Harris WS, Poston WC, Haddock CK. Tissue n-3 and n-6 fatty acids and risk for coronary heart disease events. Atherosclerosis 2007; 193: 1–10. [DOI] [PubMed] [Google Scholar]
- 14.Kark JD, Kaufmann NA, Binka F, Goldberger N, Berry EM. Adipose tissue n-6 fatty acids and acute myocardial infarction in a population consuming a diet high in polyunsaturated fatty acids. Am J Clin Nutr 2003; 77: 796–802. [DOI] [PubMed] [Google Scholar]
- 15.Jing H, Yao J, Liu X, Fan H, Zhang F, Li Z, Tian X, Zhou Y. Fish-oil emulsion omega-3 polyunsaturated fatty acids] attenuates acute lung injury induced by intestinal ischemia-reperfusion through Adenosine 5’-monophosphate-activated protein kinase-sirtuin1 pathway. J Surg Res 2014; 187: 252–61. [DOI] [PubMed] [Google Scholar]
- 16.Singer P, Shapiro H, Theilla M, Anbar R, Singer J, Cohen J. Anti-inflammatory properties of omega-3 fatty acids in critical illness: novel mechanisms and an integrative perspective. Intensive Care Med 2008; 34: 1580–92. [DOI] [PubMed] [Google Scholar]
- 17.Xue P, Yang Z, Wang X, Shi H. Omega-3 polyunsaturated fatty acids antagonize macrophage inflammation via activation of AMPK/SIRT1 pathway. PLoS One 2012; 7: e45990–e45990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernauer W, Ernenputsch I. Antagonistic effects of alpha-adrenoceptor blocking agents on arrhythmias, enzyme release, and myocardial necrosis in isolated rat hearts with coronary occlusion and reperfusion. Naunyn Schmiedebergs Arch Pharmacol 1988; 338: 88–95. [DOI] [PubMed] [Google Scholar]
- 19.Collins TJ. ImageJ for microscopy. Biotechniques 2007; 43: 25–30. [DOI] [PubMed] [Google Scholar]
- 20.Nebot C, Moutet M, Huet P, Xu J, Jadan JC, Chaudiere J. Spectrophotometric assay of superoxide dismutase activity based on the activated autoxidation of a tetracyclic catechol. Anal Biochem 1993; 14: 442–51. [DOI] [PubMed] [Google Scholar]
- 21. Aebi H. Catalase: methods in enzymatic analysis. Editorial Bergmeyer. New York: Academic Press, 1974.
- 22.Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol 1984; 105: 114–21. [DOI] [PubMed] [Google Scholar]
- 23.Hissin PJ, Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 1976; 74: 214–26. [DOI] [PubMed] [Google Scholar]
- 24.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95: 351–58. [DOI] [PubMed] [Google Scholar]
- 25.Zusterzeel PL, Mulder TP, Peters WH, Wiseman SA, Steegers EA. Plasma protein carbonyls in nonpregnant, healthy pregnant and preeclamptic women. Free Radical Research 2000; 364: 471–76. [DOI] [PubMed] [Google Scholar]
- 26.Carrasco-Pozo C, Speisky H, Brunser O, Pastene E, Gotteland M. Apple peel polyphenols protect against gastrointestinal mucosa alterations induced by indomethacin in rats. J Agric Food Chem 2011; 59: 6459–66. [DOI] [PubMed] [Google Scholar]
- 27.Deryckere F, Gannon FA. A one-hour minipreparation technique for extraction of DNA-binding proteins from animal tissues. Biotechniques 1994; 16: 405–405. [PubMed] [Google Scholar]
- 28.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959; 37: 911–17. [DOI] [PubMed] [Google Scholar]
- 29.Adkins Y, Kelley DS. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J Nutr Biochem 2010; 21: 781–92. [DOI] [PubMed] [Google Scholar]
- 30.Gilroy DW, Colville-Nash PR, McMaster S, Sawatzky DA, Willoughbt DA, Lawrence T. Inducible cyclooxygenase-derived 15-deoxy(Delta)12-14PGJ2 brings about acute inflammatory resolution in rat pleurisy by inducing neutrophil and macrophage apoptosis. FASEB J 2003; 17: 2269–71. [DOI] [PubMed] [Google Scholar]
- 31.Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, Toner M, Serhan CN. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J Immunol 2008; 181: 8677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tull SP, Yates CM, Maskrey BH, O’Donnell VB, Madden J, Grimble RF, Calder PC, Nash GB, Rainger GE. Omega-3 Fatty acids and inflammation: novel interactions reveal a new step in neutrophil recruitment. PLoS Biol 2009; 7: e1000177–e1000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castillo R, Rodrigo R, Pérez F, Cereceda M, Asenjo R, Zamorano J, Navarrete R, Villalabeitia E, Sanz J, Baeza C, Aguayo R. Antioxidant therapy reduces oxidative and inflammatory tissue damage in patients subjected to cardiac surgery with extracorporeal circulation. Basic Clin Pharmacol Toxicol 2011; 108: 256–62. [DOI] [PubMed] [Google Scholar]
- 34.Richard D, Oszust F, Guillaume C, Millart H, Laurent-Maquin D, Brou C, Bausero P, Visioli F. Infusion of docosahexaenoic acid protects against myocardial infarction. Prostaglandins Leukot Essent Fatty Acids 2014; 90: 139–43. [DOI] [PubMed] [Google Scholar]
- 35.von Schacky C, Harris WS. Cardiovascular risk and the omega-3 index. J Cardiovasc Med 2007; 8: S46–9. [DOI] [PubMed] [Google Scholar]
- 36.Jahangiri A, Leifert WR, Kind KL, McMurchie EJ. Dietary fish oil alters cardiomyocyte Ca2+ dynamics and antioxidant status. Free Radic Biol Med 2006; 40: 1592–602. [DOI] [PubMed] [Google Scholar]
- 37.Herrera EA, Farías JG, González-Candia A, Short SE, Carrasco-Pozo C, Castillo RL. Ω3 supplementation and intermittent hypobaric hypoxia induce cardioprotection enhancing antioxidant mechanisms in adult rats. Mar Drugs 2015; 13: 838–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castillo RL, Farias JG, Herrera EA, Alvarez PI, Short SE, Tapia L, Carrasco R, Sotomayor R. Effects of chronic intermittent hypoxia and polyunsaturated fatty acids on infarct size and oxidative stress markers in cardiac ischemia reperfusion. Exp Clin Cardiol 2014; 20: 3833–58. [Google Scholar]
- 39.Rodrigo R, Korantzopoulos P, Cereceda M, Asenjo R, Zamorano J, Villalabeitia E, Baeza C, Aguayo R, Castillo R, Carrasco R, Gormaz JG. A randomized controlled trial to prevent post-operative atrial fibrillation by antioxidant reinforcement. J Am Coll Cardiol 2013; 62: 1457–65. [DOI] [PubMed] [Google Scholar]
- 40.Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal 2013; 25: 1939–48. [DOI] [PubMed] [Google Scholar]
- 41.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res 2011; 21: 103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morishita R, Sugimoto T, Aoki M, Kida I, Tomita N, Moriguchi A, Maeda K, Sawa Y, Kaneda Y, Higaki J, Ogihara T. In vivo transfection of cis element “decoy” against nuclear factor-kappaB binding site prevents myocardial infarction. Nat Med 1997; 3: 894–99. [DOI] [PubMed] [Google Scholar]
- 43.Sawa Y, Morishita R, Suzuki K, Kagisaki K, Kaneda Y, Maeda K, Kadoba K, Matsuda H. A novel strategy for myocardial protection using in vivo transfection of cis element “decoy” against NFκB binding site. Circulation 1997; 96: II280–5. [PubMed] [Google Scholar]
- 44.Tähepõld T, Vaage J, Starkopf J, Valen G. Hyperoxia elicits myocardial protection through a nuclear factor kappaB-dependent mechanism in the rat heart. J Thorac Cardiovasc Surg 2003; 125: 650–60. [DOI] [PubMed] [Google Scholar]
- 45.Zeng M, Wei X, Wu Z, Li W, Li B, Zhen Y, Chen J, Wang P, Fei Y. NF-κB-mediated induction of autophagy in cardiac ischemia/reperfusion injury. Biochem Biophys Res Commun 2013; 436: 180–5. [DOI] [PubMed] [Google Scholar]
- 46.Chandrasekar B, Freeman GL. Induction of nuclear factor kB and activation protein-1 in postischemic myocardium. FEBS Lett 1997; 407: 30–34. [DOI] [PubMed] [Google Scholar]
- 47.Ono K, Matsumori A, Furukawa Y, Igata H, Shioi T, Matsushima K, Sasayama S. Prevention of myocardial reperfusion injury in rats by an antibody against monocyte chemotactic and activating factor/monocyte chemoattractant protein-1. Lab Invest 1999; 79: 195–203. [PubMed] [Google Scholar]
- 48.Moss NC, Stansfield WE, Willis MS, Tang RH, Selzman CH. IKKbeta inhibition attenuates myocardial injury and dysfunction following acute ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 2007; 293: H2248–53. [DOI] [PubMed] [Google Scholar]
- 49.Li C, Browder W, Kao RL. Early activation of transcription factor NF-kB during ischemia in perfused rat heart. Am J Physiol 1999; 276: H543–52. [DOI] [PubMed] [Google Scholar]
- 50.Rudolph V, Rudolph TK, Schopfer FJ, Bonacci G, Woodcock SR, Cole MP, Baker PR, Ramani R, Freeman BA. Endogenous generation and protective effects of nitro-fatty acids in a murine model of focal cardiac ischaemia and reperfusion. Cardiovasc Res 2010; 85: 155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu A, Ying Z, Gomez-Pinilla F. The salutary effects of DHA dietary supplementation on cognition, neuroplasticity, and membrane homeostasis after brain trauma. J Neurotrauma 2011; 28: 2113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Z, Lowry SF, Guarente L, Haimovich B. Roles of SIRT1 in the acute and restorative phases following induction of inflammation. J Biol Chem 2010; 285: 41391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshizaki T, Schenk S, Imamura T, Babendure JL, Sonoda N, Bae EJ, Oh DY, Lu M, Milne JC, Westphal C, Bandyopadhyay G, Olefsky JM. SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am J Physiol Endocrinol Metab 2010; 298: E419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circulat Res 2009; 105: 365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma NK, Mahadevan N, Balakumar P. Adenosine transport blockade restores attenuated cardioprotective effects of adenosine preconditioning in the isolated diabetic rat heart: potential crosstalk with opioid receptors. Cardiovasc Toxicol 13 2013; 13: 22–32. [DOI] [PubMed] [Google Scholar]
- 56.Xu B, Zhang J, Strom J, Lee S, Chen QM. Myocardial ischemic reperfusion induces de novo Nrf2 protein translation. Biochim Biophys Acta 2014; 1842: 1638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cao Z, Zhu H, Zhang L, Zhao X, Zweier JL, Li Y. Antioxidants and phase 2 enzymes in cardiomyocytes: chemical inducibility and chemoprotection against oxidant and simulated ischemia-reperfusion injury. Exp Biol Med (Maywood) 2006; 231: 1353–64. [DOI] [PubMed] [Google Scholar]
- 58.Anderson EJ, Thayne K, Harris M, Carraway K, Shaikh SR. Aldehyde stress and up-regulation of Nrf2-mediated antioxidant systems accompany functional adaptations in cardiac mitochondria from mice fed n-3 polyunsaturated fatty acids. Biochem J 2012; 441: 359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Araya J, Rodrigo R, Orellana M, Rivera G. Red wine raises plasma HDL and preserves long-chain polyunsaturated fatty acids in rat kidney and erythrocytes. Br J Nutr 2001;86:189–95. [DOI] [PubMed]