Abstract
Obesity and severe obesity constitute growing serious health problems reaching epidemic proportion in most countries. Interactions and relationships between obesity and bone tissue and its metabolism are complex but are more and more studied and recognized. Obesity is associated with an altered hormonal profile including particularly bone-regulating hormones like vitamin D. Bariatric surgery procedures, thanks to their effectiveness to achieve therapeutic endpoints for comorbidities associated with obesity, have had an increasing success. However, these surgeries by producing mechanical restriction and or malabsorption syndrome lead to nutritional deficiencies including vitamin D. In this review, we aim to (1) discuss the nutritional deficiency of vitamin D in the obese, (2) to summarize the different surgical options in bariatric surgery and to present the evidence concerning these procedures and their associated profile in vitamin D post-operative insufficiency, (3) to present the different recommendations in clinical practice to prevent or treat vitamin D deficiencies or insufficiencies in patients treated by bariatric surgery and finally to introduce emerging assumptions on the relationship between vitamin D, microbiota composition and circulating bile acids.
Impact statement
Obesity and severe obesity constitute growing serious health problems reaching epidemic proportion in most countries with a prevalence increasing from 6.4 in 1975 to 14.9% in 2014. This present review summarizes currently available data on vitamin D deficiencies in the obese population before and after bariatric surgery. The important evidence emerging from our evaluation confirms that obese patients are at risk of multiple nutritional deficiencies, especially vitamin D deficiency, before bariatric surgery. Our survey confirms that the precise role of the gut microbiome and its associated changes on the vitamin D metabolism after the different bariatric surgery procedures has not yet been studied. Furthermore, whether differences in the microbiota may alter the therapeutic responses to vitamin D is not known.
Keywords: Vitamin D, obesity, bone mineral density, bariatric surgery, bone health, endocrinology metabolism
Introduction
The continuous increase in the prevalence of overweight and obesity is a global phenomenon.1 Estimate trends in body mass index (BMI) in women have shown that age standardized prevalence of obesity increased from 6.4% in 1975 to 14.9% in 2014.2 Obesity is considered a very serious public health issue because it is associated with multiple co-morbidities3 including damage to organs as diverse as the heart, brain, liver, lungs, vessels, as well as to joints4 and bone skeleton.5
Epidemiology and definition of obesity
Overweight and obesity are defined by the World Health Organization (WHO)6 as abnormal or excessive fat accumulation that may affect health. The definition of overweight and obesity is based on the calculation of BMI, calculated by the weight in kilograms divided by height in m2. It defines respectively the overweight and obese in grade I, grade II, and grade III by a BMI of 25 to 29.9; 30 to 34.9; 35–39.9 and over 40 kg/m2. Obesity has become one of the most important health problems worldwide, according to WHO estimates, made in 2005, the projections anticipate that 2.3 billion adults will be overweight and more than 700 million clinically obese (http://www.who.int/mediacentre/factsheets/fs3a/eu/index.html, assessed June 2016).
The National Health and Nutrition Examination Survey of 2007–2008 placed the overall prevalence of adult obesity in the United States at 33.8%; with 32.2% for males and 35.5% in women.7 The prevalence of obesity (BMI (30 kg/m2) in Europe lies in the range of 10 to 20% of adult men and 15 to 25% among adult women; almost half of the European population is overweight or obese (BMI (≥25 kg/m2).8 In this concert of global epidemic, France is no slouch. A national epidemiological survey carried out by Inserm on overweight and obesity (weight and height declarative), indicated that 32.3% of French adults over 18 years would be overweight with 15% clinically obese.9 (http://www.roche.fr/content/dam/corporateroche_fr/doc/obepi-2012.pdf; carried out June 2016).
This latest study Ob-Epi-Roche conducted in 2012 estimated that approximately 6,922,000 people in France were obese, or 15.7%of women and 14.3% of men.9
In a national study carried out in 2006 and conducted in a population aged 18 to 74 years, the prevalence of obesity and overweight (weight and height measured) was, respectively, 16.9% and 32.4%.10
This present review summarizes currently available data on vitamin D deficiencies in the obese population before and after bariatric surgery. Data from the adult population are reviewed. Potential mechanisms explaining vitamin D deficiencies in obese patients and management of vitamin D deficiencies are discussed in the light of the most recent international recommendations. For this review, Pubmed articles were reviewed through 1 August 2014 by both authors using the search terms “obesity,” “vitamin D,” “bariatric surgery,” “vertical sleeve gastrectomy (VSG),” “vertical banded gastroplasty,” “Roux-en-Y gastric bypass (RYGB),” “laparoscopic adjustable gastric band (AGB),” “biliopancreatic diversion (BPD) with duodenal switch” and “bone”. References from the retrieved articles and publications in both authors library were also used.
Owing to the heterogeneity of existing evidence in this field, the results of this review have been structured as a narrative covering the following domains:
Nutritional deficiency of vitamin D in the obese.
Bariatric surgery.
Nutritional deficiency in vitamin D after bariatric surgery.
How to treat vitamin D deficiency?
We accordingly provided a data synthesis in each section.
Nutritional deficiency of vitamin D in the obese
An excess of calorific intake in connection with insufficient energy expenditure is at the origin of an imbalance of the energy balance and contributes to the occurrence of a state of positive energy balance and obesity. Despite a high calorific consumption, the associated nutritional quality is often poor, with low protein, vitamin, and mineral intake. Thus, the term malnutrition or state of “malnutrition, high calorie” has been proposed.11,12 It was established that obesity altered food absorption, metabolism, distribution and excretion; moreover, obesity affects the storage and availability of metabolic substrates.12,13
Many studies have been interested in the micronutritional status and vitamin intake of obese patients.11–17 The largest study took into consideration the case of 232 patients with morbid obesity status (BMI ≥40 kg/m2) evaluated for bariatric surgery.15 In this population, 48.7% of patients had a deficiency of vitamin D, vitamin B12, and zinc.15 Phosphate deficiency was highlighted in 5 to 10% of the patients; however, less than 5% of patients were deficient in magnesium.15 Prevalence figures as high as 80 to 90% of vitamin deficiency could be reported, the extent of the deficiency ranging from discreet to severe.18 Vitamin D deficiency is one of the most commonly occurring micronutrients in the obese population.11,13–16,19,20 The causes of vitamin D deficiency are multifactorial. Among the selected causes are (insufficient) low exposure to solar radiation,21 a reduction in the bioavailability of vitamin D in relation to sequestration (storage) of this fat-soluble vitamin in adipose tissue present in excess in case of obesity.22 This vitamin D deficiency could be linked to inadequate vitamin D intake by food and supplements despite overall high calorific intake.23 In addition, a decrease in hepatic production of 25-hydroxy vitamin D due to hepatic steatosis and a decrease in synthesis of vitamin D through the skin may also intervene.19,24 A US study showed that individuals with the most severe obesity were those whose ethnicity was African-American and those with low sun exposure appeared to be most at risk of deficiency in vitamin D.19 In this latest study, it was highlighted that for each increase of one kg/m2 of BMI, there was a decrease of 1.3 nanomole/l of serum 25 – OHD.19
Hyperparathyroidism is also common in obese subjects, and although this anomaly may be secondary to vitamin D deficiency, an independent association of vitamin D between parathyroid hormone and obesity has been reported.25,26 A mechanism of impaired calcium homeostasis in obesity has been raised to explain the high levels of PTH.27 These authors have highlighted a shift to the left of the calcium PTH curve in patients with morbid obesity resulting in a lowered threshold of the “set point” calcium for PTH response given.27
Potential role of vitamin D in obesity
Several studies reported an association between obesity and vitamin D deficiency.28,29 Recently, meta-analyses based on cross-sectional and observational studies strongly suggested that the prevalence of vitamin D deficiency was significantly different between control and obese group.29,30
Although the cross-sectional studies supported an increase in insulin sensitivity following vitamin D supplementation,31 the meta-analyses29,30 did not show any weight reduction in obese patients. Yet, intermediate biomarker of obesity demonstrated an inverse relationship between adiposity and low vitamin D levels.32 However, as highlighted by Ibero-Baraibar et al.,33 no causal relationship has been proved. Note also that, this relationship could be also altered by the volumetric dilution and sequestration of vitamin D in adipose tissue.34
A low-grade chronic inflammation through the activation of several signalling pathways could lead to the promotion of pro inflammatory cytokines as a consequence of obesity.35 Conversely, vitamin D has been recognized for its anti-inflammatory benefit on various immune cells type although not confirmed in randomized controlled trials.36
More debates are currently progressing on the relationship between cancer, obesity, and vitamin D.37 Yet, two studies including large sample of patients showed no significant relationship.38,39 However, more recently, Swami et al. explored that the breast cancer-ovariectomized mice model revealed that vitamin D played a role in decreasing the delay in tumor appearance and the progression of the mammary tumors through several signaling pathways.37
Bariatric surgery
Obesity and overweight lead to a decrease in life expectancy and reduced quality of life. European data indicate that 7.7% of all deaths are attributable to excess weight, which would lead to the figures of one death out of 13 each year which may be related with overweight.40 The risk of death at the age of 50 years, in men and women non-smokers whose BMI is greater than 40 kg/m2, is increased by 3.82 and 3.79, respectively.41 The rate of mortality among severely obese males is more than 12 times higher than that of young men of normal weight.42
Bariatric surgery has shown a tremendous growth in recent years because of the development of laparoscopic surgery on the one hand and on the other hand, the effectiveness of bariatric surgery in terms of sustained weight loss in time,12,43 regression of complications of obesity44,45 and a decrease in mortality.46,47
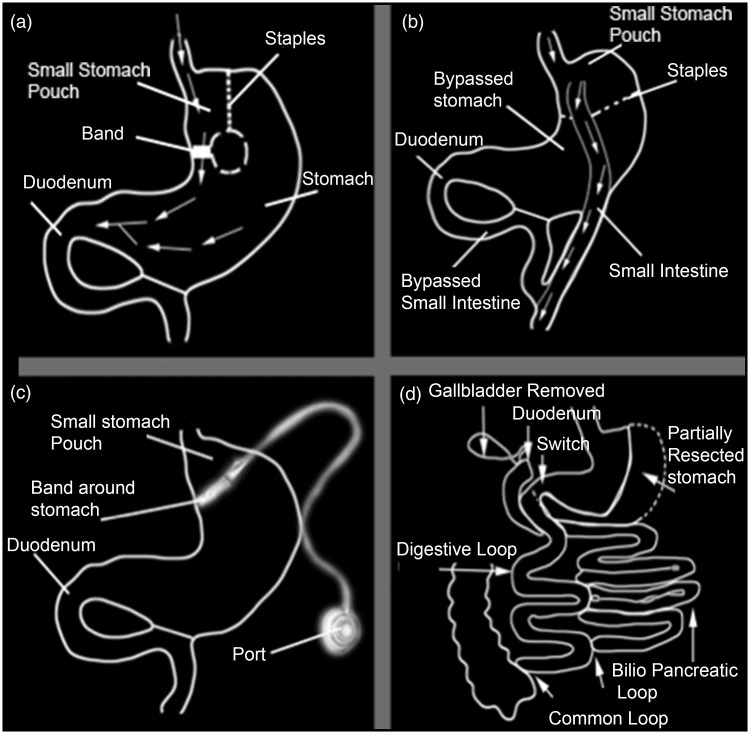
The regression of the co-morbidities associated with obesity and weight loss is a function of the type of procedure used for bariatric surgery.48 Surgical techniques are restrictive or malabsorptive by nature or even combine dietary restriction with malabsorption (Figure 1).
Figure 1.
The different types of weight loss surgery. (a) Vertical banded Gastroplasty, (b) Roux-en-Y Gastric Bypass, (c) Gastric Lap Band Surgery and (d) biliopancreatic diversion surgery
Based solely on the gastric restriction techniques, reduced food intake is achieved by reducing the stomach volume and/or delaying the emptying of the stomach. The banded adjustable gastric laparoscopy or AGB is with the vertical banded gastroplasty and the so-called longitudinal gastrectomy in sleeve or “VSG” major surgical procedures to restriction. In the event of AGB, the proximal part of the stomach is reduced (a small pocket is created) (Figure 1) which can only contain small amounts of food. In the VSG, more than 80% of the stomach is reduced (Figure 1). Nutrients quickly pass the residual stomach, leading to an alteration in intestinal hormones.
Mixed techniques associated with a gastric restriction contribute to the creation of intestinal malabsorption by creating a short circuit gastric (“gastric by-pass”) or a BPD. The “RYGB” technique, the name of the Swiss surgeon who originally performs this technique, combines restrictive and malabsorptive procedures. The restriction is associated with the creation of a small stomach pouch from the proximal part of the stomach. This pocket is anastomosed to the proximal jejunum to create a digestive tract. In this situation, the food bowl blends with the bile and secretions pancreatic in distal jejunum (Figure 1).
In the derivation BPD combined with duodenal switch, the food bowl is subject to a short circuit of most of the small intestine; the longitudinal gastrectomy is associated with an anatomose distal ileum where the food bolus mixes with digestive enzymes. In these last two procedures, the intestinal surface area available for the absorption of the food bowl and the calories is reduced, leading to malabsorption of mineral and fat-soluble vitamins including vitamin D.
Nutritional deficiency in vitamin D after bariatric surgery
Although consecutive to bariatric surgery nutritional deficiencies are well-documented,49–52 the literature emphasizes methodological weaknesses in studies addressing the phosphocalcic metabolism and bone sounding in patients who have had bariatric surgery.50,51 The limitations of the studies in terms of interpretation of data are linked most often to the weakness of samples of patients, the lack of consistency of the measurements made, out of study patients substantial rates, especially as soon as the track exceeds one year. The heterogeneity of patients studied in terms of age, gender, ethnic group, and surgical techniques is also a source of limits. Finally, in many studies, calcium and vitamin D supplementation is part of the routine clinical care and is often subject to ancillary study to the main study protocol, thus persistence and adherence to treatment with vitamin D are not evaluated.51
The type of bariatric surgery will influence the degree of weight loss, calorific deficit, and malabsorption. Thus, in the procedure known as RYGB, the longer the intestinal loop, known as the Roux loop, the shorter the common circuit, (i.e. the rest of the distal jejunum, ileum and colon), the higher the degree of malabsorption.53–55 The type and degree of vitamin deficiency and minerals are also bound to possible post-operative complications such as problems of bacterial overgrowth, food intolerance, or vomiting.
The duodenum and proximal jejunum are the main sites of absorption of calcium by passive diffusion, and on the other hand by the effects of vitamin D. Calcium deficiencies are most often the result of intolerance of patients to products containing calcium, like milk and milk products, but is also a result of the short circuit of the duodenum in the RYGB procedure.
The main causes determining vitamin D deficiency in patients after bariatric surgery are:
The initial vitamin D deficiency, prior to any surgical procedure of obese patients, already described above.
Inadequate vitamin D supplementation during rapid weight loss induced by bariatric surgery.
Bile salt deficiency associated with bariatric surgery procedures (the absorption of vitamin D requires the presence of bile salts).
Malabsorption of vitamin D sometimes due to intestinal bacterial overgrowth problems.56
The absorption of vitamin D which basically occurs next to the jejunum and ileum can be affected by the delayed blend of nutrients ingested with bile acids and pancreatic enzymes.57,58
Below, we describe the effects of the main procedures of bariatric surgery on the metabolic impact of vitamin D.
Most commonly used procedures are currently number 4: gastric ring (or AGB), the longitudinal gastrectomy or sleeve gastrectomy so-called “vertical sleeve gastrectomy (VSG), the procedure of the RYGB, the BPD with duodenal switch (Figure 1).
In the case of an AGB, it is usual to observe a weight loss in the order of 20 to 30% of the initial weight of patients59 and a loss in the range of 41 to 54% of excess weight as defined by a BMI greater than ideal, i.e. more than 25 kg/m2 weight.60 It seems that despite well-documented pre-operative vitamin D deficiency, this type of surgery does not disrupt the serum vitamin D levels that remain stable or increase, as well as the rate of PTH, which remains stable.61,62
The VSG procedure can show a weight loss in the order of 20 to 30% after two years, which equates to a loss of excess weight in the range of 45 to 64%.59,63–65 In one of the first studies that focused on the phosphocalcic metabolism in this type of procedure, it was shown that if 95% of the patients were vitamin D-deficient and had high levels of PTH in the study prior to surgery, in post-operative, 25-hydroxy vitamin D levels increased and those of PTH decreased.63 However, it should be noted that no information was available in this work concerning the use of supplementation and therapeutic adherence. A prospective study published in Spanish in patients with higher initial vitamin D found that the PTH levels did not vary and that vitamin D had increased.66
More data are available on the metabolism of vitamin D after the RYGB procedure. Typically, after this type of surgery, patients can lose up to 35% of their initial weight, which equals a loss of 62 to 75% of their excess weight.60,67–69 As early as the third month after this surgery, calcium malabsorption was observed70,71 with a reduction in the fraction of absorbed calcium.57The first studies on the effect of the RYGB on vitamin D highlighted the high rates of vitamin D deficiency.72,73 This data has led us to adopt an aggressive therapeutic attitude of vitamin D repletion. However, the observed improvements were not proportional to the proposed supplementations. For example, in a study where an increase of 200% of vitamin D intake was given (corresponding to average intakes of 658 IU per day at the beginning of study and 1698 IU per day at 12 months), vitamin concentrations remained stable.70 No increase in serum 25-hydroxy vitamin D levels was noted in a study where the daily intakes of supplementation were in the region of 5000 IU per day.74 If, respectively, lower vitamin D and higher PTH levels were observed in a study comparing the effects of the RYGB 3 aged over one year post-operative, no information on adherence to the supplementation was specified.75
The procedure for BPD associated with duodenal switch is responsible for the most weight loss and is usually reserved for patients with very severe morbid obesity (BMI ≥ 50 kg/m2). With this type of surgery, loss of excess weight can go up to 70–80%.76–78 Despite supplementation to a very good dose after this surgery, more than 50% of patients have a deficiency in vitamin D.77,79–81 Comparison of the values of 25-hydroxy vitamin D in post-operative rates in a clinical trial with the surgical procedure (RYGB versus BPD) randomization has shown deficits more pronounced in the BPD group compared to the RYGB group. In addition, the use of vitamin supplementation D was higher in the BPD versus RYGP Group.82
It has been recently reported that despite the severe vitamin D deficiency following bariatric surgery, the vitamin D supplementation proposed by guidelines83 permitted to reach wear optional levels of serum 25 (OH) D.84
How to treat vitamin D deficiency?
Serum 25-hydroxy vitamin D seems to be routinely checked before surgery.51 However, although 25-hydroxy vitamin D concentrations are lowest in obese or overweight patients or overweight than in subjects of normal or low BMI otherwise comparable, current clinical recommendations are based on the same threshold to define deficiency in vitamin D in patients of normal weight and obese subjects.84–86 The National Union of Sickness Assurance decided in January 2014 to not to authorize payment for 25-hydroxy vitamin D dosing in limited indications selected by the high authority of health (HAS). The surgical treatment of obesity in adults is one of the indications listed by the HAS.87 The proposed extension of the indications of the determination of 25-hydroxy vitamin D before and after bariatric surgery to other clinico-biological situations of mal absorption is discussed.88
Obesity defined as the presence of adult BMI 30 kg/m2 is also indicated at a dosage of 25-hydroxy vitamin D by several learned societies.89–91 In the HAS document, the quality criteria for the evaluation and improvement of practices in the case of Bariatric Surgery and support pre and post-operative patient care48 stated that the result of the determination of vitamin D should be included in the patient record. Also, stated in the same document,48 it was recommended that after malabsorptive surgery, systematic supplementation with vitamin D would be made and that supplementation should be discussed in “the clinical and biological balance” function after restrictive surgery.48
European recommendations advise biological monitoring in the case of restrictive surgery to monitor the nutritional and metabolic state on the one hand and on the other hand to prevent vitamin deficiencies and enable appropriate supplementation as necessary on a regular basis without specifying the precise rhythm.92 On the other hand, the rhythm of blood tests for determination of the 25 OHD is specified in case of gastric short circuit and BPD, respectively, on an annual basis and in 1 month, 4 months, 12 months, and then annually.92
In the past, because of the technical surgery involved, very specific bone consequences associated with deficits in vitamin D and calcium could be observed, as for example the creation of a brown tumor,93 case of osteomalacia, secondary hyperparathyroidism, but also confirmed histologically osteoporosis.94–96 The recommendations of supplementation with calcium and vitamin D in patients who are going to have bariatric surgery as well as the most recent surgical procedures now allow us to expect a better prevention of bone loss and possible fractures. The risk of fracture after bariatric surgery remains a matter of controversy; a study showed twice the risk in patients who have had bariatric surgery compared to the rates of incidence based on the general population,97 the other study showing no increase of the risk of fracture in the first two years after surgery compared to a non-operated obese population.98
There are very many recent recommendations in clinical practice focusing on patients treated with bariatric surgery84,99,100 (Table 1). While these recommendations are the subject of extreme care by their authors using all of the relevant and available data literature, certain recommended measures are not always based on medical data or evidence. This is especially true for vitamin D supplementation.
Table 1.
Pre and post-operative bone health recommendations of the post-bariatric surgery patient
| Apovian et al.86 | Endocrine society87 | AACE/TOS/ASMBS36 | |
|---|---|---|---|
| Pre-operative | Monitor for deficiencies in vitamin D and calcium If deficiencies in vitamin D: repletive doses | Monitoring and screening of vitamin D, calcium, intact PTH. Assessment of bone mineral density and composition. | No preoperative assessment of BMD by DXA outside formal clinical practice guideline by the National Osteoporosis Foundation in patients with LAGB. Appropriate nutritional evaluation including micronutrient measurements. |
| Post-operative | Monitor vitamin D, calcium, phosphorus, alkaline phosphatase levels every 6 months. DXA for bone density performed yearly until stable. | In patients with RYGB, BPD or BPD/DS, bone density measurements with use of axial (hip and spine) DXA may be indicated to monitor for osteoporosis at baseline and at about two years. | |
| Suppletive doses of vitamin D and calcium post-operatively for malabsorptive obesity surgical procedures. Doses to be adjusted by a qualified medical professional based on serum markers and measures of bone density. | Appropriate therapy for calcium and vitamin D insufficiency. Evaluation should include PTH, total calcium, phosphorus, 25-hydroxyvitamin D and 24-h urine calcium levels. |
AACE: American Association of Clinical Endocrinologists; ASMBS: American Association of Metabolic and Bariatric Surgery; BMD: bone mineral density; BPD: biliopancreatic diversion; BPD/DS: biliopancreatic diversion with duodenal switch; DXA: Dual energy X-ray absorptiometry; LAGB: laparoscopic adjustable gastric band; PTH: parathyroid hormone; RYGB: Roux-en-Y gastric bypass; TOS: The Obesity Society
The randomized trials evaluating the best diet of vitamin D in terms of effectiveness and tolerance have been worthwhile. They established that daily doses up to 800 IU are inadequate to restore good levels of vitamin D in cases of deficiency in patients who have had a procedure type RYGB.101–103 A randomized study showed that taking 50,000 IU ergocalciferol weekly gave at one year higher values of 25-hydroxy vitamin D than in the daily intake of 800 IU. Daily doses of 800, 2000, 5000 IU were given randomly to patients having also an RYGB procedure.104 In this last work, 25-hydroxy vitamin D rates increased in all patients, supplementations to 2000 and 5000 IU were associated with a greater increase of serum 25-hydroxy vitamin D. However, this work included serious limitations: small sample (n = 47), variable adherence of a group to another, many dropout of study and differences at the beginning of study for the rate of PTH and vitamin D.104 In addition, it should be noted that the variability of the required contributions from one individual to another, argues for the importance of regular monitoring of the rate of PTH, calcium and vitamin D in patients after bariatric surgery.105,106
Vitamin D deficiency on one hand and overweight or obesity on the other hand which are two possible causes of low back pain107,108 might be prevented to limit the global burden of low back pain which is associated with high disability.109 In addition, a non-linear association between vitamin D levels and obesity with mortality has been discussed highlighting the need to manage this situation.
Conclusions and perspective
Obese patients are at risk of multiple nutritional deficiencies, especially vitamin D deficiency, before bariatric surgery.
The vitamin D insufficiency is very prevalent in obese subjects. Many causes have been suggested to explain this fact, but the most important mechanism appears to be the sequestration of vitamin D in adipose tissue. The availability of vitamin D stored in the fatty tissues and the mechanisms that govern its mobilisation to the serum are still poorly elucidated.
Bariatric surgery and substantial weight losses associated with it expose even more obese patients to multiple nutritional deficiencies including vitamin D. Malabsorptive surgical procedures are associated with a higher risk of deficiency in vitamin D than those of restrictive surgery.
Revise and update recommendations from the 2008 interdisciplinary European guidelines on metabolic and bariatric surgery include 25OH Vit D3 among the laboratory tests that should be evaluated annually both after food limitations operations and after operations limiting absorption of nutriments.110
The latest recommendations of the learned companies of American Endocrinology, Obesity, and Bariatric Surgery focus on the interest of the measurement of the rate of vitamin D blood before and after bariatric surgery procedure and offer patients should be treated with 3000 IU of vitamin D daily substitution, in order to obtain 25-hydroxy vitamin D levels greater than 30 ng/ml.83
Recently, a putative role for circulating bile acids and farnesoïd-X receptor signaling (FXR) to the metabolic improvements seen after bariatric operations has been evidenced.111
In turn, both FXR signaling and modified bile acid homeostasis might be implicated in the drastic change in microbiota composition after bariatric surgery.112 The precise role of the gut microbiome and its associated changes on the vitamin D metabolism after the different bariatric surgery procedures has not yet been studied. In addition, whether differences in the microbiota may alter the therapeutic responses to vitamin D is not known.
Authors’ contributions
Both the authors contributed equally to the study concept and design and preparation of the manuscript. The authors acknowledge Mrs Nathalie Villequenault for her role in the preparation of this manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
There was no funding to support this minireview.
References
- 1.Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. WHO Technical Report Series 894, Geneva, 2000. [PubMed]
- 2.Ezzati M. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016; 387: 1377–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Prospective studies collaboration. Body-mass index and cause-specific mortality in 9000,000 adults: Collaborative analyses of 57 prospective studies. Lancet 2009; 373: 1083–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hitt HC, Mc Millen RC, Thornton-Neaves T, Koch K, Cosby AG. Comorbity of obesity and pain in a general population: Results from the Southern Pain Prevalence Study. J Pain 2007; 8: 430–6. [DOI] [PubMed] [Google Scholar]
- 5.Compston J. Obesity and fractures. Joint Bone Spine 2013; 80: 8–10. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization. Obesity and overweight. Aide-mémoire N 311 janvier 2015, www.who.int/mediacentre/factsheets/fs311/fr/index.html (accessed 1 January 2016).
- 7.Flegal KM, Sheperd JA, Looker AC, Graubard BI, Borrud LG, Ogden CL, Harris TB, Everhart JE, Schenker N. Comparisons of percentage body fat, body and mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr 2009; 89: 500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James WPT, Rigby N, Leach R. The obesity epidemic, metabolic syndrome and future prevention strategies. Eur J Cardiovasc Prev Rehabit 2004; 11: 3–8. [DOI] [PubMed] [Google Scholar]
- 9. ObEpi 2012. Enquête épidémiologique nationale sur le surpoids et l’obésité. Une enquête Inserm/Kantar Health/Roche, http://www.roche.fr/content/dam/roche_france/fr_FR/doc/obepi_2012.pdf (accessed 16 January 2016).
- 10.Vernay M, Malon A, Oleko A, Salanave B, Roudier C, Szego E, Deschamps V, Hercberg S, Castetbon K. Association of socio-economic status with overall overweight and central obesity in men and women: The French Nutrition and Health Survey 2006. BMC Pub Health 2009; 9: 215–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaidar-Person O, Person B, Szomstein S, Rosenthal RJ. Nutritional deficiencies in morbity obese patients: A new form of malnutrition? Part A: Vitamins. Obes Surg 2008; 18: 870–6. [DOI] [PubMed] [Google Scholar]
- 12.Kaidar-Person O, Person B, Szomstein S, Rosenthal RJ. Nutritional deficiencies in morbity obese patients: A new form of malnutrition? Part B: minerals. Obes Surg 2008; 18: 1028–34. [DOI] [PubMed] [Google Scholar]
- 13.Kanthakos SA. Nutritional deficiencies in obesity and after bariatric surgery. Pediatr Clin North Am 2009; 56: 1105–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaidar-Person O, Rosenthal RJ. Malnutrition in morbidity obese patients: Fact or fiction? Minerva Chir 2009; 64: 297–302. [PubMed] [Google Scholar]
- 15.Ernst B, Thurnheer M, Schmid SM, Schultes B. Evidence for the necessity to systematically assess micronutrient status prior to bariatric surgery. Obes Surg 2009; 19: 66–73. [DOI] [PubMed] [Google Scholar]
- 16.Toh SY, Zarshenas N, Jorgensen J. Prevalence of nutrient deficiencies. Nutrition 2009; 25: 1150–6. [DOI] [PubMed] [Google Scholar]
- 17.Casagrande DS, Repetto G, Mottin CC, Schneider R, Rizzolli J, Moretto M, Padoin AV, Schaan BD. Bone mineral density and nutrition profile in morbidity obese women. Obese Surg 2010; 20: 1372–9. [DOI] [PubMed] [Google Scholar]
- 18.Strohmayer E, Via MA, Yanagisawa R. Metabolic management following bariatric surgery. Mt Sinai J Med 2010; 77: 431–45. [DOI] [PubMed] [Google Scholar]
- 19.Stein EM, Strain G, Sinha N, Ortiz D, Pomp A, Dakin G, McMahon DJ, Bockman R, Silverberg SJ. Vitamin D insufficiency prior to bariatric surgery: risk factors and a pilot treatment study. Clin Endocrinol 2009; 71: 176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Signori C, Zalesin KC, Franklin B, Miller WL, McCullough PA. Effect of gastric bypass on vitamin D and secondary hyperparathyroidism. Obes Surg 2010; 20: 949–52. [DOI] [PubMed] [Google Scholar]
- 21.Compston JE, Vedi S, Ledger JE, Webb A, Gazet JC, Pilkington TR. Vitamin D status and bone histomorphometry in gross obesity. Am J Clin Nutr 1981; 34: 2359–63. [DOI] [PubMed] [Google Scholar]
- 22.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000; 72: 690–93. [DOI] [PubMed] [Google Scholar]
- 23.Hyppönen E, Power C. Hypovitaminosis D in British adults at age 45 y: Nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr 2007; 85: 860–68. [DOI] [PubMed] [Google Scholar]
- 24.Williams SE, Cooper K, Richmond B, Schauer P. Perioperative management of bariatric surgery patients: Focus on metabolic bone disease. Clev Clin J Med 2008; 75: 333–49. [DOI] [PubMed] [Google Scholar]
- 25.Grethen E, Hill KM, Jones R, Cacucci BM, Gupta CE, Acton A, Considine RV, Peacock M. Serum leptin, parathyroid hormone, 1,25-dihydroxyvitamin D, fibroblast growth factor 23, bone alkaline phosphatase, and sclerostin relationships in obesity. J Clin Endocrinol Metab 2012; 97: 1655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flores L, Osaba MJ, Andreu A, Moizé V, Rodriguez L, Vidal J. Calcium and vitamin D supplementation after gastric bypass should be individualized to improve or avoid hyperparathyroidism. Obes Surg 2010; 20: 738–43. [DOI] [PubMed] [Google Scholar]
- 27.Hultin H, Edfeldt K, Sundborm M, Hellman P. Left-shifted relation between calcium and parathyroid hormone in obesity. J Clin Endocrinol Metab 2010; 95: 3973–81. [DOI] [PubMed] [Google Scholar]
- 28.Pereira-Santos M, Costa PR, Assis AM, Santos CA, Santos DB. Obesity and vitamin D deficiency: a systematic review and metaanalysis. Obes Rev 2015; 16: 341–9. [DOI] [PubMed] [Google Scholar]
- 29.Yao Y, Zhu L, He L, Duan Y, Liang W, Nie Z, Jin Y, Wu X, Fang Y. A meta-analysis of the relationship between vitamin D deficiency and obesity. Int J Clin Exp Med 2015;8:14977–84. [PMC free article] [PubMed]
- 30.Pereira-Santos M, Costa PR, Santos CA, Santos DB, Assis AM. Obesity and vitamin D deficiency: Is there an association? Obes Rev 2016; 17: 484–484. [DOI] [PubMed] [Google Scholar]
- 31.Landrier JF, Karkeni E, Marcotorchino J, Bonnet L, Tourniaire F. Vitamin D modulates adipose tissue biology: Possible consequences for obesity? Proc Nutr Soc 2016; 75: 38–46. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez L, Ramos-Trautmann G, Diaz-Luquis GM, Perez CM, Palacios C. Vitamin D status is inversely associated with obesity in a clinic-based sample in Puerto Rico. Nutr Res 2015; 35: 287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibero-Baraibar I, Navas-Carretero S, Abete I, Martinez JA, Zulet MA. Increases in plasma 25(OH)D levels are related to improvements in body composition and blood pressure in middle-aged subjects after a weight loss intervention: Longitudinal study. Clin Nutr 2014; 34: 1010–7. [DOI] [PubMed] [Google Scholar]
- 34.Malmberg P, Karlsson T, Svensson H, Lonn M, Carlsson NG, Sandberg AS. A new approach to measuring vitamin D in human adipose tissue using time-of-flight secondary ion mass spectrometry: A pilot study. J Photochem Photobiol 2014; 138: 295–301. [DOI] [PubMed] [Google Scholar]
- 35.Enos RT, Davis JM, Velázquez KT, McClellan JL, Day SD, Carnevale KA, Murphy EA. Influence of dietary saturated fat content on adiposity, macrophage behavior, inflammation, and metabolism: composition matters. J Lipid Res 2013; 54: 152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pannu PK, Calton EK, Soares MJ. Calcium and Vitamin D in obesity and related chronic disease. Adv Food Nutr Res 2016; 77: 57–100. [DOI] [PubMed] [Google Scholar]
- 37.Swami S, Krishnan AV, Williams J, Aggarwal A, Albertelli MA, Horst RL, Feldman BJ, Feldman D. Vitamin D mitigates the adverse effects of obesity on breast cancer in mice. Endocr Relat Cancer 2016; 23: 251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kühn T, Kaaks R, Becker S, Eomois PP, Clavel-Chapelon F, Kvaskoff M, Dossus L, Tjønneland A, Olsen A, Overvad K, Chang-Claude J, Lukanova A, Buijsse B, Boeing H, Trichopoulou A, Lagiou P, Bamia C, Masala G, Krogh V, Sacerdote C, Tumino R, Mattiello A, Buckland G, Sánchez MJ, Menéndez V, Chirlaque MD, Barricarte A, Bueno-de-Mesquita HB, van Duijnhoven FJ, van Gils CH, Bakker MF, Weiderpass E, Skeie G, Brustad M, Andersson A, Sund M, Wareham N, Khaw KT, Travis RC, Schmidt JA, Rinaldi S, Romieu I, Gallo V, Murphy N, Riboli E, Linseisen J. Plasma 25-hydroxyvitamin D and the risk of breast cancer in the European prospective investigation into cancer and nutrition: a nested case-control study. Int J Cancer 2013; 133: 1689–700. [DOI] [PubMed] [Google Scholar]
- 39.Scarmo S, Afanasyeva Y, Lenner P, Koenig KL, Horst RL, Clendenen TV, Arslan AA, Chen Y, Hallmans G, Lundin E, Rinaldi S, Toniolo P, Shore RE, Zeleniuch-Jacquotte A. Circulating levels of 25-hydroxyvitamin D and risk of breast cancer: A nested case-control study. Breast Cancer Res 2013; 15: R15–R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banegas JR, Polez-Garcia E, Gutierrez-Fisac JL, Guallar-Castillón P, Rodríguez-Artalejo F. A simple estimate of mortality attributate to excess weight in the European Union. Eur J Clin Nutr 2003; 57: 201–8. [DOI] [PubMed] [Google Scholar]
- 41.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med 2006; 355: 763–78. [DOI] [PubMed] [Google Scholar]
- 42.Drenick EJ, Bale GS, Seltzer F, Johnson DG. Excessive mortality and cause of death in morbidly obese men. JAMA 1980; 243: 443–4. [PubMed] [Google Scholar]
- 43.Stefater MA, Kohli R, Inge TH. Advances in the surgical treatment of morbid obesity. Mol Aspects Med 2013; 34: 84–94. [DOI] [PubMed] [Google Scholar]
- 44.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012; 366: 1567–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, Rubino F. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012; 366: 1577–85. [DOI] [PubMed] [Google Scholar]
- 46.Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgerson J, Agren G, Carlsson LM. For the Swedish obese subjects study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007; 357: 741–52. [DOI] [PubMed] [Google Scholar]
- 47.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM, Hunt SC. Long-term mortality after gastric bypass surgery. N Engl J Med 2007; 357: 753–61. [DOI] [PubMed] [Google Scholar]
- 48.HAS – Chirurgie de l’obésité chez l’adulte – Juillet 2009, www.has-sante.fr (accessed 1 January 2016).
- 49.Soleymani T, Tejavanija S, Morgan S. Obesity, bariatric surgery, and bone. Curr Opin Rheumatol 2011; 23: 396–405. [DOI] [PubMed] [Google Scholar]
- 50.Brzozowska MM, Sainsbury A, Eisman JA, Baldock PA, Center JA. Bariatric surgery, bone loss, obesity and possible mechanisms. Obes Rev 2013; 14: 52–67. [DOI] [PubMed] [Google Scholar]
- 51.Stein EM, Silverberg S. Bone loss after bariatric surgery: Causes, consequences, and management. Lancet Diab Endocrinol 2014; 2: 165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu EW. Bone metabolism after bariatric surgery. J Bone Miner Res 2014; 29: 1507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Maria EJ. Bariatric surgery for morbid obesity. N Engl J Med 2007; 356: 2176–83. [DOI] [PubMed] [Google Scholar]
- 54.Yurcisin BM, Gaddor MM, DeMaria EJ. Obesity and bariatric surgery. Clin Chest Med 2009; 30: 539–53. [DOI] [PubMed] [Google Scholar]
- 55.Chan LN. Drug therapy-related issues in patients who received bariatric surgery (Part I). Pract Gastroenterol 2010; 85: 26–32. [Google Scholar]
- 56.Koch TR, Finelli FC. Postoperative metabolic and nutritional complications of bariatric surgery. Gastroenterol Clin North Am 2010; 39: 109–24. [DOI] [PubMed] [Google Scholar]
- 57.Riedt CS, Brolin RE, Sherrell RM, Field MP, Shapses SA. True fractional calcium absorption is decreased after Roux-en-Y gastric bypass surgery. Obesity 2006; 14: 1940–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaker JL, Norton AJ, Woods MF, Fallon MD, Findling JW. Secondary hyperparathyroidism and osteopenia in women following gastric exclusion surgery for obesity. Osteoporos Int 1991; 1: 177–81. [DOI] [PubMed] [Google Scholar]
- 59.Dixon J, Straznicky N, Lambert E, Schlaich M, Lambert G. Surgical approaches to the treatment of obesity. Nat Rev Gastroenterol Hepatol 2011; 8: 429–37. [DOI] [PubMed] [Google Scholar]
- 60.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: A systematic review and meta-analysis. JAMA 2004; 292: 1724–37. [DOI] [PubMed] [Google Scholar]
- 61.Giusti V, Gasteyger C, Suter M, Heraief E, Gaillard RC, Burkhardt P. Gastric banding induces negative bone remodelling in the absence of secondary hyperparathyroidism: Potential role of serum C telopeptides for follow-up. Int J Obes 2005; 29: 1429–35. [DOI] [PubMed] [Google Scholar]
- 62.Pugnale N, Giusti V, Suter M, Zysset E, Héraïef E, Gaillard RC, Burckhardt P. Bone metabolism and risk of secondary hyperparathyroidism 12 months after gastric bandling in obese pre-menopausal women. Int J Obes Relat Metab Disord 2003; 27: 110–16. [DOI] [PubMed] [Google Scholar]
- 63.Ruiz-Tovar J, Oller I, Priego P, Arroyo A, Calero A, Diez M, Zubiaga L, Calpena R. Short and mid-term changes in bone mineral density after laparoscopic sleeve gastrectomy. Obes Surg 2013; 23: 861–6. [DOI] [PubMed] [Google Scholar]
- 64.Huttre MM, Schirmer BD, Jones DB, Ko CY, Cohen ME, Merkow RP, Nguyen NT. First report from the American College of Surgeons Bariatric Surgery Center Network: Laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg 2011; 254: 410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Demaria EJ, Winegar DA, Pate VW, Hutcher NE, Ponce J, Pories WJ. Early postoperative outcomes of metabolic surgery to treat diabetes from sites participating in the ASMBS bariatric surgery center of excellence programas reported in the Bariatric Outcomes Longitudinal Database. Ann Surg 2010; 252: 559–66. [DOI] [PubMed] [Google Scholar]
- 66.Nogués X, Goday A, Pena MJ, Benaiges D, de Ramón M, Crous X, Vial M, Pera M, Grande L, Díez-Pérez A, Ramón JM. Bone mass loss after sleeve gastrectomy: A prospective comparative study with gastric bypass. Cir Esp 2010; 88: 103–9 (in Spanish). [DOI] [PubMed] [Google Scholar]
- 67.Schneider BE, Mun EC. Surgical management of morbid obesity. Diab Care 2005; 28: 475–80. [DOI] [PubMed] [Google Scholar]
- 68.Blackburn GL. Solutions in weight control: Lessons from gastric surgery. Am J Clin Nutr 2005; 82: 248S–52S. [DOI] [PubMed] [Google Scholar]
- 69.Everson G, Kelsberg G, Nashelsky J, Mott T. Clinical inquiries. How effective is gastric bypass for weight loss? J Fam Pract 2004; 53: 914–18. [PubMed] [Google Scholar]
- 70.Fleisher J, Stein EM, Bessler M, Della Badia M, Restuccia N, Olivero-Rivera L, McMahon DJ, Silverberg SJ. The decline in hip bone density after gastric bypass suregery is associated with extent of weight loss. J Clin Endocrinol Metab 2008; 93: 3735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coates PS, Fernstrom JD, Fernstrom MH, Schauer PR, Greenspan SL. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab 2004; 89: 1061–65. [DOI] [PubMed] [Google Scholar]
- 72.De Prisco C, Levine SN. Metabolic bone disease after gastric bypass surgery for obesity. Am J Med Sci 2005; 329: 57–61. [DOI] [PubMed] [Google Scholar]
- 73.El-Kadre LJ, Rocha PR, de Almeida Tinoco AC, Tinoco RC. Calcium metabolism in pre- and postmenopausal morbidly obese women at baseline and after laparoscopic Roux-en Y gastric bypass. Obes Surg 2004; 14: 1062–66. [DOI] [PubMed] [Google Scholar]
- 74.Stein EM, Carrelli A, Young P, Bucovsky M, Zhang C, Schrope B, Bessler M, Zhou B, Wang J, Guo XE, McMahon DJ, Silverberg SJ. Bariatric surgery results in cortical bone loss. J Clin Endocrinol Metab 2013; 98: 541–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Villarasa N, San José P, Garcia I, Gómez-Vaquero C, Miras PM, de Gordejuela AG, Masdevall C, Pujol J, Soler J, Gómez JM. Evaluation of bone mineral density loss in morbidly obese women after gastric bypass: 3-year follow-up. Obes Surg 2011; 21: 465–72. [DOI] [PubMed] [Google Scholar]
- 76.Tsiftsis DD, Mylonas P, Mead N, Kalfarentzos F, Alexandrides TK. Bone mass decreases in morbidly obese women after long limb-biliopancreatic diversion and marked weight loss without secondary hyperparathyroidism. A physiological adaptation to weight loss. Obes Surg 2009; 19: 1497–503. [DOI] [PubMed] [Google Scholar]
- 77.Hewitt S, Sovik TT, Aasheim ET, Kristinsson J, Jahnsen J, Birketvedt GS, Bøhmer T, Eriksen EF, Mala T. Secondary hyperparathyroidism, vitamin D sufficiency, and serum calcium 5 years after gastric bypass and duodenal switch. Obes Surg 2013; 23: 384–90. [DOI] [PubMed] [Google Scholar]
- 78.Feng JJ, Gagner M. Laparoscopic biliopancreatic diversion with duodenal switch. Semin Laparosc Surg 2002; 9: 125–29. [PubMed] [Google Scholar]
- 79.Newbury I, Dolan K, Hatzifotis M, Low N, Fielding G. Calcium and vitamin D depletion and elevated parathyroid hormone following biliopancreatic diversion. Obes Surg 2003; 13: 893–5. [DOI] [PubMed] [Google Scholar]
- 80.Slater GH, Ren CJ, Siegel N, Williams T, Barr D, Wolfe B, Dolan K, Fielding GA. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg 2004; 8: 48–55. [DOI] [PubMed] [Google Scholar]
- 81.Moreiro J, Ruiz O, Perez G, Salinas R, Urgeles JR, Riesco M, García-Sanz M. Parathyroid hormone and bone marker levels in patients with morbid obesity before and after biliopancreatic diversion. Obes Surg 2007; 17: 348–54. [DOI] [PubMed] [Google Scholar]
- 82.Aasheim ET, Hofso D, Sovik TT. Vitamin supplements after bariatric surgery. Clin Endocrinol 2010; 72: 134–5. [DOI] [PubMed] [Google Scholar]
- 83.Mechanick JI, Youdim A, Jones DB, Garvey WT, Hurley DL, McMahon MM, Heinberg LJ, Kushner R, Adams TD, Shikora S, Dixon JB, Brethauer S. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient-2013 update: Cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Endocr Pract 2013; 19: 337–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benhamou CL, Souberbielle JC, Cortet B, Fardellone P, Gauvain JB, Thomas T. La vitamine D chez l’adulte: Recommandations du GRIO. Presse Med 2011; 40: 673–82. [Google Scholar]
- 85.Sakhaee K, Poindexter J, Aguirre C. The effects of bariatric surgery on bone and nephrolithiasis. Bone 2016; 84: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Souberbielle JC, Body JM, Lappe JM, Plebani M, Shoenfeld Y, Wang TJ, Bischoff-Ferrari HA, Cavalier E, Ebeling PR, Fardellone P, Gandini S, Gruson D, Guérin AP, Heickendorff L, Hollis BW, Ish-Shalom S, Jean G, von Landenberg P, Largura A, Olsson T, Pierrot-Deseilligny C, Pilz S, Tincani A, Valcour A, Zittermann A. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmun Rev 2010; 9: 709–15. [DOI] [PubMed] [Google Scholar]
- 87.Souberbielle JC, Cavalier E. Supplementation, optimal status, and analytical determination of vitamin D: Where are we standing in 2013? Anticancer Agents Med Chem 2013; 13: 36–44. [PubMed] [Google Scholar]
- 88.HAS. Rapport d’évaluation technologique. Utilité clinique du dosage de la vitamine D, www.has-sante.fr/portail/upload/docs/application/pdf/2013-10/utilite_clinique_du_dosage_de_la_vitamine_d-rapport_devaluation.pdf (accessed 1 October 2013).
- 89.Souberbielle JC, Benhamou CL, Cortet B, Rousière M, Roux C, Abitbol V, Audran M, Bachetta J, Beauchet O, Blain H, Breuil V, Briot K, Brunet P, Chanson P, Cormier C, Courbebaisse M, Fardellone P, Fouque D, Friedlander G, Gauvain JB, Groussin L, Houillier P, Jacot W, Jean G, Kamenicky P, Lafage-Proust MH, Legrand E, Levy-Weil F, Linglart A, Mallet E, Marcelli C, Maruani G, Montagnon F, Personne V, Prié D, Raynaud-Simon A, Rolland Y, Salle B, Sault C, Schott AM, Thervet E, Urena-Torres P, Viard JP, Weryha G, Pierrot-Deseilligny C, Young J, Thomas T. HAS report on vitamin D measurement: don’t go from an extreme situation to another extreme situation. Press Med 2014; 4: 5–8. [DOI] [PubMed] [Google Scholar]
- 90.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96: 1911–30. [DOI] [PubMed] [Google Scholar]
- 91.Bischoff-Ferrari HA, Keller U, Burckhardt P, Quack Lötscher K, Gerber B, l’Allemand B, Laimbacher J, Bachmann M, Rizzoli R. Recommandations de la Commission fédérale de l’alimentation concernant l’apport de vitamine D. Forum Med Suisse 2012; 12: 775–8. [Google Scholar]
- 92.Rizzoli R, Boonen S, Brandi ML, Bruyère O, Cooper C, Kanis JA, Kaufman JM, Ringe JD, Weryha G, Reginster JY. Vitamin D supplementation in elderly or postmenopausal women: A 2013 update of the 2008 recommendations from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Curr Med Res Opin 2013; 4: 1–9. [DOI] [PubMed] [Google Scholar]
- 93.Fried M, Heiner V, Basdevant A, Buchwald H, Deitel M, Finer N, Greve JW, Horber F, Mathus-Vliegen E, Scopinaro N, Steffen R, Tsigos C, Weiner R, Widhalm K. Interdisciplinary European Guidelines for surgery for severe (morbid) obesity. Obes Surg 2007; 17: 260–70. [DOI] [PubMed] [Google Scholar]
- 94.Benhalima K, Mertens A, Van den Bruel A, Laga K, Vanderschueren D, Samson I, Van Damme B, Bouillon R. A brown tumor after biliopancreatic diversion for severe obesity. Endocr J 2009; 56: 263–8. [DOI] [PubMed] [Google Scholar]
- 95.Shaker JL, Norton AJ, Woods MF, Fallon MD, Findling JW. Secondary hyperparathyroidism and osteopenia in women following gastric exclusion surgery for obesity. Osteoporos Int 1991; 1: 177–81. [DOI] [PubMed] [Google Scholar]
- 96.De Prisco C, Levine SN. Metabolic bone disease after gastric bypass surgery for obesity. Am J Med Sci 2005; 329: 57–61. [DOI] [PubMed] [Google Scholar]
- 97.Goldner WS, O’Dorisio TM, Dillon JS, Mason EE. Severe metabolic bone disease as a long-term complication of obesity surgery. Obes Surg 2002; 12: 685–92. [DOI] [PubMed] [Google Scholar]
- 98.Nakamura KM, Haglind EGC, Clowes JA, Achenbach SJ, Atkinson EJ, Melton LJ, 3rd, Kennel KA. Fracture risk following bariatric surgery: A population based study. Osteoporos Int 2014; 25: 151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lalmohamed A, de Vries F, Baselier MT, Cooper A, van Staa TP, Cooper C, Harvey NC. Risk of fracture after bariatric surgery in the United Kingdom: population based, retrospective cohort study. BMJ 2012; 345: e5085–e5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Apovian CM, Cummings S, Anderson W, Borud L, Boyer K, Day K, Hatchigian E, Hodges B, Patti ME, Pettus M, Perna F, Rooks D, Saltzman E, Skoropowski J, Tantillo MB, Thomason P. Best practice updates for multidisciplinary care in weight loss surgery. Obesity 2009; 17: 871–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Heber D, Greenway FL, Kaplan LM, Livingston E, Salvador J, Still C. Endocrine and nutritional management of the postbariatric surgery patient: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2010; 39: 109–24. [DOI] [PubMed] [Google Scholar]
- 102.Goode LR, Brolin RE, Chowdhury HA, Shapes SA. Bone and gastric bypass surgery: Effects of dietary calcium and vitamin D. Obes Res 2004; 12: 40–2. [DOI] [PubMed] [Google Scholar]
- 103.Carlin AM, Rao DS, Yager KM, Parikl NJ, Kapke A. Treatment of vitamin D depletion after Roux-en-Y gastric bypass: a randomized prospective clinical trial. Surg Obes Relat Dis 2009; 5: 444–9. [DOI] [PubMed] [Google Scholar]
- 104.Da Rosa CL, Dames Olivieri Saubermann AP, Jacqueline J, Pereira SE, Saboya C, Ramalho A. Routine supplementation does not warrant the nutritional status of vitamin D adequate after gastric bypass Roux-en-Y. Nutr Hosp 2013; 28: 169–72. [DOI] [PubMed] [Google Scholar]
- 105.Goldner WS, Stoner JA, Lynden E, Thompson J, Taylor K, Larson L, Erickson J, McBride C. Finding the optimal dose of vitamin D following Roux-en-Y gastric bypass: A prospective, randomized pilot clinical trial. Obes Surg 2009; 19: 173–9. [DOI] [PubMed] [Google Scholar]
- 106.Flores L, Martinez Osaba MJ, Andreu A, Moizé V, Rodriguez L, Vidal J. Calcium and vitamin D supplementation after gastric bypass should be individualized to improve or avoid hyperparathyroidism. Obes Surg 2010; 20: 738–43. [DOI] [PubMed] [Google Scholar]
- 107.Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with persistent, non specific musculoskeletal pain. Mayon Clin Proc 2003; 78: 1463–70. [DOI] [PubMed] [Google Scholar]
- 108.Heuch I, Heuch I, Hagen K, Zwart JA. Body mass index as a risk factor for developing chronic low back pain: A follow-up in the Nord-Trondelag Health study. Spine 2013; 38: 133–9. [DOI] [PubMed] [Google Scholar]
- 109.Hoy D, March L, Brooks P, Blyth F, Woolf A, Bain C, Williams G, Smith E, Vos T, Barendregt J, Murray C, Burstein R, Buchbinder R. The global burden of low back pain: Estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014; 73: 968–74. [DOI] [PubMed] [Google Scholar]
- 110.Fried M, Yumuk V, Oppert JM, Scopinaro N, Torres AJ, Weiner R, Yashkov Y, Frühbeck G. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Facts 2013; 6: 449–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-datchary P, Myronovych A, Karns R, Wilson-Pérez HE, Sandoval DA, Kohli R, Bäckhed F, Seeley RJ. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 2014; 509: 183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Raghow R. Ménage-à-trois of bariatric surgery, bile acids and the gut microbiome. World J Diab 2015; 6: 367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]