Abstract
Background: Chondrosarcoma is one of the common malignant histologic tumors, very difficult to treat, but the concrete cause and mechanism have not yet been elucidated. The present study aimed to investigate the functional involvement of BCAR4 in chondrosarcoma and its potentially underlying mechanism. QRT-PCR and western blot were used to determine the expression of BCAR4 and mTOR signaling pathway proteins both in chondrosarcoma tissues and cells. Chondrosarcoma cell proliferation and migration were assessed by MTT assay and transwell migration assay, respectively. The expression vectors were constructed and used to modulate the expression of BCAR4 and mTOR. Chondrosarcoma xenograft mouse model was established by subcutaneous injection with chondrosarcoma cell lines. The tumor volume was monitored to evaluate the effect of BCAR4 on chondrosarcoma cell tumorigenicity. The expressions of BCAR4, p-mTOR and p-P70S6K were up-regulated in chondrosarcoma tissues and cell lines. Moreover, BCAR4 overexpression had significant promoting effect on cell proliferation and migration in chondrosarcoma cells. Furthermore, mTOR signaling pathway was epigenetically activated by BCAR4-induced hyperacetylation of histone H3. We also found that mTOR overexpression abolished the decrease of chondrosarcoma cell proliferation and migration induced by BCAR4 knockdown. In vivo experiments confirmed that BCAR4 overexpression significantly accelerated tumor growth, while the knockdown of BCAR4 significantly inhibited tumor growth. BCAR4 promoted chondrosarcoma cell proliferation and migration through activation of mTOR signaling pathway, and thus contributed to chondrosarcoma progression.
Impact statement
LncRNA BCAR4 promoted chondrosarcoma cell proliferation and migration through activation of mTOR signaling pathway, and thus contributed to chondrosarcoma progression.
Keywords: BCAR4, chondrosarcoma, proliferation, migration, mTOR signaling pathway
Introduction
Chondrosarcoma is one of the common malignant histologic tumors, very difficult to treat.1 Worldwide, the incidence of chondrosarcoma stands the third place of that of primary tumor of bone, following the multiple myeloma and osteosarcoma, and the ratio of male to female is 1.5:1.2 Recently, the five-year survival livability of patients has been improved by curative operation combined with chemotherapy, but the overall therapeutic effect is still unsatisfactory with a high fatality rate.3 The development of molecular cytogenetics and modern cytogenetics provides a new research direction for the pathogenesis of chondrosarcoma. The researches on multi-level regulation for chondrosarcoma, including the underlying transcriptional and epigenetic mechanisms, have become a more important topic.4,5 However, the concrete cause and mechanism of chondrosarcoma have not yet been elucidated.
Long non-coding RNAs (lncRNAs) are a class of nonprotein coding transcripts with more than 200 nt in length.6 Through interacting with transcriptional factors or involvement in epigenetic modification, lncRNAs regulate the expression of disease-related genes at transcriptional or posttranscriptional level.7,8 Many investigations found that lncRNAs contributed to the growth and development of malignant tumor by promoting cancer cell proliferation and migration, angiogenesis and inhibiting apoptosis.9,10 BCAR4, categorized as lncRNAs, is a novel oncogene involved in anti-oestrogen resistance in breast cancer.11 Current researches have found that BCAR4 overexpression indicated aggressiveness and prognosis of patients with breast cancer.11,12 But recent research also reported that BCAR4 was increased in osteosarcoma tissues and cells, and up-regulated BCAR4 was correlated with clinical stage and distant metastasis of patients.13 Additionally, BCAR4 also accelerated osteosarcoma progression through promoting cell proliferation and migration.14 It is therefore important to assess whether BCAR4 plays a pathogenetic role in chondrosarcoma.
It has been found that rapamycin, the inhibitor of mTOR, inhibited the growth and invasion of human chondrosarcoma cells.15 Moreover, mTOR inhibitor significantly delayed or even prevented tumor recurrence in chondrosarcoma rat model.16 Thus, it is suggested that the mechanisms uncovered in chondrosarcoma are related closely with mTOR signaling pathway. In fact, activation of mTOR signaling pathway has been considered a promoter for the genesis and development of chondrosarcoma.17 Therefore, we hypothesized that BCAR4 might be related to the pathogenic mechanism of chondrosarcoma, and the interaction between BCAR4 and mTOR signaling pathway in chondrosarcoma is also the focus in our research.
Materials and methods
Tissue collection
Chondrosarcoma tissues and matched adjacent normal tissues were obtained from 40 patients undergoing surgery at The Second Affiliated Hospital of Wenzhou Medical University, during 2009 to 2014, and informed consent was obtained from all patients. None of the patients receive any local or systemic treatment. All the tissue samples were immediately frozen in liquid nitrogen after collection and stored at −80℃. The study was approved by the Ethics Committee of The Second Affiliated Hospital of Wenzhou Medical University.
Cell culture
Three chondrosarcoma cell lines (SW1353, HS819.T, HCS-2/8) and human normal chondrocytes (C-28/I2) were purchased from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in Dulbecco Modified Eagle’s medium (DMEM) (Hyclone) supplemented with 10% fetal bovine serum (FBS) (Gibco) and 1% penicillin-streptomycin (Hyclone) at 37℃ in 5% CO2.
Western blot analysis
Total protein was extracted from chondrosarcoma cell lines or chondrocytes or tissue samples using RIPA Lysis Buffer (Beyotime Biotechnology) according to the manufacturer’s instructions. After quantification by BCA Kit (Beyotime Biotechnology), the isolated proteins (25 µg of each sample) were separated by SDS-PAGE, and then target proteins were transferred onto PVDF membrane (Invitrogen). After blocking with 5% skimmed milk, the membranes were marked with the primary antibodies, including anti-mTOR antibody (1:2000, Abcam), anti-p-mTOR antibody (1:1000, Abcam), anti-P70S6K antibody (1:5000, Abcam), anti-p-P70S6K antibody (1:500, Abcam) and anti-β-actin antibody (1:1000, Abcam), at 4℃ overnight. Then the membranes were incubated with the HRP-conjugated goat anti-rabbit IgG antibody (Abcam). Proteins on PVDF membrane were visualized using an enhanced chemiluminescence kit (Invitrogen). β-actin was used as the loading control.
RNA isolation and qRT-PCR
Total RNA was extracted from chondrosarcoma cell lines or chondrocytes or tissue samples using Trizol (Invitrogen) according to the directions of the usage of the reagent. Then total RNA was reverse transcribed into cDNA using the RNA-to-cDNA Kit (Applied Biosystems) according to the manufacturer’s instructions. Quantitative real-time PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems) in the ABI PRISM 7300 PCR system (Applied Biosystems). GAPDH was used as the control for normalization. The relative expression of BCAR4 was calculated by 2−ΔΔCt method. In the study, all specific primers were designed and synthesized by Sangon Biotech Co., Ltd. (Shanghai, China).
MTT assay
MTT assay was used to assess cell proliferation. Cells were planted in 96-well plates (Corning Costar) at 104 cells/well, and MTT solution (5 mg/ml in PBS) (Sigma) was added to each well and incubated for 4 h. After the medium was discarded, DMSO (100 µl) was added, and cellular viability was measured by absorbance of the solution at 590 nm with a microplate reader (Bio-Rad).
Transwell migration assay
Transwell chamber (Millipore) was used to perform the migration assay. The treated cells were collected by trypsinization and then seeded into the upper chamber in serum-free medium, but the lower chamber was added the medium supplemented with 10% FBS. After incubation at 37℃ with 5% CO2 for 24 h, the migrated cells were stained with crystal violet, and the number of migrated cells was counted under an inverted microscope (Olympus 600 auto-biochemical analyzer). Each experiment was performed at least three times.
Detection of the acetylation level of histone H3
Chromatin immunoprecipitation assay (ChIP) was performed with the Acetyl-Histone H3 Immunoprecipitation Assay Kit (Millipore) according to the manufacturer’s instructions. qRT-PCR was used to detect the level of immunoprecipitated DNA, and the level of product from Input was used as the internal control. The results were expressed as fold of the control. Independent experiment was conducted for five times.
Cell transfection
The expression vectors were constructed by Genechem (Shanghai) and used to modulate the expression of BCAR4 and mTOR, and empty vectors were used as the negative control. Cells were cultured in 6-well plates and transfected with expressing vectors using Lipofectamine 2000 Reagent (Invitrogen) in accordance with the manufacturer's instructions.
Tumor formation in BALB/c nude mice
Male BALB/c nude mice (4–6 weeks old and 18 ± 2 g) were purchased from the laboratory animal center of The Second Affiliated Hospital of Wenzhou Medical University. All animal experiments were in compliance with provisions of the regulation for the Care and Use of Laboratory Animals of The Second Affiliated Hospital of Wenzhou Medical University. The suspension (5 × 105 cells/ml) of HCS-2/8-pcDNA-BCAR4 cells or SW1353-si-BCAR4 cells or HCS-2/8 or SW1353 cells was prepared in phosphate buffered saline. The suspension (100 µL) was inoculated subcutaneously into the experimental mice, respectively, to establish chondrosarcoma xenograft mice model. The tumor volumes were calculated using the length (L) and width (W) measured by calipers every five days for 30 continuous days.
Statistical analysis
SPSS17.0 software system was employed to analyze the statistical analyses and to draw all graphs. All data in this study were expressed as the means ± standard deviations (SD). Differences between experimental groups were evaluated using Student’s t-test. A P value ≤ 0.05 was considered as statistically significant.
Results
Up-regulated expression of BCAR4, p-mTOR and p-P70S6K in chondrosarcoma tissues
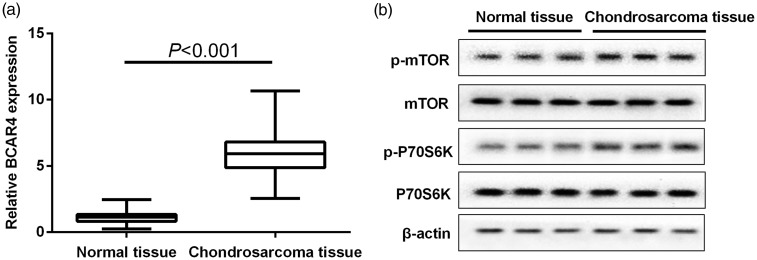
The expression of BCAR4 in chondrosarcoma tissues (n = 40) or matched adjacent normal tissues (n = 40) was quantified by qRT-PCR. As shown in Figure 1(a), BCAR4 expression in chondrosarcoma tissues was significantly higher than that of adjacent normal tissues. Furthermore, p-mTOR and p-P70S6K protein levels were increased obviously in chondrosarcoma tissues, while mTOR and P70S6K reduced correspondingly (Figure 1(b)).
Figure 1.
Up-regulated expression of BCAR4, p-mTOR and p-P70S6K in chondrosarcoma tissues. (a) The expressions of BCAR4, (b) mTOR, p-mTOR, p-P70S6K and P70S6K in chondrosarcoma tissues (n = 40) and matched adjacent normal tissues (n = 40) were quantified. GAPDH and β-actin were used as control for normalization respectively. P < 0.001 with Wilcoxon signed-rank test
Elevated expression of BCAR4, p-mTOR and p-P70S6K in chondrosarcoma cell lines
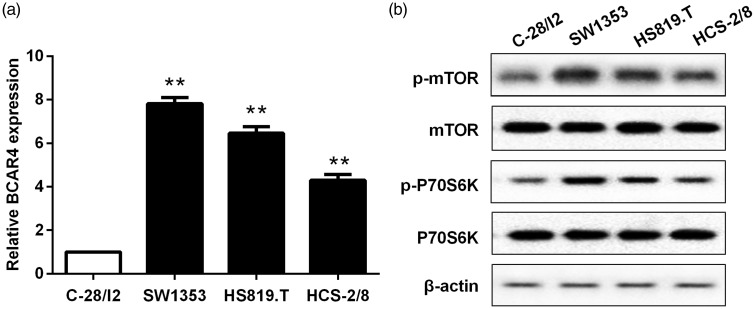
Next, endogenous expression of BCAR4 was examined in chondrosarcoma cell lines (SW1353, HS819.T and HCS-2/8) and normal chondrocytes (C-28/I2). The expression of BCAR4 was significantly elevated in chondrosarcoma cell lines, especially in SW1353 (Figure 2(a)). On the other hand, the protein expressions of p-mTOR and p-P70S6K were also enhanced in chondrosarcoma cells compared with normal chondrocytes (Figure 2(b)).
Figure 2.
Elevated expression of BCAR4, p-mTOR and p-P70S6K in chondrosarcoma cell lines. (a) The expressions of BCAR4, (b) mTOR, p-mTOR, p-P70S6K and P70S6K in chondrosarcoma cell lines (SW1353, HS819.T and HCS-2/8) were examined, with normal chondrocytes (C-28/I2) as the control. GAPDH and β-actin were used as control for normalization. respectively. **P < 0.001 vs. the control
BCAR4 promoted cell proliferation and migration of chondrosarcoma cells
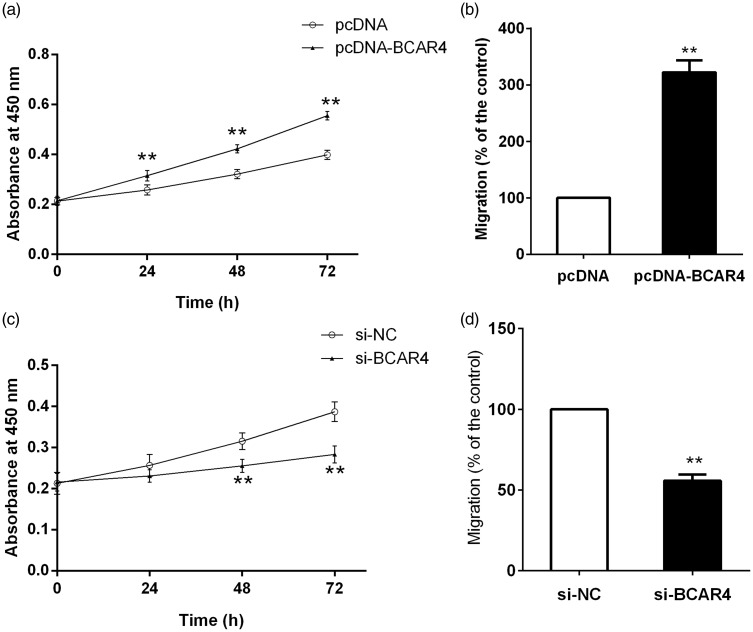
To further investigate the role of BCAR4 in chondrosarcoma cell proliferation and migration, endogenous BCAR4 expression was modulated by pcDNA-BCAR4 or si-BCAR4. It was found that BCAR4 overexpression could remarkably promote chondrosarcoma cell survival and migration (Figure 3(a) and (b)). In contrast, the survival and migration rate in chondrosarcoma cells were markedly reduced by the decrease of BCAR4 (Figure 3(c) and (d)).
Figure 3.
BCAR4 promoted cell proliferation and migration in chondrosarcoma cells. (a) Cell proliferation and (b) migration were detected in HCS-2/8 cells stably transfected with pcDNA-BCAR4 or pcDNA. (c) Cell proliferation and (d) migration were detected in SW1353 cells stably transfected with si-BCAR4 or NC. **P < 0.001
BCAR4 epigenetically activated mTOR signaling pathway in chondrosarcoma cells
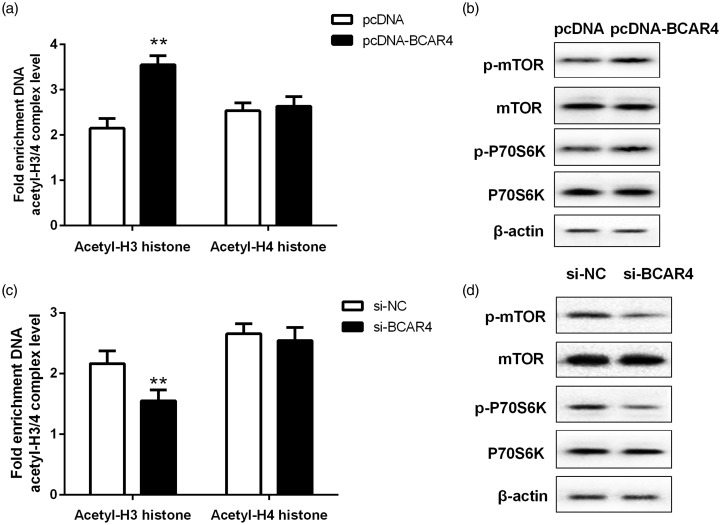
Elevated expression of BCAR4 could obviously induce hyperacetylation of histone H3 at the site of mTOR promoter (Figure 4(a)), and could also enhance the protein levels of p-mTOR and p-P70S6K (Figure 4(b)). However, we also depleted BCAR4 in SW1353 and found that the level of acetylated histone H3 in mTOR promoter was significantly decreased after BCAR4 silencing (Figure 4(c)) and was accompanied by a decrease of p-mTOR and p-P70S6K expression (Figure 4(d)).
Figure 4.
BCAR4 actived mTOR signaling pathway in chondrosarcoma cells. (a) The level of acetylated histone H3 at the site of mTOR promoter and (b) mTOR, p-mTOR, p-P70S6K and P70S6K expression were measured in HCS-2/8 cells stably transfected with pcDNA-BCAR4 or pcDNA. (c) The level of acetylated histone H3 at the site of mTOR promoter and (d) mTOR, p-mTOR, p-P70S6K and P70S6K expression were measured in HCS-2/8 cells stably transfected with si-BCAR4 or NC. **P < 0.001
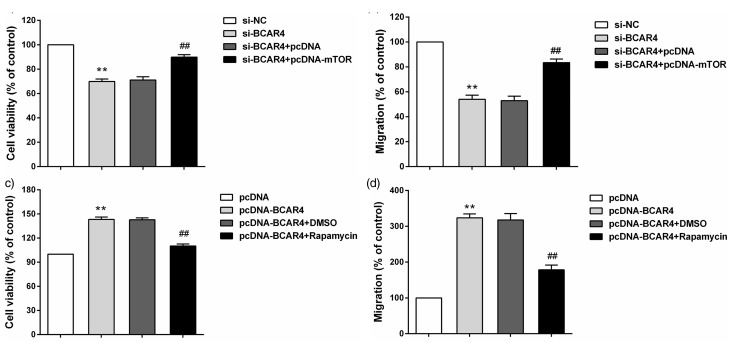
MTOR abolished the effects of BCAR4 knockdown on chondrosarcoma cell proliferation and migration
To evaluate whether the effects of BCAR4 on chondrosarcoma cell proliferation and migration were dependent on mTOR signaling pathway, we upregulated mTOR in BCAR4 stably depleted cells and inhibited mTOR in cells with BCAR4 overexpression. The results showed that mTOR overexpression released the decrease of chondrosarcoma cell proliferation and migration induced by BCAR4 knockdown (Figure 5(a) and (b)). Moreover, inhibition of mTOR could also reverse the effect of BCAR4 upregulation on chondrosarcoma cell proliferation and migration (Figure 5(c) and (d)).
Figure 5.
mTOR abolished the effects of BCAR4 knockdown on chondrosarcoma cell proliferation and migration. (a) Relative cell viability and (b) migration were measured in SW1353 cells transfected with si-BCAR4 or NC or si-BCAR4 and pcDNA or si-BCAR4 and pcDNA-mTOR. **P < 0.001 vs. si-NC, ##P < 0.001 vs. si-BCAR4 + pcDNA. (c) Relative cell viability and (d) migration were measured in HCS-2/8 cells treated with pcDNA or pcDNA-BCAR4 or pcDNA-BCAR4 and DMSO or pcDNA-BCAR4 and Rapamycin. **P < 0.001 vs. pcDNA, ##P < 0.001 vs. pcDNA-BCAR4 + DMSO
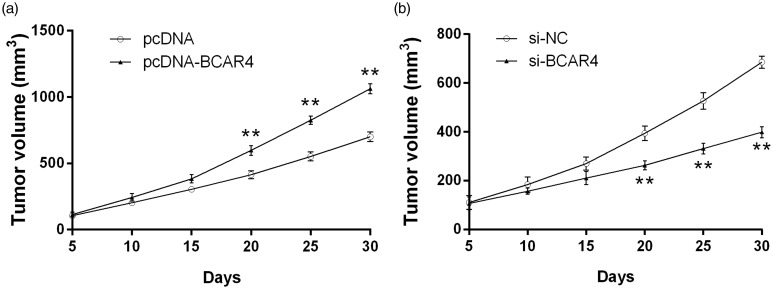
Effect of BCAR4 on chondrosarcoma cell tumorigenicity in vivo
To further investigate the biological function of BCAR4 on chondrosarcoma cell growth in vivo, cells transfected with pcDNA-BCAR4 or si-BCAR4 were injected into the nude mice, respectively. Tumor growth was continuously measured every five days for 30 days. Consistent with the expectations, BCAR4 overexpression significantly accelerated tumor growth (Figure 6(a)), while knockdown of BCAR4 significantly inhibited tumor growth (Figure 6(b)).
Figure 6.
Effect of BCAR4 on chondrosarcoma cell growth in vivo. The volume changes of subcutaneous tumors in nude mice induced by (a) CS-2/8 with BCAR4 overexpression or (b) SW1353 with BCAR4 deletion. **P < 0.001 vs. the control
Discussion
LncRNAs can exert their diverse biological functions through many mechanisms, including transcriptional regulation on target gene, post-transcriptional regulation and epigenetic regulation.18 Studies have confirmed that lncRNAs were more significantly correlated with the pathogenesis of cancer than human protein coding genes.19 Numerous studies have showed the role of lncRNAs in a wide range of human cancers,9,20 and further explorations for pathological mechanism of lncRNAs have been carried out extensively, and some results have already been made. However, there are few reports on its functional involvement and potential regulation mechanism in chondrosarcoma. The present study first identified the aberrant expression of lncRNA BCAR4 in chondrosarcoma tissues and cell lines, and it was also found that BCAR4 had significant promoting effect on cell proliferation and migration of chondrosarcoma cells, indicating its proto-oncogene role in chondrosarcoma.
BCAR4, a lncRNA with oncogenic potential, promotes breast cancer cells into an estrogen-independent and antiestrogen-resistant phenotype with rapid proliferation and progression.12 In our study, the expression of BCAR4 was significantly elevated in chondrosarcoma tissues and cell lines which suggested that up-regulated BCAR4 potentially involved in the pathogenesis of tumorigenesis and metastasis of chondrosarcoma. A recent study has found that up-regulation of BCAR4 was correlated with survival time and poor prognosis of patiens with osteosarcoma, and promoted cell invasion and metastasis.13 We identified that BCAR4 overexpression increased the proliferation and migration of chondrosarcoma cells, while the proliferation and migration were reduced after BCAR4 silencing. Collectively, this observation demonstrated that BCAR4 may serve as an oncogene and plays an important role in chondrosarcoma.
MTOR is expressed ubiquitously as a serine/threonine kinase that regulates a range of physiological activities in cells, and it is also a central regulator of many signaling pathways that responds to many physiological and pathological processes.21,22 Its function is usually mediated by the phosphorylation of its downstream target proteins, including 4E binding protein 1 (4E-BP1) and p70S6 kinase (p70S6K/S6K1) which were related to cellular processes.23,24 Additionally, the abnormal activation of mTOR signaling pathway was observed in many cancers.25 In this study, we found that the protein expressions of p-mTOR and p-P70S6K were also enhanced in chondrosarcoma tissues and cells compared with the controls, while mTOR and P70S6K reduced correspondingly. In other words, with the increase of BCAR4, mTOR signaling pathway was activated both in vitro and in vivo. Moreover, it has also been suggested that lncRNA GAS5 inhibited mTOR, and thus regulated protein synthesis, cell growth and proliferation.26 Therefore, the interaction between BCAR4 and mTOR signaling pathway in chondrosarcoma is our next research focus.
Previous studies indicated that epigenetic regulation is likely to be a key mechanism of lncRNAs in gene expression.27 Our results revealed that BCAR4 activated mTOR signaling pathway via epigenetic regulation in chondrosarcoma cells. Furthermore, mTOR overexpression reversed the decrease of chondrosarcoma cell proliferation and migration induced by BCAR4 knockdown. Also, inhibition of mTOR could also reverse the effects of BCAR4 upregulation on chondrosarcoma cell proliferation and migration. It was indicated that BCAR4 served as an oncogene to promote cell proliferation and migration in chondrosarcoma, and that in large part attributed to epigenetically activation of the mTOR signaling pathway. The results of animal model experiments further strengthened the evidence that BCAR4 promoted chondrosarcoma cell growth in vivo.
Taken together, the study indicated that high expression of BCAR4 was a prognostic factor that promoted chondrosarcoma cell proliferation and migration through activation of mTOR signaling pathway. And it also suggested that BCAR4 could be a novel marker and potential therapeutic target for chondrosarcoma.
Authors’ contributions
JK conceived of the study, and participated in its design. WL and CZ performed the experiments and collected the data. YF and YY analyzed and interpreted the data. XS wrote the article. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Xiang W, Jiang T, Guo F, Gong C, Yang K, Wu Y, Huang X, Cheng W, Xu K. Hedgehog pathway inhibitor-4 suppresses malignant properties of chondrosarcoma cells by disturbing tumor ciliogenesis. Oncol Rep 2014; 32: 1622–30. [DOI] [PubMed] [Google Scholar]
- 2.Lozano Martínez GA and Llauger RJ. Secondary chondrosarcoma: radiopathological correlation. Radiologia 2014;57:344–59. [DOI] [PubMed]
- 3.Roos E, Coevorden FV, Verhoef C, Wouters MW, Kroon HM, Hogendoorn PCW, Houdt WJV. Prognosis of primary and recurrent chondrosarcoma of the rib. Ann Surg Oncol 2016; 23: 1–7. [DOI] [PubMed] [Google Scholar]
- 4.Feinberg AP, Oshimura M, Barrett JC. Epigenetic mechanisms in human disease. Cancer Res 2002; 62: 6784–7. [PubMed] [Google Scholar]
- 5.Totoki Y, Yoshida A, Hosoda F, Nakamura H, Hama N, Ogura K, Yoshida A, Fujiwara T, Arai Y, Toguchida J. Unique mutation portraits and frequent COL2A1 gene alteration in chondrosarcoma. Genome Res 2014; 24: 1411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xin Y, Li Z, Shen J, Chan MTV, Wu WKK. CCAT1: a pivotal oncogenic long non-coding RNA in human cancers. Cell Proliferat 2016; 49: 255–260.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W, Notani D, Ma Q, Tanasa B, Nunez E, Chen AY, Merkurjev D, Zhang J, Ohgi K, Song X. Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature 2013; 498: 516–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan B, Wang ZH, Guo JT. The research strategies for probing the function of long noncoding RNAs. Genomics 2012; 99: 76–80.. [DOI] [PubMed] [Google Scholar]
- 9.Qiu JJ, Wang Y, Ding JX, Jin HY, Yang G, Hua KQ. The long non-coding RNA HOTAIR promotes the proliferation of serous ovarian cancer cells through the regulation of cell cycle arrest and apoptosis. Experiment Cell Res 2015; 333: 238–48. [DOI] [PubMed] [Google Scholar]
- 10.Weiss M, Plass C, Gerhauser C. Role of lncRNAs in prostate cancer development and progression. Biol Chem 2014; 395: 1275–90. [DOI] [PubMed] [Google Scholar]
- 11.Godinho MF, Sieuwerts AM, Look MP, Meijer D, Foekens JA, Dorssers LC, Van AT. Relevance of BCAR4 in tamoxifen resistance and tumour aggressiveness of human breast cancer. Br J Cancer 2010; 103: 1284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godinho M, Meijer D, Setyono-Han B, Dorssers LCJ, Agthoven TV. Characterization of BCAR4, a novel oncogene causing endocrine resistance in human breast cancer cells. J Cell Physiol 2011; 226: 1741–9. [DOI] [PubMed] [Google Scholar]
- 13.Ju L, Zhou YM, Yang GS. Up-regulation of long non-coding RNA BCAR4 predicts a poor prognosis in patients with osteosarcoma, and promotes cell invasion and metastasis. Eur Rev Med Pharmacol Sci 2016; 20: 4445–51. [PubMed] [Google Scholar]
- 14.Chen F, Mo J, Zhang L. Long noncoding RNA BCAR4 promotes osteosarcoma progression through activating GLI2-dependent gene transcription. Tumour Biol Journal of the International Society for Oncodevelopmental Biology & Medicine 2016;37:13403–13412. [DOI] [PubMed]
- 15.Song J, Wang X, Zhu J, Liu J. Rapamycin causes growth arrest and inhibition of invasion in human chondrosarcoma cells. J Buon 2016; 21: 244–51. [PubMed] [Google Scholar]
- 16.Perez J, Decouvelaere AV, Pointecouteau T, Pissaloux D, Michot JP, Besse A, Blay JY, Dutour A. Inhibition of Chondrosarcoma Growth by mTOR Inhibitor in an in vivo Syngeneic Rat Model. PLoS One 2012; 7: e32458.–e32458.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernstein-Molho R, Kollender Y, Issakov J, Bickels J, Dadia S, Flusser G, Meller I, Sagi-Eisenberg R, Merimsky O. Clinical activity of mTOR inhibition in combination with cyclophosphamide in the treatment of recurrent unresectable chondrosarcomas. Cancer Chemother Pharmacol 2012; 70: 855–60. [DOI] [PubMed] [Google Scholar]
- 18.Popadin K, Gutierrezarcelus M, Dermitzakis ET, Antonarakis SE. Genetic and epigenetic regulation of human lincRNA gene expression. Am J Human Genet 2013; 93: 1015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khachane AN, Harrison PM. Mining mammalian transcript data for functional long non-coding RNAs. Plos One 2009; 5: e10316–e10316.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta RA1, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, Sukumar S, Chang HY. Long noncoding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010;464:1071–6. [DOI] [PMC free article] [PubMed]
- 21.Turnquist HR, Sumpter TL, Tsung A, Zahorchak AF, Nakao A, Nau GJ, Liew FY, Geller DA, Thomson AW. IL-1beta-driven ST2L expression promotes maturation resistance in rapamycin-conditioned dendritic cells. Transplantation 2008; 86: 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon G, Marshall CA, Pappan KL, Remedi MS, Mcdaniel ML. Signaling elements involved in the metabolic regulation of mTOR by nutrients, incretins, and growth factors in islets. Diabetes 2004; 53: S225–32. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Zhu J, Cao B, Mao X. The mTOR signaling pathway is an emerging therapeutic target in multiple myeloma. Curr Pharmaceut Des 2014; 20: 125–35. [DOI] [PubMed] [Google Scholar]
- 24.Fang Y, Vilellabach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science 2001; 294: 1942–5. [DOI] [PubMed] [Google Scholar]
- 25.Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor whose frequent overexpression in multiple myeloma cells promotes their survival. Cell 2009; 137: 873–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yacqub-Usman K, Pickard MR, Williams GT. Reciprocal regulation of GAS5 lncRNA levels and mTOR inhibitor action in prostate cancer cells. Prostate 2015; 75: 693–705. [DOI] [PubMed] [Google Scholar]
- 27.Nagano T, Fraser P. Emerging similarities in epigenetic gene silencing by long noncoding RNAs. Mammalian Genome 2009; 20: 557–62. [DOI] [PubMed] [Google Scholar]