Abstract
Osteoporosis is a major public health problem associated with many factors, and it affects more than 50% of women over 50 years old. In the current study, our purpose was to investigate the effects of phosphodiestarase-5 inhibitors on osteoporosis via the nitric oxide/3′,5′-cyclic guanosine monophosphate/protein kinase G signalling pathway. A total of 50 female albino Wistar rats were separated into five groups. The first group was appointed as the healthy control group with no ovariectomy. All animals in the other groups underwent a bilateral ovariectomy. Six months after the ovariectomy, vardenafil, udenafil and tadalafil were given to the third, fourth and fifth groups, respectively, but were not administered to the positive control group (10 mg/kg per day for two months). The bone mineral density values were determined using a densitometry apparatus for all groups pre- and post-ovariectomy as well as after treatment. The levels of nitric oxide, endothelial nitric oxidesynthase, asymmetric dimethylarginine, 3′,5′-cyclic guanosine monophosphate, protein kinase G, phosphodiestarase-5, pyridinoline, deoxypyridinoline, carboxyterminal telopeptide fragments and plasma carboxy terminal propeptide of type I collagen were determined using an enzyme linked immunosorbent assay. The levels of malondialdehyde, 8-hydroxy-2-deoxy guanosine, deoxyguanosine and coenzyme Q10 were determined by a high-performance liquid chromatography assay. Additionally, the right femoral trabecular bone density and the epiphyseal plate were measured in all groups. Angiogenesis was histologically observed in the bone tissue. In addition, we determined that the inhibitors may have caused a positive impact on the increased bone mass density and reduction of bone resorption markers. We also observed the positive effects of these inhibitors on oxidative stress. In conclusion, these phosphodiestarase-5 inhibitors increase angiogenesis in bone tissue and improve the re-formation rate of bone in rats with osteoporosis.
Chemical compounds studied in this article
Udenafil (PubChem CID: 6918523); Tadalafil (PubChem CID: 110635); Vardanafil (PubCham CID: 110634).
Impact statement
The results in our study appear to establish the osteoporosis model and provide evidence of the positive effects of three separate PDE5 inhibitors (vardenafil, udenafil, and tadalafil). The positive effects of these PDE5 inhibitors are investigated and demonstrated by the bone mass density and bone resorption markers. These effects are associated with significant demonstrated antioxidant activities. Osteoporosis is a significant major public health problem especially in more aged populations. Advances in identifying and understanding new potential therapeutic modalities for this disease are significant. This study provides such an advance.
Keywords: Bone loss, tadalafil, udenafil, vardenfil, nitric oxide signalling pathway, asymmetric dimethylarginine, bone mass density, oxidative stress
Introduction
Osteoporosis is a major public health problem associated with many factors, and it affects more than 50% of women over 50 years old.1 Osteoporosis is a disease where an individual’s bone mass decreases. It is the most common reason for bone fractures, especially in postmenopausal women.2 Although there is no effective treatment for osteoporosis, there are some medications such as bisphosphonates, calcitonin, oestrogen or raloxifene that inhibit bone resorption.3 Bisphosphonates have become the dominant therapy for osteoporosis and are popular in clinical use. Although these therapeutic agents have exhibited appreciable clinical efficacy in reducing the incidence of fracture, they inhibit only bone resorption and have no effect on bone formation, which makes them not ideal for the treatment of patients with very low bone mass.4 There is clearly an urgent need for better agents for the treatment of osteoporosis.
One of the potential agents for the treatment of osteoporosis is nitric oxide (NO).5 Endogenous NO is formed from L-arginine by a reaction catalyzed by the calmodulin-dependent NO synthase (NOS) enzyme family. NOSs have three isoforms. These isoforms are neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS). There are two common pathways that mediated the effects of NO in mammalian cells: one relies on the interaction of NO with soluble guanylyl cyclase (sGC) that increases the level of 3′,5′-cyclic guanosine monophosphate/Protein kinase G (cGMP/PKG) and the second one is non-cGMP/PKG-mediated.6,7 NO has a variety of effects on different biological systems, including the inhibition of platelet aggregation, the prevention of smooth muscle proliferation, the regulation of blood flow and vascular tone and leukocyte adhesion.8,9 In addition, NO has beneficial effects on bone tissue.10,11 Therefore, NO donor compounds such as nitroglycerine and nitrates, have been used in clinical practice for several decades.10 However, the beneficial effect of NO may be limited by the induction of oxidative stress.12
Phosphodiesterase 5 (PDE5) is an enzyme that is part of the PDE class and selectively hydrolyses cGMP.13 Animal studies have shown that PDE5 is found in various tissues such as corpus cavernosum, visceral smooth muscle and skeletal muscle.14 PDE5 inhibitors act by blocking the degradative action of cGMP-specific PDE5, causing an increase in intracellular cGMP levels.15 Sildenafil is the most studied PDE5 inhibitor. We chose tadalafil, vardenafil and udenafil as PDE5 inhibitors because these inhibitors have potentially similar mechanisms of action to that of sildenafil.16 Sildenafil, vardenafil, tadalafil and udenafil are rapidly absorbed after oral administration but the half-lives of tadalafil (17.5 h) and udenafil (12.1) are longer than those of sildenafil (3.8 h) and vardenafil (3.9 h).16,17 These inhibitors are commonly used to treat erectile dysfunction. PDE5 inhibitors are also used to treat pulmonary hypertension, congestive heart failure and diabetic neuropathy.18,19 In addition, recent studies have shown that sildenafil has beneficial effects on fracture healing by enhancing bone formation in experimental animal models.20–22 This effect may be related to the beneficial effects of PDE5 inhibitors on vasodilatation,23,24 angiogenesis25,26 and the NO/cGMP/PKG signalling pathway.27
In previous studies, it has been shown that there is a positive correlation between reactive oxygen species (ROS) and osteoporosis. The decrease in bone mass and osteoporosis may occur by oxidative stress when the ROS production of the osteoclasts’ devastates the natural antioxidant defence systems.28 In previous studies, it has been shown that sildenafil reduces oxidative stress through the restraint of superoxide formation in vitro and decreases the inflammatory response by improving oxygenation in vivo.29,30
In our study, our purpose was to investigate the effects of the PDE5 inhibitors vardenafil, udenafil and tadalafil on bone tissue and oxidative stress in rats with osteoporosis via the NO/cGMP/PKG signalling pathway increasing angiogenesis. We measured the bone mass density (BMD) and levels of urine pyridinoline (PYD), deoxypyridinoline (DPD), plasma carboxy terminal propeptide of type I collagen (PICP), and carboxyterminal telopeptide fragments (CTxs) to determine the condition of osteoporosis in rats. We measured the plasma PDE5 and eNOS activities, and we also determined the levels of NO, cGMP, PKG, and asymmetric dimethylarginine (ADMA) to probe the effects of the PDE5 inhibitors on the NO/cGMP/PKG signalling pathway. We also measured the levels of malondialdehyde (MDA), coenzyme Q10 (CoQ10), 8-hydroxy-2-deoxy guanosine (8-OHdG) and deoxyguanosine (dG) to determine the oxidative damage.
Materials and methods
Chemicals
All agents and chemicals were of analytical grade and purchased from commercial suppliers. Thiobarbituric acid, 1,1,3,3-tetraethoxypropan, coenzyme Q10, dG and 8-OHdG were purchased from Sigma (T5500, T9889, C9538, 854999 and H5653, respectively, Sigma Aldrich, USA). Phosphate buffered saline and 10% formalin were purchased from Sigma (P4417 and HT501128, respectively, Sigma Aldrich, US). Osteosoft was purchased from Merck (Merck, HC 313331, made in Germany).
Animals
All animal procedures were approved by the Ethical Committee for Animal Experiments of the Yuzuncu Yil University Medical Research Centre, Van, Turkey (28 November 2013 and 2013/13 decree no). In this study, we used 50 adult female albino Wistar rats, weighing 250–300 g and born in the same week (eight months old) taken from the Yuzuncu Yil University, Experimental Animal Laboratory. These animals had never been used for breeding. The animals were randomly assigned to a sham group, the ovariectomized (OVEX) group, the OVEX + vardenafil group, the OVEX + tadalafil group and the OVEX + udenafil group. Each group and cage included 10 rats. Five cages in total were kept on a 12 h light-dark cycle with controlled temperature/humidity
Ovariectomy surgery and drug administration
During the adaptation period, the rats were fed standard commercial rat pellets (purchased from Bayramoğlu Yem, Erzurum, Turkey.). Six months before the start of the surgical procedure, the rats in the sham group underwent a laparotomy only, and the rats in the other four groups underwent total abdominal hysterectomy and bilateral ovariectomy. The rats were anesthetized with 20 mg/kg ketamine (Ketazol®, 10%/10 mL ampoules Richter Pharma, Wels, Austria) in the surgical procedure, which was injected intraperitoneally, and then a total abdominal hysterectomy and bilateral ovariectomy were performed. After six months, vardenafil (Levitra® 10 mg tablet was obtained from Bayer, Istanbul, Turkey.), tadalafil (Longis®, 10 mg tablet was purchased from Santa Pharma, Istanbul, Turkey.) and udenafil (Zydena®, 100 mg tablet was purchased from Abdi Ibrahim, Istanbul, Turkey.) were administered to the rats in the vardenafil + OVEX, tadalafil + OVEX and udenafil + OVEX groups for two months, respectively (at a dose of 10 mg/kg per day for each of the inhibitors).21 The sham and OVEX positive control groups were given a standard diet.
Samples
Urine samples (24 h) were collected from rats using metabolic cages (Kobay DH, Turkey). At the end of the eight month trial, intracardiac blood samples were taken from the rats under deep anaesthesia (5 mg xylazine + 100 mg ketamine). After the blood samples were taken, the animals were sacrificed. The blood samples for each rat were divided into two tubes with anticoagulant (ETDA). The first tubes were centrifuged at 4℃ and 3000 r/min for 10 min (plasma samples). The plasma and whole blood samples were stored at −80℃ until the study day.
Radiologic, immunohistochemical and histopathological examination
The BMD levels were determined using a dual-energy X-ray absorptiometry (DXA) instrument (Hologic® QDR-Discovery C Hologic®, Inc., Waltham, MA). Since the ex vivo bone scan needs to remove the bone tissue, the studies were performed on live intact rats. The whole body of each animal was scanned under anaesthesia before and six months after the ovariectomy as well as after the two months of drug administration.
In the histopathological examination, the trabecular bone of the femoral head tissue samples was fixed in 10% formalin, and the bone tissue was decalcified by osteosoft for 4–5 days, processed routinely, and embedded in paraffin. Then, thin sections were stained with hematoxylin and eosin for histopathologic examination. Immunohistochemical staining (IHC) was performed according to Shi et al.31 After the deparaffinization process, antigen retrieval (pH = 6.0) was applied to tissues in the microwave for 15 min. Then, to prevent endogenous peroxidase activity, sections were incubated for 10 min with a 3% H2O2 solution. The sections that were washed with phosphate-buffered saline (PBS) were incubated with the antibody CD31 (PA5-16301; Thermo Scientific, USA) for 1 h at room temperature. The sections that were washed again with PBS were stained with an Expose mouse and rabbit specific anti-polyvalent HRP detection IHC kit (CAT# TP-060-HL, Thermo Scientific, USA) as recommended by the manufacturer. Amino-ethyl carbazole (AEC) was used as a chromogen. After counterstaining with Mayer’s hematoxylin, water-insoluble glue was applied on the sections treated with water and examined using a light microscope (Leica DM1000).
Biochemical assays
The levels of urine DPD, PYD, plasma CTx, PICP, cGMP, ADMA, eNOS, PKG and PDE5 were measured using their respective commercially available ELISA (enzyme linked immunosorbent assay) kits (MyBioSource, Inc., San Diego, CA, USA). The NO levels were measured using a commercially available colorimetric assay kit (MyBioSource, Inc.). The NO and ADMA levels were expressed in µmol/L and the levels of PKG, eNOS, PDE5 CTx and PICP were expressed in ng/mL. The levels of urine PYD and DPD were expressed in nmol/mL.
The plasma MDA levels were analyzed by high-performance liquid chromatography (HPLC), as described previously.32 The MDA-thiobarbituric acid (TBA) colored complex was detected fluorometrically at an excitation wavelength of 527 nm and an emission wavelength of 551 nm. We used an Agilent 1200 series HPLC system with an autosampler, a gradient pump, a column frame, a fluorescence detector (FLD) (Agilent Technologies, Waldbronn, Germany) and a RP-C18 analytical column (150x4.6 mm, 5 µm particle size, ACE, Aberdeen Scotland). The peak of the MDA-TBA complex was calibrated using a 1,1,3,3-tetraethoxypropane standard solution, and the results were expressed as μM.
The analysis of the levels of oxidized and reduced CoQ10 (CoQ10H) were measured as described previously.33 We used an Agilent 1200 Series HPLC-ECD system with an autosampler, a gradient pump, a column frame and an electrochemical detector (ECD) (Waters, Inc., 2465 ECD, Milford, Massachusetts, USA). An octadecylsulfonate RP-Supercosil LC 18 (15 × 0.46 cm, 3 µm i.d.) analytical column (Waters, Inc.) was also used. The oxidized and total CoQ10 levels were detected using ECD at 0.35 V. CoQ10 was measured by comparing the peaks obtained from samples and the related peaks of the standard. The levels of oxidized and reduced CoQ10 were expressed in μM.
To determine 8-OHdG and dG levels, we first isolated leukocyte DNA from whole blood using a commercially available genomic DNA kit (Invitrogen, CA, USA). The DNA samples were hydrolyzed using formic acid (60%, v/v) in heat-resistant sterile tubes for 60 min at 150℃.34 The levels of 8-OHdG and dG were measured by a HPLC-ECD system and a variable wavelength detector (HPLC-UV) system, respectively, as described previously.35,36 Before measurements by HPLC, the hydrolyzed DNA samples were dissolved in the HPLC eluent (final volume: 1 mL). Twenty microliters of the final lysate were analyzed by a HPLC-ECD. A RP-C18 (RP-C18) analytical column (250 mm × 4.6 mm × 4.0 µm, Phenomenex, CA, USA) was used. The mobile phase contained 0.05 M potassium phosphate buffer at a pH of 5.5 and acetonitrile (97: 3, v/v), and the flow rate was 1 mL/min. The 8-OHdG and dG concentrations were determined based on the electrochemical reading (600 mV) and the absorbance at 245 nm, respectively. The levels of dG and 8-OHdG were quantified using standards of dG and 8-OHdG purchased from Sigma; the level of 8-OHdG is expressed as 8-OHdG molecules per 106 dG.
Statistical analysis
All data are given as a mean ± standard deviation (SD). We used the Kolmogorov–Smirnov test and equal variance F-test to investigate whether a normal distribution of data occurred. Since our data showed a normal distribution, the differences between groups were analyzed using the one-way ANOVA test. The Statistical Package for the Social Sciences (SPSS) 19.0 software Statistics was used for the statistical analyses. A P < 0.05 was considered as statistically significant.
Results
Biochemical results
We summarized the animal’s weights and the measured biochemical variables in Tables 1 and 2. There were no significant differences between the weights of rats in groups before the ovariectomy. Six months after the ovariectomy, the weights of rats in the sham group were significant higher than those in the OVEX, OVEX + vardenafil, OVEX + tadalafil, and OVEX + udenafil groups. Two months after drug administration, the weights of rats in the OVEX group were significantly lower than those in the sham, OVEX + vardenafil, OVEX + tadalafil, and OVEX + udenafil groups. The highest weights of rats were detected in the sham group. The urinary DPD level, a marker of bone resorption, in the OVEX rats was significantly higher than those in the sham, OVEX + vardenafil, OVEX + tadalafil, and OVEX + udenafil groups (P < 0.05, P < 0.005, P < 0.001 and P < 0.001, respectively) (Table 2). The urinary PYD levels, another bone resorption marker, in the OVEX + vardenafil, OVEX + tadalafil, and OVEX + udenafil groups were significantly decreased compared to those in the sham group (P < 0.05, P < 0.01, and P < 0.01, respectively) and the OVEX group (P < 0.001, P < 0.001, and P < 0.001, respectively) (Table 2). The plasma CTx level in the OVEX + tadalafil group was significantly lower than that of the OVEX group (P = 0.041). The CTx level in the OVEX group was higher than those of the sham, OVEX + vardenafil, and OVEX + udenafil groups, but the differences were not statistically significant (P > 0.05) (Table 2). When we examined bone formation by determining the level of PICP, the plasma PICP levels were significantly increased in the OVEX + vardenafil, OVEX + tadalafil, and OVEX + udenafil groups (P < 0.001). In addition, the PICP level in the sham group was significantly higher in than the OVEX group (P < 0.001) (Table 2).
Table 1.
Weights of rats in all groups
| Weight (g) (Mean ± SD) | |||
|---|---|---|---|
| Before ovariectomy BMD (g/cm2) | After six months ovariectomy BMD (g/cm2) | After treatment (g/cm2) | |
| Sham Group | 268 ± 9 | 325 ± 11 | 356 ± 13 |
| OVEX group | 271 ± 8 | 303 ± 10 | 312 ± 8 |
| OVEX + Vardenafil | 264 ± 7 | 306 ± 11 | 332 ± 11 |
| OVEX + Tadalafil | 273 ± 8 | 302 ± 10 | 324 ± 8 |
| OVEX + Udenafil | 269 ± 9 | 304 ± 9 | 339 ± 10 |
Note: All groups consumed same amount of standard rat diet.
Table 2.
Levels of the biochemical variables (mean ± SD)
| Sham | OVEX | OVEX + Vardenafil | OVEX + Tadalafil | OVEX + Udenafil | |
|---|---|---|---|---|---|
| 24 h. Urine volumes of rats | 15.8 ± 1.8a | 16.9 ± 2.5a | 13.8 ± 2.4a | 15.8 ± 2.2a | 15.3 ± 2.5a |
| PYD (nmol/day) | 2.31 ± 0.6a | 2.84 ± 0.5b | 1.55 ± 0.4c | 1.43 ± 0.2c | 1.18 ± 0.1c |
| DPD (nmol/day) | 7.09 ± 0.96a | 10.4 ± 2.7b | 5.82 ± 1.3c | 5.41 ± 1.4c | 5.44 ± 0.7c |
| PICP (ng/mL) | 75.3 ± 1.4a | 66.8 ± 0.9b | 73.3 ± 1.7a | 76.7 ± 1.4a | 78.1 ± 1.6a |
| CTx (ng/mL) | 6.15 ± 0.2a | 6.53 ± 0.3b | 6.16 ± 0.1a | 6.12 ± 0.2a | 6.14 ± 0.4a |
| eNOS (ng/mL) | 48.9 ± 0.3a | 51.7 ± 0. 6b | 53.5 ± 0.8c | 55.5 ± 0.4d | 56.55 ± 0.6d |
| PDE5 (ng/mL) | 4.77 ± 0.5a | 3.89 ± 1.1a | 2.46 ± 0.6b | 2.33 ± 0.8c | 2.27 ± 0.8d |
| NO (µmol/L) | 193.5 ± 3.9a | 219.3 ± 4.3b | 226.2 ± 5.3c | 229.7 ± 4.6d | 248 ± 5.4e |
| PKG (ng/mL) | 14.4 ± 0.3a | 14.9 ± 0.3b | 14.9 ± 0.1c | 16.4 ± 0.3d | 16.2 ± 0.2e |
| cGMP (pmol/L) | 35.5 ± 0.7a | 40.2 ± 0.5b | 41.3 ± 0.54c | 42.7 ± 0.6d | 44.2 ± 0.4e |
| ADMA(µmol/L) | 3.75 ± 1a | 2.62 ± 0.5b | 2.07 ± 0.2c | 1.45 ± 0.1d | 1.13 ± 0.2e |
| MDA (µmol/L) | 1.97 ± 0.4a | 3.8 ± 1b | 1.88 ± 0.5c | 1.95 ± 0.6a | 1.82 ± 0.4e |
| CoQ10 (µmol/L) | 0.173 ± 0.02a | 0.392 ± 0.05b | 0.213 ± 0.03c | 0.178 ± 0.03d | 0.192 ± 0.03e |
| CoQ10/CoQ10H | 0.469 ± 0.14a | 1.22 ± 0.74b | 0.622 ± 0.121c | 0.552 ± 0.07d | 0.543 ± 0.14e |
| 8-OHdG/106dG | 0.393 ± 0.172a | 1.00 ± 0.691b | 0.618 ± 0.269c | 0.526 ± 0.266d | 0.678 ± 0.09e |
Note: Different letters in the same row indicate significance between groups (P < 0.05). DPD and PYD levels were measured in urine samples. 8-OHdG and dG were measured in whole blood leucocyte samples. PICP, CTx, eNOS, PDE5, NO, PKG, cGMP, ADMA, MDA and CoQ10 levels were measured in plasma samples. PYD: pyridinoline, DPD: deoxypyridinoline, PICP: carboxyterminal propeptide of type I collagen, CTx: carboxyterminal telopeptide fragments, eNOS: endothelial nitric oxide synthase, PDE5: phosphodiesterase 5, NO: nitric oxide, PKG: protein kinase G, cGMP: cyclic guanosine monophosphate, ADMA: asymmetric dimethylarginine, CoQ10: oxidized coenzyme Q10, CoQ10H: reduced coenzyme Q10, 8-OHdG: 8-hydrocy-2′-deoxyguanosine, dG: deoxyguanosine
The PDE5 levels were decreased in the OVEX + vardenafil, OVEX + tadalafil and OVEX + udenafil groups. There was no difference between the sham and OVEX groups in terms of their PDE5 levels. We investigated the parameters of the NO/cGMP/PKG signalling pathway. The NO level in the sham group was significantly lower than for the OVEX, OVEX + vardenafil, OVEX + udenafil, and OVEX + tadalafil groups (P < 0.001). The highest NO level was found in the OVEX + udenafil group. The cGMP levels in the groups treated with the PDE5 inhibitors were significantly higher than that of the OVEX group (P < 0.001). The lowest cGMP level was detected in the sham group, and this difference was statistically significant (P < 0.001) (Table 2). We found the same results for the PKG levels. The lowest PKG level was detected in the sham group (P < 0.001). The PKG levels in the OVEX + vardenafil, OVEX + udenafil and OVEX + tadalafil groups were significantly higher than in the OVEX group (P < 0.001) (Table 2). The eNOS level in the sham group was lower than those of the OVEX, OVEX + vardenafil, OVEX + udenafil and OVEX + tadalafil groups (P < 0.001). The highest eNOS level was found in the OVEX + udenafil group. The level of ADMA, which is a physiologic eNOS inhibitor, in the sham group was significantly higher than those of the OVEX, OVEX + vardenafil, OVEX + udenafil, and OVEX + tadalafil groups (P < 0.001). The lowest ADMA level was found in OVEX + udenafil group.
In this study, we also demonstrated the status of the oxidative damage. The plasma MDA level in the OVEX group was significantly higher than those of the sham, OVEX, OVEX + vardenafil, OVEX + udenafil and OVEX + tadalafil groups (P < 0.001). The 8-OHdG/106 dG ratio in the sham group was significantly lower than for the OVEX group (P < 0.05). A higher 8-OHdG/106 dG ratio was detected in the OVEX group, and these values were statistically significantly higher than for the OVEX + vardenafil, OVEX + udenafil and OVEX + tadalafil groups (P < 0.001). The CoQ10 level in the OVEX group was significantly higher than those of the sham, OVEX + vardenafil, OVEX + udenafil, and OVEX + tadalafil groups (P < 0.001).
Results of the BMD, immunohistochemical, and histopathologic investigation
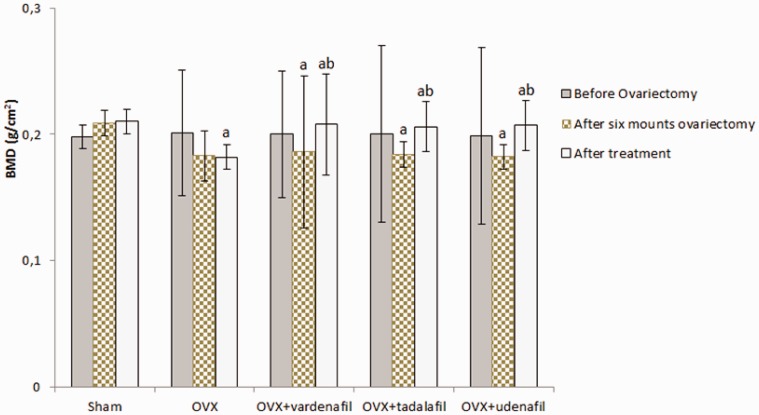
Before the ovariectomy procedures, there were no significant differences in BMD values between groups (sham group: 0.198 ± 0.009, OVEX group: 0.201 ± 0.05, OVEX + vardenafil group: 0.200 ± 0.05, OVEX + tadalafil group: 0.200 ± 0.07 and OVEX + udenafil group: 0.199 ± 0.007). Six months after the ovariectomy procedures, the BMD value in the sham group (0.209 ± 0.01) was significantly higher than those of the OVEX, OVEX + vardenafil, OVEX + tadalafil and OVEX + udenafil groups (0.183 ± 0.02, 0.186 ± 0.06, 0.184 ± 0.01 and 0.182 ± 0.01, respectively, P < 0.05). The BMD values after the treatment were significantly increased in the OVEX + vardenafil, OVEX + tadalafil, and OVEX + udenafil groups (0.208 ± 0.04, 0.206 ± 0.02 and 0.207 ± 0.02, respectively, P < 0.05) (Figure 4).
Figure 4.
The BMD values before and after an ovariectomy and after the treatments in all groups (Mean±SD)
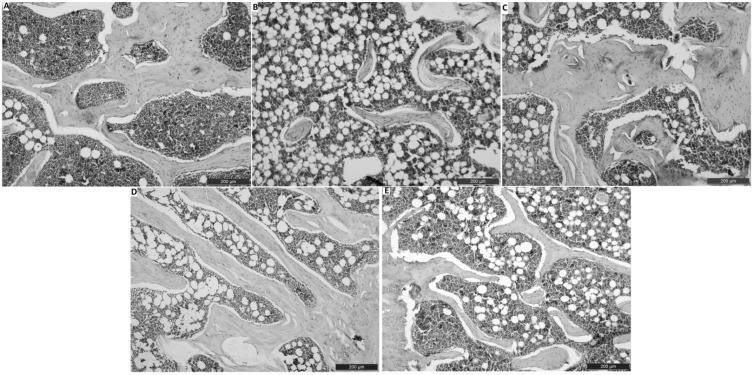
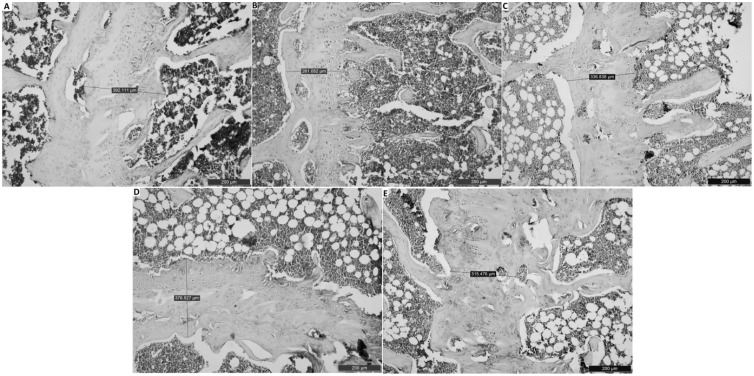
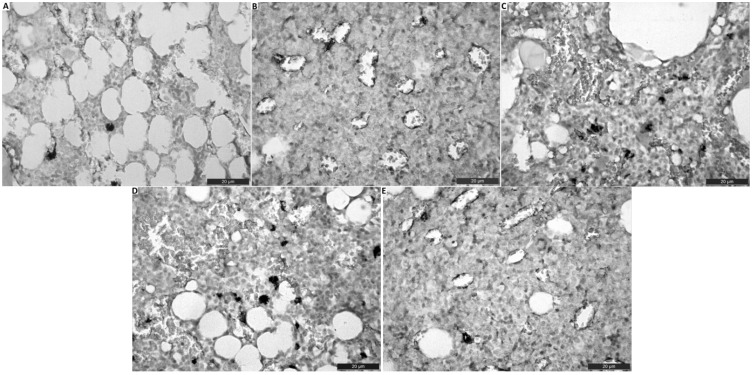
In our histopathologic examination, we detected normal histopathological architecture of the right femur in the sham group (Figures 1(a) and 2(a)). In addition, in this group, normal vascular structure was found (Figure 3(a)). In the OVEX group, atrophy in the epiphyseal bone and a degenerative cartilage layer were detected and the trabecular bone was determined to be insufficient and thin (Figures 1(b) and 2(b)). A slightly amount of angiogenesis was identified in this group (Figure 3(b)). In the OVEX + vadanefil group, there was no pathological condition found in the trabecular bone. In addition, images of the trabecular bone density and epiphyseal bone volume were very similar to those of the sham group (Figures 1(c) and 2(c)). There was significantly increased angiogenesis in the OVEX + vardenafil group (Figure 3(c)). In the OVEX + tadalafil group, the histopathological results were almost the same as for the OVEX + vardenafil group (Figures 1(d), 2(d) and 3(d)). In the OVEX + udenafil group, there was slight epiphyseal bone atrophy and thinning of the trabecular bone, but there was increased angiogenesis (Figures 1(e), 2(e), and 3(e)). The trabecular bone density, epiphyseal bone plate and angiogenesis scores are shown in Table 3.
Figure 1.
Histological images in the trabecular bone of the right femur head. The histological staining in the sham group (A), the OVX group (B), the OVX+vardenafil group (C), the OVX+tadalafil group (D), the OVX+udenafil group (E). The histological staining in the OVX+vardenafil, OVX+tadalafil and OVX+udenafil groups were more normal than that in the OVX group. Scale bar 200 µm (A-E)
Figure 2.
Histological images in the epiphyseal plate of the right femur. The histological staining in the sham group (A), the OVX group (B), the OVX+vardenafil group (C), the OVX+tadalafil group (D), the OVX+udenafil group (E). The histological staining in the OVX+vardenafil, OVX+tadalafil and OVX+udenafil groups were more normal than that in the OVX group. Scale bar 200 µm (A-E)
Figure 3.
Effect of PDE5 inhibitors on vasculogenic formation of right femur. (A)Histological staining in the sham group (A), the OVX group (B), the OVX+vardenafil group (C), the OVX+tadalafil group (D), the OVX+udenafil group (E). Effect of PDE5 inhibitors, especially udenafil on angiogenesis were much better than that of OVX group. Scale bar 20 µm
Table 3.
A comparative categorical evaluation results in trabecular bone density, epiphyseal bone plate and angiogenesis
| Trabecular bone density | Epiphyseal bone plate | Angiogenesis | |
|---|---|---|---|
| Sham | ++++ | ++++ | ++ |
| OVEX | + | + | +++ |
| OVEX + Vardenafil | ++++ | ++++ | ++++ |
| OVEX + Tadalafil | +++ | ++++ | ++++ |
| OVEX + Udenafil | +++ | +++ | +++ |
Note: The intensity of staining was scored as no staining (−), low (+), moderate (++), marked (+++), severe (++++).
Discussion
Osteoporosis is the most common human metabolic bone disease.10 Yet, there is no effective cure for osteoporosis. During the past two decades, novel therapeutic agents have emerged for the treatment of osteoporosis.
One of the potential agents is NO.5 There are many studies that demonstrate the beneficial effects of NO on bones.11 The importance of NO for the skeleton is revealed by the use of NO donors such as nitrates and nitroglycerin.11,37 However, NO is also very reactive and can act like a free radical that leads to oxidative stress. This may be a limitation of the use of NO donors to treat osteoporosis. cGMP is a cyclic nucleotide derived from guanosine triphosphate, and it acts as a secondary messenger. Its likely mechanism of the action is activation of intracellular PKG. In some studies, authors have reported that cGMP did not mediate the effects of NO on bone cells,38 but overwhelming evidence suggests otherwise.11,39 NO also has a pro-angiogenic effect via the cGMP/PKG signalling pathway.40 One of the positive effects of NO on bone may be an increase in angiogenesis because angiogenesis, which is the formation of new blood vesicles, is directly related to bone formation and healing. In the current study, our purpose was to investigate the beneficial effects of NO on bone via the cGMP/PKG signalling pathway by inhibiting PDE5 and determine the level of angiogenesis under this condition. This is the first research in the literature that examines the effects of vardenafil, udenafil, and tadalafil on the NO/cGMP/PKG signalling pathway and angiogenesis in osteoporosis and their relationship with oxidative damage.
In research on postmenopausal osteoporosis, the ovariectomized rat model is most commonly used.41 Thus, we choose this surgical procedure. First, we determined whole body BMD because the measurement of the BMD by DXA is the clinical basis for the diagnosis of osteoporosis.42 We measured the BMD levels in the rats before the ovariectomy procedures and did not find significant differences among the groups. Six months after the ovariectomy procedures, there was no markedly change in the sham group, but the BMD levels were reduced by 10–15% in the ovariectomized rats (Table 2). We determined the levels of urinary DPD, urinary PYD, and plasma CTx, which are bone resorption biomarkers, as well as plasma PICP level, which is a bone formation biomarker.43 The urinary PYD and DPD levels in the OVEX group were higher than those of the sham group. The plasma CTx level in the OVEX group was also significantly higher than that of the sham group. The plasma PICP level in the OVEX group was markedly lower than that of the sham group. Studies in the literature have found similar findings as our study.44–46 Additionally, in our histopathological investigation, it was revealed that the OVEX group had greater epiphyseal bone atrophy and a degenerative cartilage layer than the sham group (Figures 1 and 2). These data confirmed the established ovariectomy-induced osteoporosis model.
In the present study, we administered vardenafil, udenafil, and tadalafil to rats with osteoporosis for two months. After the administration of the inhibitors, we measured the levels of NO, cGMP, ADMA, eNOS, PKG, and PDE5. The PDE5 levels in the OVEX + vardenafil, OVEX + udenafil, and OVEX + tadalafil groups were significantly lower than in both the sham and OVEX groups. These data were very important for us because some studies have reported that PDE5 inhibitors did not reduce PDE5 activity. De Young et al.47 reported that the PDE5 level in the sildenafil group was higher than those of the control and vitamin E groups. This result may be associated with negative feed-back inhibition. In our study, PDE5 was successfully inhibited by the inhibitors.
In the post-menopausal period and during ovariectomy-induced osteoporosis, low oestrogen production causes an increased rate of bone resorption with bone loss particularly in the trabecular bone compartment.48 Oestrogen increases NO production by stimulating eNOS activity via the nongenomic action of the oestrogen receptors.49 In many studies, scientists have reported that NO mediates some of the effects of oestrogen on bone tissue.10,11,39. Armour et al.50 reported that eNOS-deficient mice have exaggerated bone loss after an ovariectomy. In addition, some authors have reported that NO levels increase during osteoporosis. Higashino et al.51 reported that the NO levels in individuals with various diseases such as diabetes mellitus and osteoporosis are higher than the NO levels of healthy control subjects. In our study, our findings were similar to these studies. The NO level in the OVEX group was higher than in the sham group. The elevated NO level was most likely associated with the physiological response. In addition, the NO levels in the OVEX + vardenafil, OVEX + udenafil, and OVEX + tadalafil groups were significantly higher than the NO levels in both the sham and OVEX groups. These results show that the PDE5 inhibitors contributed to the increase the NO concentration as part of the physiological response. The eNOS level in the OVEX group was also significantly higher than in the sham group. We determined that the eNOS levels in the OVEX + vardenafil, OVEX + tadalafil and OVEX + udenafil groups were significantly increased. Musicki et al.52 reported that sildenafil increased phosphorylation on Ser-1177, leading to the promotion of eNOS activity. Vardenafil, udenafil, and tadalafil probably have the same effect and increased eNOS activity in PDE5 inhibitor-treated rats. In our study, the cGMP and PKG levels in the treated groups were significantly higher than those of the sham group. In the OVEX + vardenafil, OVEX + udenafil, and OVEX + tadalafil groups, the cGMP and PKG levels were increased. These results were expected because many studies have demonstrated that PDE5 inhibitors increased the levels of cGMP and PKG.53
ADMA is an endogenous inhibitor of eNOS and some authors have proposed that ADMA negatively affects osteoblast differentiation.54,55 Gulhan et al.56 reported that there was not a significant difference between the ADMA levels in postmenopausal women with osteoporosis and postmenopausal women with non-osteoporosis. However, this study did not include a healthy control group. In our study, we determined that the ADMA level in the sham group was significantly higher than those of the OVEX, OVEX + vardenafil, OVEX + tadalafil, and OVEX + udenafil groups. In addition, the ADMA level in the OVEX group was significantly higher than those of the OVEX + vardenafil, OVEX + tadalafil, and OVEX + udenafil groups. The lowest ADMA level was found in the OVEX + udenafil group. This interesting data may explain why the eNOS levels were increased in the OVEX, OVEX + vardenafil, OVEX + tadalafil and OVEX + udenafil groups.
These changes in the NO/cGMP/PKG signalling pathway may be useful to bone tissue. Rangaswami et al.57 reported that mechanical stimulation induces osteoblast proliferation via the NO/cGMP/PKG signalling pathway. A study by Mancini et al.58 showed that cGMP-elevating agents may promote osteoblast differentiation. Histing et al.20 showed that sildenafil accelerates fracture healing, and the accelerated bone healing, including faster bone bridging in sildenefil-treated animals, may be caused by cysteine-rich 61. Kilic et al.22 showed that sildenafil citrate does not have a significant effect in the inflammatory phase, whereas it significantly accelerates bone healing in the repair phase. They also reported that this positive effect may be related to the beneficial effects of PDE5 inhibitors on vasodilatation and angiogenesis. In contrast to these studies, Gong et al.59 showed that inhibition of PDE5 decreases bone mass by inhibition of canonical Wnt signalling. Gong et al. have evaluated the effects of high doses of tadalafil (45 and 75 mg/kg daily) on osteoblastogenesis and the Wnt/β-cat signal in wild-type adult (two months) C57BL/6 mice and two-month-old SFRP1−/− knockout mice in vivo and reported that tadalafil significantly reduced the cancellous bone mass but not the cortical bone mass. In addition, Gont et al. have shown that tadalafil and vardenafil induce cGMP-dependent PKG2 and thereby reduce osteoblastic differentiation in 293T and C3H10T1/2 cell cultures in vitro. These results have shown that the use of high doses of PDE5 inhibitors may cause a decrease in bone mass.
Our study had similar findings with previous studies.20–22,57,58 The BMD levels in the OVEX + vardenafil, OVEX + udenafil, and OVEX + tadalafil groups were significantly higher after treatment than before treatment. This increase was approximately 10–11% for the treatment groups. When we examined the bone turnover parameters, we detected the positive effects of these inhibitors. The urinary DPD and PYD levels in the OVEX group were significantly higher than for the OVEX + vardenafil, OVEX + udenafil, and OVEX + tadalafil groups. The lowest PYD level was found in the OVEX + udenafil group. The plasma CTx level in the OVEX group was significantly higher than those of the OVEX + vardenafil, OVEX + udenafil, and OVEX + tadalafil groups. The lowest CTx level was found in the in OVEX + tadalafil group. The plasma PICP level in the OVEX group was significantly lower than those of the OVEX + vardenafil, OVEX + udenafil, and OVEX + tadalafil groups. The highest PICP level was found in the OVEX + udenafil group. In our histological investigation, we determined that the trabecular bone architecture and the epiphyseal bone volume in the OVEX + tadalafil group were very close to those of the sham group. Although we saw slight epiphyseal bone atrophy and thinning of the trabecular bone in the OVEX + udenafil group, the general bone architecture was better than for the OVEX group. In the OVEX + vardenafil group, we obtained similar histological results as for the OVEX + tadalafil group. We found that angiogenesis increased in all inhibitor-treated groups. The angiogenic effects of these inhibitors are very important because angiogenesis is essential for nutrition and the removal of metabolic waste in defective or normal tissue.
We also determined the oxidative damage status of each group. We measured the MDA and CoQ10 levels as well as the leukocyte 8-OHdG/106 dG ratio to investigate oxidative damage. MDA levels are widely used to assess lipid peroxidation.60 The leukocyte 8-OHdG/106 dG ratio serves as a sensitive biomarker of oxidative DNA damage.61 CoQ10 is an electron transporter in the electron transport chain (ETC). The main task of CoQ10 is to transport electrons between nicotinamide dinucleotide and succinate dehydrogenase in the ETC. CoQ10H (reduced form of CoQ10) has an antioxidant effect.62 Due to the antioxidant effect of CoQ10H, the CoQ10/CoQ10H ratio can be considered a marker to determine oxidative damage.63 There is a very close relationship between osteoporosis and oxidative stress. ROS have been related to bone loss, but the mechanisms and key players involved are uncertain. In recent years, some studies showing the relationship between osteoporosis and NADPH oxidase 4 (NOX4) have been performed.64,65 The NOX4 is a constitutively active enzymatic source of ROS. Wilson64 reported that the inhibition of NOX4 holds potential to attenuate bone loss in osteoporosis. Goettsch et al.65 reported that in vivo blockage of NOX4 activation prevented bone loss in mice, and increased bone density was detected in NOX4-knockout mice. These studies have shown that the inhibition of some enzymes such as NOX4 that are ROS sources or some molecules that have antioxidant effects, may have beneficial effects in preventing bone loss. Some studies have been performed that indicate that PDE5 inhibitors have beneficial effects on oxidative stress. Al-Amin et al.66 reported that tadalafil decreased the levels of MDA and NO in young and old rats. Fan et al.67 reported that vardenafil treatment did not change the MDA level but increased superoxide dismutase (SOD) activity in patients with pulmonary arterial hypertension. Ozgur et al.68 reported that udenafil decreased the MDA level in ischemia-reperfusion injury caused by rat testicular torsion/detorsion. Savaş et al.69 reported that tadalafil decreased the total oxidant status (TOS) and increased the total antioxidant status (TAS) in men with erectile dysfunction. In the present study, we determined the MDA levels in our groups, and our results were similar to previous studies. The MDA levels in the OVEX + tadalafil, OVEX + udenafil, and OVEX + vardenafil groups were significantly lower than in the OVEX group. There were no statistically significant differences between the sham and inhibitor-administered groups. The 8-OHdG/106 dG and CoQ10/CoQ10H ratios in the OVEX + tadalafil, OVEX + udenafil, and OVEX + vardenafil groups were significantly lower than those of the OVEX group. The 8-OHdG/106 dG ratio in the sham group was significantly lower than those of the inhibitor-administered groups. These data showed that vardenafil, tadalafil, and udenafil may have an antioxidant effect in rats with ovariectomy-induced osteoporosis. However, further studies are needed that demonstrate the relationship between PDE5 inhibitors and cytokines such as RANKL and interleucin-6, ROS production, and NOX4 activity.
The results of this study indicate that vardenafil, tadalafil, and udenafil have a positive effect on BMD in ovariectomy-induced osteoporosis and have an antioxidant effect. The NO/cGMP/PKG signalling pathway may mediate this positive effect. In addition, these inhibitors increase angiogenesis. As a result, PDE5 inhibition may be useful for bone remodeling and the treatment of osteoporosis. Future studies are needed that evaluate osteoblast apoptosis factors such as Bax, Bcl2, caspase 3 and 9, Dkk-1, and teriparatide; bone morphogenic proteins 2 and, 4; and Wnt/β-catenin signalling pathways, which affect the BMD. However, their doses should be adjusted correctly.
Authors’ contribution
HHA researched literature and conceived the study, performed biochemical measurements and, data analysis, and wrote the first draft paper. ZH was involved in animal experimental work and gaining ethical approval. SY and YB were performed the surgical protocol and histological investigation. LE helped in the clinical osteoporosis assessment of data. MRŞ analyzed all data. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Acknowledgements
We thank Dr. Abdulsamet Batur for help with the dual-energy X-ray absorptiometry (DXA) instrument. This work was supported by the Research Fund of the Yüzüncü Yıl University. Project number: 2014-TF-B068.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
The study protocol was approved by The Local Ethics Committee for Animal Experiments of Yuzuncu Yil University (28 November 2013 and 2013/13 decree no).
Guarantor
HHA
References
- 1.Bernabei R, Martone AM, Ortolani E, Landi F, Marzetti E. Screening, diagnosis and treatment of osteoporosis: a brief review. Clin Case Mineral Bone Metab: the official journal of the Italian Society of Osteoporosis, Mineral Metabolism, and Skeletal Diseases 2014; 11: 201–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson HD, Helfand M, Woolf SH, Allan JD. Screening for postmenopausal osteoporosis: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002; 137: 529–41. [DOI] [PubMed] [Google Scholar]
- 3.Siris ES, Pasquale MK, Wang YT, Watts NB. Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older, 2001–2008. J Bone Mineral Res 2011; 26: 3–11. [DOI] [PubMed] [Google Scholar]
- 4.Epstein PM. Bone and cAMP Signalling Pathway: Emerging Therapeutics. In: Bone – Metabolic Functions and Modulators. 7th ed. London: Springer, 2012, pp. 271–287.
- 5.van't Hof RJ, Ralston SH. Nitric oxide and bone. Immunology 2001; 103: 255–261.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis KL, Martin E, Turko IV, Murad F. Novel effects of nitric oxide. Annu Rev Pharmacol Toxicol 2001; 41: 203–36. [DOI] [PubMed] [Google Scholar]
- 7.Wimalawansa SJ, De Marco G, Gangula P, Yallampalli C. Nitric oxide donor alleviates ovariectomy-induced bone loss. Bone 1996; 18: 301–4. [DOI] [PubMed] [Google Scholar]
- 8.Dessy C and Ferron O. Pathophysiological roles of nitric oxide: in the heart and the coronary vasculature. Curr Med Chem 2004;3::207-16.
- 9.Wimalawansa SJ. Rationale for using nitric oxide donor therapy for prevention of bone loss and treatment of osteoporosis in humans. Skeletal Biol Med Part B 2007; 1117: 283–97. [DOI] [PubMed] [Google Scholar]
- 10.Wimalawansa SJ. Nitric oxide: novel therapy for osteoporosis. Expert Opin Pharmacother 2008; 9: 3025–44. [DOI] [PubMed] [Google Scholar]
- 11.Wimalawansa SJ. Nitric oxide and bone. Ann N Y Acad Sci 2010; 1192: 391–403. [DOI] [PubMed] [Google Scholar]
- 12.Parker JD. Nitrate tolerance, oxidative stress, and mitochondrial function: another worrisome chapter on the effects of organic nitrates. J Clinical Investigat 2004; 113: 352–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kass DA, Takimoto E, Nagayama T, Champion HC. Phosphodiesterase regulation of nitric oxide signaling. Cardiovasc Res 2007; 75: 303–14. [DOI] [PubMed] [Google Scholar]
- 14.Setter SM, Iltz JL, Fincham JE, Campbell RK, Baker DE. Phosphodiesterase 5 inhibitors for erectile dysfunction. Annals Pharmacother 2005; 39: 1286–95. [DOI] [PubMed] [Google Scholar]
- 15.Grimsley SJ, Khan MH, Jones GE. Mechanism of Phosphodiesterase 5 inhibitor relief of prostatitis symptoms. Med Hypotheses 2007; 69: 25–6. [DOI] [PubMed] [Google Scholar]
- 16.Doggrell SA. Comparison of clinical trials with sildenafil, vardenafil and tadalafil in erectile dysfunction. Expert Opin Pharmacother 2005; 6: 75–84. [DOI] [PubMed] [Google Scholar]
- 17.Kang SG, Kim JJ. Udenafil: efficacy and tolerability in the management of erectile dysfunction. Ther Adv Urol 2013; 5: 101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G. Sildenafil Use in Pulmonary Arterial Hypertension Study G. Sildenafil citrate therapy for pulmonary arterial hypertension. New Engl J Med 2005; 353: 2148–57. [DOI] [PubMed] [Google Scholar]
- 19.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med 2005; 11: 214–22. [DOI] [PubMed] [Google Scholar]
- 20.Histing T, Marciniak K, Scheuer C, Garcia P, Holstein JH, Klein M, Matthys R, Pohlemann T, Menger MD. Sildenafil accelerates fracture healing in mice. J Orthopaedic Res: official publication of the Orthopaedic Research Society 2011; 29: 867–73. [DOI] [PubMed] [Google Scholar]
- 21.Yaman F, Atilgan S, Gunes N, Agacayak S, Gunay A, Ucan MC, Bakir S, Erol B, Kose I, Atalay Y. Phosphodiesterase-5 inhibitors may facilitate bone defect recovery. Eur Rev Med Pharmacol 2011; 15: 1301–5. [PubMed] [Google Scholar]
- 22.Kilic C, Ozcan S, Acar E, Tiftikci U, Aykut S, Kilic B. Effects of sildenafil on the inflammation and repair phase of bone healing speed in rat model. Acta Medica Mediterranea 2015; 31: 1203–8. [Google Scholar]
- 23.Beltran-Gamez ME, Sandoval-Zarate J, Pulido T. [Phosphodiesterase-5 inhibitors for the treatment of pulmonary arterial hypertension]. Archivos de cardiologia de Mexico 2015; 85: 215–24. [DOI] [PubMed] [Google Scholar]
- 24.Kilickesmez K, Kucukoglu MS. [Phosphodiesterase type 5 inhibitors in the treatment of pulmonary arterial hypertension]. Anadolu kardiyoloji dergisi: AKD = the Anatolian journal of cardiology 2010; 10: 16–8. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Cao L, Ray S, Thormann U, Hillengass J, Delorme S, Schnettler R, Alt V, Bauerle T. Osteoporosis influences osteogenic but not angiogenic response during bone defect healing in a rat model. Injury 2013; 44: 923–9. [DOI] [PubMed] [Google Scholar]
- 26.Sahara M, Sata M, Morita T, Nakajima T, Hirata Y, Nagai R. A phosphodiesterase-5 inhibitor vardenafil enhances angiogenesis through a protein kinase G-dependent hypoxia-inducible factor-1/vascular endothelial growth factor pathway. Arteriosclerosis Thromb Vasc Biol 2010; 30: 1315–U89. [DOI] [PubMed] [Google Scholar]
- 27.Kalyanaraman H, Ramdani G, Joshua J, Schall N, Boss GR, Cory E, Sah RL, Casteel DE, Pilz RB. A novel, direct NO donor regulates osteoblast and osteoclast functions and increases bone mass in ovariectomized mice. J Bone Miner Res 2017; 32: 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banfi G, Iorio EL, Corsi MM. Oxidative stress, free radicals and bone remodeling. Clin Chem Lab Med 2008; 46: 1550–5. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Iturbe B, Ferrebuz A, Vanegas V, Quiroz Y, Espinoza F, Pons H, Vaziri ND. Early treatment with cGMP phosphodiesterase inhibitor ameliorates progression of renal damage. Kidney Int 2005; 68: 2131–42. [DOI] [PubMed] [Google Scholar]
- 30.Muzaffar S, Shukla N, Srivastava A, Angelini GD, Jeremy JY. Sildenafil citrate and sildenafil nitrate (NCX 911) are potent inhibitors of superoxide formation and gp91phox expression in porcine pulmonary artery endothelial cells. Br J Pharmacol 2005; 146: 109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi SR, Gu J, Krishan LK, Taiying C, Richard JC, Taylor CR. A novel approach to immunohistochemistry on routinely processed tissue sections. In: Gu J. (ed). Analitical morphology theory, applications and protocols, 1st ed Basel: Birkhäuser, 1996, pp. 1–40. [Google Scholar]
- 32.Khoschsorur GA, Winklhofer-Roob BM, Rabl H, Auer T, Peng Z, Schaur RJ. Evaluation of a sensitive HPLC method for the determination of malondialdehyde, and application of the method to different biological materials. Chromatographia 2000; 52: 181–4. [Google Scholar]
- 33.Littarru GP, Mosca F, Fattorini D, Bompadre S, Battino M. Assay of coenzyme Q10 in plasma by a single dilution step. Meth Enzymol 2004; 378: 170–6. [DOI] [PubMed] [Google Scholar]
- 34.Kaur H, Halliwell B. Measurement of oxidized and methylated DNA bases by HPLC with electrochemical detection. Biochem J 1996; 318: 21–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shigenaga MK, Aboujaoude EN, Chen Q, Ames BN. Assays of oxidative DNA-damage biomarkers 8-Oxo-2'-deoxyguanosine and 8-oxoguanine in nuclear-DNA and biological-fluids by high-performance liquid-chromatography with electrochemical detection. Oxy Radical Biol Syst Pt D 1994; 234: 16–33. [DOI] [PubMed] [Google Scholar]
- 36.Armstrong D. Free radical and antioxidant protocols, New York, NY: Humana Press, 1998. [PubMed] [Google Scholar]
- 37.Jamal SA, Hamilton CJ. Nitric oxide donors for the treatment of osteoporosis. Curr Osteoporos Rep 2012; 10: 86–92. [DOI] [PubMed] [Google Scholar]
- 38.MacIntyre I, Zaidi M, Alam AS, Datta HK, Moonga BS, Lidbury PS, Hecker M, Vane JR. Osteoclastic inhibition: an action of nitric oxide not mediated by cyclic GMP. Proc Natl Acad Sci U S A 1991; 88: 2936–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wimalawansa SJ. Rationale for using nitric oxide donor therapy for prevention of bone loss and treatment of osteoporosis in humans. Ann New York Acad Sci 2007; 1117: 283–97. [DOI] [PubMed] [Google Scholar]
- 40.Pyriochou A, Vassilakopoulos T, Zhou Z, Papapetropoulos A. cGMP-dependent and -independent angiogenesis-related properties of nitric oxide. Life Sci 2007; 81: 1549–54. [DOI] [PubMed] [Google Scholar]
- 41.Lelovas PP, Xanthos TT, Thoma SE, Lyritis GP, Dontasi IA. The laboratory rat as an animal model for osteoporosis research. Comparat Med 2008; 58: 424–30. [PMC free article] [PubMed] [Google Scholar]
- 42.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser 1994; 843: 1–129. [PubMed] [Google Scholar]
- 43.Hlaing TT, Compston JE. Biochemical markers of bone turnover - uses and limitations. Ann Clin Biochem 2014; 51: 189–202. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Lai WP, Leung PC, Wu CF, Wong MS. Short- to mid-term effects of ovariectomy on bone turnover, bone mass and bone strength in rats. Biol Pharmaceut Bull 2007; 30: 898–903. [DOI] [PubMed] [Google Scholar]
- 45.Rissanen JP, Suominen MI, Peng ZQ, Morko J, Rasi S, Risteli J, Halleen JM. Short-term changes in serum PINP predict long-term changes in trabecular bone in the rat ovariectomy model. Calcified Tissue Int 2008; 82: 155–61. [DOI] [PubMed] [Google Scholar]
- 46.Kimura S, Sasase T, Ohta T, Matsushita M. Effects of ovariectomy on bone metabolism and bone mineral density in spontaneously diabetic Torii-Lepr(fa) rats. J Veter Med Sci 2011; 73: 1025–9. [DOI] [PubMed] [Google Scholar]
- 47.De Young L, Yu D, Freeman D, Brock GB. Effect of PDE5 inhibition combined with free oxygen radical scavenger therapy on erectile function in a diabetic animal model. Int J Impot Res 2003; 15: 347–54. [DOI] [PubMed] [Google Scholar]
- 48.Bouxsein ML, Myers KS, Shultz KL, Donahue LR, Rosen CJ, Beamer WG. Ovariectomy-induced bone loss varies among inbred strains of mice. J Bone Miner Res 2005; 20: 1085–92. [DOI] [PubMed] [Google Scholar]
- 49.Mendelsohn ME, Karas RH. Rapid progress for non-nuclear estrogen receptor signaling. J Clin Investigat 2010; 120: 2277–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Armour KE, Armour KJ, Gallagher ME, Godecke A, Helfrich MH, Reid DM, Ralston SH. Defective bone formation and anabolic response to exogenous estrogen in mice with targeted disruption of endothelial nitric oxide synthase. Endocrinology 2001; 142: 760–6. [DOI] [PubMed] [Google Scholar]
- 51.Higashino H, Tabuchi M, Yamagata T, Kurita H, Miya H, Mukai H, Miya Y. Serum nitric oxide metabolite levels in groups of patients with various diseases in comparison of healthy control subjects. J Med Sci 2010; 10: 1–11. [Google Scholar]
- 52.Musicki B, Bivalacqua TJ, Champion HC, Burnett AL. Sildenafil promotes eNOS activation and inhibits NADPH oxidase in the transgenic sickle cell mouse penis. J Sex Med 2014; 11: 424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev 2010; 62: 525–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu R, Hu CP, Wu XP, Liao EY, Li YJ. Effect of age on bone mineral density and the serum concentration of endogenous nitric oxide synthase inhibitors in rats. Compos Med 2002; 52: 224–8.. [PubMed] [Google Scholar]
- 55.Peng WJ, Jiang JL, Jia SJ, Zhang XJ, Luo D, Liao EY, Deng HW, Li YJ. Asymmetric dimethylarginine is not involved in ovariectomy-induced osteopenia in rats. Comparat Med 2005; 55: 30–3. [PubMed] [Google Scholar]
- 56.Gulhan I, Kebapcilar L, Alacacioglu A, Bilgili S, Kume T, Aytac B, Gunaydin R. Postmenopausal women with osteoporosis may be associated with high endothelin-1. Gynecol Endocrinol 2009; 25: 674–8. [DOI] [PubMed] [Google Scholar]
- 57.Rangaswami H, Schwappacher R, Marathe N, Zhuang S, Casteel DE, Haas B, Chen Y, Pfeifer A, Kato H, Shattil S, Boss GR, Pilz RB. Cyclic GMP and protein kinase G control a Src-containing mechanosome in osteoblasts. Sci Signal 2010; 3: ra91–ra91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mancini L, Moradi-Bidhendi N, Becherini L, Martineti V, MacIntyre I. The biphasic effects of nitric oxide in primary rat osteoblasts are cGMP dependent. Biochem Biophys Res Commun 2000; 274: 477–81. [DOI] [PubMed] [Google Scholar]
- 59.Gong Y, Xu CY, Wang JR, Hu XH, Hong D, Ji X, Shi W, Chen HX, Wang HB, Wu XM. Inhibition of phosphodiesterase 5 reduces bone mass by suppression of canonical Wnt signaling. Cell Death Dis 2014; 5: e1544–e1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Irshad M, Chaudhuri PS. Oxidant-antioxidant system: role and significance in human body. Ind J Exp Biol 2002; 40: 1233–9. [PubMed] [Google Scholar]
- 61.Loft S, Fischernielsen A, Jeding IB, Vistisen K, Poulsen HE. 8-Hydroxydeoxyguanosine as a urinary biomarker of oxidative DNA-damage. J Toxicol Environ Health 1993; 40: 391–404. [DOI] [PubMed] [Google Scholar]
- 62.Ostman B, Sjodin A, Michaelsson K, Byberg L. Coenzyme Q10 supplementation and exercise-induced oxidative stress in humans. Nutrition 2012; 28: 403–17. [DOI] [PubMed] [Google Scholar]
- 63.Yamashita S, Yamamoto Y. Simultaneous detection of ubiquinol and ubiquinone in human plasma as a marker of oxidative stress. Anal Biochem 1997; 250: 66–73. [DOI] [PubMed] [Google Scholar]
- 64.Wilson C. Bone: oxidative stress and osteoporosis. Nate Rev Endocrinol 2014; 10: 3–3. [DOI] [PubMed] [Google Scholar]
- 65.Goettsch C, Babelova A, Trummer O, Erben RG, Rauner M, Rammelt S, Weissmann N, Weinberger V, Benkhoff S, Kampschulte M, Obermayer-Pietsch B, Hofbauer LC, Brandes RP, Schroder K. NADPH oxidase 4 limits bone mass by promoting osteoclastogenesis. J Clin Investigat 2013; 123: 4731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Al-Amin MM, Hasan SMN, Alam T, Hasan AT, Hossain I, Didar RR, Alam MA, Rahman MM. Tadalafil enhances working memory, and reduces hippocampal oxidative stress in both young and aged mice. Eur J Pharmacol 2014; 745: 84–90. [DOI] [PubMed] [Google Scholar]
- 67.Fan YF, Zhang R, Jiang X, Wen L, Wu DC, Liu D, Yuan P, Wang YL, Jing ZC. The phosphodiesterase-5 inhibitor vardenafil reduces oxidative stress while reversing pulmonary arterial hypertension. Cardiovasc Res 2013; 99: 395–403. [DOI] [PubMed] [Google Scholar]
- 68.Ozgur BC, Telli O, Yuceturk CN, Sarici H, Ozer E, Surer H, Kilinc AS, Hucumenoglu S, Eroglu M. The effect of sildenafil and udenafil on testicular damage following ischemia-reperfusion injury in rats. J Urol 2014; 192: 1272–7. [DOI] [PubMed] [Google Scholar]
- 69.Savas M, Yeni E, Verit A, Gulum M, Aksoy N, Ciftci H, Celik H, Altunkol A, Oncel H. Acute effect of phosphodiesterase type 5 inhibitor on serum oxidative status and prolidase activities in men with erectile dysfunction. Clinics (Sao Paulo) 2010; 65: 1311–4. [DOI] [PMC free article] [PubMed] [Google Scholar]