The use of vena cava filters (VCFs) has increased over the last decade without clear indication for many patients.1,2 This increase in use has been suggested to be partially motivated by upcoding for increased reimbursement, given that placement of a VCF increases the reimbursement rate for venous thromboembolism (VTE) by 250%.1
Wide variation in the use of VCFs among hospitals has been observed.3 This variation may be influenced by many factors, including the case mix of patients, hospital-level factors, and physician preferences.3 We analyzed the association of VCF use for VTE with hospital-level factors and patient variables to determine whether differences can be explained by observable factors rather than potential reimbursement upcoding.
Methods
We used inpatient discharge data from all acute care hospitals in Kentucky during the period from 2008 to 2014. These data represent all discharges in the state and include up to 25 diagnosis and procedure fields, as well as hospital variables. The University of Kentucky institutional review board approved this study and did not require informed consent because the data are publicly available and deidentified.
Diagnoses for deep vein thrombosis (DVT) or pulmonary embolism (PE) were identified.4 The use of VCFs was identified by International Classification of Diseases, Ninth Revision, Clinical Modification procedure code 38.7.4 Prophylactic VCF use without DVT or PE was excluded. Hospital-level factors included bed size, teaching or nonteaching status, and urban status. Case-mix comorbidities included cancer, chronic obstructive pulmonary disease, cerebrovascular disease, atrial fibrillation, liver disease, hypertension, heart failure, hyperlipidemia, myocardial infarction, cellulitis, trauma, diabetes, infection, renal disease, bleeding, anemia, and sepsis or septic shock based on previously published coding algorithms.4–6 Case-mix variables were entered into the model as the proportion of patients with each condition at each hospital. The ratio of VTE events attributable to PE vs DVT at each hospital was included given that VCF use is more commonly used with PE. The proportion of patients dying or transferring and the percentage of patients undergoing surgery, thrombolysis, or embolectomy were also included.6
A final linear model included the percentage of patients who received a VCF as the dependent variable and controlled for all covariates. Model assumptions were inspected, including plots of the predicted values and the fitted model residuals. The use of VCFs was plotted by year, and the overall trend from 2008 to 2014 was evaluated. All analyses were conducted using JMP Pro 12 (SAS Institute). Statistical significance was assessed at P < .05.
Results
Seventy hospitals were included in the analysis, and of the 84 357 patients with VTE who were discharged from a hospital, 7337 (8.7%) received a VCF. Overall, the percentage of patients who received a VCF was 10% in 2008 and decreased to 7.5% by 2014 (P < .001 for all trends) (Figure). In the hospitals in Kentucky, the percentage of patients who received a VCF ranged from 0% to 15.2%, with a mean (SD) percentage of 6.1% (4.4%) (median, 6.7%; coefficient of variation, 0.73). The variation among hospitals was consistent throughout the time period. In adjusted analysis, VCF use was most strongly associated with case mix, mainly the PE:DVT ratio. Other case-mix variables associated with increased VCF use were atrial fibrillation (scaled estimate, 1.28 [95% CI, 0.12–2.69]) and cancer (scaled estimate, 3.83 [95% CI, 1.36–6.31]) (Table). The model fit the data well, with R2 = 0.97 and normally distributed residuals. Restricting the sample to hospitals with at least 50 patients with PE or DVT who were discharged, we observed similar results with R2 = 0.99.
Figure. Trend in Vena Cava Filter (VCF) Use.
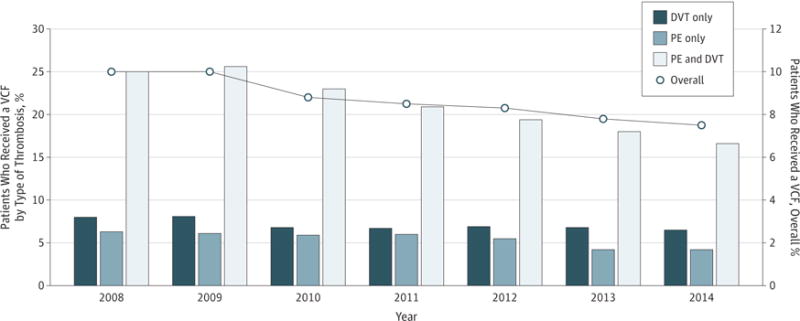
Tests for trend showed a 25% decrease in overall use (P < .001), a 33% decrease (P < .001) for pulmonary embolism (PE) alone or with deep vein thrombosis (DVT), and a 19% decrease (P < .001) for DVT alone. The line above the bar graphs is based on the overall percentage of patients who received a VCF (right axis).
Table.
Scaled Regression Estimates of Covariates Predicting Percentage of Patients With PE and/or DVT Receiving an Inferior Vena Cava Filter
| Term | Scaled Estimatea (95% CI) | P Value |
|---|---|---|
| Intercept | 6.29 (5.87–6.71) | <.001 |
| PE:DVT ratio | 7.96 (6.61–9.30) | <.001 |
| Embolectomy | 2.80 (1.22–4.37) | .001 |
| Cancer | 3.83 (1.36–6.31) | .003 |
| Thrombolysis | −2.17 (−3.70 to −0.64) | .01 |
| Trauma | −2.00 (−3.82 to −0.18) | .03 |
| COPD | −1.75 (−3.44 to −0.06) | .04 |
| 76–135 Beds | −0.73 (−1.44 to −0.01) | .05 |
| Atrial fibrillation | 1.28 (0.12–2.69) | .05 |
| ≥276 Beds | 1.08 (−0.07 to 2.24) | .05 |
| Cerebrovascular disease | 0.97 (−0.10 to 2.05) | .08 |
| Metropolitan area | −0.51 (−1.08 to 0.06) | .08 |
| Proximal DVT | −2.21 (−4.83 to 0.41) | .10 |
| Liver disease | −0.73 (−2.21 to 0.75) | .33 |
| Cellulitis | 0.69 (−0.94 to 2.31) | .40 |
| Renal disease | 0.69 (−1.00 to 2.39) | .41 |
| Rural area | 0.34 (−0.49 to 1.16) | .42 |
| Diabetes | 0.47 (−0.78 to 1.72) | .45 |
| Infection | 0.56 (−1.03 to 2.15) | .48 |
| Surgery | −0.89 (−3.43 to 1.65) | .48 |
| Heart failure | 0.36 (−0.79 to 1.51) | .53 |
| Micropolitan area | 0.17 (−0.44 to 0.79) | .56 |
| ≤75 Beds | −0.27 (−1.29 to 0.75) | .60 |
| Transfer rate | 0.40 (−1.15 to 1.96) | .60 |
| Hypertension | 0.69 (−2.35 to 3.73) | .65 |
| Myocardial infarction | −0.26 (−1.44 to 0.91) | .65 |
| Death | −0.24 (−1.36 to 0.88) | .67 |
| Unstable | 0.35 (−1.95 to 2.65) | .76 |
| 136–275 Beds | −0.09 (−0.75 to 0.58) | .79 |
| Sepsis or septic shock | −0.24 (−2.08 to 1.60) | .79 |
| Concurrent bleeding | −0.12 (−1.27 to 1.02) | .83 |
| Nonteaching | −0.07 (−0.69 to 0.56) | .84 |
| Teaching | 0.07 (−0.56 to 0.69) | .84 |
| Metastatic cancer | −0.16 (−2.95 to 2.63) | .91 |
| Hyperlipidemia | −0.09 (−2.05 to 1.86) | .92 |
| Anemia | −0.01 (−1.50 to 1.48) | .99 |
Abbreviations: COPD, chronic obstructive pulmonary disease; DVT, deep vein thrombosis; PE, pulmonary embolism.
Nominal variables are expanded to each category. Continuous variables are centered on the mean and scaled by the range/2. Estimates are ordered by significance in the model.
Discussion
These results showed a wide distribution in the use of VCFs for VTE in Kentucky that is explained almost completely by the patient case mix and hospital characteristics. The lack of residual variation among hospitals after controlling for these variables suggests that there may not be substantial overuse of VCFs to increase reimbursement. However, there may still be a systematic overuse of VCFs given the conflicting guidelines and the lack of apparent indications for many patients in a prior study.1 Additional work is needed to determine whether the rate of VCF use is appropriate.
Acknowledgments
Funding/Support: The project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1TR000117.
Role of the Funder/Sponsor: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Brown and Talbert had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Brown.
Acquisition, analysis, or interpretation of data: Both authors.
Drafting of the manuscript: Brown.
Critical revision of the manuscript for important intellectual content: Both authors.
Statistical analysis: Brown.
Administrative, technical, or material support: Talbert.
Study supervision: Talbert.
Conflict of Interest Disclosures: Dr Brown is the Humana-Pfizer Fellow at the University of Kentucky. No other disclosures are reported.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Additional Information: Data were collected by the Kentucky Cabinet for Health and Family Services, Office of Health Policy, and provided by the University of Kentucky Center for Clinical and Translational Science Enterprise Data Trust.
References
- 1.Sarosiek S, Crowther M, Sloan JM. Indications, complications, and management of inferior vena cava filters: the experience in 952 patients at an academic hospital with a level I trauma center. JAMA Intern Med. 2013;173(7):513–517. doi: 10.1001/jamainternmed.2013.343. [DOI] [PubMed] [Google Scholar]
- 2.Spencer FA, Bates SM, Goldberg RJ, et al. A population-based study of inferior vena cava filters in patients with acute venous thromboembolism. Arch Intern Med. 2010;170(16):1456–1462. doi: 10.1001/archinternmed.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White RH, Geraghty EM, Brunson A, et al. High variation between hospitals in vena cava filter use for venous thromboembolism. JAMA Intern Med. 2013;173(7):506–512. doi: 10.1001/jamainternmed.2013.2352. [DOI] [PubMed] [Google Scholar]
- 4.White RH, Zhou H, Kim J, Romano PS. A population-based study of the effectiveness of inferior vena cava filter use among patients with venous thromboembolism. Arch Intern Med. 2000;160(13):2033–2041. doi: 10.1001/archinte.160.13.2033. [DOI] [PubMed] [Google Scholar]
- 5.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 6.Stein PD, Matta F, Alrifai A, Rahman A. Trends in case fatality rate in pulmonary embolism according to stability and treatment. Thromb Res. 2012;130(6):841–846. doi: 10.1016/j.thromres.2012.07.011. [DOI] [PubMed] [Google Scholar]


