Abstract
Prolonged P-wave duration, a marker of left atrial abnormality, is associated with myocardial fibrosis, atrial fibrillation (AF), and all-cause death. It is not known if prolonged P-wave duration is associated with sudden cardiac death (SCD) in the general population. We aimed to evaluate whether prolonged P-wave duration is independently associated with SCD risk in the Atherosclerosis Risk in Communities Study, a community-based prospective cohort study. We included 15,321 participants in our analysis (age: 54.2±5.7, 55.2% women, 26.4% black). Prolonged P-wave duration was defined as maximum P-wave duration >120 ms and was determined from 12-lead electrocardiograms (ECGs) obtained during 4 exams (1987–1999). SCD was physician adjudicated and defined as a sudden, pulseless condition in a previously stable individual without evidence for non-cardiac cause of death. We used Cox proportional hazard models to assess the association between prolonged P-wave duration and SCD, adjusting for cardiovascular risk factors and conditions including atrial fibrillation. During a mean follow-up of 12.5 years (1987–2001), 268 SCDs were identified. The multivariable hazard ratio (95% CI) of prolonged P-wave duration for SCD was 1.70 (1.31–2.20). This association was attenuated, but remained significant after updating covariates to the end of follow-up with a hazard ratio of 1.35 (1.04–1.76). In conclusion, prolonged P-wave duration is independently associated with increased risk of SCD in the general population. This association is independent of AF and is only partially mediated by shared cardiovascular risk factors.
Keywords: Sudden Cardiac Death, Ventricular Tachycardia, Electrocardiogram, P-wave duration, atrial fibrillation, ventricular fibrillation
Introduction
Most sudden cardiac deaths (SCDs) occur as a result of ventricular tachyarrhythmias secondary to undiagnosed coronary heart disease in community-dwelling individuals. Electrocardiogram (ECG)-based SCD risk assessment has relied on markers reflecting abnormal ventricular conduction.1 There is growing evidence, however, to support a link between markers of abnormal atrial conduction, such as atrial fibrillation (AF) or abnormal P-wave terminal force in V1, and SCD.2,3 Prolonged P-wave duration (>120ms)4 is an accepted marker of left atrial abnormality that reflects impaired inter-atrial conduction, possibly secondary to fibrotic remodeling and/or chamber enlargement.5,6 As such, it has been linked with electromechanical dysfunction and poor left atrial contractility.7 Prolonged P-wave duration has been independently associated with incidence and recurrence of AF,8–12 embolic stroke, cardiovascular death, and all-cause death.13–16 We hypothesized that prolonged P-wave duration is independently associated with SCD in the general population and tested our hypothesis in the Atherosclerosis Risk in Communities Study, a community-based prospective cohort study.
Methods
The Atherosclerosis Risk in Communities Study was designed to identify and evaluate risk factors, etiology, and clinical manifestations of atherosclerotic coronary heart disease in the general population. Between 1987 and 1989, 15,792 men and women aged 45–64 years were recruited and enrolled from four United States communities (Washington County, MD; Forsyth County, NC; Jackson, MS; and suburban Minneapolis, MN). Participants underwent an initial baseline exam and four follow-up exams, the last in 2011–13. In between study visits, participants (or proxy) have been contacted annually by telephone to ascertain information on hospitalizations and deaths. Further, active community-wide surveillance of local hospitals has been performed to identify additional hospitalizations and cardiovascular events. For our analysis, we used follow-up data through 2001 as SCD ascertainment was completed through 2001 at the time of analysis. Approval for the study was obtained from the institutional review board on human research at each participating institution and all participants provided informed consent. Further details regarding outcome ascertainment procedures, study design, and population statistics have been previously described.17
We considered all 15,792 participants at the baseline visit and excluded those with missing covariates (n=89), missing ECG data (n=242), prevalent AF (n=37), and those who were not white or black from all study sites, and nonwhite from Minneapolis and Washington County (due to small sample size; n=103) resulting in a final cohort of 15,321 participants.
ECGs obtained during the first four study visits (1987–1998) were recorded on MAC PC Personal Cardiographs (Marquette Electronics Inc. Milwaukee, WI) and processed at the EPICORE Center (University of Alberta, Edmonton, Alberta, Canada) and EPICARE Center (Wake Forest University, Winston-Salem, NC) during the early and late study phases, respectively. P-wave duration was measured from the conclusion of the T-P segment (P wave onset) to return to baseline (PR interval). For biphasic P-waves, P-wave duration encompassed both positive and negative deflections from baseline. P-wave duration was measured from any single lead and prolonged P-wave duration was present if the maximum P-wave duration in any lead was >120 ms on a standard 12-lead ECG.
All fatal coronary heart disease events through 2001 were reviewed by an independent panel of physicians to identify SCD. Deaths were classified as definite SCD, possible SCD, not SCD, and unclassifiable. Definite SCD was defined as a sudden pulseless condition presumed to be of cardiac origin in a previously stable individual without evidence of non-cardiac cause of death. Possible SCD was defined as an unwitnessed death in a previously stable (<24 hours) individual without other evidence indicating non-cardiac origin for instantaneous death. All deaths classified as SCD had to occur outside of the hospital or in the emergency room. For our analysis, SCD was defined as definite or possible SCD.
The covariates included in our analysis were age, sex, race, study center, educational level, smoking status, prevalent coronary heart disease, heart failure, diabetes, hypertension, beta-adrenergic receptor blocker use, digoxin use, use of anti-arrhythmic drugs, left ventricular hypertrophy, body mass index, and AF. Baseline demographic data, medication use (beta-adrenergic receptor blockers, anti-arrhythmic drugs, and digoxin), and medical history were obtained by study staff from participants during the study visit. Prevalent coronary heart disease was defined as a self-reported history of myocardial infarction, coronary artery bypass grafting, percutaneous coronary intervention, or ECG signs of coronary heart disease. Heart failure was defined as stage 3 “manifest heart failure” by the Gothenburg criteria or self-reported diagnosis of heart failure. Left ventricular hypertrophy was defined by the Cornell ECG criteria. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or self-reported history of anti-hypertensive medical therapy. Diabetes was defined as a fasting (minimum of 8 hours) glucose ≥126 mg/dL, non-fasting glucose ≥200 mg/dL, self-reported use of oral hypoglycemic agents or insulin, or self-reported physician diagnosis of diabetes. Smoking status was self-reported. Participants were classified as current smokers and non-current smokers. Education level was also self-reported and classified as less than high school, high school degree or vocational school, or more than high school. AF was ascertained from study visit ECGs, hospital discharge records, and death certificates as previously described.18
Person-years at risk were calculated from the date of baseline visit until the date of SCD, other death, loss to follow-up, or end of follow-up, whichever occurred first. For those with incident prolonged P-wave duration, time between baseline and prolonged P-wave duration diagnosis was considered as non-prolonged P-wave duration follow-up. The analysis was based on data obtained from 1987–2001.
We initially explored the association between P-wave duration and SCD using restricted cubic splines (Figures 1 and 2). To calculate the hazard ratios and 95% confidence intervals of prolonged P-wave duration for SCD, we used Cox proportional hazard models with prolonged P-wave duration as a time-dependent exposure. We constructed 4 models. To evaluate for confounding, covariates were adjusted to the time point just before the development of prolonged P-wave duration, censorship, or SCD, whichever occurred first (Models 1 and 2). To evaluate for mediation, covariates adjusted to the time point just before the end of follow up (Models 3 and 4). Model 1 was adjusted for age, race, sex, and study center. Models 2 and 3 were additionally adjusted for education level, smoking status, body mass index, systolic and diastolic blood pressure, use of antihypertensive medication, diabetes, coronary heart disease, left ventricular hypertrophy, heart failure, use of anti-arrhythmic drugs, use of digoxin, and use of beta adrenergic receptor blockers. Model 4 was additionally adjusted for AF. We performed sex- and race-stratified analyses using Model 2.
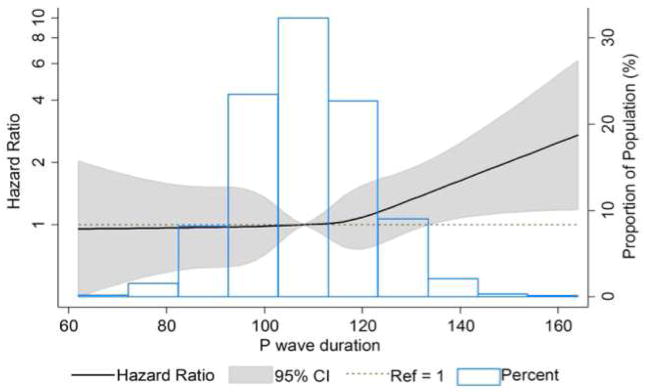
Figure 1.
Cubic spline for baseline P-wave duration and incident sudden cardiac death adjusted for baseline age, race, sex, study center, educational level, smoking status, body mass index, systolic and diastolic blood pressure, use of antihypertensive medications, diabetes, coronary heart disease, left ventricular hypertrophy, heart failure, use of anti-arrhythmic medications, use of digoxin, and use of beta-blocker medications.
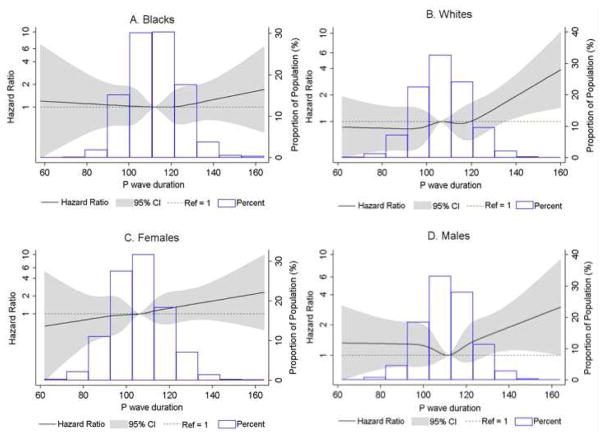
Figure 2.
Sex- and race-stratified cubic splines for baseline P-wave duration and incident sudden cardiac death adjusted for baseline age, race, sex, study center, educational level, smoking status, body mass index, systolic and diastolic blood pressure, use of antihypertensive medications, diabetes, coronary heart disease, left ventricular hypertrophy, heart failure, use of anti-arrhythmic medications, use of digoxin, and use of beta-blocker medications.
The proportional hazards assumption was assessed with scaled Schoenfeld residuals for both graphical and numerical tests, time interaction terms, and inspection of log negative log survival curves. Modeling assumptions were not violated in any model. Statistical analysis of data was performed using SAS version 9.3 (SAS Institute Inc., Cary, NC) and STATA 13.0 (StataCorp LPCollege Station, TX). All P values reported were 2-sided, and statistical significance threshold was chosen as 0.05.
Results
We identified 268 SCDs in our cohort of 15,321 participants over a mean follow up period of 12.5 years. There were 2,273 participants with prevalent prolonged P-wave duration at visit 1 and 2,406 incident cases of prolonged P-wave duration during follow up. Table 1 lists the baseline characteristics of our cohort. Participants with prolonged P-wave duration were more likely to be black and male compared with participants with normal P-wave duration. They also had significantly higher prevalence of hypertension, coronary heart disease, diabetes, left ventricular hypertrophy, and heart failure.
Table 1.
Baseline Participant Characteristics by P-Wave Duration Status, Atherosclerosis Risk in Communities Study, 1987–2001
| Characteristic | Normal PWD (n=10,624) | Prolonged PWD (N=4,697) | P-Value |
|---|---|---|---|
| Age (years) † | 53.8 ± 5.7 | 55.0 ± 5.7 | <0.0001 |
| Women | 6,392 (60.2%) | 2069 (44.1%) | <0.0001 |
| Black | 2464 (23.2%) | 1584 (33.7%) | <0.0001 |
| Education ≥ high school | 8,230 (77.5%) | 3469 (73.9%) | <0.0001 |
| Current smoker | 2954 (27.8%) | 1050 (22.4%) | <0.0001 |
| Body mass index (kg/m2) † | 27.0 ± 5.1 | 29.3 ± 5.6 | <0.0001 |
| Diabetes | 563 (5.3%) | 393 (8.4%) | <0.0001 |
| Hypertension medications | 2711 (25.5%) | 1954 (41.6%) | <0.0001 |
| Systolic blood pressure (mmHg) † | 119.4 ± 18.1 | 125.5 ± 19.9 | <0.0001 |
| Diastolic blood pressure, (mmHg) † | 72.6 ± 10.8 | 76.2 ± 11.7 | <0.0001 |
| Heart failure | 415 (3.9%) | 293 (6.2%) | <0.0001 |
| Coronary heart disease | 387 (3.6%) | 346 (7.4%) | <0.0001 |
| Incident atrial fibrillation | 442 (4.2%) | 447 (9.5%) | <0.0001 |
| Left ventricular hypertrophy | 151 (1.4%) | 185 (3.9%) | <0.0001 |
| Use of Digoxin | 106 (1.0%) | 111 (2.4%) | <0.0001 |
| Use of Beta-blockers | 811 (7.6%) | 786 (16.7%) | <0.0001 |
| Use of Anti-arrhythmics | 49 (0.5%) | 66 (1.4%) | <0.0001 |
Data are presented as mean ± standard deviation
Abbreviations: PWD (P-wave duration)
Figure 1 shows the distribution of P-wave duration in our cohort and the association between prolonged P-wave duration and SCD modeled as a restricted cubic spline. We observed a threshold effect as the positive association between P-wave duration and SCD risk was observed only for P-wave duration >110 ms. SCD risk increased almost linearly with increasing P-wave duration above this threshold. The results were similar when stratified by sex and race (Figure 2).
Table 2 shows the results of our Cox proportional hazards models. Prolonged P-wave duration was significantly associated with an increased risk of SCD in the demographically adjusted model (Model 1). This association remained significant after adjustment for cardiovascular risk factors (Model 2). The association between prolonged P-wave duration and SCD was attenuated, but remained significant after updating covariates in Model 2 to the time point just before the end of follow-up (Model 3) and after additional adjustment AF updated to the time point just before the end of follow-up (Model 4). The results of our race- and sex- stratified analysis using Model 2 are shown in Table 3. We did not find any significant sex- or race-based interactions with respect to SCD risk.
Table 2.
Risk of Sudden Cardiac Death by P-Wave Duration, Atherosclerosis Risk in Communities Study, 1987–2001.
| Normal PWD (n=10,624) | Prolonged PWD (n=4697) | p-value | |
|---|---|---|---|
| SCD Events | 153 | 115 | |
| Person-years | 145,989 | 45,784 | |
| Incidence Rate per | 1.05 (0.89–1.22) | 2.51 (2.08–3.00) | |
| 1000 person years (95% CI) | |||
| HR (95% CI) | |||
| Model 1‡† | 1 (Reference) | 2.05 (1.60–2.64) | <0.001 |
| Model 2§† | 1 (Reference) | 1.70 (1.31–2.20) | <0.001 |
| Model 3¶ | 1 (Reference) | 1.35 (1.04–1.76) | 0.02 |
| Model 4* | 1 (Reference) | 1.32 (1.02–1.72) | 0.04 |
Covariates are updated until the development of prolonged PWD, censoring, or SCD incidence, whichever occurred first
Cox proportional hazards model adjusted for age, race (blacks vs. whites), sex, and study center
Model 1 + adjustment for educational level, smoking status, body mass index, systolic and diastolic blood pressure, use of antihypertensive medication, diabetes, coronary heart disease, left ventricular hypertrophy, heart failure, use of anti-arrhythmic medications, use of digoxin, and use of beta-blocker medications.
Model 2 with covariates updated to end of follow up
Model 3 + adjustment for atrial fibrillation updated to end of follow up
Abbreviations: CI (Confidence Interval), HR (Hazard Ratio), PWD (P-Wave Duration), SCD (Sudden Cardiac Death)
Table 3.
Risk of Sudden Cardiac Death by Prolonged P-wave Duration Stratified by Sex and Race, Atherosclerosis Risk in Communities Study, 1987–2001.
| Normal PWD HR (95% CI) |
Prolonged PWD HR (95% CI) |
p-value | ||
|---|---|---|---|---|
| Male | Model 2‡† | 1 (Reference) | 1.71 (1.25–2.36) | 0.0009 |
| Female | Model 2‡† | 1 (Reference) | 1.72 (1.10–2.69) | 0.02 |
| Sex Interaction | Model 2‡† | 0.50 | ||
| Black | Model 2‡† | 1 (Reference) | 1.40 (0.94–2.09) | 0.10 |
| White | Model 2‡† | 1 (Reference) | 1.98 (1.41–2.77) | <0.001 |
| Race Interaction | Model 2‡† | 0.16 |
Covariates are updated until the development of prolonged PWD, censoring, or SCD incidence, whichever occurred first
Cox proportional hazard model adjusted for age, study center, educational level, smoking status, body mass index, systolic and diastolic blood pressure, use of antihypertensive medication, diabetes, coronary heart disease, left ventricular hypertrophy, heart failure, use of anti-arrhythmic medications, use of digoxin, and use of beta-blocker medications. When stratified by race, model was also adjusted for sex. When stratified by sex, model was also adjusted for race.
Abbreviations: CI (Confidence Interval), HR (Hazard Ratio), PWD (P-Wave Duration), SCD (sudden cardiac death)
Discussion
In this large, biracial cohort of community-dwelling individuals, we found that prolonged P-wave duration was associated with an increased risk of SCD independent of cardiovascular risk factors and conditions including AF. Further, we did not find any interactions in race- and sex-stratified analyses..
Prolonged P-wave duration is a marker of left atrial abnormality reflecting electromechanical dysfunction7 and structural remodeling of the atrium.5,6 It has been independently associated with incident AF 8,10–12 and recurrent AF after pulmonary vein isolation.9 Prospective cohort studies have demonstrated that incident AF carries an independent risk of death from cardiovascular disease and all causes.2,19 AF can reduce the threshold for ventricular arrhythmias by directly reducing refractoriness20 and generating pro-arrhythmic short-long-short sequences.21 In a meta-analysis of the data from the Atherosclerosis Risk in Communities Study and the Cardiovascular Health Study, AF was independently associated with 2.47-fold (95% confidence interval, 1.95–3.13) increased risk of SCD.2
Prolonged P-wave duration has been associated with cardiovascular and all-cause mortality in the National Heath and Nutrition Examination Survey.13 However, the association between prolonged P-wave duration and SCD has not been previously evaluated. To the best of our knowledge, our study is the first prospective analysis linking prolonged P-wave duration to SCD in the general population independent of cardiovascular risk factors and AF.
We considered several mechanisms to explain our findings. First, this association may have been mediated by development of cardiovascular risk factors such as coronary heart disease, heart failure, or even AF. To assess for this possibility, we repeated our primary analysis with covariates, including AF, updated to the time point just before the end of follow-up. The association between prolonged P-wave duration and SCD was attenuated, but remained significant. This may suggest that the increased risk of SCD attributed to prolonged P-wave duration was not entirely mediated by the cardiovascular risk factors and conditions that we adjusted for in our models.
Second, prolonged P-wave duration may reflect an arrhythmogenic myocardial substrate in the atrium and ventricle. In fact, the degree of interstitial left ventricular fibrosis on cardiac magnetic resonance imaging has been linearly associated with both increasing P-wave duration (5.4 ms/decile of fibrosis) and increasing negative P-wave terminal force in V1 (−0.76 mV*ms/decile of fibrosis).22 AF has also been associated with left ventricular fibrosis in a dose-dependent manner; left ventricular fibrosis was more severe in permanent AF compared with paroxysmal AF.23 Certainly, fibrotic remodeling can represent a common substrate between atrial and ventricular arrhythmias and may, in part, explain the relationships between markers of abnormal atrial conduction and SCD.2,3
ECG makers reflecting abnormal ventricular conduction have been independently associated with SCD.1 Our findings contribute to the evidence supporting a link between abnormal atrial conduction and SCD.2,3 If our findings are validated in independent cohorts, markers of abnormal atrial conduction and left atrial abnormality should be investigated for the purposes of ECG-based SCD risk assessment. Effective SCD risk models will likely require a multi-marker approach given the diverse cascade of events that lead to unstable ventricular tachyarrhythmias.
The principal strengths of our study include a large biracial cohort with long follow-up duration, extensive measurement of covariates, and physician-adjudication of SCD. Some limitations should be noted. First, although we adjusted for multiple potential confounders in our analyses, we cannot exclude residual confounding by imperfectly measured and unmeasured factors. Second, our final cohort excluded 331 participants due to missing ECG and covariate data. This number was small compared to our cohort size and likely random in nature. Thus we do not expect it has significantly biased our results.
Acknowledgments
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Dr. Chen is supported by R01HL126637. The authors thank the staff and participants of the Atherosclerosis Risk in Communities Study for their important contributions.
Footnotes
Disclosure Statement: The authors have no disclosures. There are no conflicts of interest and no relationships with industry to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Narayanan K, Chugh SS. The 12-lead electrocardiogram and risk of sudden death: Current utility and future prospects. Europace. 2015;17:ii7–ii13. doi: 10.1093/europace/euv121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen LY, Sotoodehnia N, BůŹková P, Lopez FL, Yee LM, Heckbert SR, Prineas R, Soliman EZ, Adabag S, Konety S, Folsom AR, Siscovick D, Alonso A. Atrial fibrillation and the risk of sudden cardiac death: the atherosclerosis risk in communities study and cardiovascular health study. JAMA Intern Med. 2013;173:29–35. doi: 10.1001/2013.jamainternmed.744. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3578214&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tereshchenko LG, Henrikson CA, Sotoodehnia N, Arking D, Agarwal S, Siscovick D, Post W, Solomon S, Coresh J, Josephson M, Soliman E. Electrocardiographic deep terminal negativity of the P wave in V(1) and risk of sudden cardiac death: the Atherosclerosis Risk in Communities (ARIC) study. J Am Hear Assoc. 2014:3. doi: 10.1161/JAHA.114.001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayés De Luna A, Platonov P, Cosio FG, Cygankiewicz I, Pastore C, Baranowski R, Bayés-Genis A, Guindo J, Viñolas X, Garcia-Niebla J, Barbosa R, Stern S, Spodick D. Interatrial blocks. A separate entity from left atrial enlargement: A consensus report. J Electrocardiol. 2012;45:445–451. doi: 10.1016/j.jelectrocard.2012.06.029. Available at: http://dx.doi.org/10.1016/j.jelectrocard.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 5.Ariyarajah V, Mercado K, Apiyasawat S, Puri P, Spodick DH. Correlation of left atrial size with P-wave duration in interatrial block. Chest. 2005;128:2615–2618. doi: 10.1378/chest.128.4.2615. [DOI] [PubMed] [Google Scholar]
- 6.Redfearn DP, Lane J, Ward K, Stafford PJ. High-resolution analysis of the surface P wave as a measure of atrial electrophysiological substrate. Ann Noninvasive Electrocardiol. 2006;11:12–19. doi: 10.1111/j.1542-474X.2006.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goyal SB, Spodick DH. Electromechanical dysfunction of the left atrium associated with interatrial block. Am Heart J. 2001;142:823–827. doi: 10.1067/mhj.2001.118110. [DOI] [PubMed] [Google Scholar]
- 8.Magnani JW, Johnson VM, Sullivan LM, Gorodeski EZ, Schnabel RB, Lubitz Sa, Levy D, Ellinor PT, Benjamin EJ. P wave duration and risk of longitudinal atrial fibrillation in persons <60 years old (from the Framingham heart study) Am J Cardiol. 2011;107:917–921. doi: 10.1016/j.amjcard.2010.10.075. Available at: http://dx.doi.org/10.1016/j.amjcard.2010.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caldwell J, Koppikar S, Barake W, Redfearn D, Michael K, Simpson C, Hopman W, Baranchuk A. Prolonged P-wave duration is associated with atrial fibrillation recurrence after successful pulmonary vein isolation for paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2014;39:131–138. doi: 10.1007/s10840-013-9851-1. [DOI] [PubMed] [Google Scholar]
- 10.Bayés de Luna A, Cladellas M, Oter R, Torner P, Guindo J, Martí V, Rivera I, Iturralde P. Interatrial conduction block and retrograde activation of the left atrium and paroxysmal supraventricular tachyarrhythmia. Eur Heart J. 1988;9:1112–8. doi: 10.1093/oxfordjournals.eurheartj.a062407. Available at: http://www.ncbi.nlm.nih.gov/pubmed/3208776. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal YK, Aronow WS, Levy Ja, Spodick DH. Association of interatrial block with development of atrial fibrillation. Am J Cardiol. 2003;91:882. doi: 10.1016/s0002-9149(03)00027-4. [DOI] [PubMed] [Google Scholar]
- 12.Ariyarajah V, Apiyasawat S, Fernandes J, Kranis M, Spodick DH. Association of Atrial Fibrillation in Patients With Interatrial Block Over Prospectively Followed Controls With Comparable Echocardiographic Parameters. Am J Cardiol. 2007;99:390–392. doi: 10.1016/j.amjcard.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 13.Magnani JW, Gorodeski EZ, Johnson VM, Sullivan LM, Hamburg NM, Benjamin EJ, Ellinor PT. P wave duration is associated with cardiovascular and all-cause mortality outcomes: the National Health and Nutrition Examination Survey. Heart Rhythm. 2011;8:93–100. doi: 10.1016/j.hrthm.2010.09.020. Available at: http://dx.doi.org/10.1016/j.hrthm.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorbar M, Levrault R, Phadke JG, Spodick DH. Interatrial block as a predictor of embolic stroke. Am J Cardiol. 2005;95:667–668. doi: 10.1016/j.amjcard.2004.10.059. [DOI] [PubMed] [Google Scholar]
- 15.Vepsalainen T, Laakso M, Lehto S, Juutilainen a, Airaksinen J, Ronnemaa T. Prolonged P wave duration predicts stroke mortality among type 2 diabetic patients with prevalent non-major macrovascular disease. BMC Cardiovasc Disord. 2014;14:168. doi: 10.1186/1471-2261-14-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chhabra L, Srinivasan I, Sareen P, Anand C, Spodick DH. Interatrial block - a novel risk factor for acute mesenteric ischemia. Indian J Gastroenterol. 2012;31:191–194. doi: 10.1007/s12664-012-0194-0. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22763894. [DOI] [PubMed] [Google Scholar]
- 17.The ARIC Investigators. The Atherosclerosis Risk In Communities (ARIC) Study: Design and Objectives. Am J Epidemiol. 1989;129:687–702. Available at: http://aje.oxfordjournals.org/cgi/content/abstract/129/4/687. [PubMed] [Google Scholar]
- 18.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–7. doi: 10.1016/j.ahj.2009.05.010. Available at: http://www.sciencedirect.com/science/article/pii/S0002870309003627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 20.Denes P, Wu D, Dhingra R, Pietras R, Rosen K. The effects of cycle length on cardiac refractory periods in man. Circulation. 1974;49:32–41. doi: 10.1161/01.cir.49.1.32. [DOI] [PubMed] [Google Scholar]
- 21.Denker S, Lehmann M, Mahmud R, Gilbert C, Akhtar M. Facilitation of ventricular tachycardia induction with abrupt changes in ventricular cycle length. Am J Cardiol. 1984;53:508–515. doi: 10.1016/0002-9149(84)90022-5. [DOI] [PubMed] [Google Scholar]
- 22.Tiffany Win T, Ambale Venkatesh B, Volpe GJ, Mewton N, Rizzi P, Sharma RK, Strauss DG, Lima Ja, Tereshchenko LG. Associations of electrocardiographic P-wave characteristics with left atrial function, and diffuse left ventricular fibrosis defined by cardiac magnetic resonance: The PRIMERI Study. Hear Rhythm. 2015;12:155–162. doi: 10.1016/j.hrthm.2014.09.044. Available at: http://linkinghub.elsevier.com/retrieve/pii/S1547527114010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shantsila E, Shantsila A, Blann AD, Lip GYH. Left ventricular fibrosis in atrial fibrillation. Am J Cardiol. 2013;111:996–1001. doi: 10.1016/j.amjcard.2012.12.005. [DOI] [PubMed] [Google Scholar]