Abstract
Insecticide resistance in German cockroaches (Blattella germanica (L.)) has been a barrier to effective control since its first documentation in the 1950s. A necessary first step toward managing resistance is to understand insecticide susceptibility profiles in field-collected strains so that active ingredients (AIs) with lowest resistance levels can be identified. As a first step in this study, diagnostic concentrations (DCs) were determined for 14 insecticide AIs based on lethal concentrations that killed 99% or 90% of the individuals from a susceptible lab strain (JWax-S). Next, cockroaches were collected from two low-income multifamily housing complexes in Danville, IL, and Indianapolis, IN, and used to establish laboratory strains. These strains were screened against the 14 AI-DCs in vial bioassays, and susceptibility profiles were determined by comparing percent mortalities between the field strains relative to the JWax-S strain. Results revealed lowest resistance of field strains to boric acid, abamectin, dinotefuran, clothianidin, thiamethoxam, and chlorfenapyr. For the AIs hydramethylnon and imidacloprid, field strains did not display survivorship different than the lab strain, but >90% mortality was never achieved. Lastly, both field strains displayed resistance to indoxacarb, fipronil, acetamiprid, beta-cyfluthrin, bifenthrin, and lambda-cyhalothrin, but at varying levels. These results satisfy two objectives. First, baseline monitoring DCs were established for 14 insecticides presently registered for use against cockroaches, which represents a useful resource. Second, our findings reveal insecticide AIs with lowest resistance levels for use in forthcoming field studies that will investigate impacts of different insecticide deployment strategies on resistance management and evolution in cockroach field populations.
Keywords: insecticide resistance, pyrethroid, resistance management, fipronil, indoxacarb
Low-income multifamily apartments are commonly infested with German cockroaches (Blattella germanica L.) (Dictyoptera: Blattellidae). Cockroach allergens in these environments are associated with negative health effects such as asthma and rhinitis in residents, especially children (Wang et al. 2008, Celmeli et al. 2016, Do et al. 2016). To a great extent, asthma morbidity in sensitive children can be affected more by exposure to cockroach allergens than dust mite or pet allergens (Gruchalla et al. 2005). Bacterial pathogens have also been isolated from German cockroaches trapped in houses and food processing facilities and thus they can be potential vectors for foodborne pathogens (Kopanic et al. 1994, Tatfeng et al. 2005, Menasria et al. 2014). Therefore, cockroach control practices have become part of the pest management routines for housing and food handling facilities. Cockroach baits have been used in integrated pest management (IPM) as a cost-effective control strategy (Schal and Hamilton 1990, Wang and Bennett 2006). Despite the initial success of baiting in controlling cockroach populations and reducing resident exposure to insecticides, recent reports indicate cockroaches have developed resistance to newer bait AIs (Gondhalekar and Scharf 2012, Gondhalekar et al. 2013, Ko et al. 2016a). Historically, German cockroaches have been able to develop extensive resistance to different insecticides (Cochran 1995). Insecticide resistance in laboratory and field-collected German cockroaches has been reported for decades (Bennett and Spink 1968; Koehler and Patterson 1986; Cochran 1987; Umeda et al. 1988; Cochran 1989; Hemingway et al. 1993a, 1993b; Valles and Yu 1996; Lee et al. 1996; Scharf et al. 1997; Scharf et al. 1998; Wei et al. 2001; Holbrook et al. 2003; Gondhalekar et al. 2011, 2013; Ko et al. 2016a; Naqqash et al. 2016). In total, German cockroaches have shown resistance to 43 active ingredients (AIs) (Whalon et al. 2016), and therefore, resistance management has become a priority in any cockroach IPM program.
Insecticide resistance in cockroaches varies between populations collected from different regions, as they may have resistance to some AIs but not others (Cochran 1989, Georghiou 1994) depending on selection history (Scharf et al. 1997). Knowledge of resistance profiles in a cockroach population is a key for making informed pest management decisions for preventing insecticide resistance evolution (Gondhalekar and Scharf 2013, Zhu et al. 2016). Insecticide resistance in German cockroaches can be classified into the two broad groups of physiological and behavioral resistance (Cochran 1995, Silverman and Bieman 1993, Wu et al. 1998, Wang et al. 2004, Liu et al. 2006, Zhu et al. 2016). Here we focus on physiological rather than behavioral (bait aversion) aspects of resistance in German cockroaches.
Discriminating dose- or concentration-based approaches have been used successfully for detecting fipronil and indoxacarb resistance in German cockroach field strains (Holbrook et al. 2003, Gondhalekar et al. 2011, 2013). The research presented here targeted the problem in urban pest management posed by cockroach resistance, using an unprecedented resistance monitoring-based approach that included 14 AIs and a glass vial bioassay method. Extensive prior research documented the vial bioassay method (e.g., Scharf et al. 1999), which works based on cockroach tarsal contact with insecticide residues and ingestion via tarsal grooming (Scharf et al. 1995). Diagnostic concentration (DC) bioassays are less labor-intensive and require fewer insects in comparison to the conventional resistance ratio method, which initially requires generation of concentration- or dose-mortality data for LC or LD estimation (Gondhalekar et al. 2013). The goals of this study were to 1) develop DCs for commonly used cockroach insecticide AIs, and 2) test the DCs against cockroach strains collected from two multifamily housing sites in advance of field studies. Based on the screening results, we are able to recommend insecticide AIs for subsequent resistance management studies to be conducted at the two field sites. More generally, our findings also provide diagnostic concentration information that can be used to help pest managers make more informed insecticide choices and help researchers to study resistance evolution under field conditions.
Materials and Methods
Insects and Chemicals
Three German cockroach strains were used. The Johnson Wax strain (JWax-S) has been maintained in the laboratory for >80 yr without insecticide exposure and was used as a standard susceptible strain. Two field strains were collected from multifamily housing sites in Danville, IL (D-IL strain), and Indianapolis, IN (I-IN strain), during December 2014 and March 2015. Full human subjects research approval was granted by the Purdue University Institutional Review Board (Protocol number 1411015460R001). The field strains were collected from multiple apartments across each site and pooled to establish laboratory “meta” populations. These populations were maintained without insecticide selection pressure. Colonies were reared in Ziploc plastic containers (44.3 by 30 by17 cm3/15.14 liter; S.C. Johnson Inc., Racine WI, USA) with screened lids and held in a controlled-environmental chamber at 26 ± 1 °C and a photoperiod of 12:12 (L:D) h. Cardboard for shelter, rodent diet (number 8604; Harlan Teklad, Madison, WI), and water were provided ad libitum to the rearing boxes. Bioassay experiments were done with 1–2-wk-old adult males. To obtain enough adult males of appropriate age for experiments, rearing containers were established with gravid adult females and mixed-age nymphs. Adult male cockroaches were separated out of these containers at the beginning of every week and aged for an additional week before using in insecticide bioassays. All bioassays with field strains were performed after three to four generations (within 12 mo after collection).
Technical grade gel bait and spray product AIs used in vial bioassays were purchased from ChemService (West Chester, PA), Fisher Scientific (Pittsburgh, PA), or Sigma-Aldrich (St. Louis, MO). These AIs included indoxacarb (99.1% purity), abamectin (98.3%), boric acid (99.9%), beta-cyfluthrin (99.5%), bifenthrin (99%), lambda-cyhalothrin (99.5%), fipronil (98.3%), dinotefuran (98.4%), imidacloprid (99.4%), acetamiprid (99.5%), clothianidin (99.5%), thiamethoxam (99.5%), chlorfenapyr (99.1%), and hydramethylnon (99.5%). These AIs were selected because they are currently registered for use in cockroach control products. Three cockroach gel baits, InVict Gold (imidacloprid 2.15%; Rockwell Lab Ltd, Kansas City, MO), Maxforce Professional Insect Control (hydramethylnon 2.15%; Bayer, Research Triangle Park, NC), and Magnetic (boric acid 33.3%; Nisus Co., Rockford, TN) were purchased from Univar (Indianapolis, IN) for follow-up testing in no-choice feeding bioassays.
Vial Bioassay for JWax-S LC and DC Estimates
As a first step in the resistance monitoring process, the JWax-S strain was prescreened against the 14 AIs listed above to determine lethal concentrations (LCs) and DCs. Insecticides and test concentrations are outlined in Supp. Table 1 [online only]. Bioassays were conducted in 30-ml Shell vials (25 by 95 mm; Kimble Chase, Vineland, NJ). The internal surface of the vials (71.67 cm2) was treated with 0.5 ml insecticide dilutions. Approximately 1 cm from the top of the vial was left untreated, as it would be covered by a cotton plug. Insecticide dilutions were made in acetone with the exception of boric acid, which was dissolved in methanol and used immediately after preparation. Insecticide solutions were mixed thoroughly before being applied to each vial. After addition of insecticide solutions, vials were rotated manually for 1 min and then on a nonheating hotdog roller (Nostalgia Products LLC, Green Bay, WI) placed in a fume hood. Complete evaporation of acetone or methanol required ∼30 min. Vials treated with acetone or methanol only were used as controls. Adult male cockroaches held in plastic cups were anesthetized on ice before transferring to individual vials in groups of 10. Glass vials were plugged with cotton balls to prevent escape. Treated and control vials were kept vertically in controlled-environmental chambers with atmospheric conditions similar to those used for rearing. Concentration–mortality data for the JWax-S strain was generated by testing 8–18 concentrations for each insecticide AI (Supp.Table 1 [online only]). For each insecticide concentration, 4–12 replicates were performed depending on the consistency of mortality responses. Mortality was recorded every 24 h up to 72 h. Owing to the slower speed of action of boric acid and hydramethylnon, mortality was scored up to 96 h. Insects were considered dead if they were knocked down on their backs and unable to recover on their feet or walk.
To determine LC90 and LC99 estimates for each insecticide, JWax-S concentration–mortality data were analyzed using the PROC PROBIT function in SAS 9.4 (SAS Institute 2012-2013). Control mortality was accounted for in probit analysis by the method of Abbott (Abbott 1925). To improve probit model estimation, multiple concentrations were tested to achieve symmetrically spaced mortality around 50%, and to increase precision of LC90/LC99 estimates, multiple concentrations providing 75–100% mortality were also tested (Robertson et al. 1984). The LC90 or LC99 estimates and their corresponding 95% fiducial limits (FLs) for each insecticide were used as reference values and then, they were checked against JWax-S concentration–mortality data for selecting baseline DCs. The lowest concentrations that provided 90 or 99–100% mortality (raw data) in the JWax-S strain were also taken into consideration while determining DCs. If 100% mortality was not achieved in the JWax-S strain when exposed to any AI, or in cases where 99% mortality would require an excessively high diagnostic concentration (i.e., for boric acid, hydramethylnon, and imidacloprid), LC90 values were used to determine DCs. Justification for using 72- or 96-h mortality data and different LC values is explained in more detail under Results and Discussion.
Vial Diagnostic Bioassay to Determine Susceptibility Profiles of Cockroach Strains to AIs
To establish insecticide susceptibility profiles in the field-collected D-IL and I-IN strains, adult males were tested at DCs determined for 14 AIs. As a positive control, adult males of the susceptible JWax-S strain were also tested at the respective DCs in parallel with field strains. Diagnostic insecticide concentrations were prepared in either acetone or methanol, and 10 replications were performed for each strain and insecticide. Ten replications of acetone or methanol treated vials for each strain were used as controls. Mortality was scored as described above.
For comparing mortality variation between the three strains (JWax-S, D-IL, and I-IN), percentage mortality from diagnostic bioassays with individual AIs were arcsine transformed and analyzed by two-way factorial ANOVA in Statistica 13 (Dell Inc. 2015) followed by a post hoc Tukey’s HSD test.
Follow-Up No-Choice Feeding Bioassays With Commercial Gel Baits Containing Boric Acid, Hydramethylnon, and Imidacloprid
Three gel bait products were screened against the JWax-S, I-IN, and D-IL strains to determine if similar mortality levels could be achieved as seen in AI-DC assays. The gel baits included InVict Gold (imidacloprid), Maxforce Professional Insect Control (hydramethylnon), and Magnetic (boric acid). Procedures as described previously in other studies were used with small modifications (Wang et al. 2004, Gondhalekar et al. 2011). Plastic containers (17.8 by 17.8 by 6 cm3/0.739 liter) were used (Glad boxes Clorox Co., Oakland, CA). These bioassays were conducted in a no-choice format in which no competing food was provided. Polystyrene weighing dishes (Fisher Scientific, Pittsburgh, PA) filled with 0.5 g gel bait, a water cup, and cardboard shelter were provided in each container. For controls, the gel bait was replaced with 0.5 g rodent diet. Seven to ten 1–2-wk-old adult males were starved for one day before assaying. To prevent escape, container walls were lightly greased with petroleum jelly and mineral oil (2:3) and containers were closed tightly with lids containing a central meshed opening (3 cm diameter). Five replications were done for each strain–treatment combination. Mortality was checked every 24 h until 100% mortality was achieved in all strains. To assure no recovery occurred, all assay boxes were kept 72 h after 100% mortality was achieved. For comparing variation among strains, mortality data were analyzed by multivariate analysis of variance (MANOVA) in Statistica 13 (Dell Inc. 2015), followed by univariate tests of significance for each day. To determine LT50, LT90, and LT99 estimates, time–mortality data were analyzed using the PROC PROBIT function in SAS 9.4 (SAS Institute 2012-2013). Control mortality was accounted for in probit analysis by the method of Abbott (Abbott 1925).
Results
Vial LC and DC Estimates for the JWax-S Strain
Although cockroach mortality data were collected every 24 h for 3–4 d, only 72 h and 96 h results were used for probit analysis, as these times provided data that were best fit to the probit model (Supp. Figs. 1–6 [online only]). LC50, LC90, and LC99 estimates, DCs, and other probit model parameters for each insecticide are presented in Tables 1 and 2. For most insecticides, DCs were chosen based on JWax-S 72 h concentration–mortality data, and corresponding LC99 values and 95% FLs (Table 1); however, owing to the use of different strategies for determining DCs for boric acid, hydramethylnon, and imidacloprid (Table 2), results for these AIs are presented separately in the Special cases section below.
Table 1.
Vial LC (µg vial−1) estimates for JWax-S adult male German cockroaches and diagnostic concentrations (DCs) (µg vial−1) chosen for establishing susceptibility profiles for field strains
| Active ingredient | n | Slope (± SE) | LC50 (95% FL) | LC90 (95% FL) | LC99 (95% FL) | χ2(df) | P-value | DC |
|---|---|---|---|---|---|---|---|---|
| Abamectin | 483 | 5.7 (±0.6) | 0.9 (0.8–0.9) | 1.4 (1.3–1.6) | 2.2 (1.9–2.7) | 12.6 (8) | 0.1261 | 2 |
| Dinotefuran | 984 | 2.7 (±0.3) | 2.0 (1.5–2.5) | 6.0 (4.9–7.9) | 14.5 (10.4–25.1) | 32.1 (12) | 0.0013 | 20 |
| Clothianidin | 480 | 1.1 (±0.1) | 2.3 (1.5–3.3) | 34.1 (22.4–59.6) | 305.8 (149.6–861.0) | 10.0 (10) | 0.4368 | 200 |
| Thiamethoxam | 440 | 1.6 (±0.4) | 0.8 (0.3–1.5) | 5.4 (2.9–19.4) | 24.8 (9.4–359.7) | 25.2 (9) | 0.0028 | 30 |
| Acetamiprid | 450 | 2.1 (±0.3) | 64.2 (39.2–95.0) | 263.4 (168.5–553.5) | 832.9 (427.3–3017.0) | 20.0 (7) | 0.0056 | 1000 |
| Chlorfenapyr | 440 | 2.6 (±0.3) | 1.0 (0.8–1.3) | 3.1 (2.5–4.5) | 7.8 (5.3–14.4) | 1.8 (9) | 0.9942 | 14 |
| Indoxacarb | 552 | 2.1 (± 0.2) | 2.7 (2.2–3.3) | 11.1 (8.8–14.9) | 35.4 (24.7–57.3) | 9.8 (9) | 0.3638 | 30 |
| Fipronil | 856 | 2.7 (±0.7) | 0.02 (0.01–0.03) | 0.1 (0.0–0.2) | 0.2 (0.1–1.3) | 116.2 (11) | <0.0001 | 0.1 |
| Beta-Cyfluthrin | 343 | 4.2 (±0.0.7) | 0.3 (0.2–0.3) | 0.6 (0.5–0.8) | 1.1 (0.8–1.8) | 5.0 (5) | 0.4185 | 1 |
| Bifenthrin | 331 | 5.1 (±0.8) | 0.6 (0.5–0.8) | 1.1 (0.8–1.9) | 1.8 (1.2–4.1) | 12.8 (5) | 0.0256 | 2 |
| Lambda-Cyhalothrin | 492 | 3.3 (±0.6) | 0.2 (0.1–0.2) | 0.4 (0.3–0.8) | 0.9 (0.5–2.6) | 27.2 (7) | 0.0003 | 1 |
All assays lasted for 72 h.
FL stands for fiducial limit.
Table 2.
Vial LC (mg vial−1) estimates for JWax-S adult male German cockroaches and diagnostic concentrations (DCs) (mg vial−1) chosen for establishing susceptibility profiles for field strains to boric acid, hydramethylnon, and imidacloprid
| Active ingredient | Daya | n | Slope (± SE) | LC50 (95% FL) | LC90 (95% FL) | LC99 (95% FL) | χ2(df) | P-value | DC |
|---|---|---|---|---|---|---|---|---|---|
| Boric acid | 4 | 440 | 2.4 (± 0.6) | 20.5 (7.5–30.9) | 68.8 (45.4–196.0) | 184.5 (94.1–1,852.0) | 34.5 (9) | <0.0001 | 60 |
| Hydramethylnon | 4 | 480 | 1.1 (± 0.2) | 1.8 (0.8–3.1) | 26.7 (11.6–163.4) | 244.6 (59.4–6,885.0) | 15.4 (8) | 0.0513 | 16 |
| Imidacloprid | 3 | 1071 | 0.7 (±0.1) | 0.2 (0.1–0.4) | 13.2 (4.9–80.3) | 394.2 (68.1–11,510.0) | 53.6 (15) | <0.0001 | 7 |
Day refers to the time point at which LC values are estimated.
FL stands for fiducial limit.
Susceptibility Profiles of Cockroach Strains to AIs Determined Using LC99 DCs
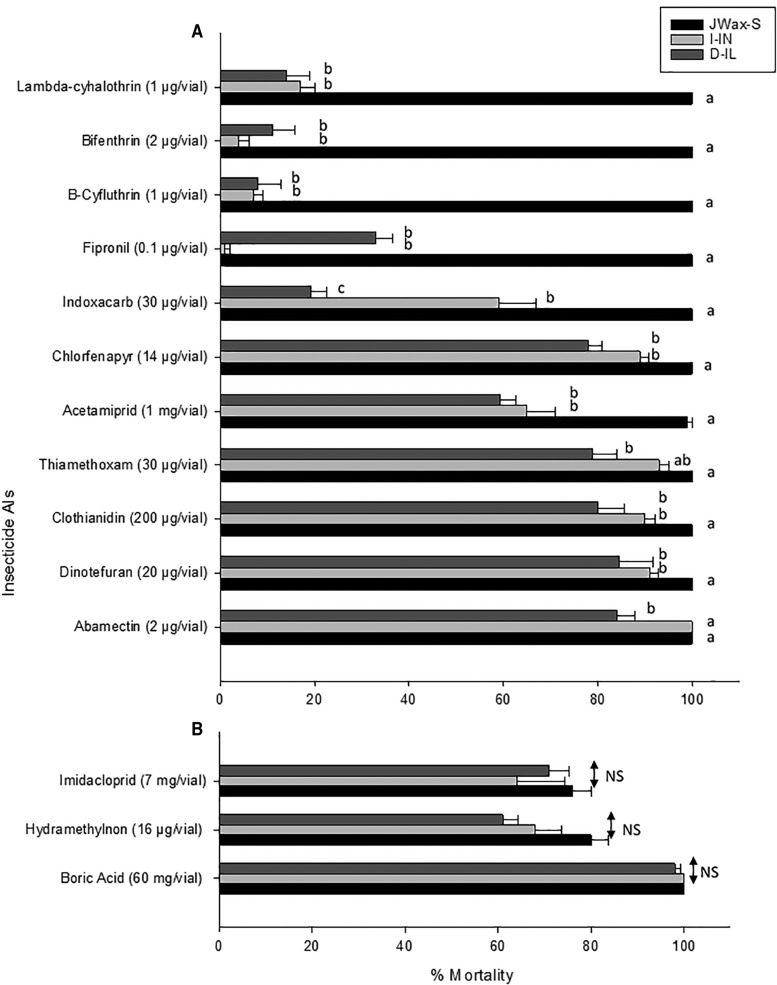
Percent mortality comparisons for the three strains for 14 AIs at their respective DCs are detailed in Fig. 1. Two-way factorial ANOVA results are summarized in Supp.Table 2 [online only]. Exposure to abamectin, dinotefuran, clothianidin, and thiamethoxam DCs resulted in high mortality of both field strains. Complete mortality was achieved in the JWax-S and I-IN strains when they were exposed to the abamectin DC; however, mortality in the D-IL strain (84.4%) was significantly lower (Fig. 1A;Supp. Fig. 1 [online only]). Average mortality for the I-IN strain was 90–93% when exposed to the dinotefuran, clothianidin, or thiamethoxam DCs. Lower mortality in the range of 79–84% was achieved for the D-IL strain when exposed to the same DCs as above with dinotefuran, clothianidin, and thiamethoxam (Fig. 1A;Supp.Fig. 2 [online only]). There were significant mortality differences among strains when tested against DCs of dinotefuran, clothianidin, and thiamethoxam.
Fig 1.
Insecticide susceptibility profiles in two field-collected German cockroach strains (I-IN and D-IL) when exposed to 14 AIs in vial bioassays at (A) LC99 diagnostic concentrations or (B) LC90 diagnostic concentrations. Statistical analysis (Tukey’s HSD test; P < 0.05) was performed in comparison to the susceptible JWax-S strain. For each AI, strains (shown as bars) with different letters are significantly different, P < 0.05 (Tukey’s HSD test). NS indicates a lack of statistical significance between strains. ANOVA results are shown in Supp. Table 2 [online only].
Acetamiprid and chlorfenapyr DC assays resulted in 60–90% mortality in the field strains, while 99–100% mortality was achieved in JWax-S. When exposed to acetamiprid at its DC, only 65 ± 6% and 59 ± 7% mortality were achieved in I-IN and D-IL strains. For chlorfenapyr, significantly lower mortality was observed in the I-IN and D-IL field strains (89 ± 2% and 78 ± 5% mortality, respectively) compared with JWax-S (Fig. 1A).
Finally, I-IN strain mortality when exposed to indoxacarb was 59 ± 8%, but it was <20% for fipronil, beta-cyfluthrin, bifenthrin, and lambda-cyhalothrin (1 ± 1, 7 ± 2, 4 ± 2, and 17 ± 3% mortality, respectively). Mortality in the D-IL stain was <20% in all instances with indoxacarb, fipronil, beta-cyfluthrin, bifenthrin, and lambda-cyhalothrin DCs (19 ± 5, 33 ± 5, 8 ± 3, 11 ± 4, and 14 ± 3% mortality, respectively; Fig. 1A).
Special Cases: Susceptibility Profiles of Cockroach Strains to Boric Acid, Hydramethylnon, and Imidacloprid as Determined Using LC90 DCs
Diagnostic concentration determinations for boric acid, hydramethylnon, and imidacloprid were done with slight modifications from that detailed above. Boric acid and hydramethylnon concentration–mortality data for JWax-S were scored after 96 h (Table 2 and Supp.Table 2 [online only]; Fig. 1B;Supp. Fig. 6 [online only]). JWax-S LC90 values and 95% FLs were used in boric acid and hydramethylnon DC determinations. In the case of imidacloprid, 72-h mortality data were used; however, imidacloprid treatment with high technical AI concentrations of up to 8 mg vial−1 did not result in 100% mortality of the JWax-S lab strain. Hence, a concentration of 7 mg vial−1 that was within the LC90 95% FLs was selected as the imidacloprid DC (Table 2).
Boric acid killed the highest proportions of the field strains among all insecticides at its DC. There were no significant differences among lab and field strain mortality levels with boric acid; i.e., 100% mortality was achieved for the JWax-S and I-IN strains, while 98 ± 4% mortality was obtained in the D-IL strain (Fig. 1B;Supp.Fig. 6 [online only]). Mortality was comparatively lower for all three strains with imidacloprid (JWax-S: 76 ± 4%, I-IN: 64 ± 10%, and D-IL: 71 ± 6%) and hydramethylnon (JWax-S: 78 ± 7, I-IN: 59 ± 18, and D-IL: 50 ± 12%) at their DCs of 7 and 16 mg vial − 1, respectively (Fig. 1B;Supp.Fig. 6 [online only]). When assayed with either imidacloprid or hydramethylnon, there were no statistically significant differences among the three strains based on Tukey’s HSD tests.
No-Choice Feeding Bioassay Results With Commercial Boric Acid, Hydramethylnon, and Imidacloprid Gel Baits
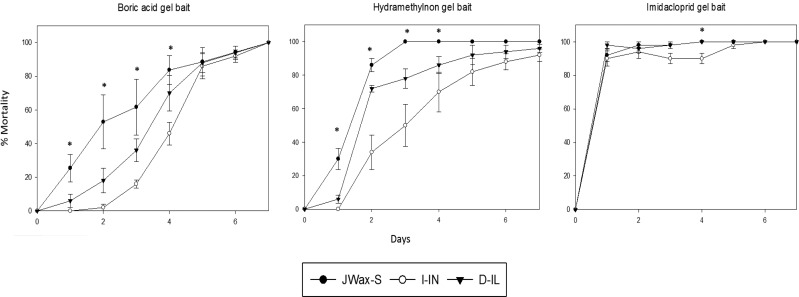
Percent mortality up to 7 d is shown for all three strains when provided gel baits containing boric acid, hydramethylnon, or imidacloprid (Fig. 2). Based on multivariate tests of significance, there were no differences in overall mortality among different strains when fed gel baits containing boric acid (P= 0.1774) or imidacloprid (P= 0.0904); however, when provided hydramethylnon gel bait, field strains survived significantly longer than the JWax-S strain (P = 0.0375). LT50 values for different gel baits determined through probit analysis ranged between 0.05 to 3.9 d for the JWax-S, I-IN, and D-IL strains (Supp. Table 3 [online only]). When exposed to Magnetic (boric acid), strain–mortality levels were significantly different across the first four assay days based on univariate results [day 1: (F= 6.5(2, 12)P= 0.01), day 2: (F= 6.5(2, 12)P= 0.01), day 3: (F= 4.8(2, 12)P= 0.03), and day 4: (F= 4.8 (2, 12)P= 0.03)], but 100% mortality was still achieved after 7 d for all strains. Magnetic LT50 values were significantly different for the JWax-S and both field strains, but not LT90 or LT99 (Supp. Table 3 [online only]). Maxforce Pro (hydramethylnon) had significantly different mortality among strains on days 1–4 as well [day 1: (F= 16.43(2, 12)P= 0.0004), day 2: (F= 17.24(2, 12)P= 0.0003), day 3: (F= 9.71(2, 12)P= 0.0031), and day 4: (F= 4.07(2, 12)P= 0.0447)], with LT50, LT90, and LT99 values also being different (Supp. Table 3 [online only]). With InVict Gold (imidacloprid) gel bait, >90% mortality was achieved for all strains within 1 d after starting assays. Owing to recovery of cockroaches, there were differences among strain–mortality levels on day 4 [F= 14.04(2, 12)P= 0.0007], but LT50, LT90, and LT99 values for InVict Gold analysis were not different among strains (Supp. Table 3 [online only]).
Fig. 2.
Assessment of commercial boric acid, hydramethylnon, and imidacloprid gel bait efficacy when tested on susceptible and two field-collected German cockroach strains (JWax-S, I-IN, and D-IL, respectively). In each day, asterisks indicate significantly different mortality among strains at P < 0.05 probability level (one-way MANOVA: univariate results).
Discussion
There is currently a pressing need for sustainable resistance management programs for German cockroaches in public housing that can 1) effectively manage populations and 2) delay insecticide resistance evolution. One of the critical elements in the success of resistance management programs for cockroaches is knowledge of their resistance status to different AIs. In this study, after first developing new DCs for a wide range of currently available AIs, susceptibility profiles were determined for cockroaches collected from low-income multifamily housing sites in Danville, IL, and Indianapolis, IN, by extensive laboratory screening against 14 AIs. Both field strains have exposure histories to multiple insecticides from different classes over the past 5 yr. Vial bioassay results showed that boric acid and abamectin killed the highest proportions of these populations followed by dinotefuran, clothianidin, thiamethoxam, and chlorfenapyr. Boric acid results were validated with gel bait feeding assays, which also supported the potential for reduced efficacy of hydramethylnon and imidacloprid gel baits. Conversely, both field strains displayed moderate to high levels of resistance to indoxacarb, fipronil, acetamiprid, and all pyrethroids tested (beta-cyfluthrin, bifenthrin, and lambda-cyhalothrin).
Susceptibility Profiles of Field-Collected Strains Versus a Laboratory Susceptible Strain
Susceptibility profiles for the I-IN and D-IL strains were determined to identify candidate insecticide AIs for use in resistance management studies to be conducted at both housing sites. Vial bioassays were used to monitor physiological resistance to the AIs, which is considered the most common category of resistance in cockroaches (Scharf et al. 1998, Wang et al. 2004, Gondhalekar et al. 2013). Insecticide resistance can progress to problem levels quickly once physiological resistance is present at detectable levels (Ffrench-Constant & Roush 1990), even for baits. This assay method also can be executed quickly and with its combination of contact and ingestion exposure, it simulates cockroach exposure to insecticides in the field (Gondhalekar et al. 2011). Similar results were obtained when the JWax-S strain was exposed to indoxacarb using vial LC-DC bioassay in other studies, which highlights the reproducibility of results by using this method (Gondhalekar et al. 2011, 2013). LC99 DCs were used for susceptibility assessment in the JWax-S, I-IN, and D-IL strains in initial AI assays. Diagnostic concentration estimates, however, were slightly different and relied upon LC90 DCs for boric acid, hydramethylnon, and imidacloprid as tested in subsequent assays. Owing to the slower-acting nature of boric acid and hydramethylnon, 96-h concentration–mortality data were used for LC determinations with these AIs. Additionally, LC90s (as supported by consultation of raw data) were used for DC assays with boric acid and hydramethylnon because using LC99s would result in extremely high DCs which were not feasible for routine testing. In imidacloprid DC determination assays, 100% mortality was not achieved for either lab or field strains, apparently due to imidacloprid’s activity as a weak partial agonists at the nicotinic acetylcholine receptor, low contact toxicity, and knock-down recovery (Kaakeh et al. 1997, Tan et al. 2007); therefore, the 72 h LC90 value was used in imidacloprid DC assays. Commercial baits containing boric acid, hydramethylnon, and imidacloprid were also used in no-choice feeding bioassays to test if similar results could be achieved compared with vial bioassays (see below).
When exposed to abamectin the I-IN and D-IL field strains displayed high mortality. For decades, abamectin efficacy has been investigated, and it has been shown effective for German cockroach control (Cochran 1985, Cochran 1990, Koehler et al. 1991, Ross 1993, Appel and Benson 1995). Additionally, cockroach females exposed to abamectin failed to reproduce (Cochran 1985, Koehler et al. 1991). However, more recent studies also showed low levels of abamectin resistance in field-collected cockroach strains (Wang et al. 2004).
Among the neonicotinoids tested, three were moderately active against the field strains (dinotefuran, clothianidin, and thiamethoxam), whereas acetamiprid was the least effective, and in the case of imidacloprid 100% mortality was not achieved in any strain. Similar results were obtained by Tan et al. (2007), who found that clothianidin and dinotefuran killed 77 and 90% of German cockroaches tested while imidacloprid and acetamiprid caused 0 and 20% mortality. Insecticidal activities of neonicotinoids were grouped previously based on their relative maximum levels of acetylcholine (ACh)-mediated current production (Tan et al. 2007). Imidacloprid only caused 20–25% of the maximum ACh current (Tan et al. 2007), which might explain the recovery of German cockroaches after being exposed to imidacloprid in the present study.
Chlorfenapyr acts via disruption of ATP production and loss of energy, and thus has a different mode of action from neurotoxic insecticides (Raghavendra et al. 2011). Chlorfenapyr insecticidal activity has been tested on German cockroaches (Ameen et al. 2000, Sims and Appel 2007) and low levels of resistance (5.7-fold) have previously been observed in a field-collected strain Gondhalekar et al. 2011).
Indoxacarb and fipronil were initially shown as effective AIs for controlling German cockroaches (Appel 2003, Wei et al. 2001); however, in the present study only low mortality was observed in both the I-IN and D-IL strains when exposed to indoxacarb and fipronil. Our findings are consistent with other studies showing significant indoxacarb (Gondhalekar et al. 2013, Ko et al. 2016a) and fipronil (Holbrook et al. 2003, Chai and Lee 2010, Gondhalekar et al. 2011) resistance in German cockroaches. Resistance to indoxacarb and fipronil in the I-IN and D-IL strains are likely the result of prior selection pressure. These findings are consistent with application records at the Indianapolis and Danville sites, and the higher-level market sales of cockroach baits containing indoxacarb and fipronil than any other bait in the United States for years preceding our study (Curl 2011).
When exposed to pyrethroids (i.e., beta-cyfluthrin, bifenthrin, and lambda-cyhalothrin), both field strains showed uniform high resistance. Reports of widespread pyrethroid resistance in German cockroaches and associated control failures are abundant (Valles and Yu 1996, Wei et al. 2001, Limoee et al. 2006, Chai and Lee 2010). Cochran previously showed pyrethroid-resistant German cockroaches could be selected within six generations by exposing a lab susceptible strain to permethrin or fenvalerate (Cochran 1987). However, resistance build-up was even faster in field strains already possessing low-level pyrethroid resistance (Cochran 1987, Scharf et al. 1997, 1998).
Susceptibility Profiles of Cockroaches for Technical Grade Boric Acid, Hydramethylnon, or Imidacloprid and Associated Commercial Gel Baits
With respect to boric acid, similar results were obtained in both no-choice feeding bioassays and vial bioassays. With no evidence of resistance, inorganic compounds such as boric acid were used regularly as a dust treatment for German cockroaches even before World War II (Ebeling 1995). Boric acid has been tested in the lab and field for controlling German cockroaches (Zurek et al. 2003, Gore and Schal 2004, Gore et al. 2004), resulting in 90% population reductions (Gore and Schal 2004).
On the other hand, for hydramethylnon, vial bioassay and no-choice feeding bioassay results were different. When exposed to technical grade hydramethylnon, no difference was observed in mortality of the JWax-S and field strains. In the case of formulated hydramethylnon gel bait, 100% mortality was achieved in JWax-S in 72 h, but it took significantly longer to achieve complete mortality for the field strains (Fig 2, Supp. Table 3 [online only]). This latter result suggests the potential for reduced hydramethylnon efficacy in the field against the I-IN and D-IL field strains. Ko et al. (2016a, 2016b) also recently reported evidence of hydramethylnon resistance in German cockroaches for the first time, as well as cross-resistance with indoxacarb. In the case of imidacloprid, similar results were obtained in vial and no-choice feeding bioassays with gel bait. For technical imidacloprid assays, mortality was not different between the lab and field strains, which is a similar result to that obtained previously (Chai and Lee 2010). However, with technical imidacloprid 100% mortality could not be achieved, suggesting the potential for reduced imidacloprid efficacy. These results may also suggest less suitability of vial bioassays for assessing imidacloprid toxicity, but DC vial assays effectively predicted the results of no-choice bait feeding bioassays. Overall, commercial gel baits tested in this study were toxic to susceptible and field strains of cockroaches and 100% mortality was eventually achieved (although taking longer with hydramethylnon and boric acid; Fig. 2 and Supp. Table 3 [online only]) probably owing to highly palatable bait matrices and higher amounts of insecticides acquired through high bait consumption.
Summary and Conclusions
As already established through prior research, vial bioassays are an easy and cost-effective technique for comparing susceptibility profiles of laboratory and field-collected cockroach strains. Although the I-IN and D-IL strains displayed susceptibility to some AIs and clear resistance to others, resistance can potentially be rapidly selected in any population once resistance alleles for any AI are present. For example, prior results showed only three generations of selection with pyrethroid or organophosphate insecticides are required to significantly increase resistance levels in a lab–field hybrid German cockroach strain (Scharf et al. 1998). Determining the effects of different insecticide deployment strategies on resistance evolution, such as use of single products versus product rotations or mixtures would provide useful information for developing sustainable resistance management strategies for German cockroaches. Even though susceptibility and resistance of field cockroaches against different AIs was observed in our study, effective control of cockroaches might still be achievable by commercially available chemicals because they are highly palatable and contain extremely high insecticide concentrations (Holbrook et al. 2003, Gondhalekar and Scharf 2012, Gondhalekar et al. 2013, Ko et al. 2016a). However, this possibility does not preclude judicial pesticide use to preserve efficacy of presently available pesticide resources (Gondhalekar and Scharf 2013).
In conclusion, the three major outcomes of this work are as follows. First is the determination of susceptibility profiles for cockroach field populations collected from two public housing sites. Abamectin and boric acid were two of the most effective insecticide AIs identified. Among neonicotinoids, the AIs dinotefuran, clothianidin, and thiamethoxam were the most active against field strain cockroaches. The highest resistance levels observed were for fipronil, indoxacarb, and all pyrethroids tested. Second, another outcome of this work is the development of DCs for a number of currently registered AIs for use against cockroaches. These DCs should have wide utility for resistance monitoring purposes, both in support of pest management programs and resistance management studies. Third, field studies already in progress are testing the most-active AIs noted above by comparing their effectiveness in different deployment schemes (single product, rotation, or mixture) at the two housing sites. These field studies are expected to reveal the relative effects of the different deployment strategies on population suppression, as well as resistance and cross-resistance evolution in response to selection pressures.
Supplementary Material
Acknowledgments
This research was funded by grant INHHU0026-14 from the United States Department of Housing and Urban Development and conducted with human subjects research approval granted by the Purdue University Institutional Review Board (protocol number 1411015460R001). Additional supplementary support came from the O.W. Rollins / Orkin Endowment in the Department of Entomology at Purdue University. We thank the Indianapolis (Indianapolis, IN) and Danville (Danville, IL) housing authorities, contracted pest control personnel, and especially tenants for their cooperation with cockroach collections.
References Cited
- Abbott W. S. 1925. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18: 265–267. [Google Scholar]
- Ameen A., Kaakeh W., Bennett G.. 2000. Integration of chlorfenapyr into a management program for the German cockroach. J. Agric. Urban Entomol. 17: 135–142. [Google Scholar]
- Appel A. G. 2003. Laboratory and field performance of an indoxacarb bait against German cockroaches. J. Econ. Entomol. 96: 863–870. [DOI] [PubMed] [Google Scholar]
- Appel A. G., Benson E. P.. 1995. Performance of abamectin bait formulations against German cockroaches. J. Econ. Entomol. 88: 924–931. [DOI] [PubMed] [Google Scholar]
- Bennett G. W., Spink W. T.. 1968. Insecticide resistance of German cockroaches from various areas of Louisiana. J. Econ. Entomol. 61: 426–431. [DOI] [PubMed] [Google Scholar]
- Celmeli F., Yavuz S. T., Turkkahraman D., Simsek O., Kılınc A., Sekerel B. E.. 2016. Cockroach (Blattella germanica) sensitization is associated with coexistence of asthma and allergic rhinitis in childhood. Pediatr. Allergy Immunol. Pulmonol. 29: 38–43. [Google Scholar]
- Chai R. Y., Lee C. Y.. 2010. Insecticide resistance profiles and synergism in field populations of the German cockroach from Singapore. J. Econ. Entomol. 103: 460–471. [DOI] [PubMed] [Google Scholar]
- Cochran D. G. 1985. Mortality and reproductive effects of avermectin B1 fed to German cockroaches. Entomol. Exp. Appl. 37: 83–88. [Google Scholar]
- Cochran D. G. 1987. Selection for pyrethroid resistance in the German cockroach. J. Econ. Entomol. 80: 1117–1121. [DOI] [PubMed] [Google Scholar]
- Cochran D. G. 1989. Monitoring for insecticide resistance in field-collected strains of the German cockroach. J. Econ. Entomol. 82: 336–341. [DOI] [PubMed] [Google Scholar]
- Cochran D. G. 1990. Efficacy of abamectin fed to German cockroaches resistant to pyrethroids. J. Econ. Entomol. 83: 1243–1245. [DOI] [PubMed] [Google Scholar]
- Cochran D. G. 1995. Insecticide resistance. Understanding and controlling the German cockroach. Oxford University Press, New York, pp.171–192. [Google Scholar]
- Curl G. 2011. A strategic analysis of the US structural pest control industry. Specialty Product Consultants, LLC, Mendham, NJ. [Google Scholar]
- Dell Inc. 2015. Dell Statistica (data analysis software system), version 13. Software.dell.com.
- Do D. C., Zhao Y., Gao P.. 2016. Cockroach allergen exposure and risk of asthma. Allergy 71: 463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeling W. 1995. Chapter 9: Inorganic insecticides and dusts, pp. 193–230. Understanding and controlling the German cockroach. Oxford University Press, New York. [Google Scholar]
- Ffrench-Constant R. H., Roush R. T.. 1990. Resistance detection and documentation: Relative roles of pesticidal and biochemical assays, pp. 4–38. InRoush R.T., Tabashnik B. E. (eds.), Pesticide Resistance in Arthropods. Chapman and Hall, New York, NY. [Google Scholar]
- Georghiou G. P. 1994. Principles of insecticide resistance management. Phytoprotection 75: 51–59. [Google Scholar]
- Gondhalekar A. D., Scharf M. E.. 2012. Mechanisms underlying fipronil resistance in a multiresistant field strain of the German cockroach. J. Econ. Entomol. 49: 122–131. [DOI] [PubMed] [Google Scholar]
- Gondhalekar A. D., Scharf M. E.. 2013. Preventing resistance to bait products. July: 42-46 (http://www.pctonline.com/pct0713-preventing-resistance-bait-products.aspx).
- Gondhalekar A. D., Song C., Scharf M. E.. 2011. Development of strategies for monitoring indoxacarb and gel bait susceptibility in the German cockroach. Pest Manag. Sci. 67: 262–270. [DOI] [PubMed] [Google Scholar]
- Gondhalekar A. D., Scherer W., Saran R. K., Scharf M. E.. 2013. Implementation of an indoxacarb susceptibility monitoring program using field-collected German cockroach isolates from the United States. J. Econ. Entomol. 106: 945–953. [DOI] [PubMed] [Google Scholar]
- Gore J. C., Schal C.. 2004. Laboratory evaluation of boric acid-sugar solutions as baits for management of German cockroach infestations. J. Econ. Entomol. 97: 581–587. [DOI] [PubMed] [Google Scholar]
- Gore J. C., Zurek L., Santangelo R. G., Stringham S. M., Watson D. W., Schal C.. 2004. Water solutions of boric acid and sugar for management of German cockroach populations in livestock production systems. J. Econ. Entomol. 97: 715–720. [DOI] [PubMed] [Google Scholar]
- Gruchalla R. S., Pongracic J., Plaut M., Evans R., Visness C. M., Walter M., Crain E. F., Kattan M., Morgan W. J., Steinbach S., et al. 2005. Inner City Asthma Study: Relationships among sensitivity, allergen exposure, and asthma morbidity. J. Allergy Clin. Immunol. 115: 478–485. [DOI] [PubMed] [Google Scholar]
- Hemingway J., Small G. J., Monro A. G.. 1993a. Possible mechanisms of organophosphorus and carbamate insecticide resistance in German cockroaches from different geographical areas. J. Econ. Entomol. 86: 1623–1630. [DOI] [PubMed] [Google Scholar]
- Hemingway J., Dunbar S. J., Monro A. G., Small G. J.. 1993b. Pyrethroid resistance in German cockroaches: resistance levels and underlying mechanisms. J. Econ. Entomol. 86: 1931–1938. [PubMed] [Google Scholar]
- Holbrook G. L., Roebuck J., Moore C. B., Waldvogel M. G., Schal C.. 2003. Origin and extent of resistance to fipronil in the German cockroach, Blattella germanica (L.). J. Econ. Entomol. 96: 1548–1558. [DOI] [PubMed] [Google Scholar]
- Kaakeh W., Reid B. L., Bohnert T. J., Bennett G. W.. 1997. Toxicity of imidacloprid in the German cockroach (Dictyoptera: Blattellidae), and the synergism between imidacloprid and Metarhizium anisopliae (Imperfect Fungi: Hyphomycetes). J. Econ. Entomol. 90: 473–482. [Google Scholar]
- Ko A. E., Bieman D. N., Schal C., Silverman J.. 2016a. Insecticide resistance and diminished secondary kill performance of bait formulations against German cockroaches. Pest Manag. Sci. 72: 1778–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko A. E., Schal C., Silverman J.. 2016b. Cross resistance between hydramethylnon and indoxacarb in German cockroaches (Blattella germanica). In Proceedings, National Conference on Urban Entomology, 22nd–25th May 2016, Albuquerque, NM.
- Koehler P. G., Patterson R. S.. 1986. A comparison of insecticide susceptibility in seven nonresistant strains of the German cockroach, Blattella germanica. J. Med. Entomol. 23: 298–299. [Google Scholar]
- Koehler P. G., Atkinson H., Patterson R. S.. 1991. Toxicity of abamectin to cockroaches (Dictyoptera: Blattellidae, Blattidae). J. Econ. Entomol. 84: 1758–1762. [DOI] [PubMed] [Google Scholar]
- Kopanic R. J., Sheldon W. Jr., Wright C. G.. 1994. Cockroaches as vectors of Salmonella: Laboratory and field trials. J. Food Prot. 57: 125–135. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Yap H., Chong N. L., Lee R.S.T.. 1996. Insecticide resistance and synergism in field collected German cockroaches in Peninsular Malaysia. Bull. Entomol. Res. 86: 675–682. [Google Scholar]
- Limoee M., Ladonni H., Enayati A. A., Vatandoost H., Aboulhasani M.. 2006. Detection of pyrethroid resistance and cross-resistance to DDT in seven field-collected strains of the German cockroach, Blattella germanica (L.). J. Biol. Sci. 6: 382–387. [Google Scholar]
- Liu N., Zhu F., Xu Q., Pridgeon J. W., Gao X.. 2006. Behavioral change, physiological modification, and metabolic detoxification: mechanisms of insecticide resistance. Acta Entomol. Sin. 49: 671. [Google Scholar]
- Menasria T., Tine S., Souad E., Mahcene D., Moussa F., Benammar L., Mekahlia M. N.. 2014. A survey of the possible role of German cockroaches as a source for bacterial pathogens. J. Adv. Sci. Appl. Engl. 1: 67–70. [Google Scholar]
- Naqqash M. N., Gökçe A., Bakhsh A., Salim M.. 2016. Insecticide resistance and its molecular basis in urban insect pests. Parasitol. Res. 1–11. [DOI] [PubMed] [Google Scholar]
- Raghavendra K., Barik T. K., Sharma P., Bhatt R. M., Srivastava H. C., Sreehari U., Dash A. P.. 2011. Chlorfenapyr: A new insecticide with novel mode of action can control pyrethroid resistant malaria vectors. Malar. J. 10: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. L., Smith K. C., Savin N. E., Lavigne R. J.. 1984. Effects of dose selection and sample size on the precision of lethal dose estimates in dose–mortality regression. J. Econ. Entomol. 77: 833–837. [Google Scholar]
- Ross M. H. 1993. Laboratory studies on the response of German cockroaches to an abamectin gel bait. J. Econ. Entomol. 86: 767–771. [Google Scholar]
- SAS Institute 2002-2013. SAS/STAT 13.1 User’s Guide. SAS Institute, Cary, NC. [Google Scholar]
- Schal C., Hamilton R. L.. 1990. Integrated suppression of synanthropic cockroaches. Annu. Rev. Entomol. 35: 521–551. [DOI] [PubMed] [Google Scholar]
- Scharf M. E., Bennett G. W., Reid B. L., Qui C.. 1995. Comparisons of three insecticide resistance detection methods for the German cockroach. J. Econ. Entomol. 88: 536–542. [Google Scholar]
- Scharf M. E., Kaakeh W., Bennett G. W.. 1997. Changes in an insecticide-resistant field population of German cockroach after exposure to an insecticide mixture. J. Econ. Entomol. 90: 38–48. [Google Scholar]
- Scharf M. E., Neal J. J., Bennett G. W.. 1998. Changes of insecticide resistance levels and detoxication enzymes following insecticide selection in the German cockroach, Blattella germanica (L.). Pest. Biochem. Physiol. 59: 67–79. [Google Scholar]
- Scharf M. E., Meinke L. J., Siegfried B. D., Wright R. J., Chandler L. D.. 1999. Carbaryl susceptibility, diagnostic concentration determination, and synergism for US populations of western corn rootworm. J. Econ. Entomol. 92: 33–39. [Google Scholar]
- Silverman J., Bieman D. N.. 1993. Glucose aversion in the German cockroach, Blattella germanica. J. Insect. Physiol. 39: 925–933. [Google Scholar]
- Sims S. R., Appel A. G.. 2007. Linear alcohol ethoxylates: insecticidal and synergistic effects on German cockroaches and other insects. J. Econ. Entomol. 100: 871–879. [DOI] [PubMed] [Google Scholar]
- Tan J., Galligan J. J., Hollingworth R. M.. 2007. Agonist actions of neonicotinoids on nicotinic acetylcholine receptors expressed by cockroach neurons. Neurotoxicology 28: 829–842. [DOI] [PubMed] [Google Scholar]
- Tatfeng Y. M., Usuanlele U., Orukpe A., Digban A. K., Okodua M., Oviasogie F., Turay A. A.. 2005. Mechanical transmission of pathogenic organisms: the role of cockroaches. J. Vector Borne Dis. 42: 129–134. [PubMed] [Google Scholar]
- Valles S. M., Yu S. J.. 1996. Detection and biochemical characterization of insecticide resistance in the German cockroach. J. Econ. Entomol. 89: 21–26. [Google Scholar]
- Umeda K., Yano T., Hirano M.. 1988. Pyrethroid-resistance mechanism in German cockroach, Blattella germanica (Orthoptera: Blattellidae). Appl. Entomol. Zool. 23: 373–380. [Google Scholar]
- Wang C., Bennett G. W.. 2006. Comparative study of integrated pest management and baiting for German cockroach management in public housing. J. Econ. Entomol. 99: 879–885. [DOI] [PubMed] [Google Scholar]
- Wang C., Scharf M. E., Bennett G. W.. 2004. Behavioral and physiological resistance of the German cockroach to gel baits. J. Econ. Entomol. 97: 2067–2072. [DOI] [PubMed] [Google Scholar]
- Wang C., El-Nour M.M.A., Bennett G. W.. 2008. Survey of pest infestation, asthma, and allergy in low-income housing. J. Commun. Health 33: 31–39. [DOI] [PubMed] [Google Scholar]
- Wei Y., Appel A. G., Moar W. J., Liu N.. 2001. Pyrethroid resistance and cross-resistance in the German cockroach, Blattella germanica (L). Pest Manag. Sci. 57: 1055–1059. [DOI] [PubMed] [Google Scholar]
- Whalon M. E., Mota-Sanchez M., Hollingworth R. M.. 2016. Arthropod resistant to pesticides database (ARPD). Available online: http://www.pesticideresistance.org/display.php?page=species&arId=215 (accessed 7 November 2016).
- Wu D., Scharf M. E., Neal J. J., Suiter D. R., Bennett G. W.. 1998. Mechanisms of fenvalerate resistance in the German cockroach, Blattella germanica (L.). Pestic. Biochem. Physiol. 61: 53–62. [Google Scholar]
- Zhu F., Lavine L., O’Neal S., Lavine M., Foss C., Walsh D.. 2016. Insecticide Resistance and Management Strategies in Urban Ecosystems. Insects 7: 2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurek L., Gore J. C., Stringham S. M., Watson D. W., Waldvogel M. G., Schal C.. 2003. Boric acid dust as a component of an integrated cockroach management program in confined swine production. J. Econ. Entomol. 96: 1362–1366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.