Abstract
Over the past decade in North America and Europe, winter losses of honey bee (Hymenoptera: Apidae) colonies have increased dramatically. Scientific consensus attributes these losses to multifactorial causes including altered parasite and pathogen profiles, lack of proper nutrition due to agricultural monocultures, exposure to pesticides, management, and weather. One method to reduce colony loss and increase productivity is through selective breeding of queens to produce disease-, pathogen-, and mite-resistant stock. Historically, the only method for identifying desirable traits in honey bees to improve breeding was through observation of bee behavior. A team of Canadian scientists have recently identified markers in bee antennae that correspond to behavioral traits in bees and can be tested for in a laboratory. These scientists have demonstrated that this marker-assisted selection (MAS) can be used to produce hygienic, pathogen-resistant honey bee colonies. Based on this research, we present a beekeeping case study where a beekeeper’s profit function is used to evaluate the economic impact of adopting colonies selected for hygienic behavior using MAS into an apiary. Our results show a net profit gain from an MAS colony of between 2% and 5% when Varroa mites are effectively treated. In the case of ineffective treatment, MAS generates a net profit benefit of between 9% and 96% depending on the Varroa load. When a Varroa mite population has developed some treatment resistance, we show that MAS colonies generate a net profit gain of between 8% and 112% depending on the Varroa load and degree of treatment resistance.
Keywords: honey bee, marker-assisted selection, economics, Varroa
Honey bees (Apis mellifera L.) play a critical role in our agricultural food system, with an estimated 35% of our diet dependent on honey bee pollination (Klein et al. 2007). Canadian beekeepers operate ∼720,000 honey bee colonies (Nagamuthu 2016) that pollinate many valuable crops in Canada including canola, which contributed $4.4 billion to the Canadian economy in 2013 (Page and Darrach 2016). In 2015, honey bees produced >95.3 million pounds of honey, with a total value of $232 million CDN (Nagamuthu 2016). Although the demand for honey as well as pollinator-dependent agricultural production continues to increase (Brittain et al. 2013), beekeepers have been struggling with significant colony losses in North America, Europe, and globally (Sumner and Boriss 2007; vanEngelsdorp et al. 2007, 2008; Aizen and Harder 2009; Bacandritsos et al. 2010; van der Zee et al. 2012; Canadian Association of Professional Apiculturists [CAPA] 2016).
Canadian honey bee colony winter losses have been documented since 2003, with provincial average winter losses ranging from 10% to 58% (Currie et al. 2010, van der Zee et al. 2012, CAPA 2016). Recent scientific studies have pointed to complex and interactive causes of colony loss (Currie et al. 2010, Guzman-Novoa et al. 2010, Potts et al. 2010, vanEngelsdorp et al. 2013). Among the detrimental pests and pathogens, the Varroa mite is known to be one of the most damaging to colony health (Higes et al. 2007, Johnson et al. 2009, Videau et al. 2011, Henry et al. 2012, Bee Informed Partnership [BIP] 2015a). When untreated colonies exceed treatment thresholds for Varroa, there is reduced honey production and lower winter survival compared with treated colonies (Currie and Gatien 2006). Untreated colonies also exhibit reduced brood area and population size resulting in lower population growth (Ostermann and Currie 2004), which affects key metrics used to determine a grade and corresponding rental fee for colonies used in pollination services (Sagili and Burgett 2011).
Honey bees, like other social insects, live in high densities within a small nest environment, making them particularly vulnerable to disease and pathogens owing to the ease of transmission among nest mates (Schmid-Hempel 1998). As a result, honey bees have evolved, to varying degrees of effectiveness, individual and colony-level defense mechanisms. Social immunity within a honey bee colony can manifest as behaviors such as grooming or hygienic behavior (Spivak and Reuter 2001, Evans et al. 2006). Hygienic behavior is a heritable social immunity response in honey bees that confers disease resistance to the colony by eliminating brood pathogens from the hive environment (Spivak and Reuter 2001). Colonies with queens selected for hygienic behavior have shown lower levels of Varroa infestation than control colonies when left untreated, with comparable honey and brood production (Spivak and Reuter 2001, Guarna et al. 2016). Selective breeding of honey bees is currently used by a small subset of queen breeders who choose traits based on behaviors exhibited by bees in the field (field-assisted selection, or FAS). There are cost, resource, and efficacy barriers that limit the widespread adoption of field-based testing, resulting in few breeders engaging in FAS and even fewer achieving accurate and effective results (Spivak and Gilliam 1998, Pernal et al. 2012). Field testing for hygienic behavior, in particular, relies on a trait-specific test that cannot be used to test for other traits such as honey production or aggression, making the testing process for multiple traits very resource intensive.
An alternative to field testing is the use of molecular diagnostics, specifically marker-assisted selection (MAS), which uses molecular markers to aid the identification of colonies carrying specific traits of interest or the lack of undesirable traits (e.g., aggression). Marker-assisted selection in honey bees is based on proteomic markers and has the potential to provide more rapid selection pressure in queen breeding than FAS, as it would enable breeders to test a larger number of colonies, encompassing and maximizing genetic diversity in the selection pool. Once additional markers are identified, marker tests can include a number of different traits simultaneously (such as hygienic behavior, honey production, and gentleness) and can be assayed by multiple reaction monitoring in the same analysis at no increased cost (Parker et al. 2014). Once a heritable trait has been identified and linked to particular protein markers by researchers, beekeepers can collect and send samples of bees to a diagnostic lab for testing. The results can then be used to screen for potential breeder colonies according to the beekeeper’s own breeding priorities or selection index.
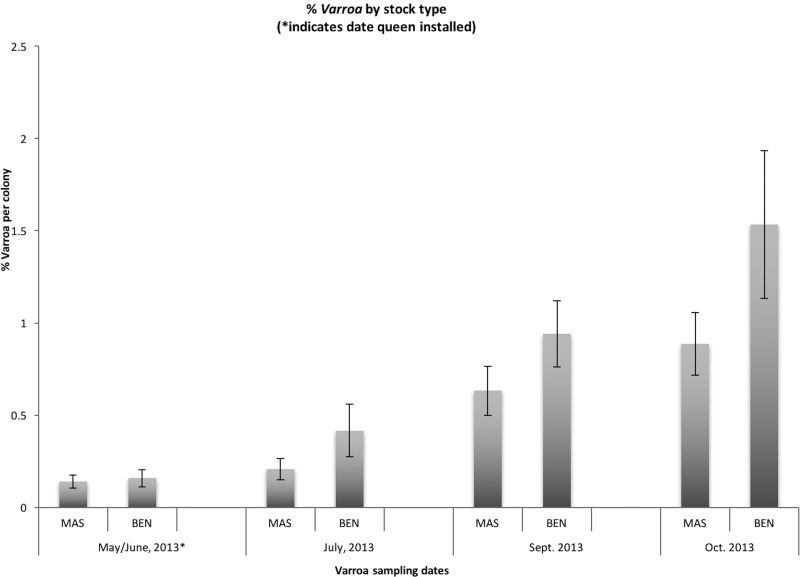
Marker-assisted selection is a new technology in beekeeping at the early stages of development. There is continued research being conducted through the Bee ‘Omics project to further confirm the validity of MAS as a useful integrated pest management (IPM) tool for Canadian beekeepers. This paper presents a case study that evaluates the economic potential of using MAS in a beekeeper’s operation to increase the level of hygienic behavior observed in colonies. Our economic analysis was conducted following a series of scientific experiments during the BeeIPM research project that showed MAS colonies have greater resistance to Varroa through increased winter survival compared with benchmark stocks (Guarna et al. 2016). To complement the scientific evaluation of the honey bee stocks, we conducted a stock evaluation in cooperating commercial beekeepers’ apiaries. This evaluation included over 400 colonies at 12 different operations in Western Canada. Each of the 12 producers were given ∼10 MAS colonies and 10 benchmark (BEN) colonies, and these colonies were managed according to standard commercial practice within each producer’s operation. The producers worked with our field teams to weigh honey output and collect samples for Varroa testing. Figure 1 shows the results for Varroa-infestation levels in these colonies. Marker-assisted selection and BEN colonies were sampled for Varroa at each indicated date. At the time the queen was introduced (in May and June), there was no difference in Varroa levels between the MAS and BEN stocks. By July, the MAS colonies, which were now carrying the progeny of the hygienic queen, showed lower levels of Varroa infestation compared with the BEN stocks, a result that continued throughout the season.
Fig. 1.
Percent Varroa infestation per colony (mean ± SEM) by sampling period and stock type (MAS or BEN). MAS colonies n = 147 (July), 133 (September), 95 (October); BEN colonies n = 127 (July), 109 (September), 64 (October). Unpaired t-test assuming unequal variances, July: 166 df, P = 0.086; September: 207 df, P = 0.084; October: 86 df, P = 0.0699.
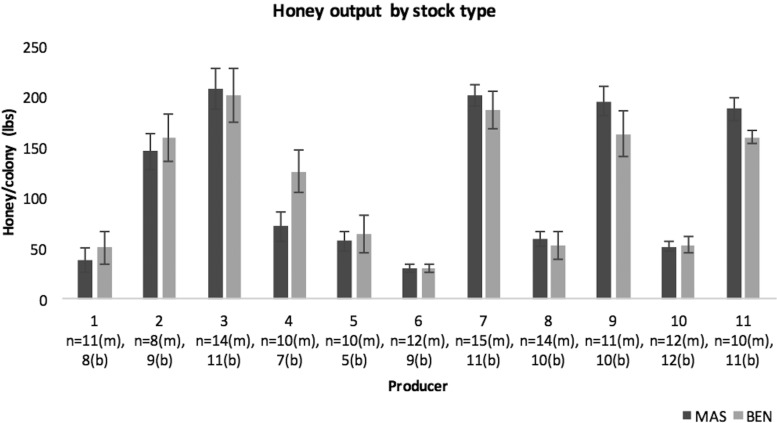
One of the concerns with using a selective breeding tool that targets social immunity such as hygienic behavior is the potential trade-off with desirable traits such as honey production. Honey output was measured in experimental colonies and found to be equivalent across all stocks (Guarna et al. 2016). To examine the potential for a hygienic behavior versus honey production trade-off in field conditions, the producer-managed colonies were also weighed for honey production during the summer of 2013. As each province and each producer has specific regional characteristics that contribute to a honey bee colony’s honey production potential, these honey results are presented by producer in Fig. 2. In nine of the 11 operations with honey production data, we observed no significant difference in honey output between our BEN stocks and our MAS stocks. Benchmark stocks for producer 4 generated more honey than the same operation’s MAS stocks, whereas BEN stocks for producer 11 generated less honey than the same operation’s MAS stocks.
| Producer |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| df | 14 | 14 | 20 | 11 | 6 | 15 | 16 | 14 | 15 | 18 | 15 |
| P | 0.277 | 0.329 | 0.419 | 0.021 | 0.375 | 0.495 | 0.247 | 0.345 | 0.123 | 0.426 | 0.028 |
Fig. 2.
Honey production by producer (mean ± SEM) for MAS and benchmark stocks. Unpaired t-test assuming unequal variances: df and P values by producer reported as: Producer 1: df 14; p= 0.277, Producer 2: df: 14, p=0.329, Producer 3: df: 20, p= 0.419, Producer 4: df: 11, p= 0.021, Producer 5: df: 6, p= 0.375, Producer 6: df: 15, p= 0.495, Producer 7: df: 16, p= 0.247, Producer 8: df: 14, p= 0.345, Producer 9: df: 15, p= 0.123, Producer 10: df: 18,p=0.426 and Producer 11: df: 15,p= 0.028.
To evaluate the net benefit of MAS, we present a Canadian beekeeping case study based on these experimental and field results, where MAS is shown to be a profitable new IPM tool. For this analysis, we describe a Canadian beekeeper who has 40 colonies in one apiary. Half of the colonies are dedicated honey-producing colonies with no commercial contract for pollination and half are commercial pollinating colonies that have one pollination rental contract per year per hive with a hybrid canola seed company and a relatively lower honey output. At the beginning of the season, the beekeeper has the choice to introduce queens having been identified as having hygienic behavior traits using MAS into some of his colonies.
The beekeeper monitors all of his colonies for Varroa, as suggested by the Canadian industry-produced recommended practices for IPM (Eccles et al. 2016). Provincial recommendations and studies suggest that treated colonies with Varroa infestations above the treatment threshold have better outcomes than nontreated colonies (Currie 2008; Guzman-Novoa et al. 2010; BIP 2015a,b; Canadian Provincial Government Treatment Recommendations [CPGTR] 2015; Nasr 2015). Half of the colonies in our case study are identified by the beekeeper as having a lower level of Varroa that is just above the treatment threshold. These colonies experience moderate deterioration if left untreated. The other half of the colonies are identified as having a higher level of Varroa infestation that causes significant deterioration if left untreated. When the beekeeper monitors his MAS colonies for Varroa at the time that the queens are established, the colonies exhibit the same Varroa levels as non-MAS colonies, as the queen has not had time to propagate and the workers are not yet her progeny. Based on our experimental results, however, that predict that these innately resistant MAS colonies will effectively mitigate Varroa infestations, the beekeeper’s MAS colonies require less treatment and experience less deterioration than non-MAS colonies if left untreated.
After the colonies are monitored, the beekeeper has the choice of whether or not to treat any given colony for Varroa. If the beekeeper chooses to treat the Varroa, we assume that he or she follows the proper label use directions and treatment is effective in controlling the mite to realize the colony’s honey and pollination potential. The treatment chosen by the beekeeper can be any combination of chemicals or natural substances that meets the requirements for Varroa treatment. Because untreated Varroa infestations lead to morbidity and premature mortality (Fries et al. 2006), an untreated colony infested with a higher Varroa load is unlikely to survive through the season and we explore the economic impact from this untreated deterioration, mortality, and cost of colony replacement. We assume that the colonies that must be replaced generate reduced profit before they die (according to their deteriorated levels of productivity).
Each of the beekeeper’s colonies thus generates profit subject to the following criteria: a honey or pollinating colony; higher or lower Varroa infestation; led by a hygienic MAS queen or not; and treated for Varroa or left untreated. Colony profit is calculated based on the colony’s honey and pollination revenue and its ability to mitigate the deterioration caused by the Varroa mite, which is a function of the initial Varroa-infestation level, treatment effectiveness, and the colony’s innate resistance. Our variables are parameterized based on industry data from Statistics Canada, industry and peer-reviewed study data, and our own cooperating producer data set. Subsequent calculations explore the economic consequences of varying degrees of treatment resistance developing in the Varroa mite population.
Materials and Methods
A Beekeeper Case Study
Beekeeper profit for colony i:
Where Πiv is profit for colony i with Varroa infestation v, is honey revenue, is pollination rental revenues, is operation and maintenance costs, is a targeted Varroa treatment cost, is the deterioration from a lack of effective Varroa treatment, is the Varroa load, and is the rate of treatment resistance in the Varroa mite population. α(0,1) is introduced only when there is evidence of treatment resistance developing in the Varroa mite population. Honey and pollination revenues reflect output from a fully productive colony. Any net productivity loss comes from the Varroa load and deterioration parameters.
Honey price is consistent with the Statistics Canada average honey price for 2015 (Statistics Canada [Stats Can] 2015) and can also be derived from calculating the $/lb for honey from the 2014 data in Page and Darrach 2016, where the total value of honey was CDN$201, 620 for 81,556 pounds of honey. This price does not account for the dramatic fall in prices in the fall 2016 (http://www.cbc.ca/news/canada/calgary/canadian-beekeepers-face-plummeting-prices-1.3746822, accessed 28 February 2017), and so we also calculate the economic impact of this lower honey price of $1.13/lb. Quantity of honey produced by a focused honey-producing colony is derived from a 2011 study in Alberta, where the average colony produced 143 lbs of honey (Laate 2013), and from our Canadian honey bee producer data and discussions collected during the BeeIPM project. Our 12 cooperating producers managed a mixture of honey-producing and commercial pollinating colonies, with an average honey output of just over 110 lbs per colony. Some of the high yield honey producers yielded well over 200 pounds. In this case study, our fully productive focused honey-producing colony yields 200 pounds, whereas our commercial pollinator yields 100 pounds. The pollination rental fee received from a canola grower for a healthy colony in Alberta is CDN$150 (Canadian Honey Council [CHC] 2016).
The total production cost to keep a colony in Alberta was estimated by Laate (2013) as being $230CDN. This figure does not explicitly include supplemental targeted treatment costs for pathogens and diseases, which we estimate at $13CDN as an average annual supplemental treatment cost for a number of different Varroa treatment options including labor (Apiguard [thymol], ApiLife Var [thymol + essential oils], THYMOVAR [thymol], Apivar [amitraz], CheckMite+ [coumaphos], Apistan [fluvalinate, formic acid fumigation, oxalic acid], and Hopguard [potassium salt of hop beta acids]; Brushy Mountain Bee Farms [BMBF] 2016, Dadant 2016, Mannlake 2016). For innately resistant MAS colonies, there is no treatment necessary at lower Varroa levels of 2%, and at higher loads of 20%, MAS colonies are assumed to require half the number of treatments ($6.50).
Varroa levels in our field experiments are measured as a percent of Varroa infestation per colony (number of mites per 100 bees). Varroa levels and economic thresholds vary by season and region, with spring thresholds for treatment in the Canadian Prairies at 1% (Currie 2008). This threshold indicates the level of Varroa infestation above which an untreated colony will deteriorate and suffer economic consequences. In our case study, both the lower Varroa level (2%) and higher Varroa level (20%) are above the treatment threshold at the time of monitoring and treatment. In our colony profit calculations, MAS and non-MAS colonies are exposed to both 2% and 20% Varroa infestations. Untreated MAS colonies are more resistant and thus able to reduce Varroa levels and mitigate harm more effectively than untreated non-MAS colonies, which are taken into account in each colony’s deterioration calculations. When we calculate apiary profit, we assume that our beekeeper has chosen to replace some of the non-MAS colonies with MAS colonies that have already reduced their Varroa load to 2% and do not require treatment, and are thus, not susceptible to treatment resistance.
The deterioration parameter, reflects a given colony’s ability to mitigate its Varroa challenge in the absence of effective treatment. The greater the Varroa infestation in a colony, the greater the economic consequences when a colony is not treated effectively. Currie and Gatien (2006) showed that honey production alone can fall by as much as 76% per colony owing to a lack of Varroa treatment when lower Varroa levels of only 2% were identified in the spring. Their results also showed that at significantly higher Varroa levels of 21%, honey output fell by over 4000% per untreated colony. Our deterioration variable in the case study transforms the Varroa-infestation level into a loss of colony productivity, which includes increased morbidity, decreased brood production, decreased population growth, and decreased honey production. For our case study, we parameterize the deterioration variable to capture just a portion of this potential loss given Varroa levels similar to Currie and Gatien’s experiments. Untreated non-MAS colonies in our case study experience a 15% decrease in total productivity (= 7.5) at 2% Varroa levels and a total productivity decrease of 40% (= 2) when challenged with Varroa levels of 20%. Innately resistant untreated MAS colonies experience no productivity loss at lower Varroa levels and a 10% productivity loss (= 0.5) when higher Varroa levels of 20% are identified at the time of monitoring. The economic impact of colony mortality is calculated separately from this productivity loss. As significant untreated Varroa leads to mortality (Fries et al 2006, Currie and Gatien 2006), in our case study, both non-MAS and MAS colonies with untreated Varroa loads of 20% do not survive the season and must be replaced.
Resistance in Varroa mites to acaracide treatments is becoming more common with increasing concern about the associated colony health and beekeeper costs (Spreafico et al. 2001, Goodwin et al. 2005, Pettis 2004, Hillesheim et al. 1996). To account for some treatment resistance, we add a treatment resistance variable, , that generates some deterioration of the colony’s productivity when treatment is not fully effective (equals 1, we have full resistance to the treatment in the Varroa mite population). As some treatment resistance develops, the treated colony deteriorates, eventually becoming equal to the no treatment case when we have full resistance. We allow treatment resistance to be 0%, 25%, 50%, and 75% in our case study.
In the case study, we assume that with the development of treatment resistance in the Varroa mite population, the treated non-MAS colonies are unable to survive when treatment resistance reaches 50%, when they are challenged with a 2% Varroa load, and when treatment resistance reaches 25% with a higher 20% Varroa load. For MAS colonies, as no treatment is necessary at lower 2% Varroa levels, MAS colonies survive regardless of treatment resistance, and with higher 20% Varroa levels, treated MAS colonies are considered unable to survive when treatment resistance reaches 75%. When a colony with higher Varroa is not treated effectively, that colony is unlikely to survive the season and will generate reduced profit according to the deteriorated levels of productivity and then will be replaced. The cost of replacing the colony is assumed to be the market cost of purchasing a nucleus colony or a package of bees (1 kg of workers and a mated queen), which can range from $180–$230 (Laate 2013, Urban Bee Supplies [UBS] 2017, Valley Beekeeping Supply [VBS] 2017). For our calculations, the cost of replacement is $180.
Experimental data show colonies that are bred for hygienic behavior exhibit lower levels of Varroa (Spivak and Reuter 2001) and greater survival (Guarna et al. 2016), ultimately requiring less or no targeted Varroa treatment depending on levels of infestation. Our MAS colonies are left untreated under lower Varroa pressure, and with higher Varroa infestation, we apply half of the typical targeted Varroa treatment in a given year (one treatment at $6.50 per colony). Beekeepers would likely apply a reduced number of treatments in a given year as opposed to half a dose per treatment. The current market cost of MAS testing is $3 per queen.
Results
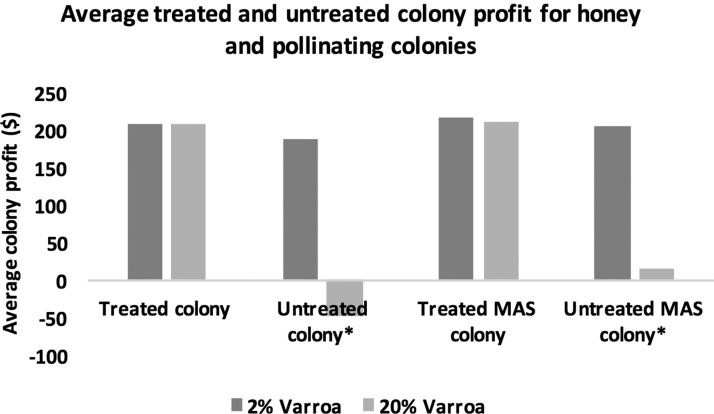
We present colony profit results as an average of a focused honey-producing colony’s profit and a commercial pollinating colony’s profit, except in the case of honey price variability which has differential effects on our two types of colony. Apiary profit is calculated based on the profit of 40 colonies, with a varying number of resistant MAS colonies replacing at-risk non-MAS colonies. Effective Varroa treatment has an average net profit benefit for non-MAS honey and pollinating colonies of 9.7% when Varroa levels are 2% (Fig. 3). When faced with higher Varroa loads, untreated colonies do not survive the season and as a result, these colony profits subtract the cost of colony replacement, resulting in a net profit benefit of treatment increasing to 128% when Varroa levels are 20%. Honey and pollinating MAS colonies challenged with a 2% Varroa load show an average net profit benefit of 4.8% when compared with treated non-MAS colonies (owing to a lack of treatment cost for MAS colonies), and an even smaller net profit increase of 1.7% when faced with a 20% Varroa load for which MAS colonies do require treatment. When our beekeeper chooses not to treat his colonies for Varroa, we see an average net profit increase of 8.9% for the untreated honey and pollinating MAS colonies challenged with a 2% Varroa level compared with untreated non-MAS colonies. When these honey and pollinating colonies are faced with a 20% Varroa load, untreated MAS colonies show an average net profit benefit of 96% compared with untreated non-MAS colonies.
Fig. 3.
Honey and pollinating colony average profits for treated and untreated Varroa infestations of 2% and 20% with non-MAS and MAS colonies. *Untreated colonies with 20% Varroa loads do not survive and are replaced.
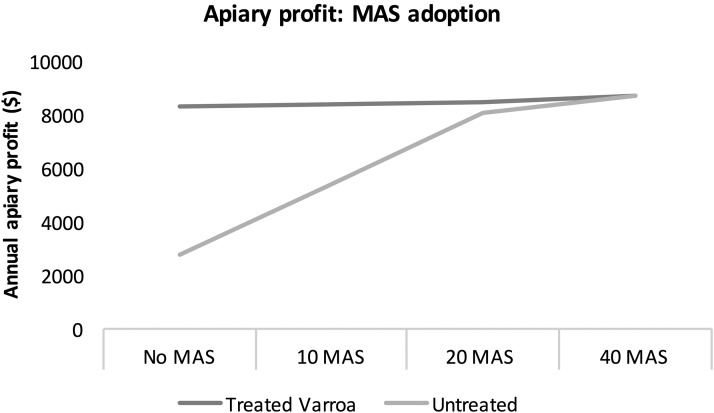
Figure 4 shows the net profit effect at an apiary level for a beekeeper who increases the number of resistant MAS colonies with 2% Varroa levels in his yard. The beekeeper first replaces his non-MAS colonies that are challenged with 20% Varroa levels, and then once all these high-risk colonies are replaced, the non-MAS colonies with 2% Varroa are replaced. Table 1 outlines the distribution of colonies for this calculation. An apiary of 40 untreated MAS colonies generates over 210% more profit than an apiary with 40 untreated non-MAS colonies. The net profit gain from adopting MAS colonies and replacing untreated non-MAS colonies with high Varroa loads decreases after the number of MAS colonies reaches half the apiary (20 colonies). Once the beekeeper replaces his weakest non-MAS colonies that are challenged with 20% Varroa and are unable to survive without treatment, the element of colony survival is taken out of the equation, as the remaining non-MAS colonies with lower 2% Varroa loads and the MAS colonies are all able to mitigate mite loads and survive the season.
Fig. 4.
Forty-colony apiary profit with gradual adoption of MAS colonies replacing weakest (highest Varroa loads) existing non-MAS colonies. Untreated colonies with high Varroa loads do not survive the season and are replaced.
Table 1.
Distribution of colonies adopted in a 40-colony apiary
| 2% Varroa |
20% Varroa |
|||
|---|---|---|---|---|
| Commercial pollinator | Honey producer | Commercial pollinator | Honey producer | |
| 0 MAS colonies | 10 non-MAS | 10 non-MAS | 10 non-MAS | 10 non-MAS |
| 10 MAS colonies | 15 (10 non-MAS + 5 MAS) | 15 (10 non-MAS + 5 MAS) | 5 non-MAS | 5 non-MAS |
| 20 MAS colonies | 20 (10 non-MAS + 10 MAS) | 20 (10 non-MAS + 10 MAS) | 0 | 0 |
| 40 MAS colonies | 20 MAS | 20 MAS | 0 | 0 |
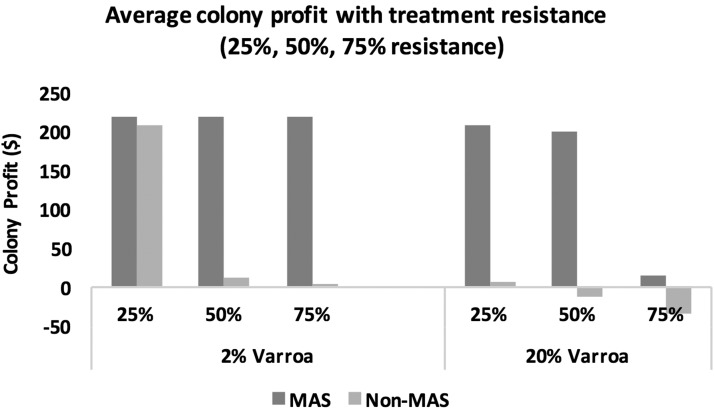
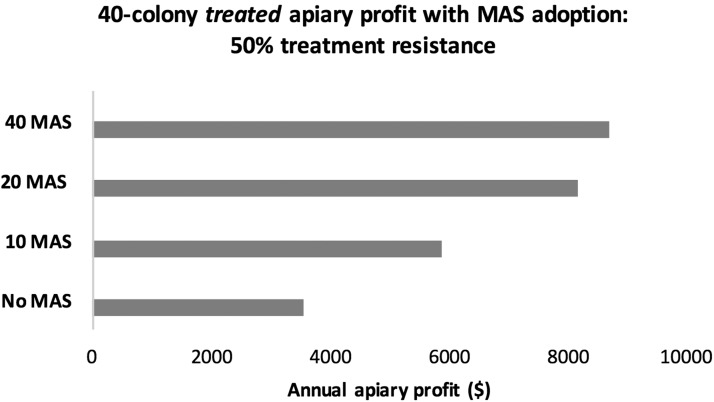
When there is 25% treatment resistance within a honey or pollinating colony’s Varroa population in our case study, we see an average net profit gain of 8.4% with Varroa loads of 2% and a 102% average net profit gain under Varroa loads of 20% for treated MAS colonies compared with treated non-MAS colonies (Fig. 5). At 50% treatment resistance, the average net profit gain for a treated MAS colony reaches 99.3% at Varroa loads of 2% and 112.6% at Varroa loads of 20%, when compared with a treated non-MAS colony. When there is 75% treatment resistance within a colony, there is a 77.5% average net profit gain for treated honey and pollinating MAS colonies compared with treated non-MAS colonies. At an apiary level with 50% treatment resistance overall, Fig. 6 shows a net profit gain of 145% from replacing 40 treated non-MAS colonies with 40 innately resistant MAS colonies.
Fig. 5.
Average honey and pollinating colony profit for treated and untreated Varroa infestations of 2% and 20%, with varying levels of treatment resistance within the Varroa mite population for MAS and non-MAS colonies. Non-MAS colonies are now unable to survive when treatment resistance reaches 50% when faced with a 2% Varroa load, and when treatment resistance reaches 25% with a higher 20% Varroa load. For MAS colonies, as no treatment is necessary at lower Varroa levels, MAS colonies survive regardless of treatment resistance, and with higher Varroa levels, treated MAS colonies are considered unable to survive when treatment resistance reaches 75%.
Fig. 6.
A 40-colony apiary (half honey producers and half commercial pollinators) with gradual MAS colony adoption when treatment resistance develops in the Varroa mite population at 50%. In this simulation with 50% treatment resistance, all treated and untreated non-MAS colonies with 2% and 20% Varroa loads are replaced. Marker-assisted selection colonies are replaced at 20% Varroa loads. *Untreated colonies with 20% Varroa loads do not survive and are replaced.
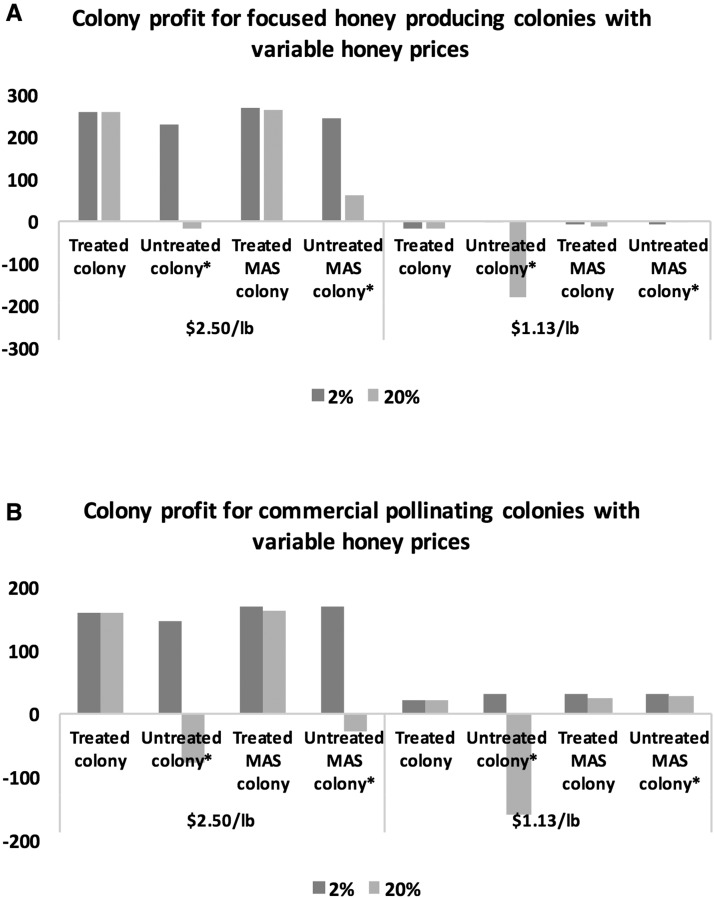
When we take into account the recent drop in honey prices in Canada from $2.50/lb to $1.13/lb, both the commercial pollinating colonies and the focused honey producers experience significant drops in profit. At the lower honey price, we see negative profits for all focused honey-producing colonies at all Varroa levels, with the highest profit of −$3.40 generated by an untreated non-MAS colony subjected to a 2% Varroa load (Fig. 7a). Given the lower honey price, with a 2% Varroa level, there is no longer an economic incentive to treat colonies or to invest in MAS colonies. However, at a 20% Varroa level, a focused honey-producing non-MAS colony still generates a net profit gain from treatment of over 90%, highlighting the economic benefit of treatment at high levels of infestation. As well, an untreated focused honey-producing MAS colony with a 20% Varroa level generates 97% more profit than an untreated non-MAS colony, pointing to the value of innate resistance as a risk mitigator in the face of ineffective treatments and market fluctuations. For commercial pollinating colonies, we see marginally higher profits with a similar pattern: 30% net decrease in profit from treating a non-MAS colony at lower Varroa-infestation levels; and a 116% net profit increase from adopting an untreated commercial pollinating MAS colony compared with an untreated non-MAS pollinator at higher Varroa levels (Fig. 7b).
Fig. 7.
(a,b) Colony profit for MAS and non-MAS focused honey-producing colonies and commercial pollinating colonies with variable honey prices and treatment decisions as well as low and high Varroa-infestation levels.
Discussion
Marker-assisted selection testing could provide beekeepers with a valuable tool for improving the accuracy and efficiency of their efforts to breed stronger, more resistant colonies in the face of Varroa or other pathogen challenges while also reducing their vulnerability to treatment resistance. This case study showed that given that there is a 10–128% net profit benefit from treatment for Varroa infestations of 2% and 20%, MAS honey bee colonies that are innately resistant to Varroa will add value to a beekeeping operation of between 9% and over 96% when there is a lack of treatment. Innate resistance could play an important role as a risk mitigation tool when treatment resistance threatens to develop in a Varroa mite population, increasing net colony profits by between 8% and 112% depending on the level of treatment resistance. We see profit gains of over 200% for our beekeeper in the case study from replacing weaker non-MAS colonies that are not able to effectively manage their higher Varroa loads with stronger more resistant MAS colonies. With a greater number of stronger colonies that have an innate resistance to Varroa, treatment becomes less critical, as these colonies are better able to survive without effective acaracide or other treatment.
When a colony is faced with a Varroa load of 20% and honey prices fall, both treatment and MAS adoption are still able to mitigate the negative effects of Varroa and minimize the decrease in profits. However, when a focused honey producer or a commercial pollinating colony is challenged with a 2% Varroa load and honey prices fall to less than half of their previous level, it is no longer profitable to treat these colonies or to adopt MAS colonies. As treatment becomes less profitable under these conditions, however, there is a negative externality as more untreated colonies will populate a beekeeper’s apiary, which will spread the Varroa infestation to other colonies and potentially result in greater than anticipated deterioration and mortality. Fries and Camazine (2001) suggest that selecting for healthier, more disease-resistant colonies in an apiary reduces the risk of intercolony transmission of pathogens, whereas others have shown that healthier colonies are also assumed to resist or absorb the impact of negative elements more effectively than weaker colonies (Cornman et al. 2012, Cavigli et al. 2015). There are hidden economic consequences resulting from a lack of treatment that need to be evaluated carefully by a beekeeper. As well, the greater the number of strong, resistant colonies in bee apiaries, the lower the operating costs for beekeepers and the larger supply of pollinator strength colonies available for crop pollination. In the long run, as colony health and strength increase, we are likely to see greater average honey production and higher pollination rental grades and rates for beekeepers. As well, when MAS testing comes to market and is adopted by an increasing number of diagnostic labs (most university protein mass spectrometry core labs can offer these types of analyses with little effort by just adding in honey bee proteins), the cost per test will fall, further increasing MAS colony profits for beekeepers.
Our parameterization of the deterioration variable likely captures only a fraction of the productivity impact from a lack of effective treatment, which would mean potentially even greater profit gains from adopting MAS colonies. The assumption built into our case study that MAS colonies require some treatment when faced with higher Varroa loads may further underestimate the economic impact of MAS when treatment resistance develops in the Varroa mite population. On-going research may reveal that MAS colonies have enough Varroa resistance that they do not require any treatment and thus are not at all susceptible to the development of treatment resistance. As well, our simulation assumes that non-MAS colonies challenged with low Varroa loads are able to survive through the season. However, some nonresistant colonies may be unable to withstand even low levels of Varroa above the treatment threshold and need replacing, resulting in even greater profit differentials between MAS and non-MAS colonies.
This case study also focuses on the isolated economic impact of adopting queens with the hygienic behavior (HB) trait identified through MAS in one beekeeper’s apiary. As current and future research progresses, MAS breeding could be shown to have much broader implications for the honey bee industry, namely, that a) multiple traits can be identified simultaneously at no increased cost, resulting in greater economic, social, and colony health benefits for the beekeeper. For example, increased revenues from higher honey production alongside decreased mortality from Varroa mites and less aggression in the bees; b) screening of many colonies at one time becomes possible with MAS; c)MAS enables screening breeder queens for markers developed in other countries, such as screening New Zealand queens for Canadian winterability; and finally d) MAS screening could provide a valuable insurance policy for beekeepers who are faced with mitigating the risk of impending treatment failure for a number of pathogens, diseases, and pests.
Marker-assisted selection breeding tools have the potential to increase colony profit and reduce mortality, which could contribute positively to the continued viability of the apiculture industry globally, particularly in the face of increased Varroa infestations and the risk of treatment resistance. More research into the effects of hygienic behavior and more specific Varroa-sensitive hygiene (VSH) would allow us to better predict the impact of the HB or VSH trait on highly infected colonies. As well, further scientific inquiry into the genomics of honey bees and the correlations between trait identification, expression, treatment resistance, and the marginal benefit to the beekeeper is necessary. Finally, expanding the selective breeding queen supply sector in Canada and globally through knowledge translation to industry about selective breeding tools is essential for optimal adoption.
Acknowledgments
This work was supported by funding from Genome Canada, Genome British Columbia, Genome Alberta, The British Columbia Honey Producers Association through the Boone–Hodgson–Wilkinson Fund, the British Columbia Blueberry Council, the University of British Columbia, the University of Manitoba, the Alberta Crop Industry Development Fund, the US Department of Agriculture, and Agriculture and Agri-Food Canada through the BeeIPM project (107BEE).
References Cited
- Aizen M. A., Harder L.. 2009. The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Curr. Biol. 19: 915–918. [DOI] [PubMed] [Google Scholar]
- Bacandritsos N., Granato A., Budge G., Papanastasiou I., Roinioti E., Caldon M., Falcaro C., Gallina A., Mutinelli F.. 2010. Sudden deaths and colony population decline in Greek honey bee colonies. J. Invertebr. Pathol. 105: 335–340. [DOI] [PubMed] [Google Scholar]
- (BIP 2015a) Bee Informed Partnership National Management Survey Results 2015a. (https://beeinformed.org/results/the-bee-informed-partnership-national-management-survey-2014-2015, accessed 28 February 2017)
- (BIP 2015b) Bee Informed Partnership National Management Survey Results 2015b. (https://beeinformed.org/results-categories/national-management/, accessed 28 February 2017)
- (BMBF) Brushy Mountain Bee Farms 2016. (http://www.brushymountainbeefarm.com/searchprods.asp, accessed 28 February 2017)
- Brittain C., Williams N., Kremen C., Klein A. M.. 2013. Synergistic effects of non-Apis bees and honey bees for pollination services. Proc. R. Soc. London, Ser. B. 280: 20122767. doi: 10.1098/rspb.2012.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (CAPA 2016) Canadian Association of Professional Apiculturists wintering losses. 2016. Annual Colony Loss Reports: CAPA Statement on Honey Bee Losses in Canada: (2007-2016). (http://capabees.org/capa-statement-on-honey-bees/, accessed 28 February 2017)
- Cavigli I., Daughenbaugh K. F., Martin M., Lerch M., Banner K., Garcia E., Brutscher L. M., Flenniken M. L.. 2015. Pathogen prevalence and abundance in honey bee colonies involved in almond pollination. Apidologie 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (CHC 2016) Canadian Honey Council. 2016. (http://www.honeycouncil.ca/honey_industry_overview.php., http://www.honeycouncil.ca/industry.php#stats, accessed 28 February 2017)
- Cornman R. S., Tarpy R., Chen Y., Jeffreys L., Lopez D., Pettis J. S.. 2012. Pathogen webs in collapsing honey bee colonies. PLoS ONE 7: e43562. doi:10.1371/journal.pone.0043562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (CPGTR) Canadian Provincial Government Treatment Recommendations. 2015. Varroa mite controls. (http://www2.gov.bc.ca/assets/gov/farming-natural-resources-and-industry/agriculture-and-seafood/animal-and-crops/animal-production/bee-assets/api_fs221.pdf, accessed 28 February 2017) (Recommendations for Management of Honey Bee Diseases and Pests in Alberta 2014-2015 (http://www1.agric.gov.ab.ca/$Department/deptdocs.nsf/all/prm13239/$FILE/2014-recommendations.pdf, accessed 28 February 2017)) Disease control recommendations 2015 (http://publications.gov.sk.ca/documents/20/84007-60f10eb7-cee7-481e-a4f0-6d1003bbad52.pdf, accessed 28 February 2017) 2013 Recommendations for administering antibiotics and acaracides to honey bees (http://gov.mb.ca/agriculture/crops/production/pubs/2013_recommendations_may14_final1.pdf, accessed 28 February 2017) (http://techtransfer.ontariobee.com/index.php?action=display&cat=52&v=76, accessed 28 February 2017) 2014 Ontario treatment recommendations for honey bee disease and mite control (http://www.omafra.gov.on.ca/english/food/inspection/bees/2014-treatment.htm#varroa, accessed 28 February 2017)
- Currie R. W., Gatien P.. 2006. Timing acaricide treatments to prevent Varroa destructor (Acari: Varroidae) from causing economic damage to honey bee colonies. Can. Entomol. 138: 238–252. [Google Scholar]
- Currie R. W. 2008. Economic threshold for Varroa on the Canadian Prairies. (http://capabees.org/content/uploads/2013/02/varroathreshold.pdf, last accessed February 28, 2017)
- Currie R. W., Pernal F., Guzman N. E.. 2010. Honey bee colony losses in Canada. J. Apic. Res. 49: 104–106. [Google Scholar]
- Dadant 2016. (https://www.dadant.com/catalog/medications/m001261-apistan-c-10, accessed 28 February 2017)
- Eccles L., Kempers M., Gonzalez R. M., Thurston D., Borges D.. 2016. Canadian best management practices for honey bee health: Industry analysis and harmonization. Bee Health Round Table, Agriculture and Agri-Food, Canada. (http://www.honeycouncil.ca/images2/pdfs/BMP_manual_-_Les_Eccles_Pub_22920_-_FINAL_-_low-res_web_-_English.pdf, accessed 28 February 2017)
- Evans J. D., Aronstein K., Chen Y. P., Hetru C., Imler J. L., Jiang H., Kanost M., Thompson G. J., Zou Z., Hultmark D.. 2006. Immune pathways and defense mechanisms in honey bees, Apis mellifera. Insect Mol. Biol. 15: 645–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries I., Camazine S.. 2001. Implications of horizontal and vertical pathogen transmission for honey bee epidemiology. Apidologie 32: 199–214. [Google Scholar]
- Fries I., Imdorf A., Rosenkranz P.. 2006. Survival of mite infested (Varroa destructor) honeybee (Apis mellifera) colonies in a Nordic climate. Apidologie 37: 564–570. [Google Scholar]
- Goodwin R. M., Taylor A., McBrydie H. M., Cox H. M.. 2005. Base levels of resistance to common control compounds by a New Zealand population of Varroa destructor. N. Z. J. Crop Hortic. Sci. 33: 347–352. [Google Scholar]
- Guarna M. M., Hoover S., Huxter E., Higo H., Moon K.-M., Domanski D., Bixby M., Melathopoulos A. P., Ibrahim A., Peirson M., et al. 2016. Expression biomarkers used for the selective breeding of complex polygenic traits. (http://biorxiv.org/content/early/2016/09/19/076174, accessed 28 February 2017) [DOI] [PMC free article] [PubMed]
- Guzman-Novoa E., Eccles L., Calvete Y., Mcgowan J., Kelly P. G., Correa-Benitez A.. 2010. Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie 41: 443–450. [Google Scholar]
- Henry M., Rollin O., Aptel J., Tchamitchian S., Beguin M., Requier F., Rollin O., Decourtye A.. 2012. A common pesticide decreases foraging success and survival in honey bees. Science 336: 348–350. [DOI] [PubMed] [Google Scholar]
- Higes M., Garcia-Palencia P., Martin-Hernandez R., Meana A.. 2007. Experimental infection of Apis mellifera honey bees with Nosema ceranae (Microsporidia). J. Invertebr. Pathol. 94: 211–217. [DOI] [PubMed] [Google Scholar]
- Hillesheim E., Ritter W., Bassand D.. 1996. First data on resistance mechanisms of Varroa jacobsoni (OUD.) against tau-fluvalinate. Exp. Appl. Acarol. 20: 283–296. [Google Scholar]
- Johnson R. M., Ellis M., Mullin C. A., Frazier M.. 2009. Pesticides and honey bee toxicity-USA. Apidologie 41: 312–331. [Google Scholar]
- Klein A. M., Vaissiere E., Cane J. H., Steffan-Dewenter I., Cunningham S. A., Kremen C., Tscharntke T.. 2007. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. London, Ser. B. 274: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laate E. 2013. Economics of beekeeping in Alberta 2011. Economics Branch, Economics and Competitiveness Division, Alberta Agriculture and Rural Development. (http://www1.agric.gov.ab.ca/$department/deptdocs.nsf/all/agdex14472/$file/821-62.pdf, accessed 28 February 2017)
- Mannlake 2016. (https://www.mannlakeltd.com/shop-all-categories/hive-colony-maintenance/medications-treatments-herbicides/varroa-mites, accessed 28 February 2017)
- Nagamuthu N. 2016. Statistical overview of the Canadian Honey and Bee Industry 2015., Horticulture and Cross Sectoral Division, Market Analysis and Information Section, Agriculture and Agri-Food Canada. To request a copy: (http://www.agr.gc.ca/eng/industry-markets-and-trade/statistics-and-market-information/by-product-sector/horticulture-industry/horticulture-sector-reports/?id=1368482338314, accessed 28 February 2017)
- Nasr M. 2015. Recommendations for management of honey bee diseases and pests in Alberta, 2014-2015 Alberta Agriculture and Rural Development. (http://www1.agric.gov.ab.ca/$Department/deptdocs.nsf/all/prm13239/$FILE/2014-recommendations.pdf, accessed 28 February 2017)
- Ostermann D., Currie R. W.. 2004. The effect of formic acid formulations on honey bee, Apis mellifera L., colonies, and the influence of colony and ambient conditions on formic acid concentration in the hive. J. Econ. Entomol. 97: 1500–1508. [DOI] [PubMed] [Google Scholar]
- Page S., Darrach M.. 2016. Statistical overview of the Canadian Honey and Bee Industry and the economic contribution of honey bee pollination 2013–2014. Horticulture and Cross Sectoral Division Agriculture and Agri-Food Canada. (http://www.agr.gc.ca/resources/prod/doc/pdf/1453219857143-eng.pdf, accessed 28 February 2017)
- Parker C. E., Domanski D., Percy A. J., Chambers A. G., Camenzind A. G., Smith D. S., Borchers C. H.. 2014. Mass spectrometry in high-throughput clinical biomarker assays: Multiple reaction monitoring. Top. Curr. Chem. 336: 117–137. doi: 10.1007/128_2012_353. [DOI] [PubMed] [Google Scholar]
- Pettis J. 2004. A scientific note on Varroa destructor resistance to coumaphos in the United States. Apidologie 35: 91–92. [Google Scholar]
- Pernal S. F., Sewalem A., Melathopoulos A. P.. 2012. Breeding for hygienic behavior in honeybees (Apis mellifera) using free-mated nucleus colonies. Apidologie 43: 403–416. [Google Scholar]
- Potts S. G., Biesmeijer C., Kremen C., Neumann P., Schweiger O., Kumin W. E.. 2010. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 25: 345–353. [DOI] [PubMed] [Google Scholar]
- Sagili R. R., Burgett D. M.. 2011. Evaluating Honey Bee Colonies for Pollination A Guide for Commercial Growers and Beekeepers A Pacific Northwest Extension Publication Oregon State University, University of Idaho, Washington State University PNW 623, (https://catalog.extension.oregonstate.edu/pnw623, accessed 28 February 2017)
- Schmid-Hempel P. 1998. Parasites in Social Insects. Princeton University Press, Princeton, NJ. [Google Scholar]
- Spivak M., Gilliam M.. 1998. Hygienic behaviour of honey bees and its application for control of brood diseases and Varroa Part II. Studies on hygienic behaviour since the Rothenbuhler era. Bee World 79: 169–186. [Google Scholar]
- Spivak M., Reuter G. S.. 2001. Resistance to American foulbrood disease by honey bee colonies Apis mellifera bred for hygienic behavior. Apidologie 32: 555–565. [Google Scholar]
- Spreafico M., Eördegh F. R., Bernardinelli I., Colombo M.. 2001. First detection of strains of Varroa destructor resistant to coumaphos. Results of laboratory tests and field trials. Apidologie 32: 49–55. [Google Scholar]
- (Stats Can 2015) Statistics Canada 2015. (http://www.statcan.gc.ca/daily-quotidien/151209/dq151209c-eng.htm, accessed 28 February 2017)
- Sumner D., Boriss H.. 2007. The Case of the Empty Hives. Science 316: 970–975. [DOI] [PubMed] [Google Scholar]
- (UBS 2017) Urban Bee Supplies 2017. (http://www.urbanbeesupplies.ca/packagedbeesandnucs.html, accessed 28 February 2017)
- (VBS 2017) Valley Beekeeping Supply 2017. (http://www.valleybeekeeping.com/store/categories.php?category=Nucs/Ontario-Nucs, accessed 28 February 2017)
- van der Zee R., Pisa L., Andonov S., Brodschneider R., Charrière J.-D., Chlebo R., Coffey M. F., Crailsheim K., Dahle B., Gajda A., et al. 2012. Managed honey bee colony losses in Canada, China, Europe, Israel and Turkey, for the winters of 2008-9 and 2009-10. J. Apic. Res. 51: 100–114. [Google Scholar]
- vanEngelsdorp D., Cox-Foster D., Frazier M., Ostiguy N., Hayes J.. 2007. Preliminary Report: First Revision. Harrisburg, PA, USA: Pennsylvania Department of Agriculture; 2007. Fall-dwindle Disease: Investigations into the Causes of Sudden and Alarming Colony Losses Experienced by Beekeepers in the Fall of 2006; p. 22. (http://maarec.cas.psu.edu/pressReleases/FallDwindleUpdate0107.pdf, accessed 28 February 2017)
- vanEngelsdorp D., Hayes J., Underwood R., Pettis J.. 2008. A survey of honey bee colony losses in the U.S., Fall 2007 to Spring 2008. PLoS ONE 3: e4071.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vanEngelsdorp D., Tarpy R., Lengerich E., Pettis J.. 2013. Idiopathic brood disease syndrome and queen events as precursors of colony mortality in migratory beekeeping operations in the eastern United States. Prev. Vet. Med. 108: 225–233. [DOI] [PubMed] [Google Scholar]
- Videau C., Diogon M., Aufauvre J., Fontbonne R., Vigues B., Brunet J.-L., Texier C., Biron D. G., Blot N., El Alaoui H., et al. 2011. Exposure to sublethal doses of Fipronil and Thiacloprid highly increases mortality of honey bees previously infected by Nosema ceranae. PLoS ONE 6: e21550. DOI: 10.1371/journal.pone.0021550. [DOI] [PMC free article] [PubMed] [Google Scholar]