Abstract
Background
The most appropriate management of Henoch-Schönlein Purpura (HSP) nephritis with nephrotic-range proteinuria remains uncertain. The aim of this study was to evaluate the clinical therapeutic effects of mycophenolate mofetil and low-dose steroid in Henoch-Schönlein purpura nephritis (HSPN) with nephrotic-range proteinuria and pathological classification less than IV in children.
Material/Methods
The clinical effects of MMF and low-dose steroid therapy were studied in children with Henoch-Schönlein purpura nephritis manifested with nephrotic-range proteinuria, normal kidney function, and <50% crescents or sclerosing lesions on renal biopsy. We enrolled 32 boys and 29 girls with nephrotic-range proteinuria, normal kidney function, and pathological classification less than IV on renal biopsy. We treated 41 cases (67.2%) with mycophenolate mofetil and low-dose prednisone combined therapy and 20 cases (32.8%) were treated with full-dose prednisone alone.
Results
Short-term response was significantly different between 2 groups (χ2=4.371, P=0.037), while no significant difference was found in long-term prognosis (χ2=0.419, P=0.522) after follow-up. The ROC curve showed that the most appropriate cutoff value was 30.67 μg·h/ml for MPA-AUC and the area under the ROC curve was 0.731, with 85.2% sensitivity and 64.3% specificity.
Conclusions
Mycophenolate mofetil and low-dose prednisone combined therapy is a reasonable treatment choice which can promote the remission of proteinuria without increasing obvious adverse reactions in pediatric HSPN with nephrotic state and pathological classification less than grade IV. MPA-AUC more than 30 μg·h/ml was an appropriate value for MMF in the combined therapy with MMF and steroid for treating children with HSPN.
MeSH Keywords: Child; Mycophenolic Acid; Purpura, Schoenlein-Henoch; ROC Curve
Background
Henoch-Schönlein purpura (HSP) is the most common childhood vasculitis, characterized by a palpable purpuric rash, arthritis, gastrointestinal involvement, and nephritis [1–3]. Kidney involvement occurs in 20–60% of patients, and the severity of renal involvement determines the long-term prognosis. Patients presenting with nephrotic-range proteinuria usually have the highest risk of poor long-term renal outcome [1,4,5]. However, the most appropriate management of HSP nephritis (HSPN) with nephrotic-range proteinuria remains uncertain.
Mycophenolate mofetil (MMF) is an immunosuppressant which selectively blocks T and B lymphocyte proliferation in vivo. It was previously used to prevent and treat allograft rejection due to its great treatment efficacy and limited liver and kidney toxicity [6,7]. In addition, it can reduce risk of de novo malignancies after liver transplantation [8]. Recently, the efficacy of MMF in several glomerular diseases has been confirmed by numerous reports [9,10] and it is recommended by the 2012 KDIGO guidelines. However, few studies have evaluated the value of MMF in HSPN [11–13].
In this study, the clinical effects and prognosis of MMF and low-dose steroid combined therapy were evaluated in Chinese pediatric HSPN patients with nephrotic-range proteinuria with normal kidney function and pathological classification less than grade IV.
Material and Methods
Patients
We enrolled 61 children who were hospitalized in the Department of Nephrology, the Children’s Hospital of Zhejiang University School of Medicine (Hangzhou, China) from Jan 2012 to Jan 2015. The inclusion criteria were pediatric HSPN patients with nephrotic-range proteinuria, normal kidney function, and <50% crescents or sclerosing lesions on renal biopsy. Because of the severe proteinuria, patients were given renal biopsy 1 month after the HSP nephritis was confirmed. Each patient’s family gave written informed consent for a renal biopsy and the collection of clinical data in follow-up. Renal tissue was obtained by needle biopsy under ultrasound guidance. The biopsy findings were graded according to the ISKDC classification [14]. Patients with ISKDC grade more than grade III were excluded from this study. Initial clinical data such as age, sex, renal function, albumin, white blood cell, 24-h proteinuria, and follow-up data were obtained from medical records. The creatinine clearance from the serum creatinine and height was estimated by the Schwartz formula [15].
Treatment protocol
Patients who met the inclusion criteria were divided into 2 groups: either administration of CellCept® plus low-dose prednisone (the MMF+GC group) or full-dose prednisone alone (the GC group). Patients in the MMF+GC group (n=41) received oral CellCept® at a dosage of 20–30 mg/kg a day with a course of about 1 year and the dose was not changed during the first 6 months unless the drug had intolerable adverse effects such as severe gastrointestinal reaction, drug allergy, and severe liver or kidney function damage. Prednisone at a dosage of 1 mg/kg·d (maximum daily dose 30 mg) was given for 4 weeks, after which prednisone was tapered off gradually within 6–9 months. We reduced the prednisone dose by about 5 mg/m2 every 4 weeks until the end of the study. In the GC group (n=20), the patients were treated with 2 mg/kg·d of prednisone (maximum daily dose 60 mg) alone for 4 weeks, reduced to 1 mg/kg for 4 weeks, and then tapered off gradually within 6–9 months. Patients in both groups also received ACEI and supportive therapies.
A 3-point abbreviated pharmacokinetic profile for MPA exposure was performed 1 month after MMF therapy, based on MPA plasma concentrations before oral intake of MMF (C0) and 30 min (C0.5) and 2 h (C2) thereafter. The MPA-AUC0–12h was calculated using the formula: MPA-AUC0–12h=7.75+(6.49*C0)+(0.76*C0.5)+(2.43*C2) [16,17].
Definitions for clinical outcomes
The patients were followed up (median: 23 months, range: 12~44 months). Short-term (6 months) and long-term outcomes were evaluated. Clinical outcome was graded as A, B, C, and D according to Meadow’s criteria [18]. Category A was favorable outcomes, while categories B, C, and D were unfavorable outcomes.
Statistical analysis
The data were analyzed using SPSS 17.0 (SPSS Inc., Chicago, IL). Continuous data are expressed as means ± standard deviation (χ̄±s), and the means between the groups were generated for comparison using the t test. Categorical data were analyzed using the χ2 test. MPA-AUC was plotted on an ROC curve. The area under the ROC was calculated to classify the MPA-AUC as either normal (proteinuria remission) or malignant (proteinuria persistent) according to the result of 6-month clinical observation. The most appropriate cutoff values were established as the ones with higher result of the sum of sensitivity and specificity. Survival analysis was performed using the Kaplan-Meier method. Survival curves were compared using the log-rank test. A P value less than 0.05 was considered as statistically significant.
Results
Baseline patient characteristics
The clinical characteristics of the 61 patients are shown in Table 1. There were 32 boys and 29 girls. The age at disease onset ranged from 3 to 15 years. There were 41 cases (67.2%) treated with MMF and low-dose prednisone (MMF+GC group) and 20 cases (32.8%) with full dose prednisone alone (GC group). No significantly difference were found in initial clinical data such as 24-h proteinuria, serum albumin, white blood cell, hemoglobin, platelet, serum creatinine, and eGFR in patients in the 2 groups. While all patients had skin lesions, gastrointestinal involvement and arthritis were determined in 54.1% and 60.7%, respectively. Extra-renal manifestations at admission were not significantly different between the 2 groups.
Table 1.
Baseline clinical characteristics and pathological characteristics of the patients in the MMF and control groups.
| Characteristics | Group | P-value | |
|---|---|---|---|
| MMF+GC | GC | ||
| Cases, n | 41 | 20 | |
| Male/female, n | 23/18 | 9/11 | 0.415 |
| Age (year) | 7.61±2.87 | 7.79±2.88 | 0.82 |
| Time from discovery to biopsy (days) | 21.29±7.37 | 17.60±8.29 | 0.083 |
| 24 hr proteinuria (mg/m2) | 96.69±50.12 | 82.15±29.36 | 0.236 |
| Serum albumin (g/L) | 36.45±5.41 | 37.98±4.44 | 0.278 |
| Serum creatinine (μmol/L) | 44.00±10.09 | 45.47±11.37 | 0.610 |
| White blood cell (×109 L−1) | 10.30±3.98 | 8.83±2.16 | 0.126 |
| Hemoglobin (g/L) | 129.05±10.54 | 124.15±12.44 | 0.114 |
| Platelet (×109 L−1) | 345.71±96.35 | 335.70±79.25 | 0.689 |
| eGFR (ml/min/1.73 m2) | 146.87±32.73 | 139.11±47.53 | 0.458 |
| IgA (mg/dl) | 2.03±0.57 | 1.94±0.42 | 0.538 |
| Extra renal manifestations at admission, n (%) | |||
| Palpable purpura | 41/41 | 20/20 | 1 |
| Gastrointestinal involvement | 21/41 (51.2%) | 12/20 (60%) | 0.518 |
| Arthritis | 24/41 (58.5%) | 13/20 (65%) | 0.628 |
| Follow-up time (month), median (range) | 23.29±6.25 | 27.1±10.13 | 0.075 |
Pathological characteristics of the patients
Pathological characteristics of renal biopsy in the 2 groups are shown in Table 2. Grade III was the most common histological type (33/61, 54.1%). Moderate mesangial proliferation (37/61, 60.7%) and mild Interstitial infiltrates (56/61, 91.8%) were the most common renal pathological lesions. No significant differences in the pathological characteristics between the 2 groups were found.
Table 2.
Initial pathological characteristics of renal biopsy.
| Characteristics | Group | P-value | |
|---|---|---|---|
| MMF+GC | GC | ||
| Cases, n | 41 | 20 | |
| Pathological type (ISKDC), n | |||
| I | 1 | 1 | 0.933 |
| II | 17 | 9 | |
| IIIa | 10 | 4 | |
| IIIb | 13 | 6 | |
| Proportion of glomeruli with (%) | |||
| Crescent | 23/41 (56.1%) | 10/20 (50%) | 0.654 |
| Global sclerosis | 2/41 (4.9%) | 1/20 (5%) | 0.984 |
| Mesangial proliferation, n | |||
| Mild | 10 | 9 | 0.251 |
| Moderate | 27 | 10 | |
| Severe | 4 | 1 | |
| Glomerular segmental necrosis, n | 1 | 2 | 0.248 |
| Interstitial infiltrates, n | |||
| Absent | 2 | 2 | 0.728 |
| Mild (≤25%) | 38 | 18 | |
| Moderate (25–50%) | 1 | 0 | |
Short-term response to therapy and long-term follow-up
The short-term and long-term outcomes in both groups (prednisone with or without mycophenolate mofetil therapy) are listed in Table 3. Significant differences were found in short-term response between the 2 groups (χ2=4.371, P=0.037), but no significant differences were found in long-term follow-up (χ2=0.419, P=0.522).
Table 3.
Comparison of short- and long-term outcome in two groups with moderately severe Henoch- Schönlein purpura nephritis.
| Group | n | Short-term outcome (6 months) | Long-term outcome (median: 23 months, min ≥12 months) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | A | B | C | D | ||
| MMF+GC | 41 | 26 | 13 | 1 | 1 | 28 | 11 | 1 | 1 |
| GC | 20 | 7 | 10 | 2 | 1 | 12 | 6 | 2 | 0 |
| Total | 61 | 36 | 20 | 3 | 2 | 40 | 17 | 3 | 1 |
Chi-square test showed significant difference on short-term outcome in two groups (χ2=4.371, P=0.037) while no statistic difference were found on long-term prognosis (χ2=0.419, P=0.522).
The description of MPA-AUC and ROC curve analysis
In the MMF+GC group, the MPA-AUC was 34.53±8.86μg·h/mL, ranging from 19.04 to 63.74 μg·h/mL and the short-term follow-up showed 26 of 41 children (63.4%) were in remission. The predictive value of the MPA-AUC for remission on the short-term follow-up was assessed by ROC curve analysis, which showed the most appropriate cutoff was 30.67 μg·h/ml for MPA-AUC (Figure 1). At the cutoff value of 30.67 μg·h/ml, the area under ROC curve was 0.731, with 85.2% sensitivity and 64.3% specificity. Comparison of short-term outcome with MPA-AUC <30.67 μg·h/ml and ≥30.67 μg·h/ml revealed a significant difference (χ2=8.744, P=0.03).
Figure 1.
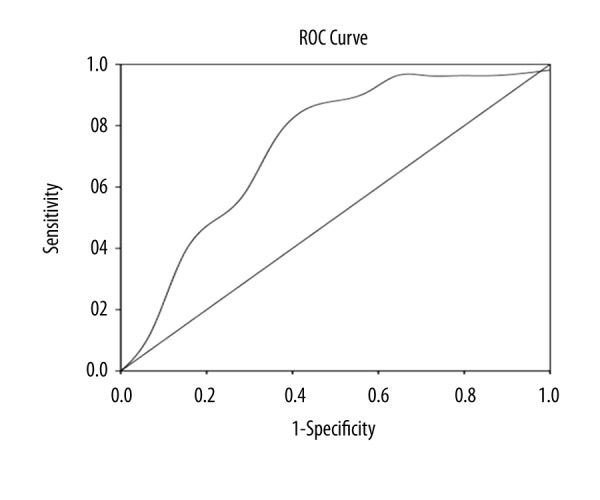
The ROC curve of MPA-AUC for short-term outcomes of MMF and GC treatment in children with HSPN. The predictive value of MPA-AUC in short-term outcomes of MMF and GC treatment in HSPN children were evaluated by receiver operating characteristics curve. The area under the ROC curve of MPA-AUC was 0.731 with the optimal cut-off value of μg·h/ml (sensitivity: 85.2%; specificity: 64.3%).
Survival curve analysis
Difference in long-term prognosis (the remission of proteinuria) between the 2 groups was analyzed by Kaplan-Meier curves; however, no significant difference was found (χ2=1.112, P=0.292) (Figure 2).
Figure 2.
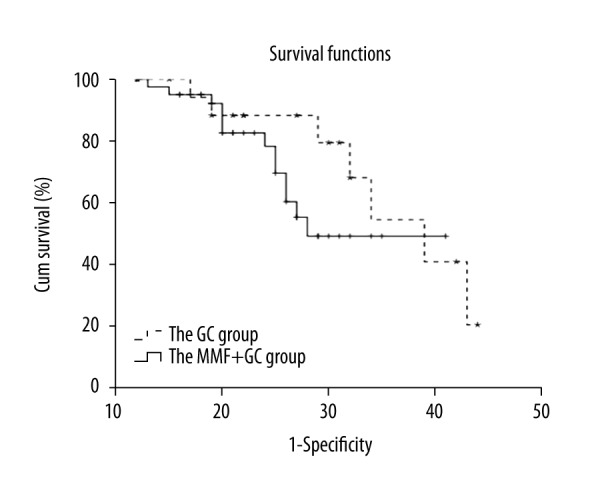
Survival analysis of GC group and MMF+GC group by Kaplan-Meier plot. The children were treated with GC or MMF+GC and followed up. Clinical outcome was graded according to Meadow’s criteria. Grade A was considered as good outcome while Grade B–D were considered as bad outcomes. Kaplan-Meier plot and log-rank test were used to compare clinical outcomes between the 2 treatment groups.
Adverse effects and extra-renal manifestations during follow-up
The adverse effects of prednisone or prednisone plus MMF are listed in Table 4. Hypertension was defined as an average systolic or diastolic blood pressure >95th percentile for age, sex, and height on at least 3 separate occasions. No significant differences were found between the 2 groups.
Table 4.
Repo rts of side effects in children with moderately severe Henoch-Schönlein purpura nephritis in two groups.
| MMF+GC | GC | P-value | |
|---|---|---|---|
| Number | 41 | 20 | |
| Adverse events (%) | 13 (31.7%) | 7 (35%) | 0.509 |
| Infection | 9 | 6 | |
| Urinary tract | 3 | 1 | |
| Bronchitis | 4 | 3 | |
| Encephalitis | 0 | 1 | |
| Herpes zoster | 2 | 0 | |
| Verruca vulgaris | 0 | 1 | |
| Abnormal liver function | 2 | 0 | |
| Hypertension | 1 | 1 | |
| Leukopenia | 1 | 0 |
During the follow-up, the extra-renal manifestations in the MMF+GC group included 3 episodes of repeating purpura, 1 of arthritis, and 2 of stomach ache. In the GC group, 1 case with repeating purpura and 1 with arthritis were found. The results suggested that no significant differences existed between the 2 groups (χ2=0.253, P=0.615).
Discussion
The degree of renal involvement is an important indicator for the prognosis of HSP, and nephrotic-range proteinuria is regarded as a risk factor for poor long-term renal outcomes. Glucocorticoid (GC) is the fundamental therapy for HSP with severe complications, including severe abdominal pain, arthralgia, or central nervous system involvement. Although GC is widely used in children presenting with nephrotic-range proteinuria or acute nephritis, there are very limited data to support the use of GC in children with established nephritis of any severity [19].
The role of immunosuppressive agents in the treatment of HSPN is uncertain. The treatment of severe HSPN with methylprednisolone and urokinase pulse therapy [20], cyclophosphamide [21,22], cyclosporine A [23], azathioprine, and other medications have been described in previous studies. However, these reports are either single-case studies or small case series, and the limited data make it unclear whether these have any role in HSP nephritis.
Mycophenolate mofetil is a common and effective immunosuppressant previously used to prevent and treat allograft rejection [6]. Recently, the efficacy of MMF in several kidney disease, such as primary nephrotic syndrome [16,24], focal segmental glomerulosclerosis, and lupus nephritis, had been confirmed by numerous reports [9,10]. In HSP nephritis, mycophenolate mofetil was considered to be a promising therapeutic agent in patients unresponsive to steroids [25,26] or severe HSP nephritis with crescent [27,28]. A single-center, retrospective analysis of 12 children with HSPN with nephrotic-range proteinuria treated with MMF [12] revealed all patients had negative proteinuria and normal renal function in the 14-month follow-up, and no relapses or serious adverse effects were noted. In the present study, we evaluated the curative effect and outcome of MMF with low-dose steroid on pediatric HSPN patients manifesting nephrotic-range proteinuria and pathological classification less than grade IV, and found that MMF plus low-dose prednisone can accelerate the remission of proteinuria in short-term observation, although no differences in long-term outcome were found. Although we found no significant differences between the 2 groups in adverse effects during the follow-up, the effect of using a smaller dose of corticosteroid on the growth and development of children cannot be ignored, which may not have shown up in the relatively short follow-up period in our study.
In addition, the duration of the nephrotic states is related to long-term outcomes in HSP patients with nephrosis. Hitoshi [5] retrospectively studied 42 children with HSPN who presented with a nephrotic state during the early phase of the disease for 2.8–14.2 years. Among 22 patients with a nephrotic duration >3 months, 13 had unfavorable outcomes (3 grade C and 10 grade D outcomes), and multivariate logistic regression analyses revealed that a nephrotic state of >3 months was the only independent predictor of long-term outcomes, which was also supported by Coppo’s study [4]. Similar results in studies of IgA nephropathy also support this notion [29,30]. Achieving remission of proteinuria as soon as possible may provide maximum protection of renal function and improve the prognosis. We did not find differences in long-term outcomes between the 2 groups in this study, which may be related to the limited follow-up time and small sample size.
MMF is quickly absorbed in the gastrointestinal tract and metabolized to MPA, which has a key biochemical function in vivo. MPA-AUC0–12h is considered the criterion standard for monitoring of MPA, which is a reflection of exposure to the drug over the entire dosing period. Although it is generally agreed [31–33] that MPA-AUC should be controlled at 30–60 μg·h/mL in an organ transplant patient, the appropriate range of MPA-AUC0–12h in pediatric glomerular disease is still controversial. Few studies have reported on MMF exposure in children with nephrotic syndrome. A study on 24 children with nephrotic syndrome showed that the upper boundary of MPA-AUC0–12h should be more than 60 ug.h/mL to ensure safe and effective treatment [34]. Another study on children with frequently relapsing nephrotic syndrome [16] revealed that patients with low MPA-AUC (<50 μg.h/ml) experienced 1.4 relapses per year compared with 0.27 relapses per year in those with high exposure (MPA-AUC>50 μg.h/ml; P<0.05). However, the appropriate range of MPA-AUC0–12h in HSPN had not been determined. Our study revealed that MPA-AUC more than 30.67 μg·h/ml was a reasonable cutoff value for treating children with HSPN. The distributions of short-term outcomes with MPA-AUC less than or more than 30 μg·h/ml are shown in Table 5. There was a significant difference between the 2 groups (χ2=8.744, P=0.03).
Table 5.
Comparison short-term outcome with different MPA-AUC range.
| Group | n | Short-term outcome (6 months) | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| MPA-AUC <30.67 μg·h/ml | 13 | 4 | 8 | 1 | 0 |
| MPA-AUC ≥30.67 μg·h/ml | 28 | 22 | 5 | 0 | 1 |
| Total | 41 | 26 | 13 | 1 | 1 |
χ2=8.744, P=0.03.
Previous studies have found that glucocorticoid was not effective for purpura in HSP. A few cases reports showed that MMF may be an acceptable treatment for skin rash or gastrointestinal symptoms. In the long-term follow-up of the present study, we did not find a difference in extra-renal manifestations between the 2 groups, regardless of the small sample size.
The present study has several limitations. First, because the drugs for treatment were selected by the patients and/or their parents, the possibility of selection bias cannot be ignored. The patients we observed were all Asians, and the effect of MMF may vary in different populations. In addition, the sample size was small and the time of follow-up was limited. Further studies with larger sample sizes and longer follow-up may draw more reliable conclusions.
Conclusions
In summary, MMF is an immunosuppressor that can be used in treating the nephrotic state and pathological classification less than grade IV for HSPN patients. It can help to reduce the dosage of glucocorticoid and promote the remission of proteinuria without increasing obvious adverse reactions in short-term follow-up. MPA-AUC more than 30 μg·h/ml is a reasonable value in the combined therapy with MMF and steroid for treating children with HSPN.
Acknowledgements
We thank all patients and their family members for participating in this study.
Abbreviations
- ACEI
angiotensin-converting enzyme inhibitors
- AUC
area under curve
- GC
glucocorticoid
- HSP
Henoch-Schönlein purpura
- HSPN
Henoch-Schönlein purpura nephritis
- MMF
mycophenolate mofetil
Footnotes
Conflict of Interests
The authors declare no conflict of interest.
Source of support: This study was supported by the National Natural Foundation of China (81470939, 81270792), the Specialized Research Fund for the Doctoral Program of Higher Education (20120101110018), the Science Foundation of Zhejiang Educational Committee (Y201636381), and the Natural Science Foundation of Zhejiang Province (LH14H050002, LY15H050001)
References
- 1.Lau KK, Suzuki H, Novak J, Wyatt RJ. Pathogenesis of Henoch-Schonlein purpura nephritis. Pediatr Nephrol. 2010;25:19–26. doi: 10.1007/s00467-009-1230-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang C, Scaramangas-Plumley D, Nast CC, et al. A Case of Henoch-Schonlein purpura associated with rotavirus infection in an elderly Asian male and review of the literature. Am J Case Rep. 2017;18:136–42. doi: 10.12659/AJCR.901978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gulati G, Siv J, Ware AE. Bullous skin lesions in an adult male: A diagnostic dilemma. Am J Case Rep. 2015;16:215–19. doi: 10.12659/AJCR.893218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coppo R, Andrulli S, Amore A, et al. Predictors of outcome in Henoch-Schonlein nephritis in children and adults. Am J Kidney Dis. 2006;47:993–1003. doi: 10.1053/j.ajkd.2006.02.178. [DOI] [PubMed] [Google Scholar]
- 5.Wakaki H, Ishikura K, Hataya H, et al. Henoch-Schonlein purpura nephritis with nephrotic state in children: predictors of poor outcomes. Pediatr Nephrol. 2011;26:921–25. doi: 10.1007/s00467-011-1827-8. [DOI] [PubMed] [Google Scholar]
- 6.Allison AC. Mechanisms of action of mycophenolate mofetil. Lupus. 2005;14(Suppl 1):s2–8. doi: 10.1191/0961203305lu2109oa. [DOI] [PubMed] [Google Scholar]
- 7.Zegarska J, Hryniewiecka E, Zochowska D, et al. Mycophenolic acid metabolites acyl-glucuronide and glucoside affect the occurrence of infectious complications and bone marrow dysfunction in liver transplant recipients. Ann Transplant. 2005;20:483–92. doi: 10.12659/AOT.894954. [DOI] [PubMed] [Google Scholar]
- 8.Aguiar D, Martinez-Urbistondo D, D’Avola D, et al. Conversion from calcineurin inhibitor-based immunosuppression to mycophenolate mofetil in monotherapy reduces risk of de novo malignancies after liver transplantation. Ann Transplant. 2017;22:141–47. doi: 10.12659/aot.901556. [DOI] [PubMed] [Google Scholar]
- 9.Baudouin V, Alberti C, Lapeyraque AL, et al. Mycophenolate mofetil for steroid-dependent nephrotic syndrome: A phase II Bayesian trial. Pediatr Nephrol. 2012;27:389–96. doi: 10.1007/s00467-011-2006-7. [DOI] [PubMed] [Google Scholar]
- 10.van Husen M, Kemper MJ. New therapies in steroid-sensitive and steroid-resistant idiopathic nephrotic syndrome. Pediatr Nephrol. 2011;26:881–92. doi: 10.1007/s00467-010-1717-5. [DOI] [PubMed] [Google Scholar]
- 11.Han F, Chen LL, Ren PP, et al. Mycophenolate mofetil plus prednisone for inducing remission of Henoch-Schonlein purpura nephritis: A retrospective study. J Zhejiang Univ Sci B. 2015;16:772–79. doi: 10.1631/jzus.B1400335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du Y, Hou L, Zhao C, et al. Treatment of children with Henoch-Schonlein purpura nephritis with mycophenolate mofetil. Pediat Nephrol. 2012;27:765–71. doi: 10.1007/s00467-011-2057-9. [DOI] [PubMed] [Google Scholar]
- 13.Ren P, Han F, Chen L, et al. The combination of mycophenolate mofetil with corticosteroids induces remission of Henoch-Schonlein purpura nephritis. Am J Nephrol. 2012;36:271–77. doi: 10.1159/000341914. [DOI] [PubMed] [Google Scholar]
- 14.Counahan R, Winterborn MH, White RH, et al. Prognosis of Henoch-Schonlein nephritis in children. Br Med J. 1977;2:11–14. doi: 10.1136/bmj.2.6078.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–63. [PubMed] [Google Scholar]
- 16.Gellermann J, Weber L, Pape L, et al. Mycophenolate mofetil versus cyclosporin A in children with frequently relapsing nephrotic syndrome. J Am Soc Nephrol. 2013;24:1689–97. doi: 10.1681/ASN.2012121200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pawinski T, Hale M, Korecka M, et al. Limited sampling strategy for the estimation of mycophenolic acid area under the curve in adult renal transplant patients treated with concomitant tacrolimus. Clin Chem. 2002;48:1497–504. [PubMed] [Google Scholar]
- 18.Mir S, Yavascan O, Mutlubas F, et al. Clinical outcome in children with Henoch-Schonlein nephritis. Pediatr Nephrol. 2007;22:64–70. doi: 10.1007/s00467-006-0278-0. [DOI] [PubMed] [Google Scholar]
- 19.Zaffanello M, Fanos V. Treatment-based literature of Henoch-Schonlein purpura nephritis in childhood. Pediatr Nephrol. 2009;24:1901–11. doi: 10.1007/s00467-008-1066-9. [DOI] [PubMed] [Google Scholar]
- 20.Kawasaki Y, Suzuki J, Nozawa R, et al. Efficacy of methylprednisolone and urokinase pulse therapy for severe Henoch-Schonlein nephritis. Pediatrics. 2003;111:785–89. doi: 10.1542/peds.111.4.785. [DOI] [PubMed] [Google Scholar]
- 21.Tarshish P, Bernstein J, Edelmann CM., Jr Henoch-Schonlein purpura nephritis: Course of disease and efficacy of cyclophosphamide. Pediatr Nephrol. 2004;19:51–56. doi: 10.1007/s00467-003-1315-x. [DOI] [PubMed] [Google Scholar]
- 22.Kawasaki Y, Suzuki J, Suzuki H. Efficacy of methylprednisolone and urokinase pulse therapy combined with or without cyclophosphamide in severe Henoch-Schoenlein nephritis: A clinical and histopathological study. Nephrol Dial Transplant. 2004;19:858–64. doi: 10.1093/ndt/gfg617. [DOI] [PubMed] [Google Scholar]
- 23.Ronkainen J, Autio-Harmainen H, Nuutinen M. Cyclosporin A for the treatment of severe Henoch-Schonlein glomerulonephritis. Pediatr Nephrol. 2003;18:1138–42. doi: 10.1007/s00467-003-1245-7. [DOI] [PubMed] [Google Scholar]
- 24.Kang Z, Li Z, Duan C, et al. Mycophenolate mofetil therapy for steroid-resistant IgA nephropathy with the nephrotic syndrome in children. Pediatr Nephrol. 2015;30:1121–29. doi: 10.1007/s00467-014-3041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikibakhsh AA, Mahmoodzadeh H, Karamyyar M, et al. Treatment of severe henoch-schonlein purpura nephritis with mycophenolate mofetil. Saudi J Kidney Dis. 2014;25:858–63. doi: 10.4103/1319-2442.135182. [DOI] [PubMed] [Google Scholar]
- 26.Nikibakhsh AA, Mahmoodzadeh H, Karamyyar M, et al. Treatment of complicated henoch-schonlein purpura with mycophenolate mofetil: A retrospective case series report. Int J Rheumatol. 2010;2010:254316. doi: 10.1155/2010/254316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dede F, Onec B, Ayli D, et al. Mycophenolate mofetil treatment of crescentic Henoch-Schonlein nephritis with IgA depositions. Scand J Urology Nephrol. 2008;42:178–80. doi: 10.1080/00365590701571514. [DOI] [PubMed] [Google Scholar]
- 28.Muzaffar M, Taj A, Sethi N, Kaw D. Rapidly progressing glomerulonephritis secondary to henoch-schonlein purpura treated with mycophenolate mofetil: A case report with atypical etiology and presentation. Am J Ther. 2010;17:e163–66. doi: 10.1097/MJT.0b013e3181b0a713. [DOI] [PubMed] [Google Scholar]
- 29.Pozzi C, Andrulli S, Del Vecchio L, et al. Corticosteroid effectiveness in IgA nephropathy: Long-term results of a randomized, controlled trial. J Am Soc Nephrol. 2004;15:157–63. doi: 10.1097/01.asn.0000103869.08096.4f. [DOI] [PubMed] [Google Scholar]
- 30.Bartosik LP, Lajoie G, Sugar L, Cattran DC. Predicting progression in IgA nephropathy. Am J Kidney Dis. 2001;38:728–35. doi: 10.1053/ajkd.2001.27689. [DOI] [PubMed] [Google Scholar]
- 31.van Gelder T, Le Meur Y, Shaw LM, et al. Therapeutic drug monitoring of mycophenolate mofetil in transplantation. Ther Drug Monit. 2006;28:145–54. doi: 10.1097/01.ftd.0000199358.80013.bd. [DOI] [PubMed] [Google Scholar]
- 32.Shaw LM, Kaplan B, DeNofrio D, et al. Pharmacokinetics and concentration-control investigations of mycophenolic acid in adults after transplantation. Ther Drug Monit. 2000;22:14–19. doi: 10.1097/00007691-200002000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Mathew BS, Fleming DH, Annapandian VM, et al. A reliable limited sampling strategy for the estimation of mycophenolic acid area under the concentration time curve in adult renal transplant patients in the stable posttransplant period. Ther Drug Monit. 2010;32:136–40. doi: 10.1097/FTD.0b013e3181cd550f. [DOI] [PubMed] [Google Scholar]
- 34.Sobiak J, Resztak M, Ostalska-Nowicka D, et al. Monitoring of mycophenolate mofetil metabolites in children with nephrotic syndrome and the proposed novel target values of pharmacokinetic parameters. Eur J Pharm Sci. 2015;77:189–96. doi: 10.1016/j.ejps.2015.06.017. [DOI] [PubMed] [Google Scholar]


